Abstract
Traditional Chinese medicines (TCM) contain multi-interactive compounds that have been used for treatment of peri-menopausal syndrome and have become a new phytoestrogens resource. The QiBaoMeiRan formula (QBMR), including Polygoni Multiflori Radix, Angelicae Sinensis Radix, Achyranthis Bidentatae Radix, Semen Cuscutae, Fructus Lycii, Poria, and Fructus Psoraleae, has been used clinically for treating osteoporosis in post-menopausal women by virtue of its kidney-invigorating function. However, no evidence base links QBMR to estrogen replacement therapy. In this study, we undertook a characterization of estrogenic activity of QBMR using ovariectomized (OVX) rats. OVX rats were treated with QBMR at doses of 0.875, 1.75, and 3.5 grams/kg per day for 8 weeks. QBMR treatments demonstrated significant estrogenic activity, as indicated by vaginal cornification, reversal of atrophy of uterus, vagina, and mammary gland, and up-regulation of estrogen receptor α (ERα) and estrogen receptor β (ERβ) expression in the reproductive target tissues, where ERβ up-regulation was stronger than that of ERα. Meanwhile, treatment with QBMR significantly increased adrenal weight and serum estradiol levels and tended to decrease serum follicle-stimulating hormone (FSH) and luteinizing hormone (LH) levels in a dose-dependent manner. Moreover, QBMR significantly decreased weight gain and rectal temperature increase caused by ovariectomy, and the largest changes in rectal temperature were found at the lowest dose. The data suggest that QBMR's estrogenic responses show tissue variation that reflects different affinities of ERs for QBMR components. This study demonstrates that QBMR activity is mediated through estrogenic components and provides an evidence base for QBMR treatment of post-menopausal symptoms.
Introduction
Estrogen is a key female regulatory hormone related to multiple physiological processes. Estrogen deficiency is known to cause many physical disorders in post-menopausal women, including short-term symptoms such as hot flushes, night sweats, palpitations, irritability, and anxiety. Chronic conditions include osteoporosis, cardiovascular disease, colon cancer, and dementia.1 Hormone replacement therapy (HRT) has often been prescribed to peri- and post-menopausal women to combat the somatic and cognitive symptoms of menopause. However, HRT is thought to increase the risk of breast cancer and cause other undesirable side effects, including breast tenderness and uterine bleeding.2,3 Therefore, post-menopausal women have tended to use phytoestrogens, such as natural isoflavonoids from herbal medicines, as alternatives.4 Phytoestrogens are similar both structurally and functionally to mammalian estrogens, but with reportedly lower side effects compared with synthetic HRT.5,6 Traditional Chinese medicines (TCM) containing multi-interactive compounds, which have been used for centuries in China for treatment of peri-menopausal syndrome, have attracted the attention of researchers regarding a TCM formula as a new phytoestrogens resource. Estrogen decline in older people and in post-menopausal women has been explained as a kidney deficiency in the TCM theory and can be treated or protected by using kidney-invigorating agents.7,8 QiBaoMeiRan formula (QBMR) is recorded in the Chinese Pharmacopeia of 2010,9 and has been used in traditional Chinese medicine since the Ming Dynasty (14th century CE) because of its functions of invigorating the kidney and replenishing bone. Today, QBMR with clear ingredients is commonly used clinically for treatment of osteoporosis, particularly in post-menopausal woman,10 but its mechanism of action remains uncharacterized, in particular its link to estrogen replacement therapy. In this study, we describe estrogenic effects of QBMR using ovariectomized (OVX) rats as models by observing estrous cycle, rectal temperature, reproductive hormone levels, measuring the histologic structure changes, and estrogen receptor α (ERα) and estrogen receptor beta (ERβ) expression in reproductive target tissues, as part of an ongoing effort to identify novel and potent agents for the prevention and treatment of post-menopausal syndromes.
Materials and Methods
Herbal preparation
QBMR was prepared as described in the Chinese Pharmacopeia of 2010. Briefly, the seven ingredients, Polygoni Multiflori Radix (128 grams), Angelicae Sinensis Radix (32 grams), Achyranthis Bidentatae Radix (32 grams), Semen Cuscutae (32 grams), Fructus Lycii (32 grams), Poria (32 grams), and Fructus Psoraleae (16 grams), were pulverized to a fine powder, suspended in distilled water to a concentration of 0.35 gram/mL, and mixed well before administration. The representative chemical compositions of stilbene glucoside (0.059%), emodin (0.0031%), physcion (0.002%), β-ecdysone (0.00048%), ferulic acid (0.00044%), quercetin (0.0026%), psoralen (0.0017%), isopsoralen (0.0014%), and goji polysaccharides (0.03%) in QBMR were determined by high-performance liquid chromatography (HPLC) analysis.
Animals and treatment
Ten-week-old female Sprague–Dawley rats (Experimental Animal Center of Academy of Military Medical Sciences, PR China) maintained normal 5-day estrous cycles as confirmed by daily vaginal epithelial cell smear testing until ovariectomy was performed. Dorsal ovariectomy was performed under general anesthesia using 0.3 mg/kg of chloral hydrate. All OVX rats were checked by daily vaginal epithelial cell smear analysis, in which 5 consecutive days of leukocytes were indicative of constant diestrus and successful ovariectomy. In sham-operated negative controls, fat near the ovary was removed. The rats were randomly assigned to six groups: OVX without treatment (OVX, n=10), sham operated (sham, n=10), OVX rats treated with 0.11 mg/kg estradiol valerate (EV, n=10), and OVX rats treated with QBMR intra-gastrically at a daily dose of 0.875 gram/kg, 1.75 grams/kg, or 3.5 grams/kg for 6 days per week for 8 weeks. Dose calculations followed guidelines correlating dose equivalents between humans and laboratory animals, on the basis of ratios of body surface area.11 Untreated control OVX rats and sham-operated rats received distilled water only. All animals were maintained on a 12-hr light/dark cycle under constant temperature (24±2°C) and humidity (55±5%) and allowed free access to food and water. All procedures for consideration of animal welfare were reviewed and approved by China Academy of Traditional Chinese Medicine.
Monitoring vaginal cornification, rectal temperature, and body weight
Rats were monitored by vaginal epithelial cells smear testing after every administration during the 8-week period. The vaginal lavage was fixed with 95% ethanol for 10 min and stained with Methylene Blue for 10 min. Vaginal epidermal cells were observed by microscopy, and keratinized vaginal cells were taken as being indicative of estrus.12 Rectal temperature was monitored using an electrothermometer at 2 hr after daily administration, and body weight was detected by animal body weight scale every week.
Analysis of tissue and serum
Animals were sacrificed by decapitation after 8 weeks of treatment. In the sham-operated group, two rats were in the diestrus stage and eight rats were in the estrus stage. Blood was collected from the abdominal aorta for analysis of estradiol (E2), follicle-stimulating hormone (FSH), and luteinizing hormone (LH) levels by enzyme-linked immunosorbent assay (ELISA) (Beijing Xinfangcheng Biotechnology, China). The sensitivities of the three ELISA assays were 1.0 pg/mL, 1.0 mIU/mL, and 1.0 ng/mL, respectively, and the antibody did not cross-react with other estrogen-like substances. All of the intra-assay and inter-assay variations of each hormonal assay were less than 9% and 15%, respectively. The uterus and adrenal gland were removed and weighed. The left horns of the uterus, the vagina, and the mammary gland were stored at −80°C and the right horns of the uterus, the rest of vagina, and the mammary gland were fixed with 4% polyoxymethylene for 24 hr. All samples were embedded in paraffin and prepared for cross sections. Sections 4 mm thick were cut, mounted, and stained with Hematoxylin & Eosin (H&E) for microscopy. The observation of uterine endometrium thickness, number of uterine glands, vaginal epithelial layer, and thickness of the mammary gland epithelium was performed on a selected single slide in each animal.
Immunohistochemistry
Tissue sections 4 mm thick of uterus, vagina, and mammary gland were mounted on polylysine-coated slides. The paraffin sections were de-waxed by routine method and incubated for 10 min with 3% hydrogen peroxide (H2O2). Each section was incubated with blocking serum (Vectastain ABC Kit) at room temperature for 30 min and then with primary rabbit anti-ERα antibody (dilution 1/30, ab37438, Abcam Biotechnology, UK) and a rabbit anti-ERβ (dilution 1/30, ab3577, Abcam Biotechnology, UK), respectively, overnight at 4°C. Sections incubated in phosphate-buffered saline (PBS) without antibody served as negative controls. After incubation with biotinylated secondary antibody, sections were incubated with avidin–biotin complex reagent containing horseradish peroxidase for 30 min. The sections were then stained with 3,3′-diaminobenzidine (DAB) (Sigma).13 The Image-Pro Plus 6.0 System image analysis system was used for quantitative analysis.
Western blot
Uterus, vagina, and mammary gland were re-suspended in lysis buffer (50 mM Tris, pH 8.0, 150 mM NaCl, 5 mM EDTA, 0.1% sodium dodecyl sulfate [SDS], 0.5% NP-40) containing 10 mM phenylmethylsulfonyl fluoride (PMSF) and 2 mg/mL aprotinin. The protein was obtained to detect the levels of ERα and ERβ in target tissue by western blotting. The western blot protocol and semi-quantitative analysis were carried out as described.14 The antibody of rabbit anti-ERα polyclonal antibody (dilution 1/100, ab37438, Abcam Biotechnology, UK) or mouse anti-ERβ monoclonal antibody (dilution 1/1000, ab37438, Abcam Biotechnology, UK) was used. All experiments were done in triplicate. The relative quantity of each antibody was measured by Alpha Ease FC (Fluorchem FC2) software. The density ratio of protein to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was calculated from the band density.
Real-time quantitative PCR
After treatments, total RNA of uterus, vagina, and mammary gland was extracted with TRIzol reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. The total RNA (2 μg) was reverse transcribed to cDNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems Foster City, CA) according to the manufacturer's manual. The specific transcripts were quantified by quantitative real-time PCR using a Quanti Tect SYBR Green PCR Kit (QIAGEN K.K., Tokyo, Japan) and analyzed with an ABI 7500 real-time PCR system (Applied Biosystems, USA). Gene-specific primers were designed by DNAMAN 7.0 and used for ERα (amplified size, 124 bp; forward, GAGGCACACAAACTCTTCTCC; reverse, ACTTGCT CTTGGACAGGAATCA), ERβ (amplified size, 106 bp; forward, GCCCTTGTTACTGATGTGC C; reverse, GGTCTGGG TGATTGCGAAGA), and β-actin (amplified size, 239 bp; forward, GAGCCACCAATCCACACAGA; reverse, AGACCTCTATGCCAAC ACAGT). The mRNA levels of ERα and ERβ were normalized to β-actin mRNA levels. PCR was performed as 95°C for 10 min, followed by 40 cycles of 95°C for 30 sec, and 60°C for 1 min. The quantification data were analyzed with ABI Prism analysis software. The relative mRNA expression was calculated with the comparative cycle threshold (Ct) method.15
Statistics analysis
The software of SPSS version 11.0 for Windows (SPSS Inc, Chicago, IL) was used for statistical analysis. All data were expressed as mean±standard deviation and were analyzed by one-way analysis of variance (ANOVA) followed by least significant difference (LSD) or the Dunnett T3 test. Differences were considered statistically significant when p was less than 0.05.
Results
Vaginal cornification
To characterize the estrogenic activity of QBMR, we treated OVX rats with QBMR and compared the activity with the synthetic estrogen EV. The estrous cycle of all rats was monitored by inspection of vaginal epithelial cell smears. As shown in Fig. 1, smears of the vaginal epithelial cells from OVX rats consisted of leukocytes, which are indicative of constant diestrus (Fig. 1a). In contrast, the vaginal cells from the OVX rats treated with EV or QBMR (0.875, 1.75, or 3.5 grams/kg) became keratinized after treatment for about 10 days, indicating that the status of estrus in the OVX rats was restored (Fig. 1b, c). All of the sham-operated rats had an estrous cycle in general (not shown).
FIG. 1.
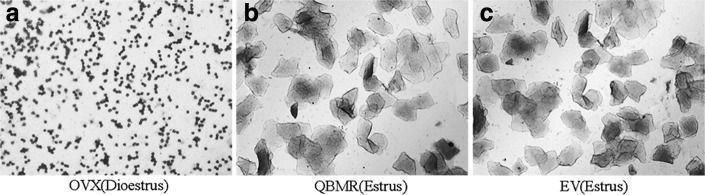
The effects of QiBaoMeiRan formula (QBMR) on the estrous cycle. Vaginal epithelial cell smears were taken at the seventh day from an ovariectomized (OVX) rat untreated (a), a rat treated with QBMR (b), and a rat treated with estradiol valerate (EV) (c). Smears from the untreated OVX rat consisted of leukocytes, confirming a state of diestrus. Smears from the OVX rat treated with QBMR or EV showed a large number of keratinized epithelial cells, indicating that both QBMR and EV can restore the estrous cycle of OVX rats.
Body, uterine, adrenal gland weights, and rectal temperature
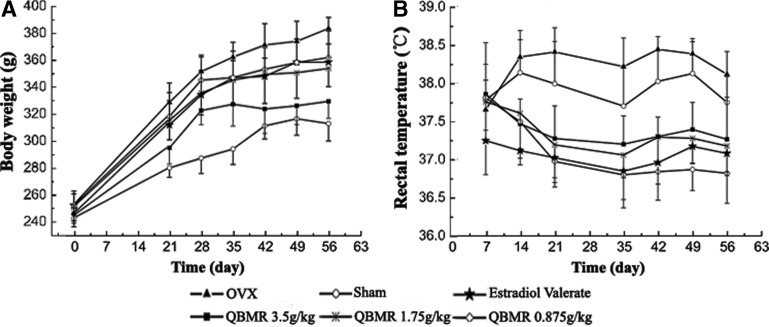
The rats from all six groups had similar initial mean body weights. At the end of the study, the mean body weight of rats in the OVX group was significantly higher than that of the sham-operated group. EV treatment completely prevented the increase in body weight associated with E2 deficiency from the fourth post-operative week and with a 23% decrease compared with untreated OVX rats at the eighth week (Fig. 2A). QBMR treatment of OVX rats had significant effects on body weight gain. There was a trend of decreasing weight with an increasing dose of QBMR, and QBMR at a dose of 3.5 grams/kg significantly decreased body weight gain from the third post-operative week and with an 39% decrease compared with untreated controls at the eighth week (p<0.001).
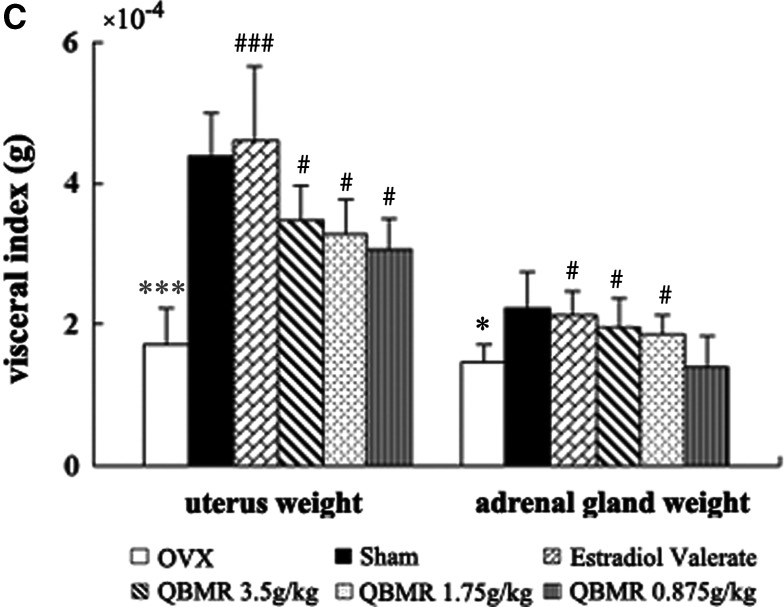
FIG. 2.
The effects of QiBaoMeiRan formula (QBMR) on body weight, rectal temperature, uterus weight, and adrenal gland weight. (A) Body weight was measured once a week for 8 weeks. (B) Rectal temperature was measured 2 hr after administration of the treatment. (C) The weights of uterine and adrenal glands were measured at the end of the 8-week treatment period. Data are the mean standard deviation (SD) of samples from 10 rats. p values are for the one-way analysis of variance (ANOVA) comparing treatment group with untreated ovariectomized (OVX) rat. (***) p<0.001 and (*) p<0.05, compared with the sham-operated group; (###) p<0.001 and (#) p<0.05, compared with the OVX group.
Rectal temperature was significantly increased in OVX rats compared with sham-operated rats, which suggested that OVX rats had hot flushes. Treatment of EV or QBMR at any dose resulted in significant decreases in rectal temperature (Fig. 2B) from the second post-operative week, and the largest changes in rectal temperature were found at the lowest dose of QBMR among the TCM treatment groups.
As expected, the mean uterine weight of OVX animals was significantly lower than that of sham-operated controls; EV treatment significantly increased uterine weight and induced 2.7-fold higher levels than those of untreated OVX rats. QBMR at any of the three doses significantly increased uterine weight in a dose-dependent manner; QBMR at a dose of 3.5 grams/kg treatment induced a two-fold increase in uterine weight of OVX rats, which was comparable to sham-operated control (Fig. 2C). QBMR treatment of OVX rats had modest stimulatory effects on adrenal gland weight, the higher doses, 3.4 grams/kg and 1.75 grams/kg treatment, resulted in a significant difference (p<0.05) with the largest change (p<0.05) a 35% increase in weight compared with untreated OVX rats.
Levels of serum E2, LH, and FSH
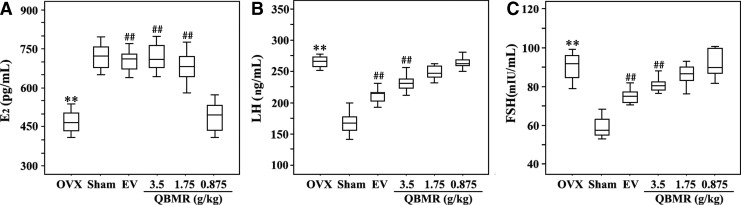
Ovariectomy is expected to result in lower levels of serum E2 and higher levels of LH and FSH compared to sham-operated rats. Treatment of OVX rats with EV and 3.5 and 1.75 grams/kg QBMR significantly raised levels of circulating E2 50%, 50%, and 45%, respectively, compared to those of OVX rats, which were comparable to the sham-operated controls (Fig. 3A). QBMR treatment of OVX rats had modest suppressive effects on LH and FSH levels (Fig. 3B, C). There was a trend of decreasing with an increasing dose of QBMR, but only the highest dose, 3.5 grams/kg, resulted in a significant difference in LH and FSH (both p<0.01) compared with untreated OVX controls, respectively.
FIG. 3.
The effects of QiBaoMeiRan formula (QBMR) on serum estradiol (E2), luteinzing hormone (LH), and follicle-stimulating hormone (FSH) in ovariectomized (OVX) rats. Serum levels of E2 (A), LH (B), and FSH (C) were measured at the end of the treatment period. Data are the mean standard deviation (SD) of samples from 10 rats. p values are for the one-way analysis of variance (ANOVA) comparing treatment group with untreated OVX rats. (**) p<0.01, compared with sham-operated; (##) p<0.01, compared with OVX group.
Histology of uterus, vagina, and mammary gland
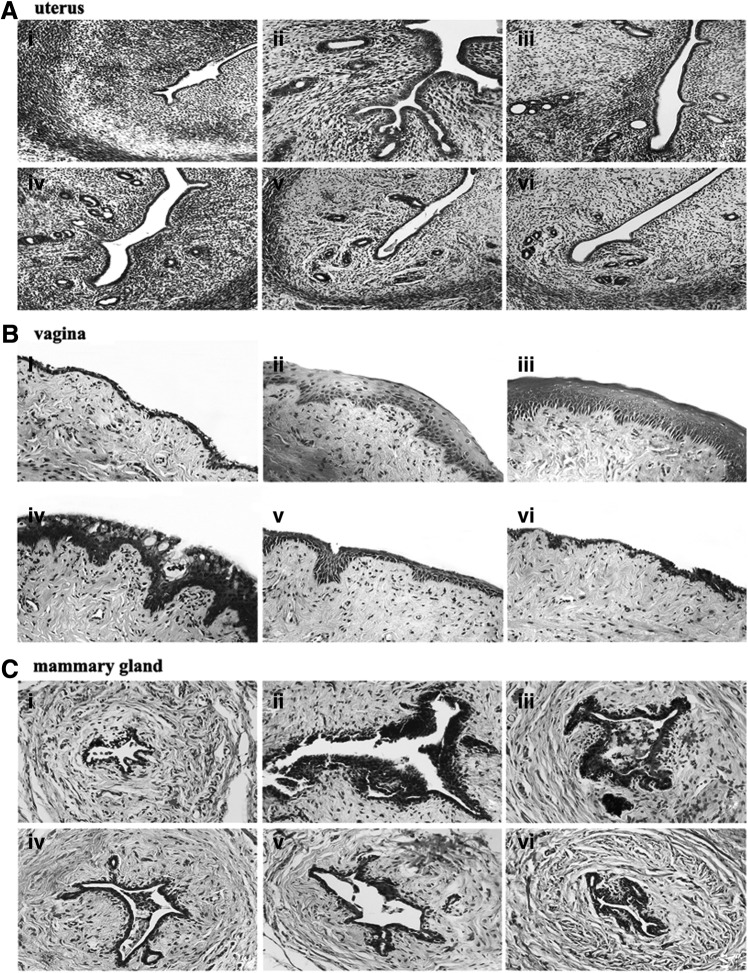
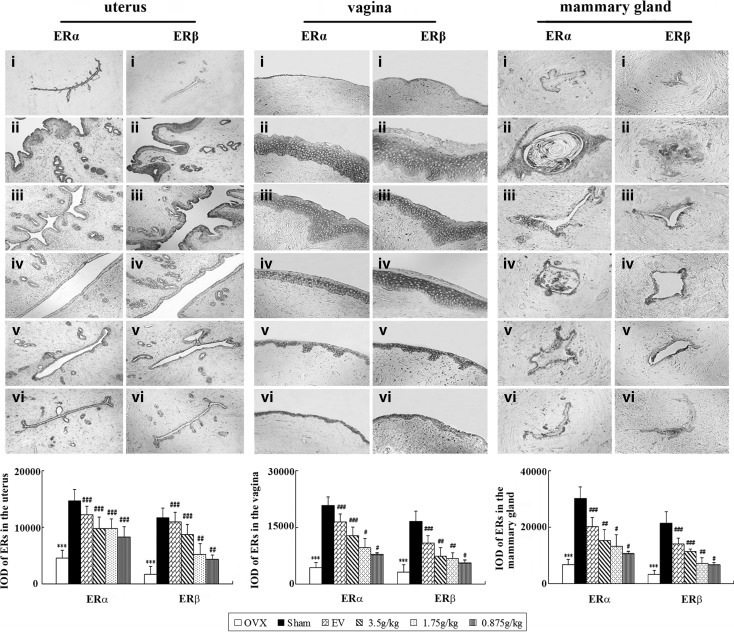
Histological analysis of uterine sections revealed significant atrophy in the uterus of untreated OVX rats (Fig. 4Ai), as indicated by obvious degeneration of the cavities, endometrium, and secretory glands. Treatment of OVX rats with EV or QBMR substantially restored uterine morphology (Fig. 4Aii–iv), as indicated by thickening of the uterine endometrium, increased number of glands, and more extended glandular cavities compared with untreated OVX samples. Thus, QBMR had a similar ability to EV to reverse the atrophy caused by ovariectomy.
FIG. 4.
The effects of QiBaoMeiRan formula (QBMR) treatment on the histology of uterus (A), vagina (B), and mammary gland (C) in the ovariectomized (OVX) rats. Representative photomicrographs taken at 200×magnification of uterus and 400×magnification of vagina and mammary gland sections from each treatment group are shown: (i) untreated OVX rat; (ii) sham-operated rat; (iii) OVX rat treated with estradiol valerate; and OVX rat treated with (iv) 3.5 grams/kg, (v) 1.7 grams/kg, (vi) 0.85 grams/kg QBMR.
Figure 4B shows microscopy preparations of representative vagina from one animal per treatment group compared with a sham-operated rat. The vaginal epithelium of the OVX rat was atrophic (Fig. 4Bi), as indicated by only two to three cell layers being present, and these were composed of flattened cells with no cornification. The EV-treated animals (Fig. 4Biii) displayed typical squamous multi-layered epithelial layers with cornification. Treatment with QBMR 3.5 grams/kg or 1.75 grams/kg increased epithelial thickness and also the number of cell layers. Cytoplasmatic vacuolizations were clearly present in the upper layers only in the 3.5 grams/kg QBMR group. The 0.875 gram/kg QBMR treatment slightly augmented epithelium thickness, and the number of cell layers did not differ from control.
As shown in Fig. 4C, the mammary glands of the sham-operated rat are composed of connective tissue, acini, and ducts, with the epithelial cells of the acini and ducts manifesting cubic or low columns. The epithelial structures of mammary glands in OVX rats were atrophic, deep in the fat pad, with scarce clusters of densely packed terminal structures, many of which did not show clear luminal formation (Fig. 4Ci). Treatment with EV or QBMR resulted in abundant terminal epithelial structures and significantly increased mammary gland epithelium thickness (Fig. 4Civ).
Taken together, these studies in vivo provide evidence that QBMR has significant estrogenic activity, comparable to that of the synthetic estrogen EV. These data prompted further studies to elucidate the molecular basis of QBMR activity, in particular to provide evidence that the effects of QBMR are mediated through activation of ERs.
Expression of ERs subtype in uterus, vagina, and mammary gland
Representative sections of the expressions of ERα and ERβ in the uterus, vagina, and mammary gland from each group and quantitative analysis are shown in Fig. 5. The expression of ERα and ERβ in the uterus, vagina, and mammary gland of untreated OVX rats was significantly decreased compared with sham-operated group, respectively (all p<0.001), and treatment with either EV or QBMR induced a clear and comparable up-regulation of ERα and ERβ. The largest increases were found in the highest dose among the TCM treatment groups. ERs in the uterus were expressed in similar cell types in the QBMR-treated, EV-treated, or sham-operated groups, namely in the epithelial cells of the endometrium interstitial cells and smooth muscle cells. ERs in the vagina were expressed in vaginal epithelial cells, squamous cells, and smooth muscle cells. Epithelial cells in mammary gland duct positively expressed ERs. In all cell types, ER staining was predominantly cytoplasmic or cytosolic. These data further support the indication that QBMR mediates its activity in vivo through ERs.
FIG. 5.
The effects of QiBaoMeiRan formula (QBMR) treatment on the expression of estrogen receptor (ER) α and estrogen receptor (ER) β in the uterus, vagina, and mammary gland. ERα and ERβ expression was assessed by quantitative immunohistochemistry. Representative photomicrographs taken at 200×magnification of uterus and 400×magnification of vagina and mammary gland sections from each treatment group are shown: (i) Untreated ovariectomized (OVX) rat; (ii) sham-operated rat; (iii) OVX rat treated with estradiol valerate (EV); and OVX rat treated with (iv) 3.5 grams/kg, (v) 1.7 grams/kg, and (vi) 0.85 gram/kg QBMR. Data are the mean standard deviation (SD) of samples from 10 rats. p values are for one-way analysis of variance (ANOVA) comparing treatment group with untreated OVX rats. (***) p<0.001, compared with the sham-operated group; (###) p<0.001, (##) p<0.01, and (#) p<0.05, compared with the OVX group.
Protein and gene levels of ER subtypes in uterus, vagina, and mammary gland
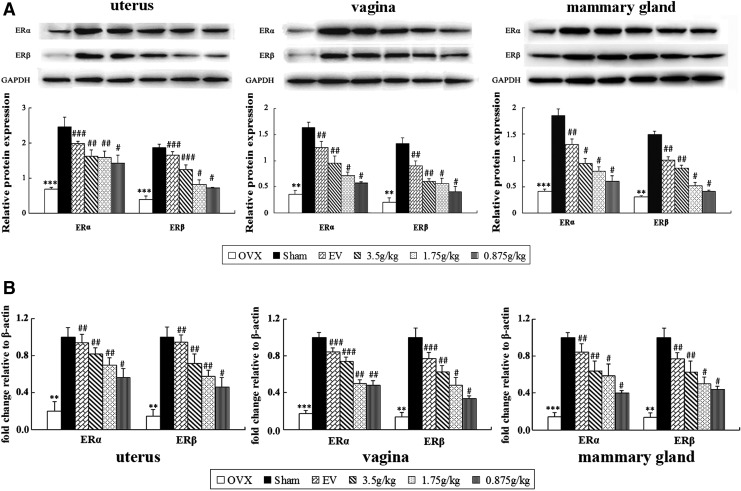
Further evidence for the interaction of the QBMR with ERs was sought by determining the effects on ER subtype expression on protein and mRNA levels in target tissues by western blotting and real-time quantitative PCR. The results as shown in Fig. 6A, B, compared with the sham-operated group, show that both protein and gene expression of ERα and ERβ were significantly decreased in the uterus, vagina, and mammary gland of OVX rats (all p<0.001). Treatment with either EV or QBMR (0.875–3.5 grams/kg) induced significant up-regulation of ERα and ERβ on protein and mRNA levels in target tissues, with a dose-dependent manner in QBMR treatment. The highest dose, 3.5 grams/kg, resulted in the largest up-regulation of protein expression with a 2.5-fold and a 3.0-fold increase in ERα (p<0.01) and ERβ (p<0.001) in the uterus, respectively, compared with untreated OVX rats. The 3.5 grams/kg QBMR treatment also up-regulated protein expression of ERα 2.6-fold (p<0.01) and ERβ 3.3-fold (p<0.01) in the vagina and increased protein expression of ERα 2.3-fold (p<0.05) and ERβ 2.8-fold (p<0.01) in the mammary gland, respectively (Fig. 6A). Meanwhile, the effects of QBMR and EV on the gene expression of ERα and ERβ were similar to those of protein levels.
FIG. 6.
The effects of QiBaoMeiRan formula (QBMR) on the expression of estrogen receptor (ER) α and estrogen receptor (ER) β at protein levels (A) and mRNA levels (B) in uterus, vagina, and mammary gland of rats. Western blot analysis and real-time PCR analyses were carried out as described in Materials and Methods. Representative blots are shown above, and quantitative analysis is shown below. Values given are the mean standard deviation (SD) of three independent experiments. (***) p<0.001, and (**) p<0.01, compared with sham-operated; (###) p<0.001, (##) p<0.01, and (#) p<0.05, compared with the ovariectomized (OVX) group.
Discussion
Considering that phytoestrogens are similar both structurally and functionally to mammalian estrogens, but with reportedly lower side effects compared with synthetic estrogens, seeking an effective phytoestrogen is an important and urgent issue in the prevention and treatment of post-menopausal syndromes. In the present study, we evaluated the estrogenic activity of QBMR using an OVX rat model. The results showed that QBMR has a potent estrogenic activity, as indicated by stimulating vaginal proliferation, antagonizing target tissue atrophy and estrogen decline in circulation caused by ovariectomy. In addition, QBMR could relieve symptoms of hot flushes and weight increase induced by estrogen decline. QBMR's estrogenic activity may be mediated by stimulating biosynthesis of estrogen and increasing the quantity of ERs in the uterus, vagina, and mammary gland.
E2, a reproductive hormone, plays a vital role in body weight control.16 In post-menopausal women, estrogen deficiency is associated with increased probability of obesity.17 Consistently, OVX rats that were deficient for estrogen and developed obesity that could be reversed by E2 replacement therapy, which decreased food intake and increased energy expenditure.18 Importantly, experimental data in vivo and in vitro have provided evidence that estrogen signaling modulates fat accumulation and body weight by binding to ERα.19–21 Our data showed that QBMR as a phytoestrogen also profoundly inhibited body weight increase and up-regulated the expression of ERα in the target tissue of OVX rats.
Using FSH and LH levels as markers of the estrogenic effect of QBMR on the hypothalamic–pituitary axis where the gonadotropin-releasing hormone (GnRH) pulse generator resides in the hypothalamus.22 The GnRH pulse generator is over-active in the absence of estrogens, which results in high serum FSH and LH release from the in pituitary of post-menopausal woman.23 Treatment with QBMR at higher doses promoted E2 release and diminished the ascending FSH and LH levels in OVX rats, suggesting either the long-loop effect of QBMR directly on the hypothalamus or the short-loop effect on the pituitary gland. In the absence of estrogens or under conditions of blocked ERs, the release of these excitatory and inhibitory neurotransmitters is exaggerated, and therefore neurotransmitters spill over to the thermo-regulatory hypothalamic neurons, resulting in hot flushes. This is why serum LH pulses and hot flushes occur often synchronously.24 Recently, others have demonstrated that OVX rats have also hot flushes,25–27 which occur as frequently as the LH pulses, and these pulses of increased skin temperature are not seen in intact animals. Similarly, the lack of estrogens in OVX rats results in high pulsatile LH release and rectal temperature that can be profoundly inhibited by QBMR, which seems to be effective in reducing the activity of this brain structure.
Estrogens are synthesized in the ovary, testis, or adrenal gland.28 The adrenal gland becomes the principal tissue for secreting estrogen after ovariectomy. The increased weight of the adrenal gland and serum estrogen concentration after treatment with QBMR suggests that at higher doses the effect of QBMR may be mediated through the hypothalamus–pituitary–adrenal axis and stimulate biosynthesis of estrogen in the adrenal gland. It is worth mentioning that the stimulation of estrogen synthesis in our OVX animals agreed with the previous studies when the OVX rats were treated with other phytoestrogens.13,29
Under physiological conditions, the biological effects of estrogen depend not only on the level of estrogen but also on the distribution and expression levels of the corresponding ERs, ERα and ERβ, in the target cell 30–32 Estrogen and ERs are involved in the physiological function regulation of the female reproductive system. In this study, QBMR significantly up-regulated the expression of ERα and ERβ in protein and gene levels in the target tissues, respectively. It is also worth mentioning that ERβ up-regulation induced by QBMR extract was stronger than that of ERα, suggesting that QBMR may bind to ERβ with higher selectivity than to ERα. Other phytoestrogens have also been reported to have a higher affinity and selectivity for ERβ33
In a recent report by Xin et al.,34 compounds isolated from one of the component herbs of QBMR, Fructus Psoraleae, showed two types of regulatory effect on ERs: The coumarins isopsoralen and psoralen acted as ERα-selective agonists, whereas the flavonoids isobavachalcone, bavachin, corylifol A, and neobavaisoflavone activated both ERα and ERβ It is likely that the ability of QBMR to up-regulate both ERs can be explained by the presence of multiple active components contained in the ingredient herbs that together exhibit polyvalent activities on ER regulation in target tissue.
Conclusion
This is the first report of estrogenic activity for the QBMR formula. QBMR is capable of stimulating vaginal cornification, interfering with the atrophy of reproductive target tissues caused by ovariectomy. In addition, QBMR could relieve symptoms of hot flushes and body weight increase induced by estrogen decline. These estrogenic effects may be mediated by stimulating biosynthesis of estrogen in circulation and increasing the quantity of ERs in the target organs. This study also provides the first evidence that QBMR can treat post-menopausal symptoms through its action as an estrogen agonist. Further studies are in progress in our laboratory to investigate the use of QBMR as an effective dietary supplement for the improvement of quality of life in menopausal women.
Acknowledgments
This work was supported by the grants from project of National Natural Science Foundation of China (81102826) and Visiting professor joint innovation projects of China Academy of Chinese Medical Sciences (ZZ070826).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Grady D. Postmenopausal hormones-therapy for symptoms only. N Engl J Med 2003;348:1839–1854 [DOI] [PubMed] [Google Scholar]
- 2.Fernandez E, Gallus S, Bosetti C, Franceschi S, Negri E, La Vecchia C. Hormone replacement therapy and cancer risk: A systematic analysis from a network of case-control studies. Int J Cancer 2003;105:408–412 [DOI] [PubMed] [Google Scholar]
- 3.Humphries KH, Gill S. Risks and benefits of hormone replacement therapy: The evidence speaks. Can Med Assoc J 2003;168:1001–1010 [PMC free article] [PubMed] [Google Scholar]
- 4.Barone M, Tanzi S, Lofano K, Scavo MP, Guido R, Demarinis L, Di Leo A. Estrogens, phytoestrogens and colorectal neoproliferative lesions. Genes Nutr 2008;3:7–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beck V, Rohr U, Jungbauer A. Phytoestrogens derived from red clover: An alternative to estrogen replacement therapy. J Steroid Biochem 2005;94:499–518 [DOI] [PubMed] [Google Scholar]
- 6.Benassayag C, Perrot-Applanat M, Ferre F. Phytoestrogens as modulators of steroid action in target cells. J Chromatogr B 2002;777:233–248 [DOI] [PubMed] [Google Scholar]
- 7.Fu XH. Treating the climacteric syndrome in the kidney theory. China J Basic Med Traditional Chinese Med 2003;9:56–57 [Google Scholar]
- 8.Shen XM, Du YH, Shi XM. The pathogenesis of climacteric syndrome and principle of acupuncture treatment based on TCM theory about brain. J Tradit Chin Med 2005;25:108–113 [PubMed] [Google Scholar]
- 9.National Pharmacopoeia Committee. China Pharmacopoeia. Chemical Industry Press, Beijing, 2010 [Google Scholar]
- 10.Liu DA, Huang SM, Yang S F, Su QQ. Qibaomeiran decoction in treatment of 76 cases of osteoporosis. Hunan J Tradit Chinese Med 1999;15:26–27 [Google Scholar]
- 11.CDER. Estimating the maximum safe starting dose in initial clinical trials for therapeutics in adult healthy volunteers. FDA. Guidance for Industry, Pharmacol Toxicol, Washington DC, 2005 [Google Scholar]
- 12.Henry LA, Witt DM. Resveratrol: Phytoestrogen effects on reproductive physiology and behavior in female rats. Horm Behav 2002;41:220–228 [DOI] [PubMed] [Google Scholar]
- 13.Novensà L, Novella S, Medina P, Segarra G, Castillo N, Heras M, Dantas AP. Aging negatively affects estrogens-mediated effects on nitric oxide bioavailability by shifting ERα/ERβ balance in female mice. PloS One 2011;6:e25335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu Y, Zhang ZJ, Geng F, Su SB, White KN, Bligh SA, Wang ZT. Treatment with Qing'E, A kidney-invigorating chinese herbal formula, antagonizes the estrogen decline in ovariectomized mice. Rejuvenation Res 2010;13:479–488 [DOI] [PubMed] [Google Scholar]
- 15.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001;29:45–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dubuc PU. Effects of estrogen on food intake, body weight, and temperature of male and female obese rat. Exp Biol Med 1985;180: 468–473 [DOI] [PubMed] [Google Scholar]
- 17.Carr MC. The emergence of the metabolic syndrome with menopause. J Clin Endocr Metab 2003;88:2404–2411 [DOI] [PubMed] [Google Scholar]
- 18.Gao Q, Mezei G, Nie Y, Rao Y, Choi CS, Bechmann I, Horvath TL. Anorectic estrogen mimics leptin's effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nat Med 2006;13:89–94 [DOI] [PubMed] [Google Scholar]
- 19.Banno R, Zimmer D, De Jonghe BC, Atienza M, Rak K, Yang W, Bence KK. PTP1B and SHP2 in POMC neurons reciprocally regulate energy balance in rat. J Clin Invest 2010;120:720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, Cooke PS. Increased adipose tissue in male and female estrogen receptor-α knockout rat. Proc Natl Acad Sci USA 2000;97:12729–12734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohlsson C, Hellberg N, Parini P, Vidal O, Bohlooly M, Ruding M, Gustafsson JA. Obesity and disturbed lipoprotein profile in estrogen receptor-α-deficient male rat. Biochem Biophys Res Commun 2000;278:640–645 [DOI] [PubMed] [Google Scholar]
- 22.Rimoldi G, Christoffel J, Seidlova-Wuttke D, Jarry H, Wuttke W. Effects of chronic genistein treatment in mammary gland, uterus, and vagina. Environ Health Perspect 2007;115:62–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wuttke W, Jarry H, Haunschild J, Stecher G, Schuh M, Seidlova-Wuttke D. The non-estrogenic alternative for the treatment of climacteric complaints: Black cohosh (Cimicifuga or Actaea racemosa). J Steroid Biochem 2014;139:302–310 [DOI] [PubMed] [Google Scholar]
- 24.Tataryn IV, Meldrum DR, Lu KH, Fruraar AM, Judd HL. LH, FSH and skin temperature during the menopausal hot flas. J Clin Endocr Metab 1979;49:152–154 [DOI] [PubMed] [Google Scholar]
- 25.Pan Y, Anthony MS, Binns M, Clarkson TB. A comparison of oral micronized estradiol with soy phytoestrogen effects on tail skin temperatures of ovariectomized rats. Menopause 2001;8:171–174 [DOI] [PubMed] [Google Scholar]
- 26.Berendsen HH, Kloosterboer HJ. Oestradiol and mirtazapine restore the disturbed tail-temperature of oestrogen-deficient rats. Eur J Pharmacol 2003;482:329–333 [DOI] [PubMed] [Google Scholar]
- 27.Puri P, Wuttke W, Seidlova-WuttkeD. 20-OH-ecdysone prevents hot flushes in ovariectomized rats. Planta Med 2012;78:109–114 [DOI] [PubMed] [Google Scholar]
- 28.Ratvych P, Soma KK, Sinchak K. Neuroprogesterone: Key to estrogen positive feedback? Brain Res Rev 2008;57:470–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xie QF, Xie JH, Dong TT, Su JY, Cai DK, Chen JP, Liu LF, Li YC, Lai XP, Karl WKTsim, Su ZR. Effect of a derived herbal recipe from an ancient Chinese formula, Danggui Buxue Tang, on ovariectomized rats. J Ethnopharmacol 2012;44:567–575 [DOI] [PubMed] [Google Scholar]
- 30.Pelletier G, Labrie C, Labrie F. Localization of oestrogen receptor alpha, oestrogen receptor beta and androgen receptors in the rat reproductive organs. J Endocrinol 2000;165:359–370 [DOI] [PubMed] [Google Scholar]
- 31.Saunders PT, Maguire SM, Gaughan J, Millar MR. Expression of oestrogen receptor beta (ER beta) in multiple rat tissues visualized by immunohistochemistry. J Endocrinol 1997;154:13–16 [DOI] [PubMed] [Google Scholar]
- 32.Shughure PJ, Lane MV, Scrimo PJ, M erchenthaler I. Comparative distribution of estrogen receptor-alpha (ER-alpha) and beta (ER-beta) mRNA in the rat pituitary, gonad, and reproductive tract. Steroids 1998;63:498–504 [DOI] [PubMed] [Google Scholar]
- 33.Oseni T, Patel R, Pyle J, Jordan VC. Selective estrogen receptor modulators and phytoestrogens. Planta Med 2008;74:1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xin D, Wang H, Yang J, Su YF, Fan GW, Wang YF, Gao XM. Phytoestrogens from Psoralea corylifolia reveal estrogen receptor-subtype selectivity. Phytomedicine 2010;17:126–131 [DOI] [PubMed] [Google Scholar]