Abstract
Context
Social anxiety disorder is thought to involve emotional hyper-reactivity, cognitive distortions, and ineffective emotion regulation. While the neural bases of emotional reactivity to social stimuli have been described, the neural bases of emotional reactivity and cognitive regulation during social and physical threat, and their relationship to social anxiety symptom severity, have yet to be investigated.
Objective
This study investigated behavioral and neural correlates of emotional reactivity and cognitive regulation in patients and controls during processing of social and physical threat stimuli.
Design
Participants were trained to implement cognitive-linguistic regulation of emotional reactivity induced by social (harsh facial expressions) and physical (violent scenes) threat while undergoing functional magnetic resonance imaging and providing behavioral ratings of negative emotion experience.
Setting
Academic psychology department.
Participants
15 adults with social anxiety disorder and 17 demographically-matched healthy controls.
Main Outcome Measures
Blood oxygen level dependent signal and negative emotion ratings.
Results
Behaviorally, patients reported greater negative emotion than controls during social and physical threat, but showed equivalent reduction in negative emotion following cognitive regulation. Neurally, viewing social threat resulted in greater emotion-related neural responses in patients than controls, with social anxiety symptom severity related to activity in a network of emotion and attention processing regions in patients only. Viewing physical threat produced no between-group differences. Regulation during social threat resulted in greater cognitive and attention regulation-related brain activation in controls compared to patients. Regulation during physical threat produced greater cognitive control-related response (i.e., right DLPFC) in patients compared to controls.
Conclusions
Compared to controls, patients demonstrated exaggerated negative emotion reactivity and reduced cognitive regulation related neural activation, specifically for social threat stimuli. These findings help to elucidate potential neural mechanisms of emotion regulation that might serve as biomarkers for interventions for social anxiety disorder.
Anxiety disorders are the most common psychiatric condition (with a lifetime prevalence of 28.8%1). Social anxiety disorder (SAD) is the most common subtype2 with 7–13.3% lifetime prevalence3. SAD is characterized by heightened anxiety and avoidance during social interactions. It has an early onset (80% of cases occur before age 184), and usually precedes other anxiety, mood, and substance abuse/dependence disorders5–7. SAD is associated with significant distress and functional impairment in work and social domains, and typically persists unless treated8–12.
Emotional Reactivity and Regulation in SAD
Models of SAD13,10,14 have highlighted the role of emotional hyper-reactivity, which is thought to arise from distorted appraisals of social situations. These maladaptive appraisals transform innocuous social cues into interpersonal threats, leading to inaccurate interpretations of self (e.g., as socially incompetent) and others (e.g., as critical judges). This induces a cascade of safety behaviors, somatic concerns, and negative emotional reactivity.
Another key feature of SAD is thought to be a failure of emotion regulation15, 16. Effective emotion regulation can reduce emotional reactions to stressful, anxiety-provoking situations17–19. Conversely, difficulties with emotion regulation have been postulated as a core mechanism underlying mood and anxiety disorders20, and accordingly, many clinical treatments focus on enhancing use of emotion regulation to modulate emotional reactivity.
It is important to distinguish among various factors that might influence effective emotion regulation. For example, individuals with SAD may have problems with emotion regulation due to (a) exaggerated emotional reactivity to all types of potential threat stimuli, (b) a general deficit in down-regulating emotional reactivity, or (c) reactivity and regulation abnormalities that are specific to social threat stimuli only. One way to examine emotion regulation in SAD is to probe regulation skills in the context of reactivity to different types of threat stimuli. Thus, in addition to social threat, we also included physical threat as a comparison condition to investigate the specificity of emotional reactivity and emotion regulation abilities in SAD.
Neuroanatomical Model of Emotional Reactivity and Regulation in SAD
Numerous functional neuroimaging investigations of both healthy and clinical populations have contributed to an emerging neuroanatomical model of emotional reactivity and regulation21–24. In this limbic-cortical model, the ventral emotion system (i.e., limbic and paralimbic areas) detects personally relevant and affectively salient stimuli. A neural signal encoding potential threat is communicated to the rostral ACC which functions to monitor emotionally salient stimuli and trigger various cognitive regulatory processes25 in the dorsal medial and lateral prefrontal cortex (PFC)21 that select, implement and monitor cognitive control strategies. While there is ample evidence for the neural bases of emotional reactivity, no published neuroimaging studies have directly investigated cognitive-linguistic regulation in SAD.
Effective communication between the dorsal regulatory system and ventral emotion system constitutes a finely balanced functional brain network that uses feedback mechanisms from the PFC to limbic regions to modulate the trajectory of an emotional response. When functioning successfully, this network confers psychological resilience, flexibility, and well-being. When not functioning optimally, the limbic-cortical network may produce acute responses that influence ongoing emotion experience, autonomic psychophysiology, cognition, and subsequent emotions.
Recent work has begun to elucidate the neural bases of emotional reactivity. This work has revealed a network of ventral emotion detection/generation-related limbic regions, including the amygdala, insula, and anterior cingulate cortex (ACC). Diverse PFC regions also have been implicated in specific dimensions of emotion processing, including valence (ventromedial and dorsomedial PFC), intensity (ventrolateral and dorsomedial PFC), and recognition (perigenual ACC)26 as well as how task instruction (e.g., passive viewing versus judgment/rating) influence neural response to emotionally-evocative stimuli27.
One common stimulus used to probe emotional reactivity in SAD is harsh facial expressions displaying, for example, anger and contempt. Such expressions can serve as a potent signal communicating social disapproval for individuals with SAD. Viewing harsh faces has been shown to reliably activate negative emotions and amygdala response in adults28–30 and adolescents31, 32 with SAD, with greater SAD symptom severity predicting stronger amygdala response33, 34. Evidence also suggests abnormal neural response in regions interconnected with the amygdala in SAD, including increased activity in insular cortex in response to angry faces30, 35, in ACC in response to disgust faces36, and in parahippocampal gyrus, left ventrolateral and medial PFC in response to harsh faces28.
Other types of social threat stimuli also have been used to probe emotional reactivity in SAD. Anticipation and delivery of a speech have been shown to robustly activate fear processing in the amygdala37 in adults with SAD38, 39. In fact, SAD patients who responded to either group cognitive-behavioral therapy or SSRI treatment demonstrated significant reduction from pre- to post-treatment in amygdala response during a speech task.39 Additionally, post-treatment amygdala signal reduction during a speech task significantly predicted reduced social anxiety symptoms at one-year follow-up.
Despite advances in understanding emotional reactivity in SAD, the neuroanatomical model for emotion regulation has yet to be tested in SAD. Understanding PFC cognitive regulatory system recruitment in SAD during social threat may elucidate a functional neural profile that clarifies etiological and maintaining factors in SAD.
The Present Study
The goal of the present study was to extend our current understanding of the neural bases of SAD by probing emotional reactivity and regulation in adults with SAD compared to demographically-matched non-psychiatric healthy controls (HC). Previous fMRI studies in HC have found greater neural responses to violent scenes.40 We included violent scenes (i.e., physical threat) as a comparison condition for harsh faces, in order to investigate differential emotion regulation for social (SAD-related) and physical (SAD-unrelated) threat. We expected to find (1) no difference in SAD and HC for emotional reactivity and regulation for physical threat, (2) greater reactivity to harsh faces in SAD than HC, and (3) deficits in regulation in SAD versus HC for social threat stimuli.
Methods
Participants
Participants were 15 (9 females) right-handed adults who met DSM-IV41 criteria for current SAD and 17 (9 females) demographically-matched, right-handed healthy controls (HC) with no lifetime history of any DSM-IV psychiatric disorders. SAD and HC did not differ significantly in gender, age, education or ethnicity (Table 1). All participants provided informed consent in accordance with Stanford University’s Human Subjects Committee guidelines.
Table 1.
Demographic and Clinical Variables
| SAD n=15 Mean ± SD |
HC n=17 Mean ± SD |
t-value, p |
Partial eta2 |
|
|---|---|---|---|---|
| Gender | 9 female | 9 females | ||
| Age | 31.6 ± 9.7 | 32.1 ± 9.3 | 1.15 | .03 |
| Education | 16.3 ± 2.1 | 16.8 ± 2.4 | 1.01 | .02 |
| Ethnicity | ||||
| -Caucasian | 53% | 65% | ||
| -Asian | 33% | 29% | ||
| -Latino | 13% | 6% | ||
| LSAS-SR | 67.6 ± 21.1 | 29.3 ± 20.9 | 24.93 *** | .47 |
| BFNE | 44.1 ± 9.4 | 32.8 ± 5.2 | 16.36 *** | .37 |
| BDI-II | 11.9 ± 11.3 | 3.4 ± 2.6 | 7.99 ** | .22 |
| STAI-S | 38.7 ± 11.3 | 30.0 ± 8.4 | 5.76 * | .17 |
| PANAS-Neg | 19.8 ± 9.6 | 13.7 ± 4.2 | 5.16 * | .16 |
| PANAS-Pos | 31.3 ± 8.03 | 33.5 ± 8.7 | 0.44 | .02 |
p<.05,
p<.01,
p<.001
LSAS-SR=Liebowitz Social Anxiety Scale–Self-Report; BFNE=Brief Fear of Negative Evaluation; BDI-II=Beck Depression Inventory–II; STAI-S=Speilberger State Trait Anxiety Inventory-State Version; PANAS =Positive and Negative Affect Scale.
Exclusion Criteria
All participants passed a MRI safety screen. Participants were excluded if they reported current use of any psychotropic medication, history of neurological or cardiovascular disorders, diabetes, hypo- or hyperthyroidism, or head trauma with loss of consciousness greater than five minutes. Both healthy and SAD participants were excluded if they had a lifetime diagnosis of a psychotic disorder, mania, hypomania, bipolar disorder, or substance/alcohol abuse. Due to potential effects on blood flow, participants were asked not to consume alcohol, recreational drugs, or pain killers during the 24-hour period before their MR scan and not to ingest caffeine at least five hours prior to the scan. Daily cigarette users were excluded from the study. SAD participants were excluded if they met criteria for any current DSM-IV Axis I psychiatric disorders other than social anxiety, generalized anxiety, agoraphobia, or specific phobia disorders.
Clinical Assessment
Clinical diagnostic assessments were conducted by a PhD-trained clinical psychologist using the Anxiety Disorder Inventory Schedule–IV (ADIS-IV42) to diagnose current and lifetime psychiatric disorders. This structured clinical interview is based on the DSM-IV, but has been extended to be more sensitive in differential diagnosis of anxiety disorders. Only SAD participants with a primary diagnosis of SAD or HC participants with no history of DSM-IV disorders were invited to participate.
As shown in Table 1, compared to HC, SAD reported greater social anxiety (Liebowitz Social Anxiety Scale, LSAS43), fear of negative evaluation (Brief Fear of Negative Evaluation, BFNE44), depressive symptoms (Beck Depression Inventory-II, BDI-II45), state anxiety (Speilberger State-Trait Anxiety Inventory, STAI46), and negative affect (Positive and Negative Affect Schedule, PANAS47).
Procedure
Before scanning, participants were trained in accordance with methods developed by Gross and Ochsner48, 49, and practiced with two stimuli (not used in the scanning experiment) per neutral, social and physical condition (a) to “just look” without trying to control or modulate their emotional reactivity, and (b) to “regulate” by actively thinking in a way that modifies the interpretation of the stimulus and thus reduces negative reactions. Specifically, they were instructed to re-interpret the content of the picture using cognitive-linguistic strategies including “This does not involve me,” “This does not influence me,” or “This does not impact me” for harsh faces, and “The person will be okay,” “The person was not really hurt,” and so forth for the violent scenes.
During MR scanning, stimuli were projected to a screen inside the head-coil that was placed six inches from the participant’s eyes. Participants provided a negative emotion rating after each trial: “How negative do you feel?” (1=not at all, 2=slightly, 3=moderately, and 4=very much). Behavioral responses were made using a custom button box and recorded using Eprime software.
Experimental Task
The task consisted of 125 trials across three 9-minute functional runs (42, 42 and 41 trials, respectively) that were randomly ordered across participants. Within each run, stimuli were presented in a pseudo-randomized sequence (no more than two instances of the same condition in a row). There were 25 trials for each of five conditions: Look Harsh Face, Regulate Harsh Face, Look Violent Scene, Regulate Violent Scene, and Look Neutral Scene. Each 12s trial consisted of an instruction (Look or Regulate) (3s), stimulus (6s), and emotion rating (3s).
Stimuli
Prior fMRI studies have shown that direct-facing angry and contemptuous facial expressions produce strong neural responses in SAD28, 33. We thus trained actors to produce harsh expressions that combined angry and contempt facial expressions according to the Facial Action Coding System (FACS)50. Stimuli consisted of color photographs showing the actor’s head against a black background. Two independent raters trained in FACS coded each face stimulus for the presence of action units associated with anger (action unit 4, drawing together of the eyebrows, and action unit 7, tightening of both upper and lower eyelids) and contempt (unilateral action unit 14, dimple-smirk with no teeth bared). Face stimuli for which both raters fully agreed on facial action units were used in the study. The final face stimulus set consisted of 25 male and 25 female unique actors with 70% Anglo-American, 10% Asian-American, 10% Latin-American, and 10% African-American actors, which were equally distributed across Look and Regulate Harsh Face conditions.
Physical threat scenes, especially those displaying violence, have been shown to capture attention and produce robust neural activation in HC40. Thus, physical threat stimuli consisted of color photos of a person being violently attacked (e.g., punched, clubbed, stabbed, burnt, shot) by one or more aggressors. These high arousal, visually complex stimuli were collected from internet sites.
Neutral scenes, used as the baseline comparison for both social and physical threat, consisted of non-arousing, non-social color photos of mundane scenes (e.g., pavement, garage door, wood siding). Neutral facial expressions were not used as a contrast to harsh faces because of evidence that individuals with SAD interpret neutral face stimuli more negatively then do HC28, 51. Examples of the three stimulus types are shown in Figure 1.
Figure 1.
Exemplars of Neutral, Harsh Faces, and Violent Scenes.
Image Acquisition
Imaging was performed on a GE 3-Tesla Signa magnet with a T2*-weighted gradient echo spiral-in/out pulse sequence52 and a custom-built quadrature “dome” elliptical bird cage head-coil. Head movement was minimized using a bite-bar and foam padding. Across three functional runs, 1,114 functional volumes were obtained from 22 sequential axial slices [TR=1500 ms, TE=30 ms, flip angle=60 degrees, FOV=22 cm, matrix=64×64, single-shot, resolution=3.438 mm2 × 5 mm]. 3D high-resolution anatomical scans were acquired using fast spin-echo SPGR (.85942 × 1.5 mm; FOV=22 cm, frequency encoding=256).
fMRI Data Preprocessing
Each functional run was subjected to preprocessing steps using AFNI53 software: co-registration, motion correction, 4 mm3 isotropic Gaussian spatial-smoothing, high-pass filtering (.011 Hz), and linear detrending. No volumes demonstrated motion in the x, y, or z directions in excess of ±0.5 mm. There was no evidence of stimulus-correlated motion, as assessed by correlations between condition-specific reference functions and x, y, z motion correction parameters (all ps>.5).
fMRI Statistical Analysis
A multiple-regression model implemented with AFNI 3dDeconvolve included baseline parameters to remove mean, linear and quadratic trends, and motion-related variance. BOLD responses during the 6s when looking or regulating were investigated using regressors (convolved with the gamma variate model54 of the hemodynamic response function) for each of the five conditions (Look Neutral Scene, Look Harsh Face, Look Violent Scene, Regulate Harsh Face, Regulate Violent Scene). fMRI BOLD signal intensity was represented as percent signal change ((MR signal per voxel per time point / mean MR signal in that voxel for the entire functional run) × 100). The differential BOLD signal between target and comparison conditions (e.g., Regulate versus Look Harsh Face) is reported as BOLD percent signal change, an effect size measure.
Individual brain maps were converted to Talairach atlas space55 and second-level group statistical parametric maps were produced according to a random-effects model. To correct for multiple comparisons, AlphaSim, a Monte Carlo simulation bootstrapping program in the AFNI library, was employed to protect against false positives56. This method uses a voxel-wise and cluster volume joint-probability threshold to establish a cluster-wise false positive cluster detection level. For all contrasts, a threshold consisting of a voxel-wise p<0.005 and cluster volume >162 mm3 (4 voxels × 3.438 mm3) protected against false positive cluster detection at p<0.01.
Results
We examined the effects of emotional reactivity and cognitive regulation on both negative emotion ratings and fMRI BOLD signal during social (harsh face) and physical (violent scene) threat. Additionally, we investigated the relationship of social anxiety symptom severity with neural and behavioral indices of emotional reactivity and regulation.
Emotional Reactivity
Behavioral responses
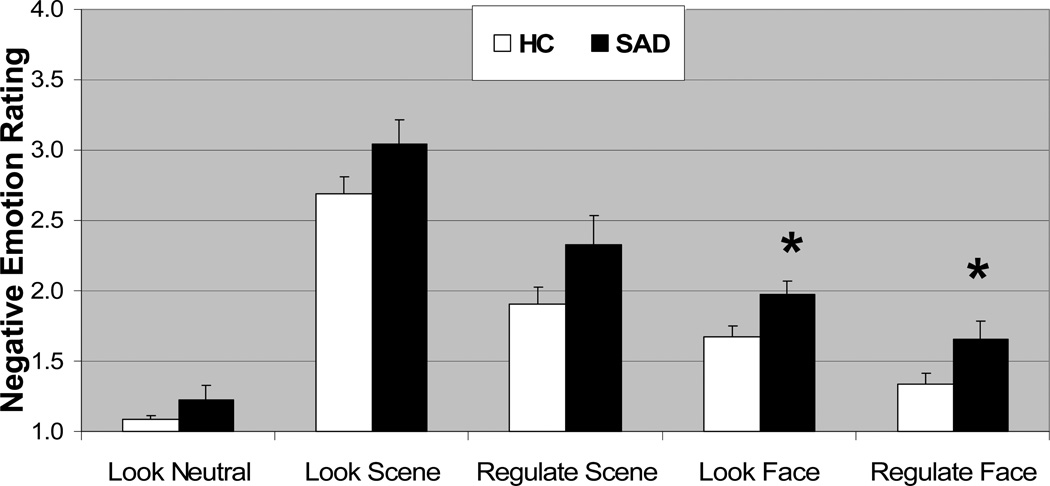
A 2 (Group: SAD, HC) × 3 (Condition: Look Neutral Scene, Look Harsh Face, Look Violent Scene) repeated-measures ANOVA of negative emotion ratings resulted in no interaction of Group × Condition, p>.42. There were main effects of group, SAD>HC, F(2,30)=7.32, p<.05, eta2 (η2) =.20, and of condition, F(2,30)=229.78, p<.001, η2=.88, with Violent Scene>Harsh Face>Neutral Scene (p<.001 for each comparison) as shown in Figure 2.
Figure 2.
Negative emotion ratings for Look Neutral Scenes, Look and Regulate Violent Scenes, and Look and Regulate Harsh Faces in SAD and HC. Negative emotion ratings after the offset of each stimulus were provided by participants in response to “How negative do you feel?” (1=not at all, 2=slightly, 3=moderately, and 4=very much).
* p < 0.05; Error bars = SEM
Neural responses
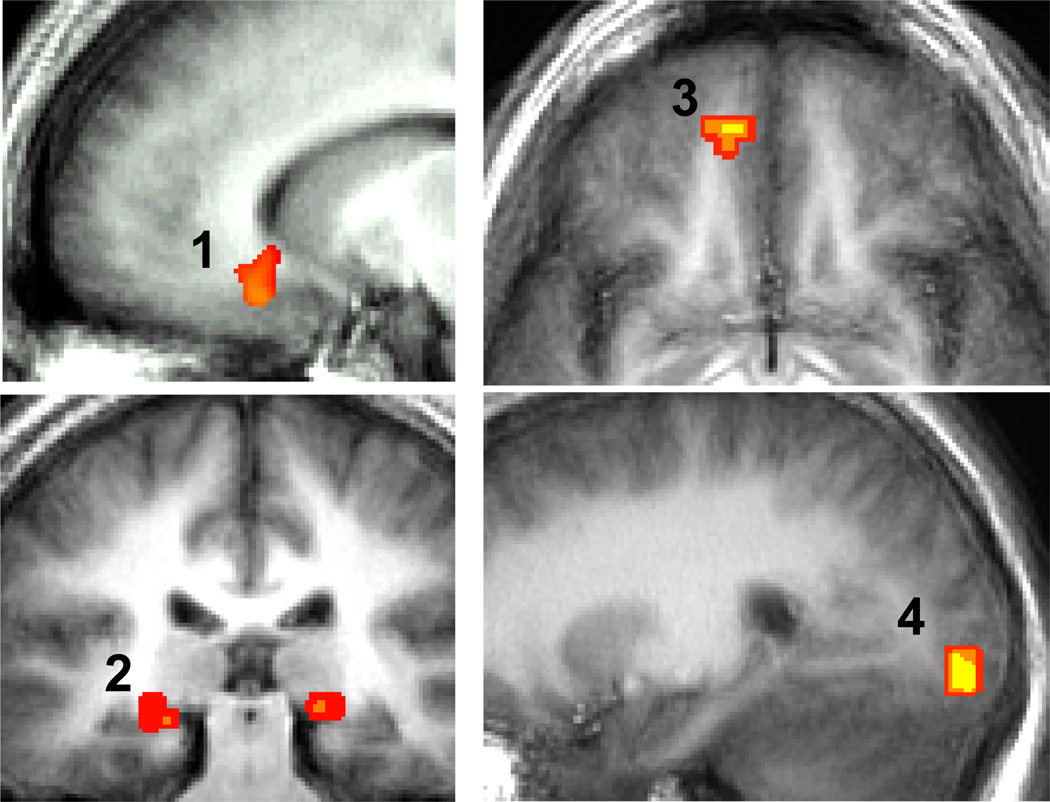
For social threat, a between-group t-test for the Look Harsh Face versus Neutral Scene contrast resulted in significantly greater BOLD responses in SAD versus HC in brain regions implicated in emotion (medial OFC, subgenual ACC, bilateral parahippocampal gyrus), ventral/dorsal visual processing (lingual and inferior occipital gyrus, superior and middle occipital gyrus, cuneus, superior parietal lobule), face-selective processing (lateral occipital cortex (LOC), but not fusiform face area (FFA)), and sensory processing (postcentral gyrus) (Table 2, Figure 3). Compared to SAD, HC had greater BOLD signal in regions implicated in attention processing (medial precuneus, left inferior parietal lobule and right supramarginal gyrus). Both groups had bilateral dorsal/extended amygdala and face-selective LOC responses for the contrast of Look Harsh Face versus Neutral Scene (Supplemental Table 1, 2). However, only SAD produced evidence of FFA responses. For physical threat, a between-group t-test for the Look Violent versus Neutral Scene found no between-group differences. Both groups had left dorsal/extended amygdala, bilateral FFA and LOC responses for the contrast of Look Violent versus Neutral Scene (Supplemental Table 3, 4).
Table 2.
Differential BOLD Responses for Look Harsh Faces versus Neutral Scenes in SAD versus HC
| Brain Regions | BA | x y z of peak |
Percent Change |
Vol (mm3) |
t- value |
|
|---|---|---|---|---|---|---|
| HC | SAD | |||||
| SAD > HC | ||||||
| Frontal Lobes | ||||||
| Medial OFC | 11 | −8 49 −9 | .05 | .22 | 244 | 4.06 |
| L Subgenual ACC | 25 | −13 25 −12 | .01 | .12 | 813 | 3.37 |
| Temporal Lobes | ||||||
| L Parahippocampal Gyrus | 28 | −21 −27 −6 | .06 | .14 | 244 | 3.56 |
| R Parahippocampal Gyrus | 28 | 19 −28 −3 | .05 | .13 | 324 | 3.14 |
| Parietal Lobes | ||||||
| L Postcentral Gyrus | 1, 3 | −55 −24 56 | −.07 | .18 | 2723 | 3.16 |
| L Postcentral Gyrus | 3 | −43 −17 49 | −.04 | .09 | 812 | 3.15 |
| L Superior Parietal Lobule | 7 | −21 −55 67 | −.03 | .18 | 203 | 3.04 |
| Occipital Lobes | ||||||
| L Middle Occipital Gyrus | 39 | −40 −74 18 | .04 | .20 | 203 | 3.60 |
| R Inferior Occipital Gyrus | 18 | 24 −89 −5 | .05 | .29 | 610 | 3.44 |
| R Lingual Gyrus | 19 | 10 −55 −2 | −.05 | .14 | 244 | 3.36 |
| R Cuneus | 18 | 20 −79 28 | .08 | .25 | 366 | 3.52 |
| HC > SAD | ||||||
| Occipital Lobes | ||||||
| L Medial Precuneus | 31 | 7 −54 35 | .8 | −.03 | 203 | 3.13 |
| L Inferior Parietal Lobule | 40 | −49 −55 48 | .12 | −.02 | 203 | 3.14 |
| R Supramarginal Gyrus | 40 | 60 −54 29 | .15 | .03 | 447 | 3.99 |
Note. t-value threshold≥3.034, voxel p<0.005, minimum cluster volume threshold ≥163 mm3 (4 voxels × 3.438 mm3), cluster-wise p<0.01. BA=Brodmann Area, ACC=anterior cingulate cortex, L=left, OFC=orbitofrontal cortex, R=right. Coordinates based on Talairach & Tournoux Deamon Atlas.
Figure 3.
SAD > HC BOLD Signal for Look Harsh Faces versus Neutral Scenes.
1. Subgenual Anterior Cingulate Cortex BA25, x=−10; 2. bilateral Parahippocampal Gyrus BA28, y=−27; 3. Medial Orbitofrontal Cortex BA11, z=−8; 4. Inferior Occipital Gyrus BA18, x=24
Emotion Regulation
Behavioral responses
A 2 (Group: SAD and HC) × 2 (Condition: Regulate Violent Scene, Regulate Harsh Face) repeated-measures ANOVA of negative emotion ratings showed no evidence of an interaction, p>.67. There was a main effect of condition, Regulate Violent Scene>Regulate Harsh Face, F(1,31)=47.19, p<.001, η2=.61, but no effect of group, p>.10. There were no group differences in the percent reduction in negative emotion following emotion regulation for social (HC=18.8%±17.3% vs. SAD=16.9%±19.0%, p>.76) or physical threat (HC=28.8%±14.8% vs. SAD=25.0%±16.3%, p>.48).
Neural Responses
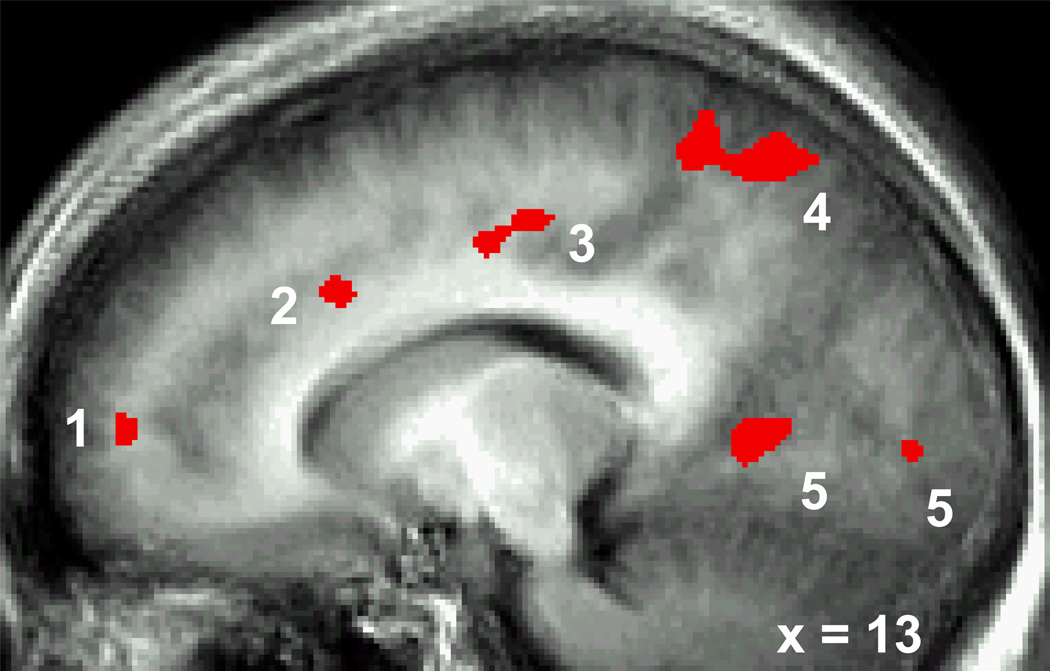
For social threat (Table 3, Figure 4), a between-group t-test of the Regulate versus Look Harsh Face contrast showed that, compared to SAD, HC produced greater BOLD responses in brain regions implicated in cognitive control (dorsolateral PFC, dorsal ACC), visual attention (medial cuneus, posterior cingulate), attention areas (bilateral dorsal parietal), and visual feature detection (bilateral fusiform, superior temporal gyrus). No brain areas showed greater BOLD responses in SAD compared to HC. For physical threat (Table 4), a between-group t-test of the Regulate versus Look Violent Scenes contrast demonstrated that, compared to HC, SAD had greater BOLD response in right mid-dorsolateral PFC, and bilateral lentiform/caudate. Compared to SAD, HC produced greater BOLD responses for Regulate versus Look Violent Scene in a motor area of right middle frontal gyrus and left superior temporal gyrus.
Table 3.
Differential BOLD Responses for Regulate versus Look Harsh Faces in SAD versus HC
| Brain Regions | BA | x y z of peak |
Percent Change |
Vol (mm3) |
t-value | |
|---|---|---|---|---|---|---|
| HC | SAD | |||||
| SAD > HC No results | ||||||
| HC > SAD | ||||||
| Frontal Lobes | ||||||
| Medial PFC | 10 | 7 51 8 | .22 | .2 | 560 | 3.55 |
| Supragenual ACC | 24 | 10 −6 43 | .12 | −.12 | 528 | 3.10 |
| L Middle Frontal Gyrus | 6 | −24 −10 46 | .12 | −.12 | 528 | 3.04 |
| R Posterior Insula | 13 | 41 −20 5 | .14 | −.13 | 284 | 3.37 |
| R Precentral Gyrus | 4 | 41 −20 60 | .13 | −.18 | 163 | 2.80 |
| Occipital Lobes | ||||||
| Medial Cuneus | 19 | −7 −89 32 | .15 | −.13 | 406 | 3.42 |
| R Lingual Gyrus | 17 | 10 −89 5 | .15 | −.16 | 366 | 3.30 |
| L Lingual Gyrus | 19 | −21 −61 1 | .12 | −.16 | 244 | 3.13 |
| Parietal Lobes | ||||||
| R Postcentral Gyrus | 3 | 45 −20 53 | .15 | −.14 | 1300 | 2.73 |
| R Posterior Cingulate, Cuneus | 30 | 14 −58 8 | .11 | −.11 | 488 | |
| R Superior Parietal Lobule | 7 | 21 −65 63 | .17 | −.10 | 2845 | 3.79 |
| R Superior Parietal Lobule | 7 | 31 −48 63 | .14 | −.15 | 488 | 3.35 |
| L Inferior Parietal Lobule | 40 | −41 −37 50 | .11 | −.16 | 244 | 2.67 |
| R Posterior Cingulate | 30 | 17 −58 15 | .10 | −.17 | 163 | 3.02 |
| L Superior Parietal Lobule | 7 | −21 −48 70 | .14 | −.15 | 163 | 3.27 |
| Temporal Lobes | ||||||
| R Fusiform Gyrus | 37 | 41 −55 −19 | .16 | −.16 | 406 | 3.30 |
| L Superior Temporal Gyrus | 42 | −58 −17 12 | .13 | −.19 | 975 | 2.66 |
| R Superior Temporal Gyrus | 42 | 62 −17 15 | .16 | −.14 | 284 | 2.78 |
| L Fusiform Gyrus | 19 | −24 −68 −19 | .18 | −.11 | 163 | 3.63 |
| R Superior Temporal Gyrus | 22 | 65 −13 5 | .16 | −.15 | 163 | 3.13 |
Note: t-value threshold≥3.034, voxel p<0.005, minimum cluster volume threshold ≥163 mm3 (4 voxels × 3.438 mm3), cluster-wise p<0.01. ACC=anterior cingulate cortex, L=left, PFC=prefrontal cortex, R=right. Coordinates based on Talairach & Tournoux Deamon Atlas.
Figure 4.
HC > SAD BOLD Signal for Regulation versus Look Harsh Faces.
1. Medial Prefrontal Cortex, 2. Supragenual ACC, 3. Posterior Cingulate, 4. Precuneus/Superior Parietal Lobule, 5. Lingual Gyrus
Table 4.
Differential BOLD Responses for Regulate versus Look Violent Scenes in SAD versus HC
| Brain Regions | BA | x y z at peak |
Percent Change |
Vol (mm3) |
t-value | |
|---|---|---|---|---|---|---|
| HC | SAD | |||||
| SAD > HC | ||||||
| Frontal Lobes | ||||||
| R Mid-Dorsolateral PFC | 9 | 21 49 29 | .00 | .16 | 463 | 3.07 |
| Subcortical | ||||||
| R Lentiform/Cuadate | 10 4 −6 | −.04 | .13 | 244 | 3.79 | |
| L Lentiform/Caudate | −10 −4 −6 | −.06 | .12 | 285 | 3.04 | |
| HC > SAD | ||||||
| Frontal Lobes | ||||||
| R Middle Frontal Gyrus/Pre-motor Cortex |
6 | 58 4 36 | .14 | −.06 | 203 | 3.11 |
| Temporal Lobes | ||||||
| L Superior Temporal Gyrus | 22 | −62 −20 5 | .17 | .00 | 263 | 3.41 |
| L Superior Temporal Gyrus | 41 | −52 −27 12 | .15 | −.02 | 263 | 3.18 |
Note: t-value threshold≥3.034, voxel p<0.005, minimum cluster volume threshold ≥163 mm3 (4 voxels × 3.438 mm3), cluster-wise p<0.01. L=left, PFC=prefrontal cortex, R=right. Coordinates based on Talairach & Tournoux Deamon Atlas.
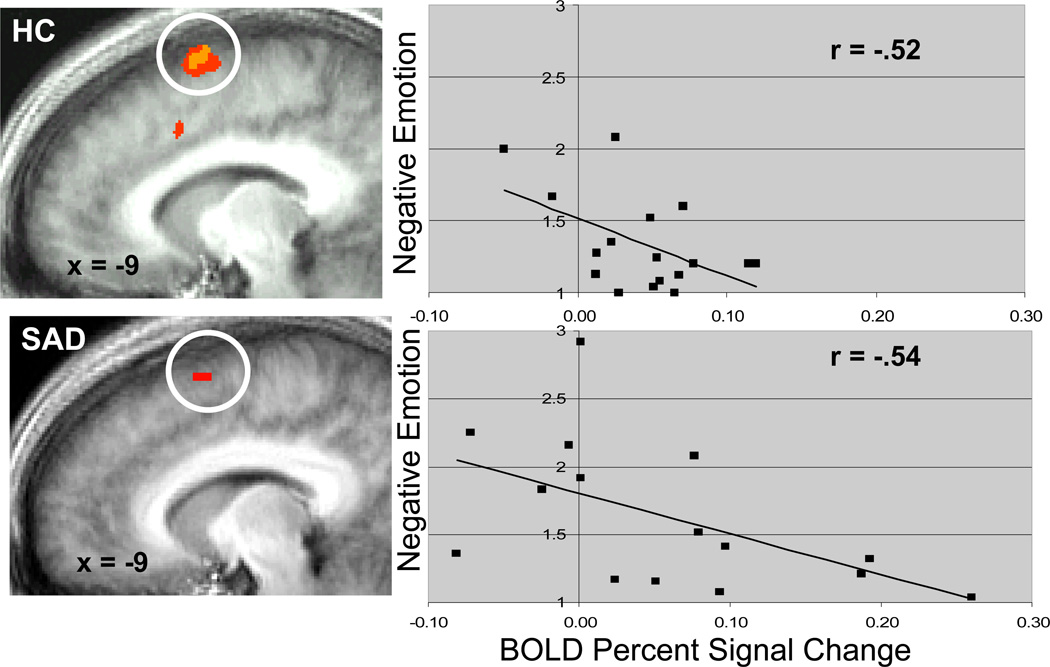
Similar regions of activation for both groups during regulation of social threat included cognitive control regions (dorsomedial PFC and right superior frontal gyrus) and linguistic regions (left inferior frontal gyrus, left supramarginal gyrus, and bilateral posterior superior temporal gyrus). In SAD and HC, greater BOLD signal in dorsomedial PFC during cognitive regulation was associated with significant reduction in negative emotion ratings (Figure 5).
Figure 5.
Dorsomedial Prefrontal Cortical BOLD Activation During Regulation Predicts Reduction in Negative Emotion Experience Ratings.
Emotional Reactivity, Regulation, and Social Anxiety Severity
Social anxiety severity (measured by the Liebowitz Social Anxiety Scale) was positively associated with BOLD signal in SAD and inversely with BOLD signal in HC during Look Harsh Face in left dorsal/extended amygdala, right middle occipital gyrus (Fisher's z-test ps<.05). When social anxiety severity was measured by the Brief Fear of Negative Evaluation, the same pattern of positive correlation in SAD and inverse correlation in HC during Look Harsh Face was observed in bilateral dorsal/extended amygdala, posterior cingulate and precuneus (Fisher's z-test ps<.05). There was no relationship of social anxiety symptom severity with BOLD responses (a) during looking at violent scenes, and (b) during emotion regulation.
Discussion
The goal of this study was to investigate the neural bases of emotional reactivity and cognitive regulation in adults diagnosed with SAD versus HC. Using both social and physical threat stimuli, we were able to examine the specificity of emotional reactivity and emotion regulation abnormalities in SAD. The primary finding was that, compared to demographically-matched HC, SAD demonstrated exaggerated negative emotion reactivity and reduced cognitive-linguistic regulation related neural activation specifically for social threat stimuli.
Emotional Reactivity
Behaviorally, compared to HC, SAD reported greater negative emotion experience for both social and physical threat, suggesting elevated emotional reactivity across these two types of threat stimuli. Neurally, while there was no between-group difference for physical threat, viewing social threat stimuli resulted in greater differential BOLD responses in SAD compared to HC in emotion21 (medial OFC, subgenual cingulate, parahippocampal gyrus), visual, face, and sensory processing brain regions. For both social and physical threat compared to neutral stimuli, both groups reported elevated negative emotion and enhanced BOLD signal in dorsal/extended amygdala, providing converging evidence for successful acute negative emotion induction. Additionally, while both groups had bilateral face-selective LOC responses for social threat, and bilateral LOC and FFA for physical threat, only SAD had FFA activation for social (harsh face) threat.
These results converge with prior findings of recognition bias and negative emotion reactivity to harsh faces in SAD28, 33, 57–59, and neural bases of emotion processing in primates60–62. Medial PFC and parahippocampal activations have been observed in a previous study of reactivity to harsh faces28 and may be related to higher-order neural representations of self-focused attention, perspective taking63, and greater emotion intensity26 that may be exaggerated in SAD. Insular responses to emotional face stimuli have also been observed in SAD29 and are implicated in interoceptive processing of bodily sensations64. Both the FFA and LOC have subregions that are highly selective to faces and different objects65, 66 which accounts for activation of these visual processing regions in both groups. However, elevated dorsal and ventral visual processing activations in general, and in the FFA specifically during harsh face processing in SAD versus HC confirms findings of enhanced visual processing in SAD for facial emotion stimuli35.
Both groups produced dorsal/extended amygdala responses to harsh (i.e., mixed anger and contempt) facial expressions presented for 6s. While several prior studies of harsh facial expression have found greater amygdala response in SAD versus HC28, 29, 33–35, the present study utilized a face displaying a mixed emotion (anger+contempt), and included longer stimulus presentation times. These stimulus parameters differentiate this study from prior studies, and may increase the likelihood that HC will, like SAD, evaluate the stimuli as threatening.
Social anxiety symptom severity was associated with significantly greater BOLD signal in response to viewing social threat (but not physical threat) in SAD versus HC in brain regions implicated in emotion (bilateral dorsal/extended amygdala)37, visual attention (posterior cingulate cortex, and right middle occipital gyrus), and attentional control (right dorsolateral PFC)67. Our findings replicate previous studies that reported an association of social anxiety symptoms and amygdala response in adults33 and adolescents32 with SAD. Furthermore, recent neural models demonstrate that fear-related amygdala activity can directly modulate attentional process68. This aligns with cognitive information processing models of SAD that propose a vigilance-avoidance pattern involving automatic allocation of attention towards potential threat immediately followed by inhibition and avoidance of the threat signals69, 70. Accordingly, due to sensitivity to social threat cues, SAD should be associated with rapid initial orientating towards facial expressions that suggest social disapproval, and then turning attention away as an overlearned protective response.
Emotion Regulation
Behaviorally, SAD and HC reported similar reductions in negative emotion following cognitive regulation for both physical and social threat. However, because of greater initial negative emotion for physical versus social threat, post-regulation negative emotion continued to be greater for physical versus social threat. This indicates that all participants were able to down-regulate negative emotion using cognitive-linguistic strategies, and that the physical threat scenes were emotionally more evocative than the social threat stimuli.
Neurally, during cognitive regulation both groups had neural activity in dorsomedial and dorsolateral PFC regions supporting cognitive regulation21 (e.g., strategy selection, implementation, monitoring) and in a linguistic network including left inferior frontal, supramarginal, and posterior superior temporal regions71. These data are consistent with prior findings of cognitive down-regulation of emotion17 and the neural bases of cognitive emotion regulation in non-psychiatric adults48, 49, 72, 73. Prior studies have also observed dissociation between self-report ratings and physiological responses during anxiety-inducing experimental tasks74, 75. Importantly, these findings demonstrate that, when cued in a controlled context, SAD can implement cognitive-linguistic regulation strategies.
Between-group analyses revealed that during regulation of social threat, compared to SAD, HC had a distributed pattern of neural activity implicated in cognitive regulation, attention, and visual processing. Specifically, during regulation of social threat, both compared to SAD and within-group, HC produced greater neural responses in both dorsomedial and dorsolateral PFC, suggesting an enhanced coordination of cognitive control circuitry not shown in SAD. Reciprocal modulation and attenuation in medial and lateral prefrontal cortex have previously been shown as a potential neural mechanism for emotion-cognitive interactions76. The differential pattern observed here in response to social threat stimuli suggests that greater emotional reactivity in SAD may be associated with enhanced medial PFC and concurrent attenuation of recruitment of dorsolateral PFC. In contrast, during regulation of physical threat, differential BOLD responses were observed in SAD in DLPFC and lentiform/caudate, and in HC in pre-motor and superior temporal cortex.
These results suggest that SAD may be less able to access and implement cognitive-linguistic emotion regulation strategies during social threat conditions, while showing relatively few differences from HC during regulation of physical threat. This supports the specificity of neural responses to disorder-relevant social threat stimuli in SAD. Furthermore, to compensate for high levels of initial reactivity, SAD may need to train in emotion regulation skills that specifically enhance the implementation and effectiveness of cognitive and attention regulation.
Implications for Psychopathology and Treatment
Exaggerated emotional reactivity and affective dysregulation are thought to be core features of many psychiatric problems20. The present study indicates that individuals with SAD (a) experience elevated negative emotion in response to social threat, (b) demonstrate the greatest difference from HC in cognitive control related brain regions during regulation of social threat, but (c) can implement emotion regulation during social and physical threat, when cued to do so.
These results suggest that SAD may be less able to access and implement cognitive-linguistic emotion regulation skills without an external cue during social threat conditions, while showing relatively no difference in neural activation from HC during emotional reactivity and regulation of physical threat. This supports the specificity of neural responses to disorder-relevant social threat stimuli in SAD. Furthermore, in order to reduce negative emotional reactivity to the same levels as HC, SAD may need to train in emotion regulation strategies that specifically enhance the implementation and effectiveness of cognitive and attention regulation.
Thus, difficulties in regulation in SAD may be to lack of skill in applying regulation strategies. If this is correct, in addition to expanding the repertoire of emotion regulation strategies, clinical interventions need to increase accessibility and effective implementation of these regulation strategies. Training in implementing emotion regulation strategies in anticipation of and during social situations should enhance both accessibility and confidence in effective regulation. Understanding how social anxiety primes or entrains brain-behavioral systems towards emotional hyper-reactivity may help patients and clinicians better appreciate the experience of “limbic override” of PFC-related regulation attempts. Training in different forms of PFC-mediated cognitive and attentional control systems, for example, inhibition of cognitive elaboration, re-allocation of attentional focus, cognitive diffusion, may result in new forms of emotion learning and self-regulation instantiated by re-setting the relative weights of limbic and PFC systems, and modulating the trajectory of emotion experience.
Limitations
The current study is limited to inferences related to only one type of social threat (harsh facial expressions) and one type of non-social threat (violence scenes). This study used the same comparison condition (non-social neutral visual scenes) for both social and physical threat. It is important to note that the neutral scenes were not matched to violent scenes or harsh faces on a range of stimulus features, including number of actors, facial expressions, and complexity. Using neutral faces from the same set of actors who displayed harsh facial expressions, and the same people in a peaceful interaction in contrast to the physically violent interactions might serve as a better matched control for the social and physical threat, respectfully, in future studies. Still, using neutral scenes had the advantage that both types of threat were compared to the same comparison condition thereby reducing possible BOLD signal variability in the baseline comparison condition. One of the complexities associated with neutral faces is that prior studies indicate that they are not perceived as neutral by individuals with SAD51. Thus, some studies have used happy, not neutral, facial expressions as the comparison condition28. Investigating emotion regulation in response to a variety of threat stimuli and adding a non-SAD psychiatric comparison group will help identify the specificity of emotional reactivity and regulation in SAD. Similarly, comparison of different types of emotion regulation (e.g., linguistic, attention, distraction, visualization) will deepen our understanding of the typology of emotion regulation strategies. Additionally, the current study examined only a short duration of emotion regulation (6s) and punctate emotion experience ratings. Future studies may benefit from examining temporal dynamics of emotional reactivity and regulation by collecting continuous measures of emotion experience over durations longer than 6s with emotionally-evocative situations that more closely reflect real-life situations. Addressing these limitations will clarify the neurobehavioral bases of emotional reactivity and regulation. This may in turn help clinical researchers and patients better understand the pre-onset risk, maintaining, and relapse prevention factors that characterize anxiety disorder.
Supplementary Material
Acknowledgments
This research was supported by NIMH Grant MH58147 to James Gross, NIMH Postdoctoral Fellowship and Mind and Life Summer Research Institute grant to Philippe Goldin, and a NARSAD award to Turhan Canli.
Philippe Goldin had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
The authors of this manuscript do not have any direct or indirect conflicts of interest to disclose.
References
- 1.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 2.Jefferys D. Social phobia: The most common anxiety disorder. Australian Family Physician. 1997;26:1061, 1064–1067. [PubMed] [Google Scholar]
- 3.Kessler RC, McGonagle KA, Zhao S, et al. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Results from the National Comorbidity Survey. Archives of General Psychiatry. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- 4.Otto MW, Pollack MH, Maki KM, et al. Childhood history of anxiety disorders among adults with social phobia: Rates, correlates, and comparisons with patients with panic disorder. Depression and Anxiety. 2001;14:209–213. doi: 10.1002/da.1068. [DOI] [PubMed] [Google Scholar]
- 5.Lampe L, Slade T, Issakidis C, Andrews G. Social phobia in the Australian National Survey of Mental Health and Well-Being (NSMHWB) Psychological Medicine. 2003;33:637–646. doi: 10.1017/s0033291703007621. [DOI] [PubMed] [Google Scholar]
- 6.Matza LS, Revicki DA, Davidson JR, Stewart JW. Depression with atypical features in the National Comorbidity Survey: Classification, description, and consequences. Archives of General Psychiatry. 2003;60:817–826. doi: 10.1001/archpsyc.60.8.817. [DOI] [PubMed] [Google Scholar]
- 7.Randall CL, Thomas S, Thevos AK. Concurrent alcoholism and social anxiety disorder: a first step toward developing effective treatments. Alcoholism, Clinical and Experimental Research. 2001;25:210–220. [PubMed] [Google Scholar]
- 8.Schneier FR, Heckelman LR, Garfinkel R, et al. Functional impairment in social phobia. Journal of Clinical Psychiatry. 1994;55:322–331. [PubMed] [Google Scholar]
- 9.Lochner C, Mogotsi M, du Toit PL, Kaminer D, Niehaus DJ, Stein DJ. Quality of life in anxiety disorders: a comparison of obsessive-compulsive disorder, social anxiety disorder, and panic disorder. Psychopathology. 2003;36:255–262. doi: 10.1159/000073451. [DOI] [PubMed] [Google Scholar]
- 10.Clark DM, Wells A. A cognitive model of social phobia. New York, NY: Guilford Press; 1995. [Google Scholar]
- 11.Rapee RM. Descriptive psychopathology of social phobia. New York: Guilford Press; 1995. [Google Scholar]
- 12.Stein MB, Kean YM. Disability and quality of life in social phobia: Epidemiologic findings. American Journal of Psychiatry. 2000;157:1606–1613. doi: 10.1176/appi.ajp.157.10.1606. [DOI] [PubMed] [Google Scholar]
- 13.Clark DM, McManus F. Information processing in social phobia. Biological Psychiatry. 2002;51:92–100. doi: 10.1016/s0006-3223(01)01296-3. [DOI] [PubMed] [Google Scholar]
- 14.Rapee RM, Heimberg RG. A cognitive-behavioral model of anxiety in social phobia. Behaviour Research And Therapy. 1997;35:741–756. doi: 10.1016/s0005-7967(97)00022-3. [DOI] [PubMed] [Google Scholar]
- 15.Hermann C, Ofer J, Flor H. Covariation bias for ambiguous social stimuli in generalized social phobia. Journal of Abnormal Psychology. 2004;113:646–653. doi: 10.1037/0021-843X.113.4.646. [DOI] [PubMed] [Google Scholar]
- 16.Hofmann SG. Cognitive mediation of treatment change in social phobia. Journal of Consulting and Clinical Psychology. 2004;72:393–399. doi: 10.1037/0022-006X.72.3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gross JJ. Emotion regulation: affective, cognitive, and social consequences. Psychophysiology. 2002;39(3):281–291. doi: 10.1017/s0048577201393198. [DOI] [PubMed] [Google Scholar]
- 18.Abelson JL, Liberzon I, Young EA, Khan S. Cognitive modulation of the endocrine stress response to a pharmacological challenge in normal and panic disorder subjects. Arch Gen Psychiatry. 2005;62(6):668–675. doi: 10.1001/archpsyc.62.6.668. [DOI] [PubMed] [Google Scholar]
- 19.Gross JJ. The Handbook of Emotion Regulation. New York: Guilford Press; 2007. [Google Scholar]
- 20.Campbell-Sills L, Barlow DH. Incorporating emotion regulation into conceptualizations and treatments of anxiety and mood disorders. In: Gross JJ, editor. Handbook of emotion regulation. New York: Guilford; 2007. pp. 542–559. [Google Scholar]
- 21.Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 22.Kim SH, Hamann S. Neural correlates of positive and negative emotion regulation. J Cogn Neurosci. 2007;19(5):776–798. doi: 10.1162/jocn.2007.19.5.776. [DOI] [PubMed] [Google Scholar]
- 23.Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biol Psychiatry. 2008;63(6):577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor SF, Liberzon I. Neural correlates of emotion regulation in psychopathology. Trends Cogn Sci. 2007;11(10):413–418. doi: 10.1016/j.tics.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Mohanty A, Engels AS, Herrington JD, et al. Differential engagement of anterior cingulate cortex subdivisions for cognitive and emotional function. Psychophysiology. 2007;44(3):343–351. doi: 10.1111/j.1469-8986.2007.00515.x. [DOI] [PubMed] [Google Scholar]
- 26.Grimm S, Schmidt CF, Bermpohl F, et al. Segregated neural representation of distinct emotion dimensions in the prefrontal cortex-an fMRI study. NeuroImage. 2006;30(1):325. doi: 10.1016/j.neuroimage.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 27.Taylor SF, Phan KL, Decker LR, Liberzon I. Subjective rating of emotionally salient stimuli modulates neural activity. Neuroimage. 2003;18(3):650–659. doi: 10.1016/s1053-8119(02)00051-4. [DOI] [PubMed] [Google Scholar]
- 28.Stein MB, Goldin PR, Sareen J, Zorrilla LT, Brown GG. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Archives of General Psychiatry. 2002;59:1027–1034. doi: 10.1001/archpsyc.59.11.1027. [DOI] [PubMed] [Google Scholar]
- 29.Yoon KL, Fitzgerald DA, Angstadt M, McCarron RA, Phan KL. Amygdala reactivity to emotional faces at high and low intensity in generalized social phobia: a 4-Tesla functional MRI study. Psychiatry Res. 2007;154(1):93–98. doi: 10.1016/j.pscychresns.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 30.Straube T, Kolassa IT, Glauer M, Mentzel HJ, Miltner WH. Effect of task conditions on brain responses to threatening faces in social phobics: An event-related functional magnetic resonance imaging study. Biological Psychiatry. 2004;56:921–930. doi: 10.1016/j.biopsych.2004.09.024. [DOI] [PubMed] [Google Scholar]
- 31.McClure EB, Monk CS, Nelson EE, et al. Abnormal attention modulation of fear circuit function in pediatric generalized anxiety disorder. Arch Gen Psychiatry. 2007;64(1):97–106. doi: 10.1001/archpsyc.64.1.97. [DOI] [PubMed] [Google Scholar]
- 32.Killgore WD, Yurgelun-Todd DA. Social anxiety predicts amygdala activation in adolescents viewing fearful faces. Neuroreport. 2005;16(15):1671–1675. doi: 10.1097/01.wnr.0000180143.99267.bd. [DOI] [PubMed] [Google Scholar]
- 33.Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME. Association between Amygdala Hyperactivity to Harsh Faces and Severity of Social Anxiety in Generalized Social Phobia. Biol Psychiatry. 2006;59(5):424–429. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 34.Evans KC, Wright CI, Wedig MM, Gold AL, Pollack MH, Rauch SL. A functional MRI study of amygdala responses to angry schematic faces in social anxiety disorder. Depress Anxiety. 2007 doi: 10.1002/da.20347. [DOI] [PubMed] [Google Scholar]
- 35.Straube T, Mentzel HJ, Miltner WH. Common and distinct brain activation to threat and safety signals in social phobia. Neuropsychobiology. 2005;52(3):163–168. doi: 10.1159/000087987. [DOI] [PubMed] [Google Scholar]
- 36.Amir N, Klumpp H, Elias J, Bedwell JS, Yanasak N, Miller LS. Increased activation of the anterior cingulate cortex during processing of disgust faces in individuals with social phobia. Biol Psychiatry. 2005;57(9):975–981. doi: 10.1016/j.biopsych.2005.01.044. [DOI] [PubMed] [Google Scholar]
- 37.LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 38.Tillfors M, Furmark T, Marteinsdottir I, et al. Cerebral blood flow in subjects with social phobia during stressful speaking tasks: A PET study. American Journal of Psychiatry. 2001;158:1220–1226. doi: 10.1176/appi.ajp.158.8.1220. [DOI] [PubMed] [Google Scholar]
- 39.Furmark T, Tillfors M, Marteinsdottir I, et al. Common changes in cerebral blood flow in patients with social phobia treated with citalopram or cognitive-behavioral therapy. Archives of General Psychiatry. 2002;59:425–433. doi: 10.1001/archpsyc.59.5.425. [DOI] [PubMed] [Google Scholar]
- 40.Bradley MM, Sabatinelli D, Lang PJ, Fitzsimmons JR, King W, Desai P. Activation of the visual cortex in motivated attention. Behav Neurosci. 2003;117(2):369–380. doi: 10.1037/0735-7044.117.2.369. [DOI] [PubMed] [Google Scholar]
- 41.Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- 42.DiNardo PA, Brown TA, Barlow DH. Anxiety Disorders Interview Schedule for DSM-IV: Lifetime version (ADIS-IV-L) Albany, NY: Graywind Publications Inc; 1994. [Google Scholar]
- 43.Liebowitz MR. Social phobia. Modern problems of pharmacopsychiatry. 1987;22:141–173. doi: 10.1159/000414022. [DOI] [PubMed] [Google Scholar]
- 44.Leary MR. A brief version of the Fear of Negative Evaluation Scale. Personality and Social Psychology Bulletin. 1983;9:371–375. [Google Scholar]
- 45.Beck ATSR, Brown GK. Manual for Beck Depression Inventory II (BDI-II) 1996 [Google Scholar]
- 46.Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- 47.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 48.Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: An FMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- 49.Ochsner KN, Ray RD, Cooper JC, et al. For better or for worse: Neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 50.Ekman P, Friesen WV, Hager JC. Facial Action Coding System (FACS) Salt Lake City, UT: A Human Face; 2002. [Google Scholar]
- 51.Cooney RE, Atlas LY, Joormann J, Eugene F, Gotlib IH. Amygdala activation in the processing of neutral faces in social anxiety disorder: is neutral really neutral? Psychiatry Res. 2006;148(1):55–59. doi: 10.1016/j.pscychresns.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 52.Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magnetic Resonance in Medicine. 2001;46:515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- 53.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 54.Cohen MS. Parametric analysis of fMRI data using linear systems methods. Neuroimage. 1997;6(2):93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- 55.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- 56.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 57.Lundh LG, Ost LG. Recognition bias for critical faces in social phobics. Behav Res Ther. 1996;34(10):787–794. doi: 10.1016/0005-7967(96)00035-6. [DOI] [PubMed] [Google Scholar]
- 58.Coles ME, Heimberg RG. Recognition bias for critical faces in social phobia: a replication and extension. Behav Res Ther. 2005;43(1):109–120. doi: 10.1016/j.brat.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 59.Mogg K, Philippot P, Bradley BP. Selective attention to angry faces in clinical social phobia. J Abnorm Psychol. 2004;113(1):160–165. doi: 10.1037/0021-843X.113.1.160. [DOI] [PubMed] [Google Scholar]
- 60.Carmichael ST, Price JL. Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol. 1996;371(2):179–207. doi: 10.1002/(SICI)1096-9861(19960722)371:2<179::AID-CNE1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 61.McDonald AJ, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1996;71(1):55–75. doi: 10.1016/0306-4522(95)00417-3. [DOI] [PubMed] [Google Scholar]
- 62.Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34(3):905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.D’Argembeau A, Ruby P, Collette F, et al. Distinct Regions of the Medial Prefrontal Cortex Are Associated with Self-referential Processing and Perspective Taking. J Cogn Neurosci. 2007;19(6):935–944. doi: 10.1162/jocn.2007.19.6.935. [DOI] [PubMed] [Google Scholar]
- 64.Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13(4):500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 65.Grill-Spector K, Knouf N, Kanwisher N. The fusiform face area subserves face perception, not generic within-category identification. Nat Neurosci. 2004;7(5):555–562. doi: 10.1038/nn1224. [DOI] [PubMed] [Google Scholar]
- 66.Grill-Spector K, Sayres R, Ress D. High-resolution imaging reveals highly selective nonface clusters in the fusiform face area. Nat Neurosci. 2006;9(9):1177–1185. doi: 10.1038/nn1745. [DOI] [PubMed] [Google Scholar]
- 67.Fan J, McCandliss BD, Fossella J, Flombaum JI, Posner MI. The activation of attentional networks. Neuroimage. 2005;26(2):471–479. doi: 10.1016/j.neuroimage.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 68.Vuilleumier P, Driver J. Modulation of visual processing by attention and emotion: windows on causal interactions between human brain regions. Philos Trans R Soc Lond B Biol Sci. 2007;362(1481):837–855. doi: 10.1098/rstb.2007.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Amir N, Foa EB, Coles ME. Automatic activation and strategic avoidance of threat-relevant information in social phobia. J Abnorm Psychol. 1998;107(2):285–290. doi: 10.1037//0021-843x.107.2.285. [DOI] [PubMed] [Google Scholar]
- 70.Mogg K, Bradley BP. A cognitive-motivational analysis of anxiety. Behav Res Ther. 1998;36(9):809–848. doi: 10.1016/s0005-7967(98)00063-1. [DOI] [PubMed] [Google Scholar]
- 71.Iacoboni M, Wilson SM. Beyond a single area: motor control and language within a neural architecture encompassing Broca's area. Cortex. 2006;42(4):503–506. doi: 10.1016/s0010-9452(08)70387-3. [DOI] [PubMed] [Google Scholar]
- 72.Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;57(3):210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 73.Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. J Neurosci. 2001;21(18):RC165. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Edelmann RJ, Baker SR. Self-reported and actual physiological responses in social phobia. Br J Clin Psychol. 2002;41(Pt 1):1–14. doi: 10.1348/014466502163732. [DOI] [PubMed] [Google Scholar]
- 75.Mauss IB, Wilhelm FH, Gross JJ. Is there less to social anxiety than meets the eye? Emotion experience, expression, and bodily responding. Cognition & Emotion. 2004;18:631–662. [Google Scholar]
- 76.Northoff G, Heinzel A, Bermpohl F, et al. Reciprocal modulation and attenuation in the prefrontal cortex: An fMRI study on emotional-cognitive interaction. Human Brain Mapping. 2004;21(3):202–212. doi: 10.1002/hbm.20002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.