Abstract
Human stem cell-derived cardiomyocytes provide a cellular model for the study of electrophysiology in the human heart and are finding a niche in the field of safety pharmacology for predicting proarrhythmia. The cardiac L-type Ca2+ channel is an important target for some of these safety studies. However, the pharmacology of this channel in these cells is altered compared to native cardiac tissue, specifically in its sensitivity to the Ca2+ channel activator S-(−)-Bay K 8644. Using patch clamp electrophysiology, we examined the effects of S-(−)-Bay K 8644 in three separate stem cell-derived cardiomyocyte cell lines under various conditions in an effort to detect more typical responses to the drug. S-(−)-Bay K 8644 failed to produce characteristically large increases in current when cells were held at −40 mV and Ca2+ was used as the charge carrier, although high-affinity binding and the effects of the antagonist isomer, R-(+)-Bay K 8644, were intact. Dephosphorylation of the channel with acetylcholine failed to restore the sensitivity of the channel to the drug. Only when the holding potential was shifted to a more hyperpolarized (−60 mV) level, and external Ca2+ was replaced by Ba2+, could large increases in current amplitude be observed. Even under these conditions, increases in current amplitude varied dramatically between different cell lines and channel kinetics following drug addition were generally atypical. The results indicate that the pharmacology of S-(−)-Bay K 8644 in stem cell-derived cardiomyocytes varies by cell type, is unusually dependent on holding potential and charge carrier, and is different from that observed in primary human heart cells.
Introduction
The L-type Ca2+ channel plays a crucial role in excitation–contraction coupling and electrical conduction in the human heart. This prominent physiological role has made the channel an attractive pharmacological target leading to the development of several classes of drugs (e.g., dihydropyridines, phenylalkylamines, and benzothiazepines) widely used to treat a variety of cardiovascular diseases.1 Conversely, inhibition or activation of the channel as an unintended off-target effect during drug development can lead to unwanted cardiac side effects, including arrhythmia and alterations in cardiac contractility. Therefore, the L-type Ca2+ channel is often used as part of a cardiac safety screen during the drug discovery process. Somewhere between these two extremes lies yet another interesting aspect of the channel, namely, inhibition of the L-type Ca2+ channel in the setting of human Ether-à-go-go-related gene (hERG) cardiac K+ channel inhibition can reduce or eliminate the potential for torsades de pointes arrhythmia that would be expected from a pure hERG channel blocking drug. This effect is evidenced in drugs such as verapamil,2 tolterodine,3 and ranolazine,4 where clinically relevant hERG inhibition is offset by concomitant L-type Ca2+ channel inhibition resulting in little, if any, proarrhythmic activity for these compounds. Recent in silico modeling efforts have also concluded that the ratio of hERG to L-type Ca2+ channel inhibition provides the best predictor of torsades de pointe in the clinic and highlights the notion that unintended inhibition of cardiac Ca2+ channels may in some cases have a beneficial role for drug development/safety.5 Recognizing this, the U.S. Food and Drug Administration has launched an initiative, whereby Ca2+ channel screening, as part of a larger preclinical safety profile, will play a greater part in predicting the proarrhythmic potential for drugs in development.6
Stem cell-derived cardiomyocytes represent a potential new tool to study cardiac ion channel pharmacology and are also expected to play an increasing role in drug development and safety testing in the future.6 Their many advantages include the fact that they are derived from human tissue sources, commercially available, easy to culture, amenable to a variety of electrophysiological techniques, and reduce the need for animal usage. Unfortunately, the stem cell-derived cardiomyocytes developed to date fail to faithfully replicate all of the electrophysiological properties of adult human cardiomyocytes displaying embryonic-like characteristics with altered numbers and types of ion channels.7–9 Therefore, detailed biophysical and pharmacological analysis of individual ion channels in these cells will be a necessary prerequisite before their routine incorporation into the drug development process. We have previously undertaken a detailed pharmacological study of the L-type Ca2+ channel in stem cell-derived cardiomyocytes.10 Although the Ca2+ channel antagonist pharmacology appeared to be intact, the response of the channel to activators, especially Bay K 8644, was uncharacteristic and unusually weak. Specifically, Bay K 8644 not only failed to produce large increase in current amplitude but also slowed both activation and inactivation kinetics of the current, effects that are opposite to what is normally observed for the drug.11,12 The aim of the present study was to compare the effects of Bay K 8644 in several different stem cell-derived cardiomyocyte cell lines and determine under what conditions, if any, a typical Bay K 8644 response could be restored.
Materials and Methods
Cell Preparation
Experimental procedures and protocols were approved by the Sanofi Institutional Animal Care and Use Committee (Bridgewater, NJ) and/or by the Waltham Biosafety Committee (Waltham, MA). Primary cardiomyocytes from guinea pigs were isolated for Ca2+ channel recordings, as previously described.10 Two different human-induced pluripotent stem cell-derived cardiomyocyte cell lines were used for Ca2+ channel recordings. Cor.4U® cardiomyocytes were purchased from Axiogenesis (Cologne, Germany), while iCell® cardiomyocytes were purchased from Cellular Dynamics International (Madison, WI). Cytiva® Plus human embryonic stem cell-derived cardiomyocytes were obtained from General Electric Healthcare (Little Chalfant, United Kingdom). All cells were cultured according to the manufacturer's instructions for single cell electrophysiology recordings similar to what we have described previously.10 Cells were seeded onto glass coverslips for electrophysiological recordings and were maintained in culture for up to 2 weeks.
Electrophysiological Recordings
All Ca2+ channel recordings were carried out at room temperature using the whole-cell patch clamp technique.13 Electrodes (1–3 MΩ resistance) were fashioned from TW-150F glass capillary tubes (WPI, Sarasota, FL) and filled with a solution containing 130 mM cesium methanesulfonate, 20 mM tetraethylammonium chloride, 1 mM MgCl2, 10 mM EGTA, 10 mM HEPES, 4 mM Tris-ATP, 0.3 mM Tris-GTP, 14 mM phosphocreatine, and 50 U/mL creatine phosphokinase, pH 7.2 with CsOH. The external solution for Ca2+ channel recording contained 137 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, and 10 mM glucose, pH 7.4 with NaOH. For some recordings, the CaCl2 was replaced with 10 mM BaCl2. For all experiments, Ca2+ channel currents were elicited at a rate of 0.2 Hz. Ca2+ channel currents were recorded using an Axopatch 200B amplifier (Danaher, Inc., Sunnyvale, CA). For longer (200 ms) pulses, currents were sampled at 4 kHz and low-passed filtered at 2 kHz. For shorter (30 ms) pulses, currents were sampled at 20 kHz and filtered at 2 kHz. Currents were analyzed using the pCLAMP suite of software (Danaher, Inc.). IC50 values were obtained by nonlinear least-squares fit of the data (GraphPad Software, Inc., San Diego, CA). The protocol for electrophysiological experiments is outlined in Table 1.
Table 1.
Electrophysiology Protocol
| Step | Parameter | Value | Description |
|---|---|---|---|
| 1 | Cell addition | 1 coverslip/chamber | Placing glass coverslip in the recording chamber |
| 2 | Seal formation | Seal resistance: >1 GΩ | Establishing a gigaohm seal between electrode tip and cell membrane |
| 3 | Whole-cell configuration | Applying gentle suction to rupture the membrane | |
| 4 | Current stabilization | 3–4 overlapping current traces | Holding cells at −40 mV (or −60 mV) and depolarizing to 0 mV for 200 ms at a frequency of 02 Hz |
| 5 | Drug addition | Various concentrations | Perfusing until reaching equilibrium |
Step Notes
1. Cells were seeded on glass coverslips and incubated at 37°C in an atmosphere of 95% air and 5% CO2 (incubation time: 4–14 days after thawing).
2. Electrodes were filled with an internal solution, which contained 130 mM cesium methanesulfonate. Twenty millimolar tetraethylammonium chloride, 1 mM MgCl2, 10 mM EGTA, 10 mM HEPES, 4 mM Tris-ATP, 0.3 mM Tris-GTP, 14 mM phosphocreatine, and 50 U/mL creatine phosphokinase, pH 7.2 with CsOH.
3. Cells were continuously perfused with an external solution, which contained 137 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, and 10 mM glucose, pH 7.4 with NaOH (for some recordings, the CaCl2 was replaced with 10 mM BaCl2). Capacity transients compensated once whole-cell configuration was established.
4. Ca2+ channel currents were recorded using an Axopatch 200B amplifier.
5. R- and S-Bay K 8644 prepared as 10 mM stock solutions in DMSO. Final DMSO concentration in all solutions, including control was 0.01%.
DMSO, dimethyl sulfoxide.
Chemicals
R-(+)- and S-(−)-Bay K 8644 were obtained from Tocris (Ellisville, MD). All other chemicals were obtained from Sigma-Aldrich (St. Louis, MO).
Results
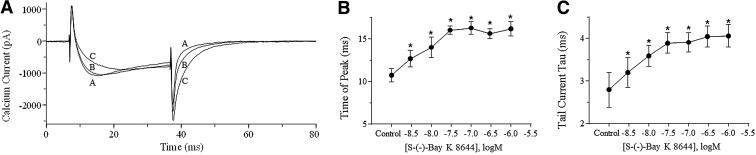
Figure 1 illustrates the effects of S-(−)-Bay K 8644 on Ca2+ channel currents in primary guinea pig myocytes. Cells were depolarized every 5 s to 0 mV from a holding potential of −40 mV. In these cells, S-(−)-Bay K 8644 produced large increases in current amplitude that reached a maximum of 295%±50% at a concentration of 100 nM (Fig. 1A, C). In addition, S-(−)-Bay K 8644 accelerated Ca2+ current inactivation (Fig. 1D) consistent with its well-known effects on the channel. Figure 1 also shows the effects of S-(−)-Bay K 8644 on Cor.4U cardiomyocytes using an identical protocol. In these cells, S-(−)-Bay K 8644 (up to 1,000 nM) had little effect on Ca2+ channel current amplitude producing a maximal increase of only 6.4%±5.7% (n=6) observed at 30 nM (Fig. 1B, C). The most pronounced effects of the drug were to slow the time to peak as well as current deactivation and inactivation. When fit with a single exponential, inactivation of the Ca2+ current measured 39±3 ms without drugs and increased in a concentration-dependent manner in the presence of ascending concentrations of S-(−)-Bay K 8644, reaching a maximum of 58±2 ms in the presence of 1,000 nM (Fig. 1D). In a separate experiment, the activity of S-(−)-Bay K 8644 on Cor.4U cells was repeated using a pulse frequency of 0.1 Hz, but results were identical to those observed at 0.2 Hz (data not shown). The effects of S-(−)-Bay K 8644 on Ca2+ channel current time to peak and deactivation were easiest to see using the short pulse (30 ms) protocol shown in Figure 2A. Unlike current amplitude, both time to peak and deactivation of the current (measured as a single exponential fit of the tail current decay) were highly sensitive to S-(−)-Bay K 8644 being significantly (P<0.05 paired t-test) prolonged at concentrations of 3 nM and higher (Fig. 2B, C). The small effect of S-(−)-Bay K 8644 on current amplitude combined with the observed changes on current kinetics is similar to what we have previously observed using iCell and Cytiva cardiomyocytes under identical conditions.10
Fig. 1.
Effects of S-(−)-Bay K 8644 on guinea pig cardiomyocytes and Cor.4U® stem cell-derived cardiomyocytes. (A) Ca2+ channel current traces from guinea pig cardiomyocytes under control conditions and following the addition of 10 and 100 nM S-(−)-Bay K 8644. Cells were held at −40 mV and depolarized for 200 ms to 0 mV every 5 s using 1.8 mM external Ca2+ as the charge carrier. (B) Ca2+ channel current traces in a Cor.4U cardiomyocyte under identical conditions described in (A). The effects of 100 and 1000 nM S-(−)-Bay K 8644 are shown. (C) Concentration–response relationships for S-(−)-Bay K 8644 on peak current in both myocyte preparations are shown. Error bars indicate the standard error of the mean (SEM), n=5–6. (D) The time constant for current inactivation (single exponential fit) is plotted against S-(−)-Bay K 8644 concentration for both myocyte preparations. Error bars indicate SEM and asterisks denote statistical significance (P<0.05, paired t-test, n=5–6).
Fig, 2.
Effects of S-(−)-Bay K 8644 on Ca2+ channel currents in Cor.4U cardiomyocytes using short pulses. Cells were held at −40 mV and depolarized to 0 mV for 30 ms every 5 s using 1.8 mM Ca2+ as the charge carrier. (A) Ca2+ channel current traces under control conditions and following the addition of 10 and 100 nM S-(−)-Bay K 8644 are indicated by the letters (A–C), respectively. Concentration-dependent effects of S-(−)-Bay K 8644 on time to peak current and on tail current decay time constants (tau) are shown in (B, C), respectively. Error bars indicate SEM and asterisks denote statistical significance (P<0.05, paired t-test, n=6).
To further explore the Bay K 8644 binding site in these cells, we tested the effects of R-(+)-Bay K 8644, a potent antagonist of the L-type Ca2+ channel.14 Figure 3A shows the effects of R-(+)-Bay K 8644 on Ca2+ channel currents elicited by 200 ms test pulses to 0 mV from a holding potential of −40 mV every 5 s. R-(+)-Bay K 8644 inhibited Ca2+ channel current in a concentration-dependent manner displaying an IC50 value of 58 nM (42–79 nM, 95% confidence limits [CL] n=7, Fig. 3B). Moreover, when we repeated this experiment in the presence of 10 nM S-(−)-Bay K 8644, the concentration–response curve for R-(+)-Bay K 8644 was shifted by approximately sixfold in the rightward direction and now measured 341 nM (309–377 nM, 95% CL, n=6, Fig. 3B).
Fig. 3.
Inhibition of Ca2+ channel currents in Cor.4U myocytes by R-(+)-Bay K 8644. (A) Ca2+ channel current traces under control conditions and following the addition of 10, 100, and 1000 nM R-(+)-Bay K 8644 are shown. Currents were elicited by 200 ms depolarizing pulse to 0 mV from a holding potential of −40 mV every 5 s using 1.8 mM Ca2+ as the charge carrier. (B) Concentration–response relationships for R-(+)-Bay K 8644 alone (open circles) or in the presence of 10 nM S-(−)-Bay K 8644 (filled triangles). R-(+)-Bay K inhibited Ca2+ channel currents with an IC50 of 58 (42–79 nM, 95% confidence limits [CL]). In the presence of 10 nM S-(−)-Bay K 8644, this value measured 341 nM (309–377 nM, 95% CL).
It has been shown in human heart cells that hyperphosphorylation of the L-type Ca2+ channel can greatly reduce the response of the channel to the agonist effects of Bay K 8644 and that this response can be restored by dephosphorylating the channel with acetylcholine pretreatment.15 To test this, we pretreated Cor.4U cells with 20 μM acetylcholine for 4–5 min before exposure to S-(−)-Bay K 8644. Acetylcholine by itself produced a 17%±4% (n=4) reduction in Ca2+ channel current amplitude. Current traces following subsequent treatment with S-(−)-Bay K 8644 are shown in Figure 4A. Acetylcholine pretreatment failed to restore the stimulatory effect of S-(−)-Bay K 8644 on current amplitude (Fig. 4B) nor did it alter the atypical effects observed on activation and inactivation kinetics. Similar results were also obtained when we repeated this protocol in the absence of ATP, GTP, phosphocreatine, and creatine phosphokinase in the patch pipette. Under this condition S-(−)-Bay K 8644 produced a concentration-dependent decrease in current amplitude, perhaps indicating enhanced current rundown with this internal solutions (Fig. 4B).
Fig. 4.
Calcium channel current responses to S-(−)-Bay K 8644 in Cor.4U cardiomyocytes following pretreatment with acetylcholine. (A) Ca2+ channel current traces under control conditions, following treatment with 20 mM acetylcholine, and subsequent treatment with 100 nM S-(−)-Bay K 8644 are shown. Currents were elicited by 200 ms depolarizing pulses to 0 mV from a holding potential of −40 mV every 5 s using 1.8 mM Ca2+ as the charge carrier. (B) Concentration–response relationships for S-(−)-Bay K 8644 on peak Ca2+ channel currents following preincubation of the cells with acetylcholine. Open circles represent data collected with a normal pipette solution, while filled triangles show data collected in the absence of ATP, GTP, phosphocreatine, and creatine phosphokinase in the pipette. Error bars indicate SEM (n=4).
The effects of altering holding potential and/or charge carrier on S-(−)-Bay K 8644 responses in Cor.4U cells are illustrated in Figure 5. Step depolarizations to 0 mV from a holding potential of −60 mV every 5 s elicited a somewhat larger response compared to that observed at the −40 mV holding potential. Under these conditions S-(−)-Bay K 8644 produced a biphasic increase in current amplitude that peaked at 40%±8% (Fig. 5A, D, n=6). Holding the cells at −40 mV and using 10 mM Ba2+ as the charge carrier (to increase the percentage of cells with large current amplitude) caused a similar biphasic concentration–response curve resulting in a maximal increase in peak current of 41%±5% (Fig. 5B, D, n=6). Under both these conditions, S-(−)-Bay K 8644 produced some increase in current amplitude in all cells tested. It was not until we combined both a −60 mV holding potential with Ba2+ as the charge carrier did we finally elicit a robust S-(−)-Bay K 8644 response in these cells. Under these conditions, S-(−)-Bay K 8644 increased current amplitude in a concentration-dependent manner in all cells tested with a maximal increase in peak current of ∼200% (Fig. 5D, n=9). Importantly, in about half of the cells tested, we were also able to observe the typical effects of S-(−)-Bay K 8644 on current kinetics that included an acceleration in the rate of current activation and inactivation (Fig. 5C).
Fig. 5.
Calcium channel current responses to S-(−)-Bay K 8644 in Cor.4U cardiomyocytes under various assay conditions. (A) Effects of 300 nM S-(−)-Bay K 8644 on currents recorded from a holding potential of −60 mV using 1.8 mM Ca2+ as the charge carrier. (B) S-(−)-Bay K 8644 (300 nM) response on calcium channel currents held at −40 mV using 10 mM Ba2+ as the charge carrier. (C) Effects of 10 and 300 nM S-(−)-Bay K 8644 on calcium channel currents recorded from a holding potential of −60 mV using 10 mM Ba2+ as the charge carrier. Note the enhancement of current activation and inactivation following addition of the drug. (D) Concentration–response curves for S-(−)-Bay K 8644 on peak Ca2+ channel currents under the various conditions. Error bars indicate SEM, n=6–9.
Using the assay conditions described in the previous paragraph, we examined the effects of S-(−)-Bay K 8644 on Ca2+ channel currents recorded in Cytiva Plus cardiomyocytes. When cells were held at −40 mV and Ca2+ was used as the charge carrier, S-(−)-Bay K 8644 had little effect on peak current producing a maximum 14%±12% increase at 30 nM (Fig. 6D, n=8). However, this response varied dramatically ranging from a purely inhibitory effect in some cells to an increase of about 90% in one cell. In all cells, a slowing of both activation and inactivation was observed as described for the Cor.4U cells. Switching the holding potential to −60 mV produced a modestly larger 21%±11% average increase in current amplitude (Fig. 6A, D), which again varied from cell to cell (range: 105% increase to 31% inhibition). In two cells, we noted acceleration of current activation and inactivation kinetics, but only at the lowest (3 nM) concentration tested, while in all other cells this effect was not observed. When the holding potential was set at −40 mV and Ba2+ was substituted for Ca2+, S-(−)-Bay K 8644 produced a biphasic concentration–response curve with a maximal increase of 43%±15% (Fig. 6B, D, n=11). In only one cell did we observe an acceleration of activation and inactivation kinetics. Like the Cor.4U cells, combining both the −60 mV holding potential and Ba2+ as the charge carrier resulted in the best response to S-(−)-Bay K 8644. Under these conditions, the drug produced a dose-dependent increase in current of approximately twofold (Fig. 6C, D). Current amplitude was consistently increased in all cells tested but unlike the Cor.4U cells, we did not observe any cells where S-(−)-Bay K 8644 accelerated current activation and inactivation kinetics.
Fig. 6.
Calcium channel current responses to S-(−)-Bay K 8644 in Cytiva® Plus cardiomyocytes. (A) Effects of 300 nM S-(−)-Bay K 8644 on currents recorded from a holding potential of −60 mV using 1.8 mM Ca2+ as the charge carrier. (B) S-(−)-Bay K 8644 (300 nM) response on Ca2+ channel currents held at −40 mV using 10 mM Ba2+ as the charge carrier. (C) Effects of 10 and 300 nM S-(−)-Bay K 8644 on Ca2+ channel currents recorded from a −60 mV holding potential using 10 mM Ba2+ as the charge carrier. (D) Concentration–response curves for S-(−)-Bay K 8644 on peak calcium channel currents under the various conditions. Error bars indicate SEM, n=8–12.
The effects of holding potential and charge carrier on the responses to S-(−)-Bay K 8644 were also examined in the iCell cardiomyocyte cell line. When the holding potential was set a −40 mV, little or no effect of S-(−)-Bay K 8644 on current amplitude was observed, regardless of the charge carrier used (Fig. 7B, D, n=6). Under both these conditions, the maximal change in current amplitude in response to the drug varied by approximately ±25% depending upon the cell tested. Using a −60 mV holding potential and Ca2+ as the charge carrier resulted in a more consistent (five out of six cells) although small maximal increase in current amplitude of 23%±11% (Fig. 7A, D, n=6). Finally, even the combination of the −60 mV holding potential with Ba2+ as the charge carrier failed to elicit a robust S-(−)-Bay K 8644 response in these cells resulting in a maximum increase in current amplitude of only about 30% (Fig. 7C, D, n=9). Under no condition did we observe acceleration of activation or inactivation kinetics in response to S-(−)-Bay K 8644 in these cells. The effects of S-(−)-Bay K 8644 on Ca2+ currents in stem cell-derived cardiomyocytes under various conditions is summarized in Table 2.
Fig. 7.
Calcium channel current responses to S-(−)-Bay K 8644 in iCell® cardiomyocytes. (A) Effects of 300 nM S-(−)-Bay K 8644 on currents recorded from a holding potential of −60 mV using 1.8 mM Ca2+ as the charge carrier. (B) S-(−)-Bay K 8644 (300 nM) response on Ca2+ channel currents held at −40 mV using 10 mM Ba2+ as the charge carrier. (C) Effects of 10 and 300 nM S-(−)-Bay K 8644 on Ca2+ channel currents recorded from a −60 mV holding potential using 10 mM Ba2+ as the charge carrier. (D) Concentration–response curves for S-(−)-Bay K 8644 on peak calcium channel currents under the various conditions. Error bars indicate SEM, n=6.
Table 2.
Effects of S-(−)-Bay K 8644 on L-Type Ca2+ Channel Currents on Cardiomyocytes Under Various Recording Conditions
| Patch clamp recording conditions | ||||
|---|---|---|---|---|
| Cell type | −40 mV holding potential, 1.8 mM Ca2+ | −60 mV holding potential, 1.8 mM Ca2+ | −40 mV holding potential, 10 mM Ba2+ | −60 mV holding potential, 10 mM Ba2+ |
| Cor.4U® cardiomyocytes | No significant increase in current amplitude; slowing of activation, inactivation, and deactivation | 40% average maximal increase in current amplitude; slowing of activation, inactivation, and deactivation | 41% average maximal increase in current amplitude; slowing of activation, inactivation, and deactivation | ∼200%, concentration-dependent increase in current amplitude; slowing of activation and inactivation in some cells; acceleration of these parameters in others; slowing of deactivation in all cells |
| Cytiva® Plus cardiomyocytes | Small (14%) maximum increase in current amplitude with variability from cell to cell; slowing of activation, inactivation, and deactivation | 21% average maximal increase in current amplitude with variability from cell to cell; slowing of activation, inactivation, and deactivation | 43% average maximal increase in current amplitude, variability not as pronounced as with Ca2+; slowing of activation, inactivation, and deactivation | 120% concentration-dependent increase in current amplitude, variability not as pronounced as with Ca2+; slowing of activation, inactivation, and deactivation |
| iCell® cardiomyocytes | No significant increase in current amplitude; slowing of activation, inactivation, and deactivation | 23% maximal increase in current amplitude; slowing of activation, inactivation, and deactivation | No significant increase in current amplitude; slowing of activation, inactivation, and deactivation | 30% average maximal increase in current amplitude; slowing of activation, inactivation, and deactivation |
| Primary cardiac myocytes (guinea pig) | ∼300% increase in current amplitude; acceleration of activation and inactivation; slowing of deactivation | |||
Discussion
We have previously shown10 that L-type Ca2+ channels in stem cell-derived cardiomyocytes respond normally to a wide variety of antagonist molecules, but fail to respond in a normal manner to the agonist effects of S-(−)-Bay K 8644. Typical responses to Bay K 8644 (Fig. 1) in a wide variety of preparations (including primary human heart cells) consist of an enhancement of Ca2+ current activation and inactivation kinetics, a large (twofold or greater) increase in peak current amplitude, and a slowing of tail current decay.11,12,15,16 Conversely, in stem cell-derived cardiomyocytes, the predominate effects of S-(−)-Bay K 8644 include a slowing of both activation and inactivation accompanied by little or no effects (or even inhibition) of current amplitude.10 The only effect of S-(−)-Bay K 8644 that is preserved is a slowing of tail current decay. It is possible that the failure of these cells to respond normally to S-(−)-Bay K 8644 resides in their origin (embryonic vs. fibroblast-derived) or is due to their well-described embryonic phenotype.7–9 However, we have failed to find significant differences between embryonic- and adult fibroblast-derived stem cell cardiomyocytes.10 Furthermore, the stimulatory effects of Bay K 8644 are preserved in a variety of embryonic heart preparations,17,18 including human fetal heart,19 indicating that the unusual effects of the drug in stem cell-derived cardiomyocytes is unlikely due directly to the embryonic nature of the cells. To further explore this phenomenon, we utilized three separate stem cell-derived cardiomyocyte cell lines combined with a variety of assay conditions in an attempt to reveal a more typical response to S-(−)-Bay K 8644 as well as to compare and contrast results obtained from these various cell lines.
S-(−)-Bay K 8644 displays high-affinity binding to the cardiac L-type Ca2+ channel20 and the binding and functional effects of the molecule are known to be critically dependent upon amino acid residues Ser1115 and Phe1112.21 It is possible that the high-affinity binding site of Bay K 8644 is lost or altered in stem cell-derived cardiomyocytes, thereby leading to a reduction in efficacy. In the absence of a clear effect on Ca2+ current amplitude, we examined the potency of S-(−)-Bay K 8644 on the time to peak current as well as inactivation and tail current kinetics in Cor.4U cells to assess the affinity of the drug for the channel. S-(−)-Bay K 8644 prolonged each of these parameters in a concentration-dependent manner with statistically significant effects observed with as little as 3 nM of the drug. The R-enantiomer of Bay K 8644 is known to be a potent antagonist of the L-type Ca2+ channel and both the R- and S-enantiomers of Bay K 8644 compete for a mutually exclusive binding site on the channel.14 Figure 3 demonstrates that high-affinity inhibition of the Ca2+ channel by R-(+)-Bay K 8644 is reliably observed in these cells. Furthermore, in the presence of 10 nM S-(−)-Bay K 8644, which by itself had no effect on current amplitude, the R-(+)-Bay K 8644 concentration–response curve was shifted by sixfold in the rightward direction, consistent with strong competitive antagonism between the two molecules. Taken together, these data indicate that high-affinity binding of both the R- and S-isomers of Bay K 8644 remains intact in the cells, but that the functional consequences of this binding are disrupted for the agonist isomer.
Adrenergic stimulation leads to phosphorylation of the L-type Ca2+ channel in the heart through the protein kinase A signaling pathway. It has been demonstrated that phosphorylation of the channel through this pathway leads to a reduced response to Bay K 8644.22,23 In these studies, phosphorylation of the channel before addition of Bay K 8644 not only reduced the effects of the drug on peak currents but also resulted in a slowing of inactivation very similar to what we observe in the stem cell-derived cardiomyocytes. It has also been shown that Ca2+ channels recorded from human cardiac myocytes obtained from failing hearts display less sensitivity to Bay K 8644, presumably due to hyperphosphorylation, and that sensitivity to the drug can be restored by means of dephosphorylation of the channel by pretreatment of the cells with acetylcholine.15 We therefore tested the ability of acetylcholine to restore normal Bay K 8644 responses in stem cell-derived cardiomyocytes. Application of 20 μM acetylcholine resulted in a 17% decrease in Ca2+ channel current amplitude, as previously reported.15 However, the subsequent addition of S-(−)-Bay K 8644 failed to produce an increase in current amplitude and neither were the aberrant channel kinetics normalized in any obvious way (Fig. 4). This was also true when ATP, GTP, phosphocreatine, and creatine phosphokinase were removed from the pipette to further enhance dephosphorylation of the channel. Therefore, we conclude that hyperphosphorylation of the L-type Ca2+ channel through the protein kinase A pathway does not contribute to the reduced effects of Bay K 8644 observed in stem cell-derived cardiomyocytes as it does in adult human cardiomyocytes.
We examined the effects of holding potential and charge carrier on S-(−)-Bay K 8644 responses in stem cell-derived cardiomyocytes. Holding potential was reduced to −60 mV and Ca2+ was replaced by Ba2+ to relieve voltage- and calcium-dependent inactivation processes, respectively. In some preparations, it has been shown that depolarized holding potentials (approximately −40 mV and higher) favor blockade of the Ca2+ channel by Bay K 8644, while at lower potentials, only agonist properties are apparent.11,24 When Ca2+ was used as the charge carrier, lowering the holding potential of the stem cell-derived cardiomyocytes to −60 mV failed to elicit a robust agonist response to S-(−)-Bay K 8644. Likewise, when Ba2+ replaced Ca2+ in the external solution and the cells were held at −40 mV, only weak agonist effects could typically be observed. Furthermore, the effects of the drug on current amplitude tended to vary considerably depending upon the particular cell tested. Only when the hyperpolarized holding potential was combined with external Ba2+ could a more consistent and robust agonist response to S-(−)-Bay K 8644 be finally revealed. Yet even under these conditions, the responses to S-(−)-Bay K 8644 varied dramatically within a given cell line and especially between cell lines from different sources. Thus, when considering current amplitude, Cor.4U myocytes responded to S-(−)-Bay K 8644 with the largest increase followed by Cytiva Plus cells, while iCell cardiomyocytes displayed little enhancement. Within the Cor.4U cell line, Bay K 8644 enhanced current activation and inactivation kinetics in about half the cells tested, but this phenomenon was not apparent in other cells and neither was it observed in the other two cell lines. We conclude that responses to Bay K 8644 in stem cell-derived cardiomyocytes are highly variable within a given cell line and between different cell lines. In addition, drug responses are abnormally dependent upon holding potential and charge carrier. The attenuation of both voltage- and calcium-dependent inactivation processes appears to be an unusual prerequisite for the Bay K 8644 activity in these cells.
Several reports, utilizing a variety of indirect measures of Ca2+ channel activity, have suggested that Bay K 8644 responses are preserved in stem cell-derived cardiomyocytes, including those used in this study. Thus, calcium transients in iCell cardiomyocytes measured using a fluorescent dye were prolonged by Bay K 8644 in the nanomolar range, while the peak of the transient showed only a modest increase requiring micromolar concentrations.25 In Cor.4U myocytes, action potential waveforms displayed a small increase in both amplitude and duration following addition of 10 μM Bay K 8644.26 Most recently, using a multielectrode array, field potential duration measured in Cytiva Plus cells was prolonged by 3–300 nM Bay K 8644.27 We believe that these findings do not reflect a normal response to Bay K 8644 in these cells, but rather arise predominantly from a concentration-dependent slowing of Ca2+ channel inactivation in response to the drug, accompanied by a slowing of deactivation and perhaps a small (20%–40%) increase in current amplitude depending upon the particular cell or cell-type used. Future studies examining the effects of Bay K 8644 in stem cell-derived cardiomyocytes should not rely on indirect measures of Ca2+ channel activity alone, but must incorporate direct measurements of channel function, such as patch clamp electrophysiology, to accurately assess responses.
In summary, we find that S-(−)-Bay K 8644 responses are significantly altered in a variety of stem cell-derived cardiomyocytes. Although high-affinity binding appears to be intact, this binding is not translated into a characteristic pharmacological effect. Only under conditions where both Ca2+- and voltage-dependent inactivation processes are reduced, can the activity of S-(−)-Bay K 8644 be significantly enhanced. Even under these conditions, the response to the drug in most cells is atypical with regard to effects on calcium channel current kinetics. Furthermore, the effects of S-(−)-Bay K 8644 vary dramatically from cell-to-cell within a given cell line as well as between lines from different sources, suggesting significant heterogeneity in the L-type Ca2+ channel in stem cell-derived cardiomyocytes. To our knowledge, stem cell-derived cardiomyocytes are the only cells that are known to respond to S-(−)-Bay K 8644 in this unusual manner. We therefore believe that S-(−)-Bay K 8644 responses could be used as a simple biomarker for the development of new cardiomyocyte cell lines with improved Ca2+ channel characteristics and perhaps phenotypes more closely resembling adult human heart cells.
Abbreviations Used
- CL
confidence limits
- DMSO
dimethyl sulfoxide.
- hERG
human Ether-à-go-go-related gene
- SEM
standard error of the mean
Disclosure Statement
The authors declare no conflict of interests.
References
- 1.Triggle DJ: The pharmacology of ion channels: with particular reference to voltage-gated Ca2+ channels. Eur J Pharmacol 1999;375:311–325 [DOI] [PubMed] [Google Scholar]
- 2.Zhang S, Zhou Z, Gong Q, Makielski JC, January CT: Mechanism of block and identification of the verapamil binding domain to HERG potassium channels. Circ Res 1999;84:989–998 [DOI] [PubMed] [Google Scholar]
- 3.Kang J, Chen X-L, Wang H, et al. : Cardiac ion channel effects of tolterodine. J Pharmacol Exp Ther 2004;308:935–940 [DOI] [PubMed] [Google Scholar]
- 4.Schram G, Zhang L, Derakhchan K, Ehrlich JR, Belardinelli L, Nattel S: Ranolazine: ion-channel-blocking actions and in vivo electrophysiological effects. Br J Pharmacol 2004;142:1300–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kramer J, Obejero-Paz CA, Myatt G, et al. : MICE models: superior to HERG model on predicting torsade de pointes. Sci Rep 2013;3:2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sager PT, Gintant G, Turner JR, Pettit S, Stockbridge N: Rechanneling the cardiac proarrhythmia safety paradigm: a meeting report from the cardiac safety research consortium. Am Heart J 2014;167:292–300 [DOI] [PubMed] [Google Scholar]
- 7.Peng S, Lacerda AE, Kirsch GE, Brown AM, Bruening-Wright A: The action potential and comparative pharmacology of stem cell-derived human cardiomyocytes. J Pharmacol Toxicol Methods 2010;61:277–286 [DOI] [PubMed] [Google Scholar]
- 8.Ma J, Guo L, Fiene SJ, et al. : High purity human-induced pluripotent stem cell-derived cardiomyocytes: electrophysiological properties of action potentials and ionic currents. Am J Physiol Heart Circ Physiol 2011;301:H2006–H2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roden DM, Hong CC: Stem cell-derivedcardiomyocytes as a tool for studying proarrhythmia a better canary in the coal mine? Circulation 2013;127:1641–1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang J, Chen X-L, Ji J, Lei Q, Rampe D: Ca2+ channel activators reveal differential L-type Ca2+ channel pharmacology between native and stem cell-derived cardiomyocytes. J Pharmacol Exp Ther 2012;341:510–517 [DOI] [PubMed] [Google Scholar]
- 11.Sanguinetti MC, Krafte DS, Kass RS: Voltage-dependent modulation of Ca channel current in heart cells by Bay K8644. J Gen Physiol 1986;88:369–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Markwardt F, Nilius B: Modulation of calcium channel currents in guinea pig single ventricular heart cells by the dihydropyridine Bay K 8644. J Physiol 1988;399:559–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ: Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch 1981;391:85–100 [DOI] [PubMed] [Google Scholar]
- 14.Wei XY, Luchowski EM, Rutledge A, Su CM, Triggle DJ: Pharmacologic and radioligand binding analysis of the actions of 1,4-dihydropyridine activator-antagonist pairs in smooth muscle. J Pharmacol Exp Ther 1986;239:144–153 [PubMed] [Google Scholar]
- 15.Chen X, Zhang X, Harris DM, et al. : Reduced effects of Bay K 8644 on L-type Ca2+ current in failing human cardiac myocytes are related to abnormal adrenergic regulation. Am J Physiol Heart Circ Physiol 2008;294:H2257–H2267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skasa M, Jungling E, Picht E, Schondube F, Luckhoff A: L-type calcium currents in atrial myocytes from patients with persistent and non-persistent atrial fibrillation. Basic Res Cardiol 2001;96:151–159 [DOI] [PubMed] [Google Scholar]
- 17.Anderson AJ, Duncan GP, Spedding M, Patmore L: Pertussis toxin-sensitive G-protein activation does not influence the response to Bay K 8644 in embryonic chick myocytes. J Cardiovasc Pharmacol 1990;16:681–683 [DOI] [PubMed] [Google Scholar]
- 18.Rampe D, Anderson B, Rapien-Pryor V, Li T, Dage RC: Comparison of the in vitro and in vivo cardiovascular effects of two structurally distinct Ca2+ channel activators, Bay K 8644 and FPL 64176. J Pharmacol Exp Ther 1993;265:1125–1130 [PubMed] [Google Scholar]
- 19.Chen L, El-Sherif N, Boutjdir M: Unitary current analysis of L-type Ca2+ channels in fetal human ventricular myocytes. J Cardiovasc Electrophysiol 1999;10:692–700 [DOI] [PubMed] [Google Scholar]
- 20.Ferrante J, Luchowski E, Rutledge A, Triggle DJ: Binding of a 1,4-dihydropyridine calcium channel activator, (-) S Bay K 8644, to cardiac preparations. Biochem Biophys Res Commun 1989;158:149–154 [DOI] [PubMed] [Google Scholar]
- 21.Yamaguchi S, Zhorov BS, Yoshioka K, Nagao T, Ichijo H, Adachi-Akahane S: Key roles of Phe 1112 and Ser1115 in the pore-forming IIIS5-S6 linker of the L-type Ca2+ channel alpha 1C subunit (CaV 1.2) in binding of dihydropyridines and action of Ca2+ channel agonists. Mol Pharmacol 2003;64:235–248 [DOI] [PubMed] [Google Scholar]
- 22.Tiaho F, Richard S, Lory P, Nerbonne JM, Nargeot J: Cyclic-AMP-dependent phosphorylation modulates the stereospecific activation of cardiac Ca channels by Bay K 8644. Pflugers Arch 1990;417:58–66 [DOI] [PubMed] [Google Scholar]
- 23.Gomez J-P, Fares N, Potreau D: Effects of Bay K 8644 on L-type calcium current from newborn rat cardiomyocytes in primary culture. J Mol Cell Cardiol 1996;28:2217–2229 [DOI] [PubMed] [Google Scholar]
- 24.Hadley RW, Hume JR: Calcium channel antagonist properties of Bay K 8644 in single guinea pig ventricular cells. Circ Res 1988;62:97–104 [DOI] [PubMed] [Google Scholar]
- 25.Cerignoli F, Charlot D, Whittaker R, et al. : High throughput measurement of Ca2+ dynamics for drug risk assessment in human stem cell-derived cardiomyocytes by kinetic image cytometry. J Pharmacol Toxicol Methods 2012;66:246–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Becker N, Stoelzle S, Gopel S, et al. : Minimized cell usage for stem cell-derived and primary cells on an automated patch clamp system. J Pharmacol Toxicol Methods 2013;68:82–87 [DOI] [PubMed] [Google Scholar]
- 27.Clements M, Thomas N: High-throughput multi-parameter profiling of electrophysiological drug effects in human embryonic stem cell derived cardiomyocytes using multi-electrode arrays. Toxicol Sci 2014;140:445–461 [DOI] [PubMed] [Google Scholar]