Abstract
Mercury in the Arctic is an important environmental and human health issue. The reliance of Northern Peoples on traditional foods, such as marine mammals, for subsistence means that they are particularly at risk from mercury exposure. The cycling of mercury in Arctic marine systems is reviewed here, with emphasis placed on the key sources, pathways and processes which regulate mercury levels in marine food webs and ultimately the exposure of human populations to this contaminant. While many knowledge gaps exist limiting our ability to make strong conclusions, it appears that the long range transport of mercury from Asian emissions is an important source of atmospheric Hg to the Arctic and that mercury methylation resulting in monomethylmercury production (an organic form of mercury which is both toxic and bioaccumulated) in Arctic marine waters is the principal source of mercury incorporated into food webs. Mercury concentrations in biological organisms have increased since the onset of the industrial age and are controlled by a combination of abiotic factors (e.g., monomethylmercury supply), food web dynamics and structure, and animal behavior (e.g., habitat selection and feeding behavior). Finally, although some Northern Peoples have high mercury concentrations of mercury in their blood and hair, harvesting and consuming traditional foods has many nutritional, social, cultural and physical health benefits which must be considered in risk management and communication.
Keywords: mercury, Arctic, momomethyl mercury, marine, biota, transormations, exchange, human exposure
1. INTRODUCTION
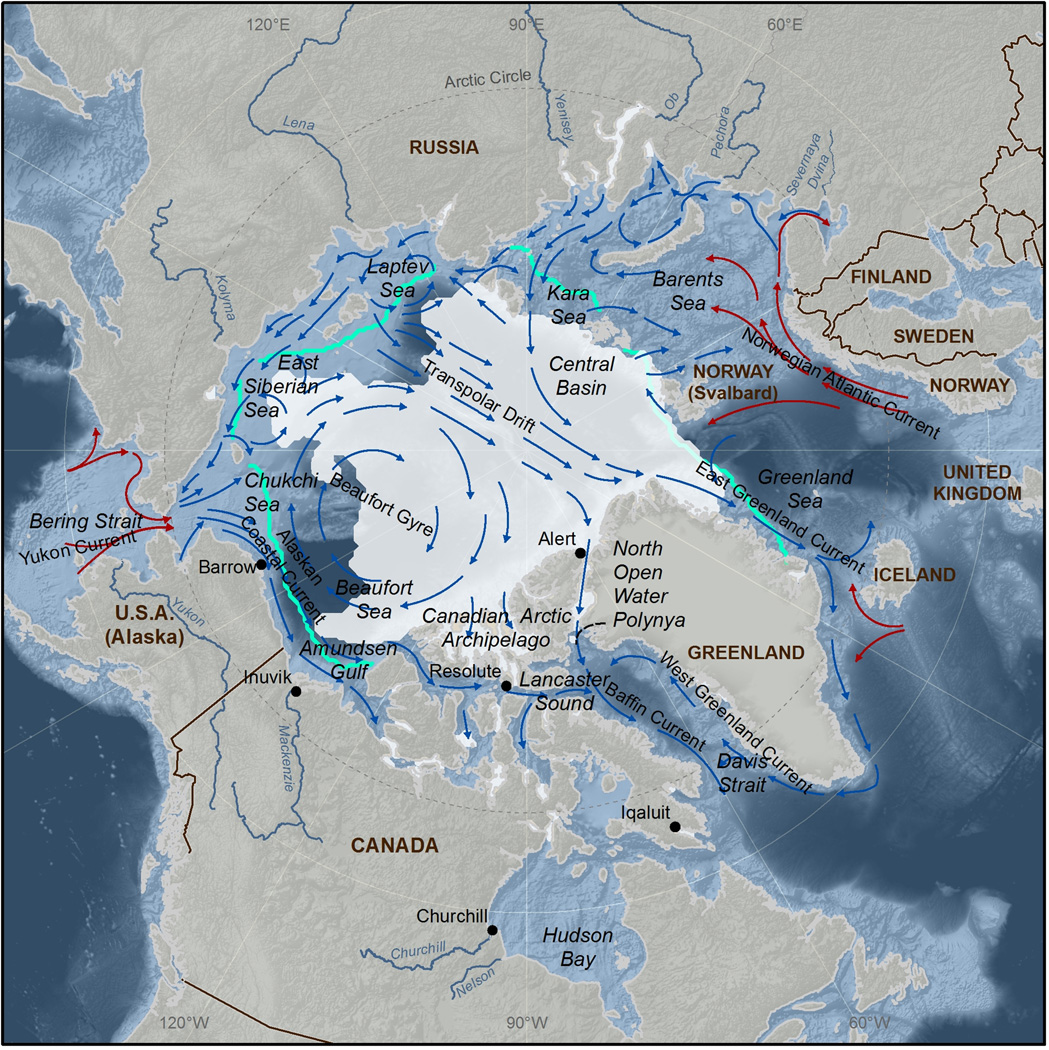
The Arctic Ocean (Figure 1), including its coastal seas, has many features differentiating it from the rest of the world’s marine ecosystems that can affect the fate of mercury (Hg) and how issues surrounding Hg contamination are viewed. Firstly, due to its extremely remote location, virtually all anthropogenic Hg inputs to the Arctic Ocean originate from long-range transport rather than point source emissions, making source attribution more challenging than in other systems. Furthermore, atmospheric processes that are unique to polar regions play an important role in controlling the deposition of atmospheric Hg to this region. A large percentage of the Arctic Ocean is ice covered for much of the year, which alters many aspects of the Hg cycle (e.g., rates of ocean-atmosphere Hg exchange and photochemical processes) and makes access for scientific study more logistically challenging. The Arctic is also inhabited by Northern Peoples, such as Inuit, who harvest and rely on marine mammals and fishes for subsistence, adding a very important human dimension to the issue of Hg contamination of Arctic marine ecosystems. Many changes have recently occurred in communities across the Arctic, and Northern Peoples are now experiencing a combination of complex stressors. These stressors include health issues associated with an increased reliance on processed foods from the south and exposure to multiple contaminants, such as Hg and persistent organic pollutants, through the consumption of traditional foods (AMAP 2011).
Figure 1.
Map of the Arctic Ocean including major seas and ocean currents (arrows; warm currents = red, cold currents = black), as well as median sea ice extent between 1979–2000 (blue line), and minimum sea ice extent in 2010 (shaded area).
Monomethylmercury (MeHg), a potent vertebrate neurotoxin that is readily bioaccumulated and biomagnified, is present in numerous Arctic marine mammals, such as ringed seals (Phoca hispida) and beluga whales (Delphinapterus leucas), at concentrations high enough to pose health risks to Northern Peoples consuming these animals as traditional country foods (AMAP 2011). In fact, >50% of mothers and women of child bearing age exceed the U.S. Environmental Protection Agency’s recommended blood Hg level of 5.8 µg L−1 in several northern populations (AMAP 2011), which is very high compared to the 50th percentile for American females of 0.720 µg L−1 (Department of Health and Human Services 2012). In some Greenland communities where diets are comprised predominantly of traditional foods, average Hg concentrations up to 40 µg L−1 have also recently (2000–2007) been observed in pregnant women, mothers, and women of child bearing age (AMAP 2011). Furthermore, there is evidence that MeHg concentrations in many marine mammals and birds have increased since industrialization (Dietz et al. 2009) and continue to increase presently in some regions of the Arctic (Rigét et al. 2011).
Until recently, Arctic Hg research has largely focused on monitoring Hg levels in top predators, such as polar bears, beluga whales and birds. Through monitoring programs such as Canada’s “Northern Contaminants Program” working in tandem with subsistence hunters from northern coastal communities to obtain samples, a moderate amount of data on Hg levels in biota has been collected. Since the discovery of Atmospheric Mercury Depletion Events (AMDEs) in 1995 (Schroeder et al. 1998), numerous atmospheric and snowpack Hg measurements have also been carried out to try attempt to understand atmospheric Hg deposition processes in the Arctic (Steffen et al. 2008). These efforts have resulted in some of the most extensive long-term data sets in the world; however, information on the key processes linking atmospheric deposition to Hg levels in top predators has only become available in the last 5–10 years with the publication of studies on Hg speciation, transformations and exchange in Arctic marine waters (Kirk et al. 2008, Lehnherr et al. 2011, Andersson et al. 2008, St. Louis et al. 2007) as well as on factors controlling bioaccumulation of Hg through food webs (Loseto et al. 2008a, Loseto et al. 2008b, AMAP 2011). An up-to-date review of Hg in Arctic marine ecosystems which tracks Hg pathways from sources to human exposure is presented here with the goal of providing a holistic viewpoint that will be informative to policy makers. Although Hg cycling in the environment is extremely complex, we attempt to identify factors that are important for MeHg production and bioaccumulation and discuss potential management mechanisms that could address some of these issues.
2. EXTERNAL INPUTS
Hg enters the Arctic Ocean via a number of different pathways, including the atmosphere, river exports and ocean currents. Some of the major questions that Arctic Hg research has recently focused on are: “How much Hg is deposited to the Arctic Ocean from atmospheric deposition? Where does this atmospheric Hg originate?” Answering these questions has been challenging because Hg emissions to the air occur from both natural and anthropogenic sources and complex exchanges of Hg occur at interfaces between the air, water and cryosphere. Most recently, progress has been made in understanding the relative contributions of Hg inputs from atmospheric deposition and river exports and we therefore provide up-to-date information on Hg sources to the Arctic Ocean, with focus on atmospheric deposition and river exports to the Arctic Ocean and identification of major knowledge gaps.
2.1 Hg in the Atmosphere
The main species of Hg in the atmosphere is gaseous elemental Hg or Hg(0), which has a long atmospheric residence time (6–12 months) and can thus undergo long-range transport (Selin 2009). In the atmosphere, Hg(0) can react with strong oxidants, such as halogen radicals, to form chemical species termed reactive gaseous Hg (RGM) and particulate Hg (PHg), both of which have relatively short atmospheric residence times and therefore are rapidly deposited to underlying surfaces, such as landscapes and water bodies (Steffen et al. 2008). In polar regions, the oxidation of Hg(0) and subsequent deposition of RGM and PHg is enhanced during polar spring by phenomena called Atmospheric Mercury Depletion Events (AMDEs). Steffen et al. (2008) reviewed and synthesized current AMDE science and concluded that the initiation of AMDEs requires Hg in the atmosphere, cold temperatures, a stable inversion layer, sunlight and reactive halogens. Sea ice or snow may provide a large pool of halogen radicals such as Br˙, BrO˙, Cl˙ and ClO˙ to catalyze the photochemical reactions. Thus, the Arctic Ocean during springtime provides the physical and chemical conditions required for atmospheric transformation and deposition of Hg. Unfortunately, because the Arctic is a large and remote region, there are still relatively few atmospheric and Hg deposition measurements directly over the Arctic Ocean (Figure 2). However, great strides have recently been made in the development of atmospheric Hg models for the Arctic (Dastoor et al. 2008, Holmes et al. 2010, Travnikov et al. 2009), and models now incorporate, for example, the role of snow chemistry in deposition and emission of Hg around the Arctic.
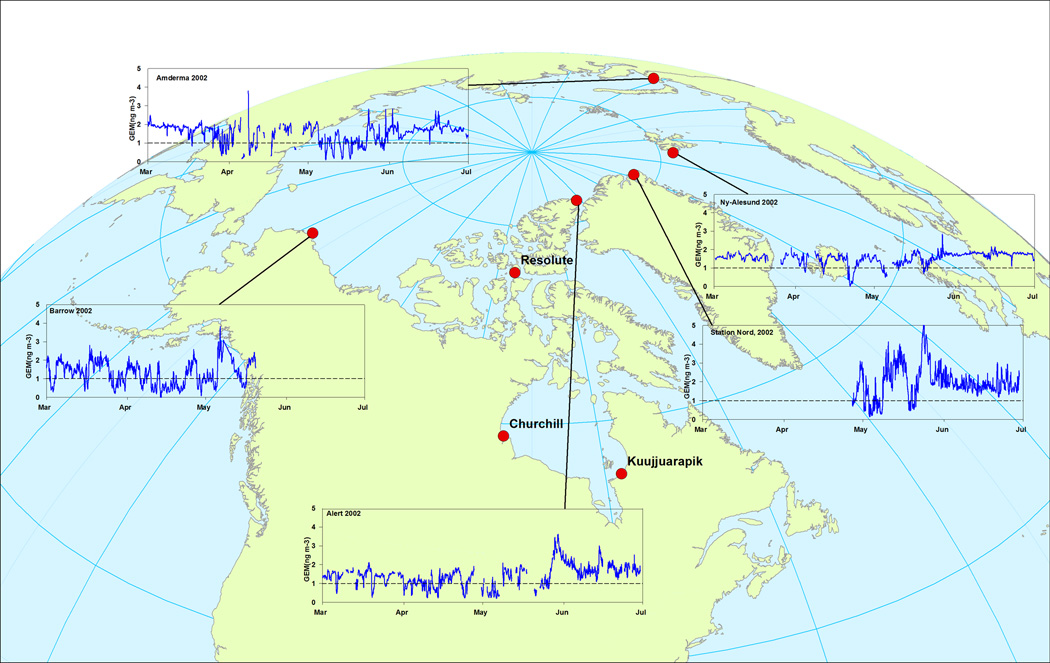
Figure 2.
Locations where atmospheric Hg speciation measurements have been carried out across the circumpolar Arctic and concentrations of Hg(0) at some of these sites (from Steffen et al. 2008).
2.1.1 Concentrations of Hg in the Arctic atmosphere and snowpack
The longest continuous measurements of atmospheric Hg have been collected at Alert, Canada, Ny Ålseund, Norway and Amderma, Russia, beginning in 1995, 2000 and 2002, respectively (Figure 2). Less continuous measurements have been collected at Barrow, Alaska), Station Nord, Greenland, and Churchill and Kuujjuarapik, Canada. In general, concentrations of Hg(0) in high Arctic and subarctic regions vary from <0.05 ng m−3 to ~3 ng m−3 and have distinct seasonal signatures which reflect a variety of processes, including the occurrence of AMDEs, the emission of Hg(0) from snowpacks, tundra and oceans, and variation in long-range transport episodes. For example, at Alert, in the high Arctic, median (± standard deviation) fall, winter, spring and summer concentration are 1.49 ± 0.11, 1.59 ± 0.17, 1.24 ± 0.53 and 1.80 ± 0.35 ng m−3, respectively. The low spring median concentration accompanied by high variation, for example, reflects that during AMDEs, atmospheric Hg(0) concentrations frequently drop <1 ng m−3 but can increase to above 1.7 ng m−3 (northern hemispheric background concentration level) between AMDEs. Generally, concentrations of RGM and PHg in Arctic air are in the low pg m−3 concentration range but increase to the low ng m−3 range during AMDEs (Steffen et al. 2008). For example, during polar night at Alert in 2005, the distribution of atmospheric GEM, RGM, and PHg was 95%, 0.63%, and 4.4%, respectively, but changed to 88.6%, 5.7% and 5.7%, respectively, during springtime AMDEs (Cobett et al. 2007).
Few measurements of atmospheric Hg species directly over the Arctic Ocean have been published to date; however available data suggest that Hg(0) concentrations are higher and more variable over the Arctic Ocean, particularly in regions with greater ice cover (median ± standard deviation; 1.82 ± 0.24 ng m−3), than over the open North Atlantic Ocean (1.53 ± 0.12 ng m−3) (Aspmo et al. 2006). Back trajectory analysis demonstrated that Hg(0) over the Arctic Ocean did not originate from Europe or North America, and as a result, it was hypothesized that elevated summer-time Hg(0) concentrations were due to reduction and subsequent emission of Hg(II) deposited to snow and ice surfaces during spring-time AMDEs or evasion directly from the ocean surface due to increased reduction potential at high latitudes during the summer (Aspmo et al. 2006). However, recent modeling efforts have suggested that the summer-time elevated atmospheric Hg(0) concentrations are due to evasion from the ocean rather than the photoreduction of Hg(II) from snow/ice surfaces, as the snowpack Hg(II) reservoir is insufficient to generate such high concentrations (Fisher et al. 2012).
Measured concentrations of total Hg (THg; includes all forms of Hg in a sample) in surface snow and meltwater vary greatly across the Arctic, ranging from 0.9 ng L−1 at Summit (Greenland) to 55 ng L−1 in Davis Straight for surface snow and from 3.5 ng L−1 in northern Sweden to 20 ng L−1 in Barrow (Alaska) for meltwater (Figure 3A and B) (Durnford and Dastoor 2011 and references therein). This variability is due both to among and within site variation in Hg deposition and post-deposition processes. For example, some studies have observed dramatic increases in surface snow Hg concentrations during AMDEs, but decreases directly following AMDEs (Kirk et al. 2006, Ferrari et al. 2005, Lindberg et al. 2002), indicative of reemission of deposited Hg(II). It is now widely recognized that a large portion of the Hg that is deposited to snowpacks is photoreduced and revolatilized (Lalonde et al. 2002, Lalonde et al. 2003, Poulain et al. 2004). From published time series of measured snowpack Hg concentrations, Durnford and Dastoor (2011) calculated a mean loss of 20–50% from surface snow within a 24-hour period due to photoreduction and revolatilization as Hg(0) (Figure 3C). In addition, Sherman et al. (2010) recently measured Hg isotope ratios in surface snow, and in Hg(0) emitted from the snowpack surface during an AMDE at Barrow. They reported large mass independent fractionation of Hg isotopes in surface snow, which they proposed is the result of Hg(II) photoreduction in surface snow. Using the isotope signature of Hg in snow, they estimated that 35–75% of deposited Hg(II) is reduced to Hg(0) and revolatilized from the snowpack (Sherman et al. 2010).
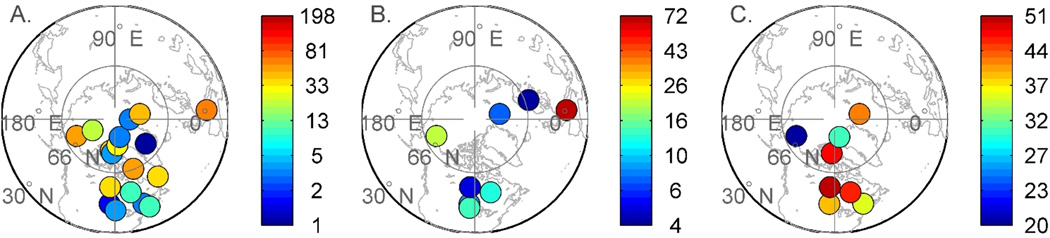
Figure 3.
Average concentrations of THg (ng L−1) in snow (A) and melt water (B) as well as calculated average mean loss of Hg from surface snow within a 24 hr period (%) (C) at various Arctic locations (Data from Durnford and Dastoor 2011).
Durnford and Dastoor (2011) recently developed a conceptual mechanism of the physical and chemical processes governing the fate of Hg in snow. It is hypothesized that all Hg(0) deposited onto snow covered surfaces is revolatilized immediately while deposited PHg is strongly retained by the snowpack. The chemical cycle of Hg(II) within the snowpack and the subsequent revolatilization of the Hg(0) produced were found to depend on snowpack characteristics and local environmental conditions. H2O2 in pH neutral snow, HO2˙, humic acids, oxalic acid and sulfite based compounds promote Hg(II) reduction within the snowpack (Munthe et al. 1991, Van Loon et al. 2000, Dommergue et al. 2003b, Gardfeldt and Jonsson 2003, Lahoutifard et al. 2005, Dommergue et al. 2007, Faïn et al. 2008). On the other hand, H2O2 in acidic snow, Br˙, Br2, O3, OH˙, alkenes and alkyl nitrates promote the oxidation of snowpack Hg(0) (Mann et al. 2005, Lahoutifard et al. 2006, Lin et al. 2006, Faïn et al. 2008). Hg(0) produced within the snowpack may be reoxidized prior to revolatilization and hence retained by the snowpack. Moreover, halides, such as Cl− and Br− stabilize Hg(II) in snow (St. Louis et al. 2007, Lalonde et al. 2003, Ferrari et al. 2004, Faïn et al. 2006, Faïn et al. 2008). Therefore, at coastal marine sites and over first year sea ice, where snowpack halogen concentrations are frequently high (Simpson et al. 2007a, Simpson et al. 2007b), the retention of deposited Hg(II) may be enhanced (Garbarino et al. 2002, Poulain et al. 2007b, St. Louis et al. 2007, Larose et al. 2010). At the onset of snow melt, Hg(0) emission to the atmosphere increases (Dommergue et al. 2003a, Faïn et al. 2007, Sommar et al. 2007, Brooks et al. 2008, Douglas et al. 2008) while the snowpack’s Hg(II) load rapidly exits the snowpack in the meltwater ionic pulse (Bales et al. 1990, Kuhn 2001, Bishop et al. 1995, Allan et al. 2001, Lindberg et al. 2002, Dommergue et al. 2010, Larose et al. 2010).
2.1.2 How much Hg is deposited to the Arctic from atmospheric sources
Net atmospheric Hg deposition in the Arctic has been estimated using measured areal snowpack loadings (Constant et al. 2007, Ferrari et al. 2005, Kirk et al. 2006, St. Louis et al. 2007, Steffen et al. 2002) and modeling (AMAP 2011, Holmes et al. 2010, Dastoor et al. 2008, Christensen et al. 2004, Ariya et al. 2004, Travnikov 2005). Areal spring-time loads of THg and MeHg in Canadian high Arctic snowpacks averaged 3.1 ± 7.0 g m−2 and 0.0034 ± 0.0021 g m−2, respectively (St. Louis et al. 2007). In Churchill, Canada, average spring-time loads calculated from THg concentrations in snow melt or year round wet precipitation collections were even lower than those in the high Arctic, ranging from 0.04–0.4 µg m−2 (Kirk et al. 2006) and 0.5–2.0 µg m−2 (Sanei et al. 2010), respectively.
Numerous models have been developed which incorporate contemporary anthropogenic emissions, natural emissions and re-emissions of both anthropogenic and natural Hg and simulate the cycling of Hg between the atmosphere and landscape (AMAP 2011). Early model estimates of Hg deposition to the Arctic ranged from 208–325 t yr−1, 20–57% of which was attributed to AMDEs (Christensen et al. 2004, Ariya et al. 2004, Travnikov 2005). However, recent deposition estimates from the GRAHM (Global/Regional Atmospheric Heavy Metals Model) and GEOS-Chem (global 3-D atmospheric composition model driven by data from the GODDARD Earth Observing System) models, which now incorporate snowpack photoreduction processes, are much lower, suggesting net annual depositional fluxes of 143 t yr−1 (Figure 4A, NCP 2012) and 60 t yr−1 (Holmes et al. 2010), respectively, for the area north of 66.5 °N. The GRAHM model also simulates atmospheric Hg(0) concentrations for the Canadian Arctic and suggests that in the western Canadian Arctic, Hg deposition is highest and Hg(0) concentrations are lowest, which is consistent with measured Hg(0) concentrations (Figure 4B) (NCP 2012). The GRAHM model also suggests that there is an increasing north to south Hg deposition gradient that may be attributed to Br˙ concentrations, proximity to emission sources and precipitation.
Figure 4.
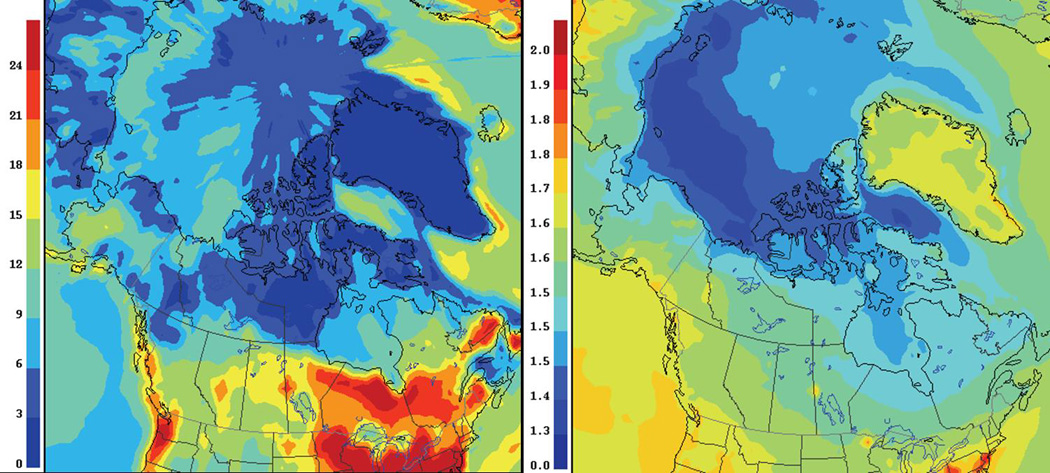
THg deposition (µg m−2yr−1; left) and surface air concentration of Hg(0) (ng m−3; right) simulated by the Global Regional Atmospheric Heavy Metals Model (GRAHM) for year 2005 (AMAP 2011).
In the Canadian high Arctic and subarctic where both measured and modeled estimates of net atmospheric deposition are available, direct measurements of Hg loads from high Arctic snowpacks (0.5–3.5 µg m−2 (St. Louis et al. 2007)), as well as meltwater (0.04–0.4 µg m−2 (Kirk et al. 2006)) and wet deposition (0.5–2.0 µg m−2 (Sanei et al. 2010) for Churchill, Manitoba tend to be lower than model simulations. For example, the GRAHM model estimates depositional fluxes of 4.5 µg m−2 for the high Arctic and 9 and 12 µg m−2 for the western and eastern subarctic, respectively (NCP 2012), while estimates obtained with the GEOS-Chem model range between 3.0 µg m−2 (Holmes et al. 2010) and 3.7 µg m−2 (Fisher et al. 2012) for the area north of 66.5° N and 70° N, respectively. Field measurements differ from model simulations because snowpack and meltwater loads, which incorporate both wet and dry net deposition, do not reflect deposition over a full 12 month cycle while wet only deposition fluxes do not include the contribution of dry deposited Hg species such as RGM and PHg. Average Hg flux values recently obtained using dated lake sediment cores from 32 locations across the Canadian Arctic are in good agreement with GRAHM modeled values, averaging 2.8 and 7.5 µg m−2 for the high and subarctic, respectively, and showing a similar negative relationship between latitude and Hg deposition (Muir et al. 2009). Regardless of the exact deposition values, it is apparent that atmospheric deposition in the Arctic is less than at temperate latitudes or in industrialized regions of the world. For example, atmospheric Hg depositional fluxes over the Gulf of Maine (northeastern U.S.) have recently been estimated at 10–12 µg m−2 (Sunderland et al. 2012). Furthermore, the reservoir of Hg in snowpacks appears to be insufficient to explain seasonal patterns in atmospheric Hg concentrations (Fisher et al. 2012), or to be the principal driver of Hg methylation and bioaccumulation in marine ecosystems.
2.1.3 Source attribution models: “What are the source regions of atmospheric Hg to the Arctic?”
Since there are no anthropogenic Hg point sources in the Arctic, determining the relative importance of different source regions is of great importance.
GEOS-Chem and GRAHM model simulations were recently used to track Hg from its point of emission to global and Arctic reservoirs, respectively (Corbitt et al. 2011, Durnford et al. 2010, AMAP 2011). GEOS-Chem model simulations suggest that anthropogenic emissions, legacy sources from the past two centuries, and natural sources each contribute approximately 1/3 of the Hg deposited to the global oceans (Corbitt et al. 2011). Hg re-emission, particularly from the oceans, plays a key role in recycling Hg, with 10%, 40% and 50% of Hg that is deposited to land, oceans and snow, respectively, being re-emitted rather than transferred to deeper reservoirs.
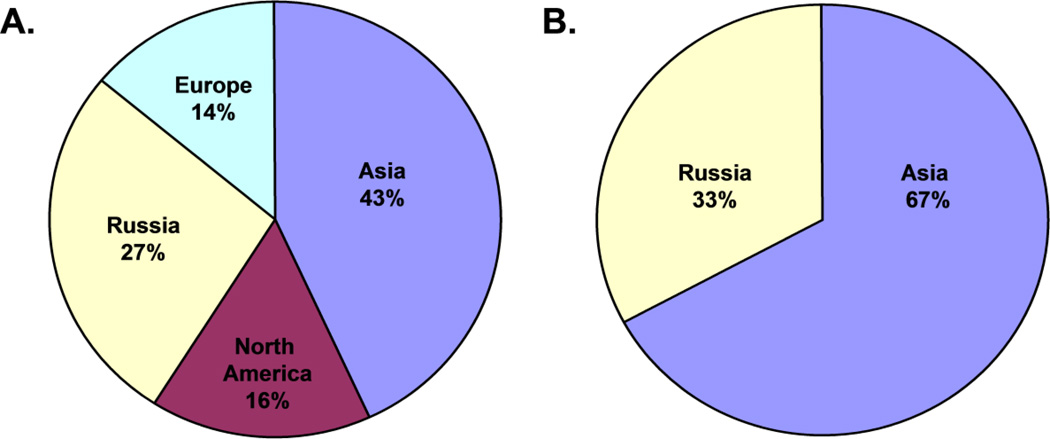
Similar to GEOS-Chem modeling results for the global oceans, GRAHM modeling suggests that ~2/3 of Hg deposited to the Arctic is natural and re-emitted Hg (with 40–45% coming from land and 30–34% from oceans) whereas 20–28% is from anthropogenic sources (Durnford et al. 2010, AMAP 2011). The contribution of anthropogenic Hg from different source regions is impacted by weather fronts, such as the Arctic front, which have an important influence on the long-range transport of aerosols (Fisher et al. 2011). For example, the Arctic front extends southward over Russia and Eurasia during the winter, facilitating the transport of air-borne contaminants from this region to the Arctic. In the spring, the polar front contracts northward, allowing East Asian sources to become more important in contributing air-borne contaminants to the Arctic (Fisher et al. 2011). The contribution of anthropogenic sources to Hg deposition is higher (28%) in the high Arctic than in the subarctic (20–21%) due to higher contributions from East Asia (15% vs. 11%) and Europe (3% vs. 2%) (Figure 5) (Durnford et al. 2010, AMAP 2011). Overall, the largest anthropogenic Hg source region to the Arctic is East Asia (10–15%) followed by Europe (2–3%), North America (2–2.5%) and South Asia (1.5–2%). This is consistent with GEOS-Chem modeling, which suggests that Asian sources are the largest source of new anthropogenic deposition to all oceans (for example, 53% in the North Atlantic and 62% in the North Pacific) (Corbitt et al. 2011). Interestingly, although North American and European sources contribute only 30% of new anthropogenic deposition to the world’s oceans, because 1/3 of current deposition is from legacy sources, a large portion of current deposition to the oceans is from legacy North American and European emissions over the last two centuries (Corbitt et al. 2011). The Task Force on Hemispheric Transport and Air Pollution recently assessed GEOS-Chem and GRAHM modeling results and it was concluded that decreasing East Asian and European emissions could effectively control Arctic Hg deposition due to the proximity of these regions to the Arctic, the prevailing atmospheric circulation patterns, and the contribution of these regions to global anthropogenic emissions (HTAP 2010).
Figure 5.
Frequency of long-range transport episodes or intercontinental transport of Hg to the high Arctic (left) and subarctic (right) from different source regions (Data from Durnford et al. 2010).
Although great strides have recently been made to improve modeled estimates of Hg deposition and source attribution to the Arctic and around the globe in general, some major model uncertainties remain (AMAP 2011, Corbitt et al. 2011). The first is atmospheric Hg emission estimates. Although there are good anthropogenic Hg emission inventories, there are large uncertainties associated with the speciation of anthropogenic Hg emissions as well as estimates of natural Hg sources and re-emissions, particularly from different ecosystem types (AMAP 2011). Thus, in the Arctic, estimates of re-emission rates from different snow and ice surfaces would be of great value. The second major limitation to current Hg models is a lack of understanding of atmospheric Hg speciation and of the chemical processes governing atmospheric reactions. Specifically, there are uncertainties in the identity of the major atmospheric Hg(0) oxidants (Br˙, Cl˙, Br2, Cl2, BrO˙, O3, OH˙ and HOCl/OCl−), although it is becoming apparent that Br˙ plays an important role (Holmes et al. 2010), as well as uncertainty in the products and rates of atmospheric Hg reactions. In the Arctic, the chemical processes governing atmospheric Hg reactions on snow, ice, and particle surfaces as well as the effect of temperature, sea ice extent, and snow and ice melt on atmospheric Hg reactions are not well understood (Cole et al. 2010). Given current knowledge gaps, models rely on observed air Hg speciation and wet and dry deposition data to constrain non-anthropogenic emission and reaction rates. However, because Arctic measurements, especially over the Arctic Ocean, are scarce, the variation in Hg deposition estimates among different models is greater in the Arctic than for most other regions. Therefore, co-located measurements of atmospheric oxidized Hg species, snowpack Hg concentrations and wet deposition, would help constrain model deposition estimates (HTAP 2010). Regardless of current limitations, models are an extremely valuable tool, not only for scaling up measurements, but also for testing hypotheses, such as the impacts of climate-induced changes or altered emission scenarios on Hg deposition in the Arctic.
2.2 Rivers and Watersheds
River discharge is another important source of both THg and MeHg to the Arctic Ocean. In the high Arctic, the most extensive measurements have been conducted on the Mackenzie and Yukon rivers, which are located in the northwestern Canadian Arctic and discharge into the Beaufort and Bering seas, respectively. Average annual exports to the Beaufort Sea from the Mackenzie River of 1200–2900 kg THg yr−1 for 2003–2005 and 7–22 kg MeHg yr−1 for 2003–2004, respectively, were reported by Leitch et al. (2007). However, Graydon et al. (2009, NCP 2012) estimated summer only (June to August) Mackenzie River exports for 2004 and 2007–2009 of 1200–3531 kg THg yr−1 and 8–21 kg MeHg yr−1, respectively. These values are equal to annual exports reported by Leitch et al. (2007) and the discrepancy between the two estimates arises because Graydon et al. (2009) accounted for the contribution to Hg exports from seasonally flooded delta lakes. Knowing that a large portion of the Mackenzie River flow and Hg export occurs during the spring freshet right after ice out (Leitch et al. 2007), it is likely that current values of annual Hg exports from the Mackenzie River are underestimates (Graydon et al. 2009). Based on year round sampling, Schuster et al. (2011) recently reported average 2001–2005 Yukon River THg exports to the Bering Sea of 4400 kg yr−1, which is much higher than those reported for the Mackenzie River despite the fact that Yukon River discharge is ~2/3 that of the Mackenzie (Schuster et al. 2011). Yukon River MeHg exports were not reported however, due to the fact that ~1/2 the MeHg samples obtained were <MDL. The authors found that ~50% of the THg export occurred during the spring freshet season, with 80% of freshet sample concentrations exceeding the U.S. EPA Hg standard for adverse chronic effects to biota, which highlights the importance of high frequency spring-time sampling. In addition, 90% of the annual THg export was particulate-bound, with dissolved and particulate organic carbon explaining 81% and 50% of the variation in dissolved and particulate Hg exports, respectively. These results highlight the importance of organic carbon in river-ocean Hg transport as well as the potentially significant impact of climate-induced permafrost degradation on the mobilization of organic carbon-bound Hg in Arctic watersheds with high percentages of permafrost cover. Exports of THg to the Kara and Laptev Seas from three Siberian rivers, the Lena, Ob, and Yenisei were estimated in 1993 to be 4000, 1300 and 700 kg yr−1 THg, respectively (Coquery et al. 1995). However, these estimates are based on a very limited number of concentration measurements taken in September when concentrations are expected to be lower, and therefore are likely underestimating true THg exports. Furthermore, these Siberian rivers account for over 1/3 of river discharge to the Arctic and play a large role in determining the magnitude of Hg exports to Arctic marine environments. Exports of Hg to Hudson Bay from several subarctic rivers have also been estimated. Based on 2005–2007 sampling, Hare et al. (2008) calculated combined THg river export to Hudson Bay of 501 kg yr−1 from the Povirnituq, Kogaluc, Nastapoca, Grande Baleine, Winisk Heyes, Nelson and Churchill rivers as well as the Baker Lake system. Based on continuous sampling from 2003–2007, Kirk and St. Louis (2009) estimated Nelson and Churchill river exports of 113 ± 52 and 37 ± 28 kg yr−1, respectively, for THg and 9 ± 4 and 4 ± 4 kg yr−1, respectively, for MeHg. The total export of Hg to Arctic marine waters from the rivers listed above is about 13 t yr−1. Using an export of riverine DOC to Arctic coastal seas of 37.7 Tg C yr−1 (Manizza et al. 2009), as well as the observed relationships between DOC and dissolved Hg, and dissolved Hg and particulate Hg in the Yukon River reported by Shuster et al. (2011), we obtain a significantly larger pan-Arctic riverine THg export to Arctic marine waters of 108 t yr−1. A much more refined approach, using a coupled ocean-atmosphere Hg model (GEOS-Chem), yielded an estimated input of 80 t yr−1 of riverine Hg to the portion of Arctic marine waters located between 70°–90° N (Fisher et al. 2012), much higher than the previous estimate of 12.4 t yr (Outridge et al. 2008). Therefore, it is apparent that the importance of rivers as a source of Hg to the Arctic Ocean was previously underestimated and that river inputs of Hg are at least as great as atmospheric deposition and deserve further consideration. Although there is very limited data for MeHg exports from Arctic Rivers, we can derive an approximate export from the THg export of 80 t yr−1 reported by Fisher et al. (2012), assuming that MeHg makes up between 0.5% (Shuster et al. 2011) and 5% (Kirk et al. 2008) of THg in Arctic rivers. This approach yields a MeHg export ranging between 0.4 and 4 t yr−1. While this represents a small input relative to the existing ocean MeHg pool, river MeHg inputs may still be important in near shore environments such as estuaries.
Although the river exports described above are informative, this dataset is still incomplete as data on MeHg and on some of the major Russian rivers is lacking and exports have often been estimated from a limited number of concentration measurements that may not reflect the seasonal variability. Future work is required to improve our understanding of the relative importance of river exports to the Arctic Ocean compared to other external sources. This could be attained by conducting multi-year studies incorporating hydrological discharge and Hg concentration data collected across seasons and including winter, ice-influenced periods and rising water periods. A better understanding of river-ocean mixing processes would be useful to understand the fate of river-derived Hg and determine how much of that Hg settles out on particles in coastal areas and how much enters the ocean water column pool, where it can be bioaccumulated into local marine food webs. Finally, it is worthy to note that MeHg concentrations in certain rivers are elevated (e.g., on average 0.18 ± 0.09 ng L−1 in the Churchill River) compared to marine waters such that organisms that feed in river estuaries may be exposed to more MeHg (Kirk and St. Louis 2009).
2.3 Ocean Circulation
Very few estimates exist of the inputs of Hg from other oceans to the Arctic region, primarily due to a lack of Hg concentration in inflowing and outflowing water masses. Outridge et al. (2008) estimated that on a yearly basis 3.9 t and 44 t of Hg enter the Arctic Ocean via the Pacific and Atlantic inflows, respectively. This large difference in Hg inputs from the Pacific and Atlantic oceans primarily reflects the water budget for the Arctic Ocean, which receives approximately four times more water from the Atlantic Ocean than from the Pacific Ocean. While Hg inputs from ocean circulation total 48 t yr−1, it was also estimated that 14 t yr−1 and 54 t yr−1 of Hg are lost from Arctic Ocean outflows into the Canadian Archipelago and the Atlantic Ocean (via the East Greenland Current), respectively, meaning that overall, ocean circulation results in a net loss of ~20 t yr−1 of Hg from the Arctic Ocean (Outridge et al. 2008). However, it is important to note that in Outridge et al. (2008) the Canadian Archipelago was not considered part of the Arctic Ocean and therefore transport of Hg from the Arctic Ocean to the Canadian Archipelago was considered as an output. If we consider the Canadian Archipelago as part of the Arctic Ocean system, the loss of Hg from ocean circulation decreases to 6 t yr−1. However, the uncertainty associated with each of the ocean circulation terms is large enough that it cannot be confidently stated whether ocean circulation is truly a sink or a source of Hg to the Arctic Ocean. It is clear, however, that ocean circulation can transport significant quantities of Hg across large distances. For example, in the eastern North Pacific, increases in THg concentrations (~3% yr−1) in intermediate waters have been reported in recent years, and THg concentrations are predicted to double by 2050, compared to 1995 (Sunderland et al. 2009). This increase is thought to be the result of increased anthropogenic emissions from Asian sources, enhanced Hg deposition to coastal waters in the western North Pacific and lateral transport across ~650 km along the North Pacific current to the eastern North Pacific (Sunderland et al. 2009), demonstrating that ocean circulation can be an important transport mechanism and source of Hg to certain regions.
2.4 Mass-Balance Considerations
To date, two mass balance Hg budgets have been assembled for the Arctic Ocean. Outridge et al. (2008) concluded that atmospheric deposition and coastal erosion were the two largest sources of Hg to the Arctic Ocean, and that sedimentation was the largest Hg sink. However, a more sophisticated modeling study recently performed (Fisher et al. 2012) suggests that rivers (80 t yr−1) are the most important source of Hg to the Arctic Ocean (70°–90° N), followed by the atmosphere (45 t yr−1 net deposition, 25 t yr−1 through direct deposition and 20 t yr−1 through meltwater inputs of atmospheric Hg deposited on snow/ice surfaces), and coastal erosion (15 t yr−1). Furthermore, Fisher et al. (2012) report that evasion of Hg from the surface ocean (90 t yr−1) and particle settling (43 t yr−1) are the most important processes removing Hg from surface ocean waters. However, it is important to understand that because of a lack of data for vast regions of the Arctic, these estimates cannot yet be validated using field measurements and represent an evolving understanding of the various Hg sources and sinks to the Arctic Ocean.
3. HG TRANSFORMATIONS AND IN-OCEAN PROCESSES
To understand the link between Hg sources (such as atmospheric deposition) and MeHg bioaccumulation through food webs, it is necessary to first understand Hg speciation in the Arctic Ocean and the processes that control the production of MeHg. There are four main species of Hg that exist in marine waters: Hg(II), Hg(0), MeHg and dimethyl Hg (DMHg). DMHg is a gaseous and toxic form of Hg, and although it has not been shown to be bioaccumulative, it is hydrophobic (Log KOW = 2.59), readily crosses artificial membranes, and uptake measurements have only been carried out with one coastal diatom species (Mason et al. 1996). Within the last 7 years, research on Hg in the Arctic marine water column has increased dramatically (i.e., St. Louis et al. 2007, Kirk et al. 2008, Andersson et al. 2008, Lehnherr et al. 2011); thus a detailed review of Hg speciation, exchange and transformation processes within the two major environmental compartments of the Arctic Ocean (water and sediment) is presented below.
3.1 Hg Speciation in Marine Waters and Sediments
The most extensive examination of Hg speciation has been carried out in marine waters of the Canadian Arctic Archipelago (Table 1) (Kirk et al. 2008, Andersson et al. 2008, Lehnherr et al. 2011, St. Louis et al. 2007). However, the types of speciation measurements made in the water column and depths sampled vary from study to study. Some studies measured all four species of Hg (Lehnherr et al. 2011), some measured the sum of methylated Hg species (MeHg + DMHg) rather than just MeHg (Kirk et al. 2008, St. Louis et al. 2007), while others reported dissolved gaseous Hg (DGM, includes both Hg(0) and DMHg) rather than Hg(0) and DMHg separately (Andersson et al. 2008). Despite the variation among studies, recent speciation measurements have provided key information on the pools of Hg(II) and MeHg available for methylation and bioaccumulation, respectively, as well as indications of zones of production and loss of various Hg species in the water column. Hg speciation data are not available for Arctic Ocean sediments; however, there is good spatial coverage of THg in sediments for the central basin of the Arctic Ocean (Gobeil et al. 1999, Macdonald et al. 1991, Asmund and Nielson 2000) as well as Hudson Bay (Hare et al. 2008).
Table 1.
Summary of concentrations (ng L−1 or pg L−1) of various Hg species, including THg, Hg(0), DGM, MMHg, DMHg, and methylated Hg, in Arctic marine waters. See text for abbreviation definitions.
| Site/location | THg (ng L−1) | Hg(0) (pg L−1) | DGM (pg L−1) | MMHg (pg L−1) | DMHg (pg L−1) | Methylated Hg (pg L−1) | Reference |
|---|---|---|---|---|---|---|---|
|
Canadian Arctic Archipelag o Chl. Max Oxycline |
0.42 ± 0. 1 5 0.37 ± 0. 0 5 |
15 ± 13 2 |
26± 25 38 ± 7 |
16± 8 32 ± 16 |
11 ± 11 36 ± 7 |
27± 19 68 ± 17 |
Lehnherr et al. 2011 |
|
Beaufort Sea Chl. Max Oxycline |
0.44 0.35 |
32 19 |
78 92 |
26 49 |
46 73 |
73 122 |
Lehnherr et al. 2011 |
|
Canadian Arctic Archipelag o Surface Mid Bottom |
0.6 ± 0. 6 0.2 ± 0. 1 0.3 ± 0. 3 |
25 ± 1 0 32 ± 1 6 36 ± 4 1 |
37 ± 1 7 98± 39 113 ± 4 6 |
7.9 ± 4.4 65 ± 37 80 ± 37 |
24 ± 9 80 ± 37 99 ± 34 |
Kirk et al. 2008 | |
|
Hudson Bay Surface Mid Bottom |
0.5 ± 0.8 0.4 ± 0.4 0.4 ± 0. 3 |
30 ± 5 33 ± 23 21 ± 2 3 |
34 ± 8 67 ± 29 73 ± 33 |
4 ± 9 34 ± 29 52 ± 37 |
22 ± 8 39 ± 17 62 ± 32 |
Kirk et al. 2008 | |
|
Cruise along the coasts of Greenland and Alaska, across the Chukchi Sea and the Arctic Ocean from Barrow to Spitsberge n Surface |
45 ± 22 | Andersson et al. 2008 | |||||
|
Resolute Passage Surface (under ice) 2004 Surface (under ice) 2005 |
0.2 ± 0.01 0.1 ± 0.03 |
129 ± 36 | 140 | 11 ± 4 | 95 ± 14 57 ± 11 |
St. Louis et al. 2007 | |
|
Wellington Channel Surface (under ice) 2005 |
0.2 ± 0.2 | 91 | St. Louis et al. 2007 | ||||
|
Jones Sound Surface (under ice) 2005 |
0.2 ± 0 | 73 ± 44 | St. Louis et al. 2007 |
3.1.1 Water column THg and Hg(0) concentrations and distribution
THg concentrations are generally low in Arctic marine waters. For example, in the Canadian Arctic Archipelago and Hudson Bay regions, low concentrations of THg have been observed throughout the water column with mean concentrations reported in various studies ranging from 0.31 ± 0.11 to 0.42 ± 0.53 ng L−1 (mean ± standard deviation) (Kirk et al. 2008, Lehnherr et al. 2011), while concentrations measured at the surface under the ice were even lower (0.14–0.24 ng L−1, St. Louis et al. 2007). The current data suggest no strong spatial patterns in THg concentrations across the Canadian Arctic, and thus in the Hg(II) availability for methylation. However, increased sampling efforts, particularly near major river inflows, may reveal regions of higher Hg(II) concentrations. In general, Arctic Ocean THg seawater concentrations are similar to those measured in the north Atlantic (0.48 ± 0.32 ng L−1) (Mason et al. 1998), north Pacific (0.23 ± 0.17 ng L−1 (Laurier et al. 2004)), Southern Ocean (0.27 ± 0.09 ng L−1) (Cossa et al. 2011), and Mediterranean (0.26 ± 0.10 ng L−1 (Kotnik et al. 2007).
In the euphotic zone (the portion of the water column exposed to light), Hg(0) is produced both from photochemical and biological Hg(II) reduction (Whalin et al. 2007, Poulain et al. 2007a, Mason et al. 1995) as well as by MeHg photodegradation (Chen et al. 2003), with wind speed and ice-cover largely dictating Hg(0) concentrations. For example, in ice free surface waters of the Canadian Arctic Archipelago and Hudson Bay region, low concentrations of Hg(0) were generally observed (25.4 ± 10.2 and 29.6 ± 5.0 pg L−1, respectively, Kirk et al. 2008), with elevated concentrations coinciding with high concentrations of chlorophyll a, a proxy for biological productivity. In contrast, Hg(0) concentrations measured under the sea ice near Resolute Bay, Nunavut were much higher (129 ± 36 pg L−1) as they represent net Hg(0) accumulation over the ice covered season (St. Louis et al. 2007).
Extensive measurements of dissolved gaseous Hg (DGM) in surface water and TGM (total gaseous Hg) in air have been carried out in the Arctic Ocean (Figure 6) (Andersson et al. 2008, see also Aspmo et al. 2006). Mean DGM concentrations were 45 ± 22 pg L−1, with lowest concentrations observed at open water sites in the Canadian Arctic Archipelago (5 pg L−1) and highest concentrations observed in ice covered areas (range 15–70 pg L−1). During passages through ice covered areas, enhanced air TGM concentrations (up to 5.2 ng m−3) were also observed, indicative of DGM evasion during ice-out due to the passage of the ship. The influence of river Hg exports on surface marine DGM concentrations was suggested by elevated DGM concentrations north of Alaska (134 pg L−1) and close to the North Pole, likely due to Russian river inputs, as well as near the Mackenzie River Delta (100 pg L−1) (Andersson et al. 2008). In the Canadian Arctic Archipelago, average surface DGM values from Kirk et al. (2008) (37 ± 17 pg L−1), which were calculated by adding Hg(0) and DMHg concentrations together, were very comparable with those reported by Andersson et al. (2008) (38 ± 19 pg L−1) (Figure 6).
Figure 6.
DGM concentrations (pg L−1) measured in surface waters of the Canadian Arctic Archipelago, Hudson Strait, Hudson Bay (Kirk et al. 2008), along the coast of Greenland, along the Alaskan coast, across the Chukchi Sea and across the Arctic Ocean going from Barrow, Alaska to Spitsbergen measured in 2005 (Andersson et al. 2008).
In the aphotic zone of the water column where light does not penetrate (below ~100–150 m), biotic reduction of Hg(II) is likely the dominant mechanism for Hg(0) production; although reductive demethylation may also occur (Mason et al. 1998). Both among and within site variation in Hg(0) concentration are generally observed, indicating that the biotic and/or abiotic processes responsible for net Hg(0) production vary from site to site and throughout the water column. For example, Hg(0) concentrations ranging from <MDL-33 pg L−1 were observed in the Canadian Arctic Archipelago and Hudson Bay (Kirk et al. 2008). Hg(0) concentrations in deeper regions of the water column are likely partially driven by primary productivity. For example, in October in the Canadian Arctic Archipelago and Beaufort Sea, Hg (0) concentrations (16 ± 13 pg L−1, Lehnherr et. al 2011) were lower than those measured in August–September in both the Canadian Arctic Archipelago and Hudson Bay (34.3 ± 29.7 pg L−1 and 26.5 ± 24.5 pg L−1, respectively, Kirk et al. 2008), coincident with lower chlorophyll a concentrations.
3.1.2 Water column MeHg and DMHg
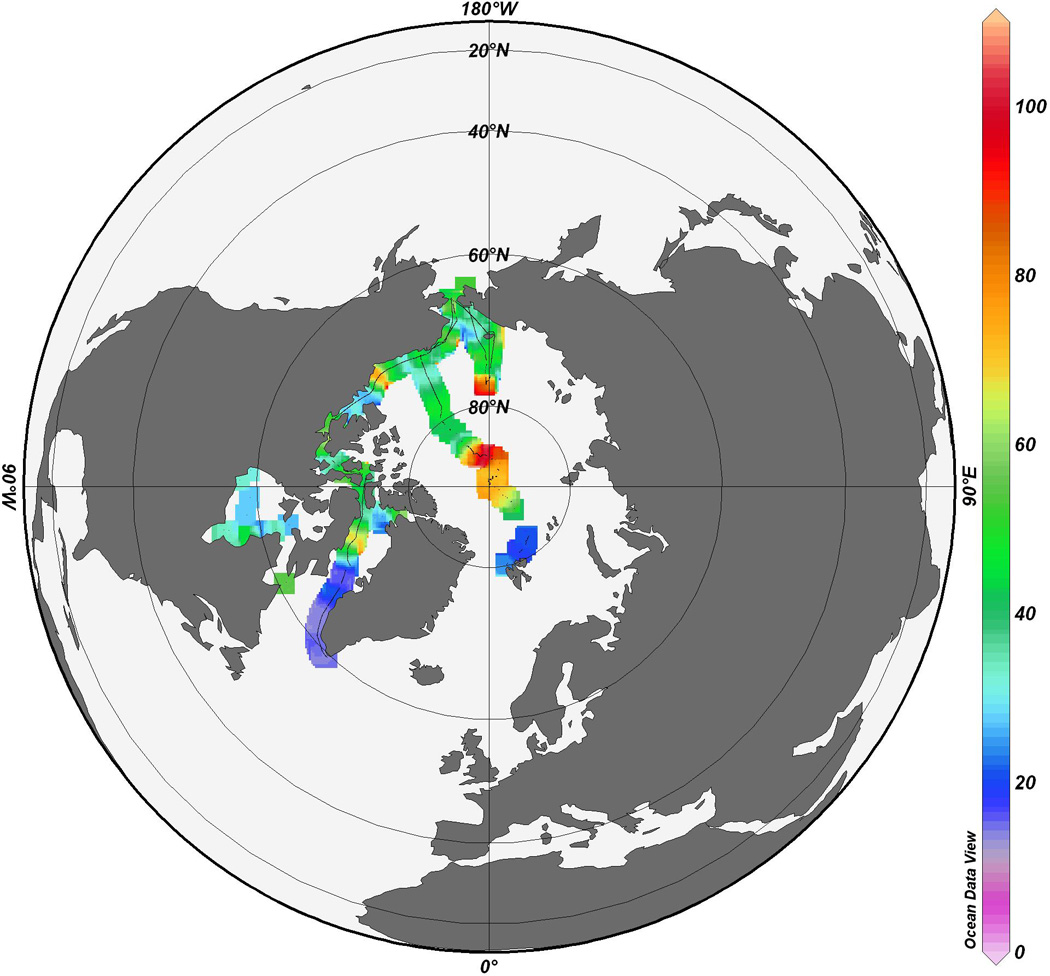
Concentrations of methylated Hg species are generally low in ice free surface marine waters as a result of loss via photodemethylation and, for DMHg, evasion. For example, in the Canadian Arctic Archipelago, concentrations of methylated Hg (MeHg + DMHg) and DMHg averaged only 24 ± 9 and 8 ± 4 pg L−1, respectively, in the surface polar mixed layer. Similarly, in Hudson Bay, concentrations of methylated Hg and DMHg in surface waters averaged 23 ± 11 and 2 ± 1 pg L−1, respectively (Figure 7). However, water column mixing may transport MeHg and DMHg from sub-surface waters up to surface waters and may thus represent an important source of MeHg to organisms feeding in the surface waters, where primary production is generally highest (Michel et al. 2006). Vertical mixing may also result in deposition of MeHg to surrounding landmasses as DMHg in surface water is evaded to the atmosphere where it undergoes photodegredation to MeHg (Sommar et al. 1996, Niki et al. 1983) prior to depositing on surrounding seascapes (Kirk et al. 2008, St. Louis et al. 2005, St. Louis et al. 2007, Pongratz and Heumann 1999). For example, two known zones of upwelling in the Canadian Arctic Archipelago and Hudson Bay region (Peel Sound and the eastern Hudson Strait) had high surface concentrations of methylated Hg (68 and 24 pg L−1, respectively) and DMHg (52 and 32 pg L−1, respectively). Ice cover also has a large impact on methylated Hg concentrations. For example, concentrations of methylated Hg and DMHg in surface waters sampled under the sea ice near Resolute Bay, Nunavut were elevated, comparable to values observed in zones of vertical mixing (Figure 7). High concentrations of MeHg and DMHg (up to ~160 pg L−1) have also been measured in surface waters of Fram Strait, and the Greenland and Barents seas (Pongratz and Heumann 1998a). However in remote regions of the north Atlantic (Mason et al. 1998) and equatorial Pacific (Mason and Fitzgerald 1993), concentrations of methylated Hg species in surface waters are generally <MDL.
Figure 7.
Average concentrations of methylated Hg (includes both MeHg and DMHg) (A) and DMHg (B) at the surface, mid, and bottom of the water column in the Canadian Arctic Archipelago and Hudson Bay (HB) in 2005 (Kirk et al. 2008), at the chlorophyll a maximum and oxycline in the Canadian Arctic Archipelago and Beaufort Sea (BS) in 2007 (Lehnherr et al. 2011) and in surface water under ice in Resolute Passage, Nunavut in 2004 (St. Louis et al. 2007).
Concentrations of methylated Hg species are often elevated in deeper regions of the water column, suggesting that they may be produced there. At mid-depths (35–250 m depending on site) of the water column in the Canadian Arctic Archipelago, for example, concentrations of methylated Hg and DMHg averaged 80 ± 37 and 65 ± 37 pg L−1, respectively, increasing to 99 ± 34 and 80 ± 37 pg L−1, respectively, at the bottom of the water column (320–808 m) (Figure 7). Similarly, in the Hudson Bay region, concentrations of methylated Hg and DMHg averaged 38 ± 17 and 35 ± 29 pg L−1, respectively, midway through the water column (20–200 m) and 69 ± 31and 61 ± 32 pg L−1, respectively, at the bottom (45–435 m). In a more recent study, concentrations of MeHg and DMHg in the Canadian Arctic Archipelago and Beaufort Sea were slightly lower at the chlorophyll a maximum (20–60 m; 18 ± 8 and 18 ± 19 pg L−1, respectively) and at the oxycline (zone of decreasing dissolved oxygen concentrations (125–327 m); 36 ± 15 and 43 ± 18 pg L−1, respectively) (Figure 7) (Lehnherr et al. 2011). However, if oxycline MeHg and DMHg concentrations reported in Lehnherr et al. (2011) are added together, values are comparable to methylated Hg concentrations reported in Kirk et al. (2008) for the Canadian Arctic Archipelago (79 ± 28 vs 80 ± 37 pg L−1) (Figure 7).
Concentrations in deep regions of the Arctic marine water column are comparable to those measured in deep waters of the equatorial Pacific (maximum MeHg and DMHg concentrations 116 and 134 pg L−1, respectively) (Mason and Fitzgerald 1993). In deep north Atlantic waters (Mason et al. 1998) average concentrations of DMHg (26 ± 16 pg L−1) were lower than those reported for the Arctic, but concentrations of MeHg there were higher, averaging 208 ± 216 pg L−1.
3.1.3 Sediment Hg
Concentrations of THg in Arctic marine sediments vary greatly, with higher values generally observed in the high Arctic compared to Hudson Bay. For example, concentrations of THg in Arctic marine sediment cores collected from the interior Arctic Ocean, Beaufort Shelf and Greenland coast in the 1980s and 1990s were 10–120, 1–130 and 4–280 ng g−1, respectively (Gobeil et al. 1999, Macdonald et al. 1991, Asmund and Nielson 2000), whereas those from Hudson Bay were 8–58 ng g−1 (Hare et al. 2010). Hudson Bay surface sediment THg concentrations also varied spatially, with highest values observed offshore and in eastern Hudson Bay and lowest values observed along the southern and western coasts. No measurements of MeHg in Arctic marine sediments have been reported.
3.2 Exchange of Hg Species Among Different Ocean Compartments
3.2.1 Evasion of Hg(0) and DMHg from the ocean surface
Arctic marine waters are a source of Hg(0) and DMHg to the atmosphere. Site to site differences in instantaneous Hg(0) fluxes are best explained by variability in the site-specific gas transfer velocity, itself a function of wind-induced turbulence and mixing. On the other hand, differences in DMHg evasion are primarily due to large site-to-site differences in surface water DMHg concentrations. For example, because Hg(0) concentrations in the Canadian Arctic Archipelago and Hudson Bay region were similar among all sites, variation in flux was driven by wind velocity and ranged between 2.6–388 ng m−2 day−1, averaging 130 ± 138 ng m−2 day−1 (Kirk et al. 2008). In contrast, surface concentrations of DMHg varied among regions, resulting in fluxes of DMHg that were greater in the Canadian Arctic Archipelago (39.7 ± 54.6 ng m−2 day−1) compared to Hudson Bay (16.9 ± 35.7 ng m−2 day−1). Average instantaneous ocean-atmosphere Hg(0) fluxes in the Canadian Arctic Archipelago and Hudson Bay are higher than those calculated previously for north Atlantic (94.7 ng m−2 day−1 (Mason et al. 2001) and 10.1 ± 8.7 ng m−2 day−1 (Andersson et al. 2011)), Mediterranean (2.8 ng m−2 day−1 (Cossa et al. 1997)) and Baltic waters (50 ng m−2 day−1 (Kuss and Scheider 2007)) likely due to lower concentrations of DMHg in surface waters, as well as year-round degassing of mid latitude oceans relative to the Arctic Ocean where Hg(0) builds up due to the persistent ice-cover. Average ocean-atmosphere DMHg fluxes were also higher than previous estimates for the Arctic (4.7 ng m−2 day−1), Antarctic (2.8 ng m−2 day−1) and Atlantic oceans (4.9 ng m−2 day−1) (Pongratz and Heumann 1999). Andersson et al. (2008) observed a wide range of DGM fluxes in the Arctic Ocean, with Hg(0) deposition (−38.4 ng m−2 day−1) observed along the west coast of Greenland and maximum DGM evasion of 2352 ng m−2 day−1 observed along the coast of Alaska, which is higher than those measured in polluted areas of the Mediterranean Sea (570 ng m−2 day−1) (Andersson et al. 2007). It is important to note that instantaneous air water Hg fluxes measured in the summer and fall (Kirk et al. 2008, Andersson et al. 2008) may differ from mean annual fluxes to the large seasonal variability in air water gas exchange imposed by the presence of sea ice.
By comparison, using the GEOS-Chem model, Fisher et al. (2012) estimated that total annual evasion of DGM from the Arctic Ocean (70°–90° N) is 90 t yr−1, which corresponds to an average flux of ~20 ng m−2 d−1. Therefore, evasion of Hg(0) from surface waters is one of the principal mechanism of Hg removal from the Arctic Ocean, along with sedimentation of Hg to the seafloor (see Section 2.4). Sea ice plays an important role in controlling Hg(0) and DMHg evasion. As noted in Section 3.1.1, concentrations of DGM are often higher in areas of extensive sea ice cover, likely due to the physical barrier to air-water gas exchange presented by sea ice and because biological Hg(II) reduction (Poulain et al. 2007a) in the Arctic is an important pathway for Hg(0) production compared to other oceans, where reduction is almost entirely photochemically driven (Soerensen et al. 2010). However, it is still not clear whether cracks and leads in the ice may allow significant gas exchange to take place even in areas that are mostly ice covered. In the absence of sea-ice, DGM concentrations are lower due to photochemically driven Hg(0) production and evasion.
3.2.2. Fluxes of THg from water to sediments
Surface sediment THg fluxes, obtained by multiplying concentrations of THg in surface sediments by sedimentation rates, were recently calculated for Hudson Bay and ranged from 1.2–5.1 ng cm−2 year−1 (Hare et al. 2010). Assuming that these fluxes reflect the focusing of particles through their transport, resuspension and deposition into quiescent areas, the Hudson Bay wide mean surface sediment Hg flux was estimated at 4.5 t yr−1 (Hare et al. 2008). Outridge et al. (2008) estimated that Hg sedimentation over the shelf and basin sediments of the Arctic Ocean totaled 108 t yr−1, although there was a great deal of uncertainty surrounding this estimate.
3.3 Methylation and Demethylation
3.3.1 Zones of MeHg production
There is a paucity of data on methylation and demethylation processes in Arctic marine environments and it was therefore recently suggested that research on the biogeochemical cycle of Hg in Arctic regions needed to focus on methylation processes within the ocean (Figure 8) (Macdonald and Loseto 2010). Studies conducted in other regions indicate that DMHg (as opposed to MeHg) is sometimes the principal methylated Hg species found in marine waters. This observation has led to the hypothesis that DMHg may be the primary product of Hg(II) methylation in seawater and that MeHg is then produced by decomposition of DMHg (Mason et al. 1995, Mason and Fitzgerald 1993). In open-ocean environments, Hg(II) methylation in the water column is likely the principal source of methylated Hg (Sunderland et al. 2009, Mason and Fitzgerald 1990, Cossa et al. 2009, Heimburger et al. 2010, Lehnherr et al. 2011), whereas in estuaries and near-shore environments sediments are an important MeHg source (Hollweg et al. 2009, Hammerschmidt et al. 2004). However, both deep-sea sediments (Ogrinc et al. 2007) and continental shelf sediments (via lateral transport) (Hammerschmidt et al. 2006) may contribute to the open-ocean water column pool of methylated Hg. In the Arctic, the possibility also exists that methylation in the cyrosphere (sea ice and overlying snow) may also contribute MeHg to surface marine waters.
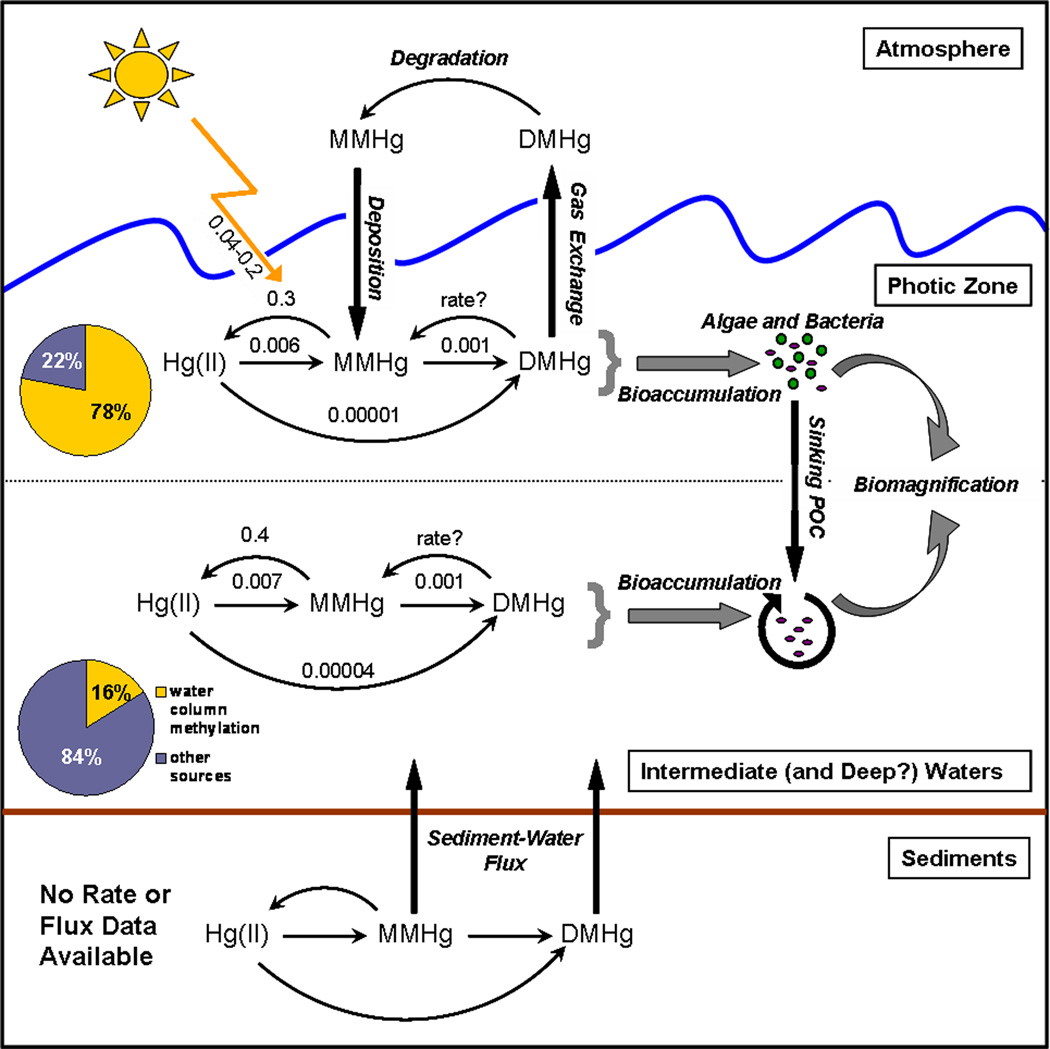
Figure 8.
Conceptual diagram of processes affecting MeHg concentrations in the water column of the Arctic Ocean. The various Hg methylation and (photo)demethylation pathways (thin arrows), each governed by their respective rate constants (k, d−1; values displayed above the arrows) along with associated biogeochemical fluxes (thick arrows), such as air–water gas exchange of DMHg and remineralization of POC and MeHg bioaccumulation/biomagnification (block arrows) (Data from Lehnherr et al. 2011).
3.3.2 Water column methylation
Both pure cultures of marine bacteria originating from Antarctic surface waters (Pongratz and Heumann 1999) and macroalgae collected from an Arctic fjord (Pongratz and Heumann 1998b) have been shown to produce MeHg and DMHg, suggesting that Hg(II) methylation can occure in the water column compartment of polar marine ecosystems. Furthermore, the correlation of DMHg and MeHg concentrations with chlorophyll a concentrations in Arctic and Antarctic marine waters supports the notion that these species have a biogenic origin in the euphotic zone (Pongratz and Heumann, 1999, Pongratz and Heumann 1998a). The vertical distribution of methylated Hg in marine waters of the Canadian Arctic Archipelago indicates that these species are also produced at sub-thermocline depths where MeHg and DMHg make up a large proportion of the THg (see Section 3.2.1 and Kirk et al. 2008). Additionally, MeHg can also be produced from the decomposition of DMHg in the water column (Mason et al. 1995).
Methylation and demethylation rate constants were recently measured in marine waters of the Canadian Arctic Archipelago (see Figure 8), demonstrating that the water column is an important site of MeHg production (Lehnherr et al. 2011). Methylation of Hg(II), resulting in the production of both MeHg and DMHg was observed during incubations of seawater samples. Additionally, DMHg was also formed by the further methylation of MeHg, although DMHg production was generally slower than MeHg production. However, there is still only a very rudimentary understanding of the interconversion between DMHg and MeHg. Therefore, the importance of DMHg as a precursor of MeHg, and by extension, a source of Hg available for bioaccumulation and biomagnification, has still not been properly quantified. For example, estimates of the rate constant of DMHg degradation to MeHg vary greatly from 2 × 10−4 d−1 to 0.2 d−1 (Mason and Sullivan 1999, Mason et al. 1995). A large proportion of aqueous methylated Hg is in the DMHg form and, therefore, quantifying the rates of interconversion between MeHg and DMHg could be the key to understanding why marine organisms can have high tissue MeHg concentrations despite low MeHg concentrations in the water.
A simple model built using the rate constants of MeHg production and degradation measured in the Canadian Arctic Archipelago was used to demonstrate that, on average, Hg(II) methylation in the water column accounts for about half (47 ± 62%) of the MeHg occurring in marine waters in the Canadian Arctic Archipelago, and is therefore the single largest source of MeHg to Arctic marine waters and food webs (Lehnherr et al. 2011). Furthermore, MeHg demethylation in the water column limits how far MeHg can be transported by ocean currents; for example, 90% of the MeHg in a particular water mass is predicted to be demethylated in the time it takes for that water mass to travel 20–200 km (Lehnherr et al. 2011). Therefore, the majority of MeHg occurring in coastal Arctic marine waters cannot originate from distant sources, highlighting the importance of Hg(II) methylation in local compartments.
Because the availability of Hg(II) limits MeHg production, the implication is that if concentrations of Hg(II) in Arctic marine waters were to increase, as a result of either increased anthropogenic inputs or environmental change, the production of MeHg in the water column from Hg(II) methylation is also likely to increase. The opposite is also expected to be true, and decreases in anthropogenic Hg inputs to the ocean should result in decreases in seawater MeHg concentrations. While increases in Hg(II) will result in more MeHg, Mason et al. (2012) conclude that MeHg in fish will respond slowly to changes in atmospheric deposition given the decadal timescale of processes, such as ocean circulation, which affect MeHg production in the open ocean. Therefore, Hg concentrations in marine biota may initially continue to increase even if atmospheric Hg deposition begins to decrease (Mason et al. 2012).
Production of MeHg in ocean compartments is also likely linked to the availability of organic carbon for microbial decomposition. Various studies have shown that the distribution of MeHg in surface/intermediate marine waters of the Pacific Ocean (Sunderland et al. 2009), Mediterranean Sea (Cossa et al. 2009), Southern Ocean (Cossa et al. 2011) and over the Canadian Arctic shelf (Lehnherr et al. 2011) is correlated to indices of organic carbon remineralization, such as apparent oxygen utilization (AOU) and inferred rates of organic carbon remineralization. In addition, others have demonstrated that MeHg and DMHg concentrations in polar oceans are correlated to chlorophyll a concentrations (Pongratz and Heumann 1998a and 1999). Within a one-year annual cycle, concentrations of methylated Hg increased and decreased with the abundance of phytoplankton in the Mediterranean Sea (Heimburger et al. 2010). Although the relationship between primary productivity and MeHg production has not been quantified in the Arctic, it is fair to say that any spatial or temporal increases or decreases in primary productivity and organic carbon respiration will also result in corresponding increases or decreases in MeHg production and uptake by biota.
3.3.3 Sediment and cryospheric methylation
There is no data for Hg(II) methylation in Arctic marine sediments, but in other regions shelf sediments have been shown to be an important source of MeHg (Hollweg et al. 2009, Hammerschmidt et al. 2004) to the water column. However it is important to note that when particle settling and sediment burial are taken into account, MeHg losses to the sediments can be bigger than the release of MeHg to the overlying water from diffusion and resuspension, meaning that sediments are not always a net source of MeHg, particularly in shallow coastal regions where particles do not have a long residence time in the water column before settling out (Sunderland et al 2010). Hollweg et al. (2009) in reviewing the literature demonstrated that the MeHg flux from coastal sediments predicted from diffusion calculations can be 1–2 orders of magnitude lower than the flux measured using benthic flux chambers and sediment core incubations, presumably because diffusion-based fluxes ignore other important contribution to the total MeHg flux. Therefore, to comprehend the role of marine sediments as MeHg sources, it is important to understand the various mechanisms that are responsible for the exchange of MeHg between the water column and sediments, including diffusion, particle settling and resuspension, as well as bioturbation which has been shown to enhance the transport of MeHg from sediments to overlying waters (Benoit et al 2009). Shelf sediments, inputs from major Arctic rivers (Graydon et al. 2009, Leitch et al. 2007, Kirk and St. Louis 2009, Coquery et al. 1995) and atmospheric deposition of MeHg following the evasion and degradation of DMHg from surface waters (St. Louis et al. 2005) likely account for the remainder of the MeHg pool in coastal Arctic waters that cannot be accounted for by water column methylation (see Section 3.3.2). Snow-phase Hg(II) methylation and snowmelt have also been proposed as potential sources of MeHg to Arctic ecosystems (Loseto et al. 2004, Larose et al. 2010, Constant et al. 2007). Measured MeHg concentrations in snow sometimes correlate with indicators of microbial activity, although methylation rates and/or MeHg exports during snowmelt have not directly been quantified. MeHg concentrations in sea ice are in the same range as what has been observed in open ocean waters (<0.090 ng L−1), and the vertical MeHg concentration profile through the snow sea ice continuum suggests a mix of sources including atmospheric, seawater and methylation in basal ice (Cossa et al. 2011). However, it is not yet possible to estimate the importance of cryospheric MeHg production to Hg bioaccumulation in marine food webs.
3.3.4 Water column demethylation
MeHg can be demethylated under both light and dark conditions as a result of photodemethylation and microbial demethylation (Lehnherr et al. 2011). While microbial demethylation is likely occurring throughout the water column, photodemethylation is limited to the euphotic zone and likely primarily to the depth to which UV radiation can penetrate in the water column (Lehnherr and St. Louis 2009). It was recently shown in laboratory experiments that the rate constant of MeHg photodemethylation is lower in seawater compared to freshwater due to a shift in MeHg speciation, as a result of higher chloride (Cl−) concentrations, to CH3HgCl, which appears to be more resistant to photodemethylation (Zhang and Hsu-Kim 2010). This is consistent with field measurements performed in the Arctic where the photodemethylation rate constant is about 3–4 times higher in freshwater ponds (3.4 × 10−3 m2 E−1, Lehnherr et al., 2012) compared to seawater (1 × 10−3 m2 E−1, Lehnherr et al. 2011). However, although the MeHg photodemethylation rate constant is slower in seawater than in freshwater, photodemethylation may still play a large role in controlling MeHg exposure in marine food webs because the euphotic zone is typically deeper in more transparent marine waters than in lakes (which tend to have more light absorbing solutes and particles). Thus, the relative losses of MeHg from photodemethylation are greater on an areal basis than what might be predicted simply by comparing surface photodemethylation rates in fresh and marine waters. This is substantiated by recent measurements of stable isotope signatures in fish tissues showing that MeHg incorporated into the pelagic marine food web of the Gulf of Mexico had undergone substantial (50%) photodemethylation (Senn et al. 2009), comparable to what was previously reported for freshwater fish (25–68%) in the U.S. (Bergquist and Blum 2007).
Photodemethylation in the Arctic exhibits strong seasonal variability due to the absence of sunlight in the winter and continuous illumination in the summer. Snow and sea ice cover also strongly control the light regime of Arctic marine waters and sea ice cover was recently shown to be the dominant control on MeHg photodemethylation in the Arctic during the spring season (early April to late June) (Point et al. 2011). Furthermore, it is thought that climate change-induced sea ice loss will increase the amount of photodemethylation taking place in polar surface marine waters (Point et al. 2011). However, it is also possible that sea ice declines will mostly change the timing of photodemethylation, and the seasonal dynamics in MeHg concentrations, rather than the total annual flux. For example, methylated Hg has been observed to build up under sea ice (St. Louis et al. 2007) such that at ice-out there is a large pool of methylated Hg potentially available to take part in photochemical reactions. Under such a scenario, photodemethylation rates, which are proportional to MeHg concentration (Lehnherr and St. Louis 2009, Sellers et al. 1996), would be high during the short ice-free season but negligible at other times. If the spatial extent and duration of ice cover continues to decline as is widely predicted, a new scenario might emerge where there is no longer a build up of methylated Hg under the ice and photodemethylation would occur at a slower rate, due to lower MeHg concentrations, but for a longer time period, resulting in little net change in the annual MeHg photodemethylation flux.
In summary, local methylation and demethylation processes control the size of the MeHg pool in marine waters and ultimately how much Hg is available for bioaccumulation and biomagnification through marine food webs. Increasingly, evidence is pointing to the importance of methylation in the water column (see also Mason et al. 2012), but with some smaller contributions from shelf sediments and rivers in coastal areas. Any processes which directly or indirectly affect methylation and demethylation rates, such as sea ice cover and the availability of Hg(II) for methylation or organic carbon for microbial respiration, will likely result in changes in Hg exposure throughout marine food webs.
4.0 FOOD WEB PROCESSES AND DRIVERS OF HG CONCENTRATIONS IN ARCTIC MARINE BIOTA
Dietary exposure, through the ingestion of prey, represents the primary means by which higher trophic level species such as marine mammals, birds and fish are exposed to MeHg. MeHg binds to proteins in organisms and increases over time in individuals (bioaccumulation) as well as by orders of magnitude with each trophic level (biomagnification). Thus, although MeHg concentrations, and the proportion of THg that is MeHg, are low at the bottom of the food web, the bioaccumulating and biomagnifying properties of MeHg result in top predators with high MeHg concentrations which make up nearly 100% of the THg present in the muscle tissue (e.g. Morel et al. 1998, Campbell et al. 2005, Loseto et al., 2008, Macdonald and Loseto 2010). Hg concentrations in higher trophic level species are controlled by numerous processes including: a) the amount of bioavailable Hg and/or MeHg at the bottom of the food web, b) species-specific processes controlling bioaccumulation and dilution and c) food web length, structure and type. Therefore, when evaluating Hg exposure rates and body burdens in higher trophic species, it is important to consider both the abiotic Hg and MeHg pools available for uptake at the bottom of the food web, as well as food web processes, such as trophic dynamics and species foraging behavior (AMAP 2011).
4.1 Bioaccumulation and Biomagnification of MeHg through Arctic Marine Food webs
Arctic marine food webs generally consist of seven functional groups spanning a total of five trophic levels, including primary producers, such as ice algae or phytoplankton, grazers, predatory invertebrates, fish, predators such as beluga whales and seals, and apex predators, which include polar bears and humans. While Arctic marine food webs and the trophic transfer of Hg within them are difficult to study due to the remoteness, seasonal ice cover constraints and the lack of suitable platforms to sample key biota (e.g. fish in the deep ocean), Hg concentrations in top predators such as polar bears, beluga, and birds are moderately available due the subsistence lifestyles of many northern coastal communities and associated monitoring programs. Because of the importance of marine mammals to human diet in the north, understanding why Hg concentrations in Arctic marine mammals are so high, and thus the food web processes that drive these concentrations, is extremely important.
4.1.1. The base of the Arctic Ocean food web
Few studies have sampled the base of Arctic marine food webs (e.g. plankton, algae, bacteria) to assess the entry of Hg to food webs. In the North Water Polynya, concentrations of THg in sea ice algae were 0.015 µg g−1 dw (n=1) (Campbell et al. 2005). Concentrations of Hg in particulate organic material (POM) were <0.02 µg g−1 dw in Lancaster Sound (Atwell et al. 1998). Calanoid copepods (e.g., Calanus glacialis and C. hyperboreus), the primary consumers of ice algae and phytoplankton (Geynrikh 1986, Springer et al. 1996, Mumm et al. 1998, Auel and Hagen 2002) had lower Hg levels in the Mackenzie estuary (0.025 µg g−1 dw) compared with offshore deep water habitat of the Beaufort Sea (0.032 µg g−1 dw) (Loseto et al. 2008a). Calanoid copepod Hg concentrations were lower in the winter season (~ 0.02 to 0.06 µg g−1 dw) than in the warmer open water season (0.04 to 0.127 µg g−1 dw) (Stern and Macdonald 2005, Loseto et al. 2008a). These values compare well to those observed in the North Water Polynya, Lancaster Sound, and off the coast of Greenland, where copepods had Hg concentrations of 0.025 (Campbell et al. 2005), 0.06 (Atwell et al. 1998), and 0.08 µg g−1 dw (Riget et al. 2007), respectively. Mysids, which are considered to be an epibenthic primary consumer as they scavenge floating detritus, had Hg concentrations similar to deep water copepods (Loseto et al. 2008a).
4.1.2. Fish and predatory invertebrates
Hg concentrations in Arctic cod (Arctogadus glacialis) collected in spring under the ice in the Amundsen Gulf/Franklin Bay averaged 0.37 µg g−1 dw and were significantly higher than those collected from the shallow coastal shelf region of the Beaufort Sea, near the Mackenzie Delta (0.16 µg g−1 dw) (Loseto et al. 2008a). Hg concentrations in other fishes of the Beaufort coastal shelf-region, including rainbow smelt (Osmerus mordax), Pacific herring (Clupea pallasi), Arctic cisco (Coregonus autumnalis) and least cisco (C. sardinella)) were similar to those observed in Arctic cod of the coastal-shelf region (0.2 µg g−1 dw) (Loseto et al. 2008a). The similarities in Hg concentrations among species in the coastal shelf-region combined with the difference observed in Arctic cod Hg concentrations between different regions highlights the potential influence of regional Hg sources and food web structure on fish Hg concentrations, however differentiating between these factors is challenging. Hg concentration differences between offshore and shelf region Arctic cod could be driven by regional Hg sources or seasonal influences on foraging and physiology that can affect bioaccumulation and/or dilution. For example, rapid growth rates can result in a ‘biodilution’ effect due to the reduced concentration over a large biomass, whereas those with slower growth rates may have higher Hg concentrations (Kidd et al. 1999, Karimi et al. 2007).
Variability in Arctic cod Hg concentrations are also observed across the circumpolar Arctic with concentrations in Lancaster Sound and North Water Polynya being considerably higher (0.2 µg g−1 dw) (Atwell et al. 1998, Campbell et al. 2005) than those measured in Kongsfjorden near Svalbard (0.05 µg g−1 dw) (Jaeger et al. 2009). Beaufort Sea benthic species, which comprise an important part of the beluga diet, had Hg levels ranging from 0.2 µg g−1 dw in flounders and predator invertebrates, such as shrimp, to 0.5 µg g−1 dw in sculpins (Loseto et al. 2008a). Sculpins were found to range from 0.24 µg g−1 dw in Lancaster Sound to 0.34 µg−1 dw near Qaanaaq and Qeqertarsuaq, western Greenland (Atwell et al. 1998, Riget et al. 2007).
4.1.3. The use of δ15N Hg relationships to investigate biomagnification
Relationships between δ15N stable isotope signatures and Hg concentration in biota are now widely used to infer species’ trophic status and define Hg transfer from prey to predator (Cabana and Rasmussen 1994). For example, Figure 9 demonstrates Hg concentrations versus δ15N signatures in different marine species of the Beaufort Sea, including copepods from near and off-shore sites, invertebrates, fish from benthic, pelagic and coastal habitats, and beluga whales (Loseto et al. 2008a). The considerable variability in Hg and δ15N observed among the invertebrates and fish likely results from a range in foraging behaviors and habitat uses. Beluga whales also showed variability in Hg and δ15N; however using a combined approach of Hg and δ15N analyses and satellite telemetry, beluga whales were split into three distinct feeding groups based on sex and age (see Section 4.2 for details) (Loseto et al. 2008a). The slopes of the Hg-δ15N regressions, which represent the Hg biomagnifications rates, ranged from 0.23 to 0.25 for the three different beluga feeding groups and their hypothesized food items. These rates are similar to those observed in marine food webs of the central and Canadian high Arctic (Atwell et al. 1998, Campbell et al. 2005), western Greenland (Riget et al. 2007) Icelandic waters (McMeans et al. 2010), and fjords of Svalbard, Norway (Jaeger et al. 2009).
Figure 9.
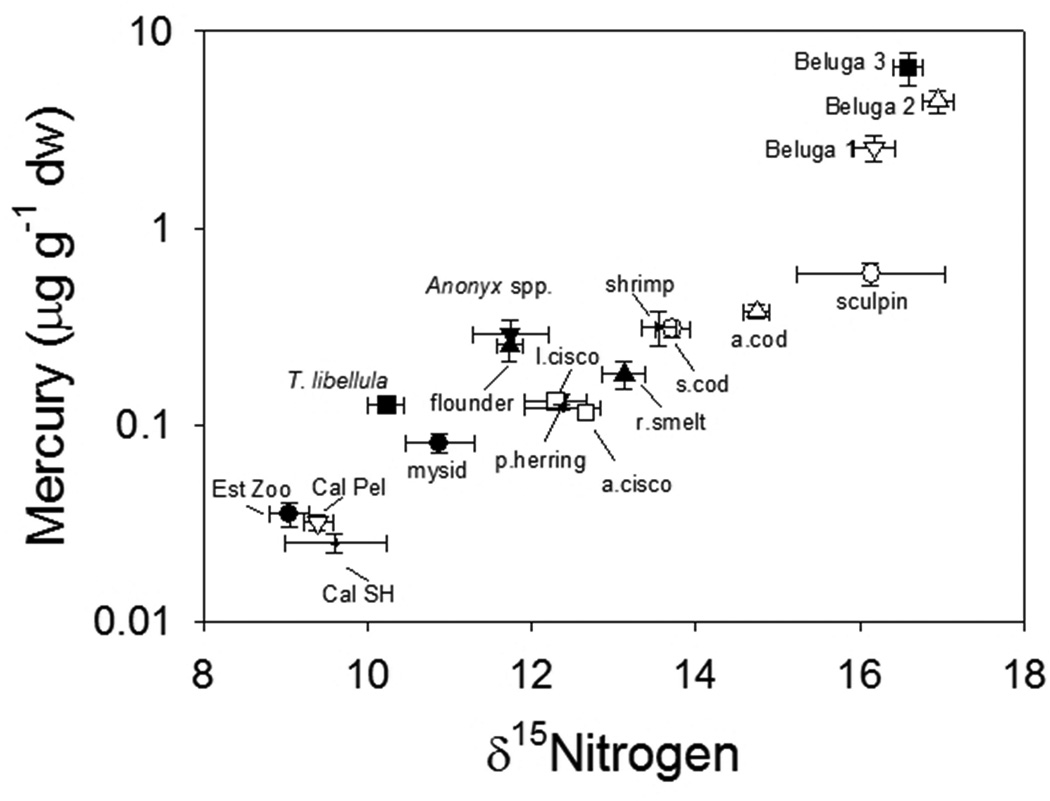
Concentrations of Hg and δ15N signatures in Beaufort Sea biota collected from coastal shelf, off shore pelagic, and benthic zones. Invertebrates collected include: calanus copepods collected from the coastal shelf (Cal SH), calunus copepods (Cal Pel) and hyperiid amphipods (Themisto libellula) from off shore pelagic zone, mixed zooplankton from near the coastline (Est Zoo), and mysids, gammarid amphipods (Anonyx spp.) and shrimp from benthic zones. Fish collected include: pacific herring, Arctic cisco, least cisco, rainbow smelt, and saffron cod from the coastal shelf, Arctic cod from the off shore pelagic zone, and flounder and sculpin from the benthic regions. Three groups of beluga whales which utilize different habitats and foraging areas are also shown: Beluga 1: shallow coastal areas; Beluga 2: along ice edges; and Beluga 3: deep off-shore waters (Date from Loseto et al. 2008).
4.2 Controls on Exposure to Predators: A case study on the Beaufort beluga whale population
Considerable research on the Beaufort Sea beluga whale population has been driven by the local Inuvialuit of the western Arctic who continue to lead a traditional lifestyle and harvest beluga whales for food (Harwood et al. 2002). Concern for both food safety and beluga health was heightened when Hg concentrations in this population significantly increased between the 1980’s and 1990’s (Lockhart et al. 2005). To try to understand the factors driving high Hg concentration in the Beaufort beluga population, studies on trophic structure and beluga ecology, both of which can influence the dominant source of MeHg that is bioaccumulated, were conducted. For example, belugas feeding in pelagic, benthic, and estuarine/shelf habitats may receive MeHg predominantly originating from water column methylation, sediment methylation, and river inputs, respectively (AMAP 2011). However, predator feeding ecology can be complicated by large home ranges and feeding variability within different groups. Using Hg and δ15N analyses of food web organisms from a variety of potential habitats, and satellite telemetry to track beluga movement, trophic structure, beluga habitat and diet preference (and thus potentially important sources of Hg to the Beaufort beluga whales) were identified (Loseto et al. 2006, Loseto 2007, Loseto et al. 2008a, Loseto et al. 2008b, Loseto et al. 2009).
4.2.1 Beluga Habitat Use and Associated Diet and Mercury Exposure
As described above (see Sections 4.1.1–4.1.3), to examine trophic structure, Hg concentrations and δ15N signatures were examined in different marine food web organisms, including lower trophic copepods from near and off-shore sites, the invertebrates that prey on them, fish from benthic, pelagic and coastal habitats, and beluga (Figure 9) (Loseto et al. 2008a). To quantify daily habitat selection of the Beaufort beluga whale population, data obtained from satellite telemetry was analyzed using the Resource Selection Function (Arthur et al. 1996). Results suggested that the Beaufort Sea beluga population sexually segregates into groups with different summer ranges based of sea ice-cover, bathymetry, and distance from shoreline. Thus three habitat groups were defined based on beluga length, sex, reproductive status, and summer habitat usage: 1) females with and without calves and small males (< 4 m), which selected shallow open-water habitats near the mainland; 2) medium length males (3.8–4.3 m) and a few females (>3.4 m) without neonates, which selected the sea ice edge habitat; and 3) large males (4–4.6 m), which selected regions of heavy sea ice cover in deep, offshore waters (Loseto et al. 2006). Intra-species habitat segregation obviously has consequences for feeding ecology, trophic structure and Hg exposure, as it relates to different energetic requirements over space and time (Stevick et al. 2002). In fact, average Hg concentrations varied greatly among the three habitat groups, and food web groups were therefore identified based on habitat usage: 1) beluga using shallow estuarine habitats had the lowest Hg concentrations (2.6 ± 0.8 µg g−1 dw) and likely fed on estuarine-shelf food webs; 2) beluga selecting the ice edge in the offshore region had mid-range Hg concentrations (4.4 ± 0.7 µg g−1 dw) and likely fed on pelagic food webs consisting largely of Arctic cod and; 3) beluga selecting regions of heavy sea ice cover over deep waters had the highest Hg concentrations (6.5 ± 0.7 µg g−1 dw) and likely fed on benthic and epibenthic food webs (Figure 9) (Loseto et al. 2008a). It is noteworthy that despite the high influx of THg and MeHg to the Mackenzie Delta (Graydon et al. 2009, Leitch et al. 2007), Hg concentrations in the estuarine shelf belugas and their prey were lowest of all groups. This illustrates that the large mass fluxes of THg and/or MeHg into the delta do not necessarily result in higher MeHg concentrations in marine waters, and, by extension, higher concentrations in marine food webs. On the other hand, belugas feeding in benthic and epibenthic food webs had the highest Hg concentrations, likely due to high Hg concentrations in prey items such as sculpin, shrimp and flounder (Loseto. 2008a).
Although the assigned feeding groups were an oversimplification of the temporal and spatial complexities of beluga movement and seasonal feeding preferences, the exercise was valuable in understanding trophic level transfers in various food webs. Results from this case study demonstrate that the somewhat predicable increase in Hg up food chains reflects the biomagnification of MeHg, increasing in concentration by approximately ten times at each trophic level, thus highlighting the importance of food chain length in driving Hg concentrations in top predators. The study also revealed differences in estuarine-shelf, pelagic and benthic food webs that may reflect trophic guild, species-specific processes, or regional Hg sources at the base of the food web (Loseto et al. 2008a). In summary, evaluating Hg concentrations in higher trophic level species need to consider both Hg sources and food web processes, including foraging behaviors and habitat usage. In the case of the Beaufort Sea beluga whale population, differences in feeding habits resulted up to a two-fold difference in Hg concentration among groups (Loseto et al. 2008a). While determining the source of MeHg at the base of the food web remains difficult, species-specific processes driving bioaccumulation and/or dilution, food web structure and predator feeding ecology are key factors to consider when examining Hg levels in biota.
5.0 SPATIAL AND TEMPORAL TRENDS OF HG IN BIOTA
Monitoring programs working in tandem with subsistence hunters from northern coastal communities to obtain samples have resulted in substantial data sets on Arctic biota, particularly for birds, seals, and polar bears. In addition, analysis of well preserved teeth, feathers and hair has allowed comparison of pre-industrial to modern-day Hg concentrations in Arctic mammals, birds and humans. Comparisons of Hg concentrations in Arctic biota to determine spatial and temporal trends can be challenging because sample sizes are often small and Hg varies largely with age and sex of the animals; however some broad-scale patterns have been well demonstrated and are described below.
5.1. Spatial Trends
Ford et al. (2005) found no circumpolar spatial trend for Hg in blue mussels (Mytelis edulis) sampled during the late 1990s from Alaska, Canada, Greenland, Iceland, the Faroe Islands and Norway; nor was any spatial pattern evident for Hg in sea run char (Salvelinus alpinus) sampled from 55 locations across the Canadian Arctic (Braune et al. 2005). The spatial distribution of Hg in seabirds is mixed. In general, Hg concentrations in seabirds from the Barents Sea (Savinov et al. 2003) were lower than in Greenland, Canada and northeast Siberia (Ford et al. 2005). Braune (2007) found that Hg in thick-billed murre (Uria lomvia) eggs from the Canadian high Arctic were higher than in murre eggs from Alaska and northern Norway and Hg concentrations in eggs of ivory gulls (Pagophila eburnea) from the Canadian high Arctic (Braune et al. 2006) were higher than in eggs from the Norwegian and Russian Arctic (Miljeteig et al. 2009) (Figure 10). However, no spatial pattern was evident for Hg in black guillemot eggs (Cepphus grylle) or northern fulmar (Fulmarus glacialis) livers sampled from the Canadian Arctic, Greenland and the Faroe Islands (Ford et al. 2005), or for black legged kittiwake (Rissa tridactyla) eggs sampled from the Canadian high Arctic and northern Norway (Braune 2007). Regional patterns were not evident in four species of eiders (Somateria spp. and Polysticta spp.) from Alaska, Arctic Canada, Arctic Russia, Greenland, the Barents Sea and northeastern Siberia (Ford et al. 2005). For migratory species, Hg exposure during the winter range may be a factor. In addition, birds of the same species may have different diets in different regions, which likely affects the patterns (or lack thereof) reported.
Figure 10.
THg concentrations in eggs of different marine birds breeding in the circumpolar Arctic. Colors indicate region (turquoise = Alaska, orange = Canadian Arctic, purple = Norway, Yellow = Russia); error bars show the maximum observed concentration and the vertical red dashed lines indicates the threshold range for reproductive effects reported in the literature (Modified from Dietz et al. 2011).
A circumpolar comparison of hepatic Hg in ringed seals (Phoca hispida) sampled from 1995 to 2001 found that Hg concentrations were highest in the central/western Canadian Arctic locations compared with those from Alaska, Greenland, Norway and Russia (Rigét et al. 2005). An earlier study (Wagemann et al. 1996) found higher Hg concentrations in liver of beluga whales (Delphinapterus leucas) from the western Canadian Arctic compared with the eastern Canadian Arctic. However, a more recent analysis suggests that regional differences in Hg concentrations between eastern and western Canadian Arctic beluga have diminished (Lockhart et al. 2005) although levels in 2009 were still relatively higher in western Arctic beluga (Stern 2010). Hepatic Hg concentrations in polar bears (Ursus maritimus) sampled from various locations across the Canadian Arctic showed higher Hg concentrations in the Beaufort Sea population compared with populations further east (Rush et al. 2008).
Latitudinal differences in the form of increasing south-to-north trends in Hg have also been observed for seabirds and marine mammals in both Greenland (Dietz et al. 1996) and the Canadian Arctic (Braune et al. 2002, Rush et al. 2008, St. Louis et al. 2011). However, as with the circumpolar comparisons, differences in availability of MeHg for bioaccumulation at the base of the food web, feeding behavior and food web structure may all be confounding factors contributing to the complexity of interpreting these geographical patterns in the absence of additional data. For example, on the basis of δ15N and δ13C signatures in polar bear hair, published δ15N signatures in particulate organic matter and sediments, and water column concentrations of MeHg, it was recently demonstrated that bears from the southern Beaufort Sea have significantly higher Hg concentrations than those from western Hudson Bay, likely due to both a longer and more pelagic food web and higher water column concentrations of methylated Hg available to initiate bioaccumulation (St. Louis et al. 2011).
5.2. Temporal Trends
Long-term data (i.e., comparing modern with historical or pre-industrial Hg concentrations) can be used to estimate the relative importance of natural and anthropogenic metal inputs in modern biota and the environment (Dietz et al. 2009). In contrast, short-term data (i.e., covering the past one to three decades) illustrate how Hg concentrations have changed in recent times (Rigét et al. 2011) and suggest likely trends in the near future.
5.2.1 Long-term trends
Numerous studies have used analysis of archived and modern teeth (Dietz et al. 2009, Eide et al. 1993, Outridge et al. 2002, Outridge et al. 2005, Outridge et al. 2009, Tvinnereim et al. 2000), hair (Dietz et al. 2006a, Hansen et al. 1989, Wheatley and Wheatley 1988) and feathers (Dietz et al. 2006b) dating back as far as 800 years to reconstruct long-term trends in Hg concentrations in Arctic animals and people. Results suggest that there has been a ten-fold increase in Hg levels in upper trophic level marine animals (beluga, ringed seal, polar bear, birds of prey) over the past 150 years with an average rate of increase of 1 to 4% per year (Figure 11, Dietz et al. 2009). It has thus been suggested that ~90% of the Hg currently present in Arctic animals originates from anthropogenic sources (Dietz et al. 2009), although the observed increases also reflect both anthropogenically and naturally-induced increases in Hg(II) methylation and bioaccumulation rates, and shifts in feeding behavior or food web structure, all of which unfortunately cannot be teased apart with current analysis.
Figure 11.
Historical trends of Hg concentrations in hard tissues of various Arctic animals, expressed as % of modern maximum annual average concentrations (Modified from Braune et al. 2011, data from Dietz et al. 2009).
5.2.2 Recent trends
Changes in Hg concentrations in Arctic biota over recent decades have not been consistent across the Arctic or among species. Hg levels in terrestrial species such as moose (Alces alces) and caribou (Rangifer tarandus) from the western Canadian Arctic (Gamberg et al. 2005) as well as caribou from western Greenland (Rigét et al. 2004), caribou/reindeer from Sweden (Odsjö et al. 2007) and mountain hare (Lepus timidus) from the Faroe Islands (Hoydal and Dam 2009) have either decreased or not changed. This same pattern holds true for a variety of freshwater fish species, primarily from Canadian Arctic lakes (Evans et al. 2005, Muir et al. 2005, Gantner et al. 2009) but also from the Faroe Islands (Hoydal and Dam 2009) and Sweden (Bignert 2002), with the exception of Hg in male burbot (Lota lota) from the Mackenzie River in Canada; where Hg concentrations appear to be increasing (Evans et al. 2005). Hg trends in Arctic marine species, however, are quite varied depending on species and location. Marine fish, such as shorthorn sculpin (Myoxocephalus scorpius) from Greenland have shown no change in Hg concentrations whereas Hg in seabird eggs has increased in some species (Braune 2007, Braune et al. 2006) but not others (Braune 2007, Helgason et al. 2008). Likewise, Hg trends in marine mammals such as walrus (Odobenus rosmarus), ringed seal, pilot whale (Globicephala melas), beluga and polar bear are quite variable, reported to be increasing in species in some areas (Aubail et al. 2010, Braune et al. 2005, Dietz et al. 2011, Lockhart et al. 2005, Rigét et al. 2007, Rush et al. 2008) and either decreasing or showing no change in others (Braune et al. 2005, Dietz et al. 2006a, Gaden et al. 2009, Hoydal and Dam 2009, Kannan et al. 2007, Lockhart et al. 2005, Rigét et al. 2004, Rigét et al. 2007).
A recent meta-analysis of 83 time-series datasets for Arctic wildlife species in Alaska, Canada, Greenland, Iceland, the Faroe Islands, Norway and Sweden examined changes in Hg concentrations over the past 7 to 38 years using a statistically rigorous methodology (Rigét et al. 2011). The review included data on marine mammals, marine and freshwater fish, marine invertebrates, seabirds and land mammals. Many of these time series are now long and powerful enough (i.e., assuming a sampling period of 10 years, a significance level of 0.05 and a statistical power of 80%) to reliably detect a temporal trend if one exists. Overall, Hg concentrations showed a recent increase in 16% of the datasets, a recent decrease in 5% of the datasets and no significant change or fluctuating trends in the remaining 79% of datasets. The average annual increases of the significantly increasing datasets ranged between +0.9 and +10 % (median +5.0 %) and the significant annual decreases ranged between −2.4 and −8.6 % (median −5.9 %). Although the annual change showed no apparent trend with longitude or latitude, the annual rate of change increased significantly from east to west.
The results from the meta analysis were generally consistent with a review of recently published literature (Rigét et al. 2011). Although there was no consistent trend across species and tissues for the entire circumpolar Arctic, there was a clear east-to-west gradient in the occurrence of recent increasing Hg trends with more time-series in the Canadian and Greenland region of the Arctic showing significant increases than in the northern Atlantic Arctic. Most of the increasing time-series were for marine-based species (i.e., marine mammals, seabirds) followed by predatory freshwater fish. The factors driving these trends likely vary among species and among areas and may include relative proximity to source regions where emissions are either decreasing or increasing and/or biological (i.e., food web) processes, possibly related to climate change.
6.0 EXPOSURE OF NORTHERN PEOPLES THROUGH THEIR TRADITIONAL DIET OF COUNTRY FOODS
The potential health impacts of elevated contaminant concentrations, including Hg, on Arctic human populations has been a concern since the mid 1990s (Kuhnlein and Chan 2000; Hansen 2000). The most at-risk population is the Inuit who are a group of culturally similar indigenous Northern Peoples inhabiting the Arctic regions of Canada (Northwest Territories, Nunatsiavut, Nunavik, Nunavut, Nunatukavut), Denmark (Greenland), Russia (Siberia) and the United States (Alaska) (ICC 2008). In fact, average human blood Hg concentrations up to 40 µg L−1, which are well above blood Hg guidelines of 5.8 µg L−1, were recently (2000–2007) observed in pregnant women, mothers, and women of child bearing age in communities where diets are comprised predominantly of traditional foods (AMAP 2011). As detailed below, data on Hg levels in Northern Peoples and their food items are available due to fairly comprehensive monitoring programs; however much is still unknown about the neurological and cardiovascular impacts of Hg exposure on humans, as well as the best approaches to communicate health risks.
6. 1. How are Northern Peoples Exposed to Hg?
The maintenance of traditional lifestyles with respect to diet composition places the Inuit at an increased risk to environmental contaminants such as Hg, compared to southern populations (Van Oostdam et al. 1999, Van Oostdam et al. 2005). The Inuit traditional diet includes species that are at the top of the food chain, such as marine mammals like seals, beluga and narwhal, which are major sources of Hg to humans (Kuhnlein and Chan 2000). Hg speciation varies within different parts of these animals and concentrations generally increase in order from fat, muscle, kidney and liver. Laird et al. (2009) measured Hg bioaccessibility using an in vitro system and demonstrated that small intestinal Hg bioaccessibility from 16 fish, wild game and marine mammal samples consumed by Inuit in northern Canada ranged between 1 and 93% and was independent of food THg concentration. Additionally, they also demonstrated that gastrointestinal microbes may affect Hg bioaccessibility of the 16 country foods, either increasing or decreasing bioaccessibility depending upon the type of food. These results indicate that gastrointestinal absorption of Hg is not likely limited by the concentration of Hg in the food, which compounds the uncertainty of exposure assessment.
6.2. Rates of Hg Intake and Hg Levels in Northern Peoples
A study conducted by the Centre for Indigenous Peoples Nutrition and Environment (CINE) at McGill University in 1999 found that the 95th percentile intake of Hg in 18 Inuit communities exceeded by over a factor of 10 the Provisional Tolerable Weekly Intake (PTWI) established by the FAO/WHO of 1.6 µg kg−1 body weight day−1 (Table 2, Kuhnlein et al. 2000). Chan et al. (2008) re-calculated the intake in 2007 using the updated Hg concentrations in traditional food but using the same food consumption intake information and found similar levels of intake (Table 2), suggesting that there was little change in average Hg concentrations in traditional foods. There was a significant regional difference in Hg intake, with the Baffin region showing the highest Hg intake levels (Table 3). This is due to the relatively high intake of marine mammals in the Baffin region. Contrary to Hg, a significant decrease of polychlorinated biphenyls (PCBs) and 1, 1-dichloro-2,2'-bis-p-chlorophenyl-ethylene (DDE) concentration in traditional foods was observed suggesting that the regulations of persistent organic pollutants (POPs) under the Stockholm Convention may be having the positive effect of decreasing POP concentrations. A similar trend was also observed in Greenland. Deutch et al. (2006) compared the dietary composition and nutrients in 177 traditional meals collected in Uummannaq municipality, northern Greenland in 1976 with 90 meals sampled in Uummannaq in 2004. They found that the percentage of local food in the diet has decreased and with it the intake of n-3 fatty acids and, after adjustment for n-3 fatty acid content in the food, they found a significant decrease of PCBs and lead, but found an increase in local food Hg concentration.
Table 2.
Population distribution of THg intake from consumption of traditional foods in 2000 and 2007 (µg kg−1 body weight day−1)a (n=1875)b
| Year | n>0 | Mean | Median | 75th | 95th | 99th | 95th/PTDI |
|---|---|---|---|---|---|---|---|
| 2000 | 1077 | 0.6 | 0.1 | 0.6 | 3.0 | 7.0 | 4.3 |
| 2007 | 1077 | 0.7 | 0.1 | 0.5 | 3.5 | 7.9 | 5.0 |
Table 3.
Mean ± SE THg exposure (µg kg−1 body weight day−1) by region for 2000 and 2007, adjusted for community size, sex, age and season (n=1875).
| Year | Inuvialuit (n=294) |
Kitikmeot (n=300) |
Kivalik (n=341) |
Baffin (n=522) |
Labrador (n=417) |
|---|---|---|---|---|---|
| 2000 | 0.26 ± 0.03 | 0.55 ± 0.08 | 0.82 ± 0.11 | 1.57 ± 0.27 | 0.41 ± 0.06 |
| 2007 | 0.26 ± 0.03 | 0.58 ± 0.09 | 0.78 ± 0.11 | 1.65 ± 0.27 | 0.41 ± 0.07 |
Biomonitoring of Hg in blood conducted in Nunavik, Canada showed a decreasing trend. In 2004, 917 adults aged between 18 and 74 were recruited in 14 communities of Nunavik to participate in a broad-scale health survey. Results were compared with data obtained in 1992, where 492 people were recruited for a similar survey in the same population (Fontaine et al. 2008). Mean blood Hg concentration was 10.3 µg L−1, which represents a 32% decrease between 1992 and 2004 (p < 0.001). Hg blood concentrations were mainly explained by age (partial r2 = 0.20; p < 0.0001), and the most important source of exposure to Hg was marine mammal meat consumption (partial r2 = 0.04; p < 0.0001) (Table 4). These results suggest that Hg intake may have decreased because of the decline in traditional food use over the last decade while the concentrations in traditional food remained unchanged. Hg can cross the placenta, and fetuses are a high-risk group because the developing brain is susceptible to the harmful effects of MeHg exposure (Oken et al. 2005). Therefore, many blood monitoring programs in the Arctic have been focusing on pregnant women. Blood Hg levels in mothers have generally decreased in almost all circumpolar regions including: parts of Alaska, Arctic Canada and northern Sweden, since the 1990s (Donaldson et al. 2010, AMAP 2011) and much of this decline is due to the decreased consumption of traditional foods. However, Hg exposure in parts of the Arctic continues to be high; i.e. over 90% of women of child bearing age in some areas of Greenland had blood Hg exceeding the guideline level of 5.8 µg L−1 (AMAP 2011).
Table 4.
Comparison of blood THg concentrations (µg L−1) in Nunavik in 1992 and 2004.
| Region | Year | N | Geometric Mean (µg L−1) | 95% Confidence Interval | |
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Nunavik | 1992 | 492 | 15.0 | 13.9 | 16.2 |
| Nunavik | 2004 | 917 | 10.3 | 9.6 | 11.0 |
Hg exposure in early childhood can present a risk to neurodevelopment. Tian et al. (2010) measured the dietary Hg exposure from traditional food among Inuit children 3 to 5 years of age in Nunavut, Canada and evaluated the association between estimated dietary Hg intake and body burden. They found that the geometric mean of children’s hair Hg was 0.66 µg g−1 and varied by region. Nearly 25% of children had hair Hg concentrations equal to or higher than the WHO reference level of 2 µg g−1. There was a significant correlation between Hg levels in children’s hair and that of the adults in the same household. For children, beluga muktuk, narwhal muktuk, ringed seal liver, fish, caribou meat and ringed seal meat together accounted for 95% of the dietary sources of Hg. Not surprisingly, there was also a significant positive association between children’s hair Hg levels and estimated dietary Hg intake.
6.3. Effects of Hg Exposure on the Health of Northern Peoples
The adverse effects of Hg on Inuit child development have been studied in a cohort study conducted in Nunavik, Canada. 110 pre-school children aged between 5–6 years old who had participated in an earlier cord blood monitoring program were recruited (Saint-Amour et al. 2006). A significant association between Hg body burden at birth and pattern-reversal visual evoked potentials (VEPs) was reported. In a follow-up study, Boucher et al. (2011) found that prenatal exposure was associated with adverse effect on recognition memory in the same cohort of Inuit children (11.3 yr). More importantly, the authors found that higher cord plasma concentrations of docosahexaenoic acid (DHA) and cord DHA concentrations had positive associations with performance on neurobehavioral assessments of memory. Cord DHA-related effects were also observed regardless of Hg and PCB exposure. These results suggest that a detailed risk-benefit assessment is needed to develop a public health advisory.
Recently, there is increasing evidence that Hg may have an adverse effect on the cardiovascular health of Inuit. In Nunavik, systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured in 732 adults during a clinical visit and pulse pressure (PP) was calculated. Pearson correlation analysis was used to study simple relationships between Hg and blood pressure parameters, while multiple regressions were carried out to control for confounding factors. Hg was correlated with SBP (r = 0.15; p < 0.0001) and PP (r = 0.17; p < 0.0001) while the correlation with DBP did not reach statistical significance. After adjusting for confounders, the association with SBP (β = 2.4; p = 0.0004) and PP (β = 1.2; p = 0.02) remained statistically significant and the association with DBP became significant (β = 1.0; p = 0.04), suggesting a negative impact of Hg on blood pressure (Valera et al. 2009).
Health end points related to Hg exposure and cardiovascular disease could be of greater relevance for public health than those related to, for example, neurotoxicity at current exposure levels. For example, the low incidence of cardiovascular disease in Inuit may be due to the high fatty acid composition of their diet, but this positive effect may be attenuated by high Hg exposure as recent studies indicate that Hg can have a negative effect on the cardiovascular system. Although the reasons are still not known, Hg may also inhibit important antioxidative mechanisms in humans and could promote the peroxidation of unsaturated fatty acids such as DHA and Eicosapentaenoic acid (EPA) (Donaldson et al. 2010).
6.4. Considerations When Developing and Communicating Consumption Advice
While traditional food consumption is the major source of contaminant exposure in the Arctic, the traditional food system of the Inuit is a vital component of their culture and makes many positive contributions to health. Traditional food contributes to social, cultural and physical health (Kuhnlein and Chan 2000). Benefits of traditional food use include lower costs, chronic disease prevention from a more nutritious diet, mental health, social-cohesion and cultural morale (Wheatley and Wheatley 2000).
Furthermore, the traditional diet includes nutrient-rich organ meats and other parts of the animal that are not normally available at the local markets or consumed (Kuhnlein et al. 2004). The process of collecting traditional foods involves great amounts of physical activity that reduce the risks of diabetes, obesity and loss of fitness (Kuhnlein et al. 2004). A recent cross-sectional health survey of Inuit (≥18 y) in 36 Arctic communities conducted in 2007–2008 showed that when traditional food was consumed, there was a higher intake of protein, protein related micronutrients, vitamins A and C and a lower intake of carbohydrates, saturated fat, fiber and a lower sodium:potassium ratio (p ≤ 0.05) (Egeland et al. 2011). Nutrition transition and food insecurity are associated with a multifaceted shift in nutrient status with implications for increased risk of diet sensitive chronic diseases (Egeland et al. 2011). Therefore, the benefits of traditional food consumption as well as the consequences of the loss of traditional foods in the diet must be considered in risk management studies.
The role of scientists and public health professionals is to protect populations from unsafe levels of contaminant exposures. However, they need to be sure their interventions will not result in an unnecessary decrease of traditional food consumption and hence loss of nutritional and cultural benefit. Further complicating the issue in the Arctic are the differing views of the risks associated with contaminants such as Hg in the food system, as risk perception and behavior are influenced by culture, social and demographic factors (Furgal et al. 2005). Therefore, it is important to contextualize the issue, identify the current concerns and identify the current conditions that will help the public build the skills and capacity needed to understand and participate in the assessment, communication and management of the issue.
7.0 SUMMARY, RESEARCH NEEDS AND IMPLICATIONS FOR POLICY
Above, we have discussed Hg sources, pathways and exposure in Arctic marine ecosystems. One of the primary objectives of current Hg research is to quantify how much Hg enters Arctic marine ecosystems from external sources and from where this Hg originates. This would in turn allow us to predict how these ecosystems might respond to, for example, reductions in anthropogenic Hg emissions brought about by new regulations or changes to the global Hg cycle as result of climate change. Another fundamental question is whether we can establish a link between atmospheric Hg deposition, which is in part controlled by anthropogenic Hg emissions, and Hg exposure to biological organisms and humans. The ability to answer this second question depends on a strong understanding of the many processes and pathways involved in the transfer of Hg from the abiotic pool into food webs, including upper trophic level species of fish and marine mammals consumed by humans. For example, we need to understand factors which control: 1) the distribution and speciation of Hg in various biogeochemical compartments, 2) the net production of the toxic and bioaccumulating form of Hg (MeHg) and finally 3) the bioaccumulation and biomagnification of MeHg through food webs.
Recent efforts have greatly improved our ability to provide answers regarding external sources of Hg to the Arctic. Rivers appear to be at least as large of a Hg source as atmospheric deposition, if not greater, and ocean circulation is likely a smaller source of Hg than rivers and the atmosphere. However, it should be noted that data on both rivers and inputs from ocean currents are still very incomplete. Inputs from rivers have likely been underestimated until recently (Fisher et al. 2012), while Hg fluxes associated with ocean circulation are highly uncertain, such that it is still not possible to determine whether ocean circulation is a net source or sink for Hg in the Arctic Ocean. Models of atmospheric Hg deposition in the Arctic reflect the best available information, having been expanded to incorporate snowpack processes such as reduction and remission of deposited Hg (Dastoor et al. 2008, AMAP 2011). Best model estimates of net atmospheric Hg deposition within the Arctic Circle are 60 t yr−1 (Holmes et al. 2010) and 143 t yr−1 (NCP 2012), although there are still very few field measurements of deposition in the high Arctic to corroborate these model results, and areal loads in snowpacks suggest that atmospheric Hg deposition may be somewhat lower (St. Louis et al. 2007). Both model simulations and field measurements suggest that atmospheric Hg deposition is less in the Arctic compared to more temperate and/or industrialized regions. It has also been determined that the largest source of atmospheric Hg to the Arctic are Asia and Russia, followed to a lesser extend by North America and Europe. However, some knowledge gaps remain. For example, most measurements of atmospheric deposition have been conducted on land at coastal sites and have limited spatial and temporal coverage. Very few data have been collected over sea ice or over the Arctic Ocean proper, even though most of the surface area within the Arctic is not land but sea ice covered ocean. Furthermore, the role of sea ice, and the impact of declining sea ice extent, on processes controlling atmospheric Hg speciation and distribution is not well understood. Finally, modeled estimates of atmospheric Hg deposition would be improved by field measurements to parameterize and calibrate models.
As data on both atmospheric Hg deposition and Hg concentrations in biota exist for some regions of the Arctic, it might be tempting to simply compare the two to determine the importance of Hg deposition in controlling Hg exposure in marine biota and thereby predict how Hg concentrations in biota might respond to changes in anthropogenic emissions. However, this would be erroneous, potentially misleading and clearly over simplistic because of the numerous complex processes linking Hg(II) deposition, Hg(II) methylation, bioaccumulation and biomagnification, for which we possess only a rudimentary understanding. For example, there are currently no measurements of MeHg production and release from Arctic marine sediments. There is also a lack of concentration data for seston/phytoplankton and many benthic organisms, limiting our understanding of how MeHg is bioaccumulated into organisms at the base of both pelagic and benthic marine food webs before being biomagnified. The knowledge gaps in our understanding of both processes and external sources mean that it is still difficult to assemble a mass balance for either THg or MeHg in the Arctic Ocean, although THg mass balances have been published using either a very small number of field observations (Outridge et al. 2008) or an ocean atmosphere coupled model (Fisher et al. 2012).
While not all of the pathways linking Hg deposition to Hg exposure in wildlife and humans are understood, it is possible to identify a few of the factors which may control MeHg production in Arctic marine ecosystems, and thus MeHg availability for bioaccumulation in local food webs. Water column methylation is likely the most important source of MeHg to Arctic marine food webs (Lehnherr et al. 2011) and is controlled by both primary productivity (Sunderland et al. 2009, Heimburger et al. 2010, Cossa et al. 2009) and Hg(II) availability (Lehnherr et al. 2011). Therefore, any increases or decreases in primary productivity and organic carbon decomposition will result in similar increases or decreases in MeHg production and uptake by biota. Furthermore, the addition of more Hg(II) to ocean systems will result in higher MeHg production and higher exposure to organisms at the base of the food web. However, it is also likely that MeHg in fish will respond slowly to changes in atmospheric Hg deposition given the timescale of oceanographic processes that affect MeHg production in the open ocean (Mason et al. 2012). As a result, Hg concentrations in Arctic marine biota may initially continue to increase even if atmospheric Hg deposition begins to decrease (Mason et al. 2012).
Hg concentrations in Arctic marine biota have increased significantly since the onset of industrialization (Dietz et al. 2009). There are also indications that Hg concentrations are higher (and increasing) in fish, marine mammal and seabird species in some areas of the Canadian Arctic and Greenland compared with the North Atlantic and European Arctic. However, the underlying factors contributing to these patterns and trends are complex. Hg exposure of top predators is controlled by food web structure and food chain length as well as by the availability of MeHg for bioaccumulation at the base of the food web. Habitat, diet and animal behaviour are also important in controlling Hg exposure, and all of these factors will likely be affected by climate change, potentially having dramatic effects on Hg exposure. Therefore, it is difficult to identify areas of high Hg levels in marine biota beyond the general patterns already described in Section 5.
Climate change has the potential to dramatically alter the Hg cycle in Arctic regions by altering deposition pathways and uptake, methylation/demethylation, food web structure and animal behaviour as a result of rising air and water temperatures and loss of sea ice. For example, changes in atmospheric conditions, sea ice coverage and salinity, and increasing air temperatures will affect the timing and magnitude of Hg deposition to the Arctic. Rising water temperatures and loss of sea ice will likely affect methylation/demethylation rates by altering primary productivity, which fuels methylation, as well as the light regime in the water column, increasing rates of photochemical transformations of Hg. Sea ice loss will also alter food web structure and indirectly Hg biomagnification by, for example, decreasing the abundance of sea-ice (sympagic) algae, and forcing other organisms which are part of the sympagic food web, such as Arctic cod, to feed more pelagically, thus altering the flow of Hg from abiotic pools into upper trophic level organisms. However, the consequences of climate change on Hg concentrations in biota and exposure to people are still largely unknown and difficult to predict. In many ways, the Arctic Ocean will be the first marine ecosystem to exhibit impacts from climate change, acting as the “canary in the coal mine” for how Hg cycling may respond to environmental changes in other oceans around the world.
Hg is impacting Arctic ecosystems and Northern Peoples are particularly at risk due to their reliance on traditional foods. This is apparent in the fact that the primary source of Hg exposure to Northern Peoples is from the consumption of traditional foods, particularly marine mammals, resulting in some individuals having Hg concentrations in their bodies that exceed health guidelines and recommendations. However, the nutritional, social, cultural and physical health benefits of harvesting and consuming traditional foods are great, and therefore, risk communication should be done with consideration of the social context surrounding this issue. Reducing the consumption of traditional food is not a long-term solution for decreasing Hg exposure in Northern Peoples, and only by reducing anthropogenic Hg emissions will it be possible to minimize Hg concentrations in Arctic ecosystems (ICC, 2008).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- AMAP. Mercury in the Arctic. Oslo: Arctic Monitoring and Assessment Programme (AMAP); 2011. AMAP Assessment 2011. [Google Scholar]
- Andersson ME, Gårdfeldt K, Jutterström S. Air–sea exchange of volatile mercury in the North Atlantic Ocean. Mar. Chem. 2011;125:1–7. [Google Scholar]
- Andersson ME, Sommar J, Gardfelt K, Lindqvist O. Enhanced concentrations of dissolved gaseous mercury in the surface waters of the Arctic Ocean. Mar. Chem. 2008;110:190–194. [Google Scholar]
- Andersson ME, Gårdfeldt K, Wängberg I, Sprovieri F, Pirrone N, Lindqvist O. Seasonal and daily variation of mercury evasion at coastal and off shore sites from the Mediterranean Sea. Mar. Chem. 2007;104:214–226. [Google Scholar]
- Ariya P, Dastoor A, Amyot M, Schroeder W, Barrie L, Anlauf K, Raofie F, Ryzhkov A, Davignon D, Lalonde J, Steffen A. The Arctic: A sink for mercury. Tellus B. 2004;56:397–403. [Google Scholar]
- Arthur SM, Manly BFJ, McDonald LL, Garner GW. Assessing habitat selection when availability changes. Ecology. 1996;77:215–227. [Google Scholar]
- Asmund G, Nielsen SP. Mercury in dated Greenland marine sediments. Sci. Total Environ. 2000;245:61–72. doi: 10.1016/s0048-9697(99)00433-7. [DOI] [PubMed] [Google Scholar]
- Aspmo K, Temme C, Berg T, Ferrari C, Gauchard PA, Fain X, Wibetoe G. Mercury in the atmosphere, snow and melt water ponds in the North Atlantic Ocean during Arctic summer. Environ. Sci. Technol. 2006;40:4083–4089. doi: 10.1021/es052117z. [DOI] [PubMed] [Google Scholar]
- Atwell L, Hobson KA, Welch HE. Biomagnification and bioaccumulation of mercury in an Arctic marine food web: insights from stable nitrogen isotope analysis. Can. J. Fish. Aquat. Sci. 1998;55:1114–1121. [Google Scholar]
- Aubail A, Dietz R, Rigét F, Simon-Bouhet B, Caurant F. An evaluation of teeth of ringed seals (Phoca hispida) from Greenland as a matrix to monitor spatial and temporal trends of mercury and stable isotopes. Sci. Total Environ. 2010;408:5137–5146. doi: 10.1016/j.scitotenv.2010.07.038. [DOI] [PubMed] [Google Scholar]
- Auel H, Hagen W. Mesozooplankton community structure, abundance and biomass in the central Arctic Ocean. Mar. Biol. 2002;140:1013–1021. [Google Scholar]
- Bales RC, Sommerfeld RA, Kebler DG. Ionic tracer movement through a Wyoming snowpack. Atmos. Environ. 1990;24:2749–2758. [Google Scholar]
- Benoit JM, Shull DH, Harvey RM, Beal SA. Effect of bioirrigation on sediment-water exchange of methylmercury in Boston Harbor, Massachusetts. Environ. Sci. Technol. 2009;43:3669–3674. doi: 10.1021/es803552q. [DOI] [PubMed] [Google Scholar]
- Bergquist BA, Blum JD. Mass-dependent and –independent fractionation of Hg isotopes by photoreduction in aquatic ecosystems. Science. 2007;318:417–420. doi: 10.1126/science.1148050. [DOI] [PubMed] [Google Scholar]
- Bignert A. Comments concerning the national Swedish contaminant monitoring programme in fresh water biota. Rapport till Naturvårdsverket 2002. 2002 [Google Scholar]
- Boucher O, Burden MJ, Muckle G, Saint Amour D, Ayotte P, Dewailly E, Nelson CA, Jacobson SW, Jacobson JL. Neurophysiologic and neurobehavioral evidence of beneficial effects of prenatal omega-3 fatty acid intake on memory function at school age. Am. J Clin. Nutr. 2011;93:1025–1037. doi: 10.3945/ajcn.110.000323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braune B, Carrie J, Dietz R, Evans M, Gaden A, Gantner N, Hedman J, Hobson K, Loseto L, Muir D, Outridge P, Rigét F, Rognerud S, Stern G, Verta M, Wang F, Wängberg I. AMAP Assessment 2011. Oslo: Mercury in the Arctic, Arctic Monitoring and Assessment Programme (AMAP); 2011. Chapter 5. Are mercury levels in Arctic biota increasing or decreasing, and why? pp. 87–114. [Google Scholar]
- Braune BM. Temporal trends of organochlorines and mercury in seabird eggs from the Canadian Arctic, 1975–2003. Environ. Pollut. 2007;148:599–613. doi: 10.1016/j.envpol.2006.11.024. [DOI] [PubMed] [Google Scholar]
- Braune BM, Mallory ML, Gilchrist HG. Elevated mercury levels in a declining population of ivory gulls in the Canadian Arctic. Marine Pollution Bulletin. 2006;52:969–987. doi: 10.1016/j.marpolbul.2006.04.013. [DOI] [PubMed] [Google Scholar]
- Braune BM, Outridge PM, Fisk AT, Muir DCG, Helm PA, Hobbs K, Hoekstra PF, Kwan M, Letcher RJ, Lockhart WL, Norstrom RJ, Stern GA, Kuryk ZZ. Persistent organic pollutants and mercury in marine biota of the Canadian Arctic: an overview of spatial and temporal trends. Sci. Total Environ. 2005;351–352:4–56. doi: 10.1016/j.scitotenv.2004.10.034. [DOI] [PubMed] [Google Scholar]
- Braune BM, Donaldson GM, Hobson KA. Contaminant residues in seabird eggs from the Canadian Arctic. II. Spatial trends and evidence from stable isotopes for intercolony differences. Environ. Pollut. 2002;117:133–145. doi: 10.1016/s0269-7491(01)00186-5. [DOI] [PubMed] [Google Scholar]
- Brooks S, Arimoto R, Lindberg S, Southworth G. Antarctic polar plateau snow surface conversion of deposited oxidized mercury to gaseous elemental mercury with fractional long-term burial. Atmos. Environ. 2008;42:2877–2884. [Google Scholar]
- Cabana G, Rasmussen JB. Modelling food chain structure and contaminant bioaccumulation using stable nitrogen isotopes. Nature. 1994;372:255–257. [Google Scholar]
- Campbell LM, Norstrom RJ, Hobson KA, Muir DCG, Backus S, Fisk A. Mercury and other trace elements in a pelagic Arctic marine food web (Northwater Polynya, Baffin Bay) Sci. Total Environ. 2005;351–352:248–263. doi: 10.1016/j.scitotenv.2005.02.043. [DOI] [PubMed] [Google Scholar]
- Chen J, Pehkonen SO, Lin CJ. Degredation of monomethylmercury chloride by hydroxyl radicals in simulated natural waters. Water Res. 2003;37:2496–2504. doi: 10.1016/S0043-1354(03)00039-3. [DOI] [PubMed] [Google Scholar]
- Christensen JH, Brandt J, Frohn LMSH. Modelling of mercury in the Arctic with then Danish Eulerian hemispheric model. Atmos. Chem. Phys. 2004;4:2251–2257. [Google Scholar]
- Cobbett FD, Steffen A, Lawson G, Van Heyst BJ. GEM fluxes and atmospheric mercury concentrations (GEM, RGM and HgP) in the Canadian Arctic at Alert, Nunavut, Canada (February–June 2005) Atmos. Environ. 2007;41:6527–6543. [Google Scholar]
- Cole A, Dastoor A, Antoniadis M, Ariya P, Morrison H. Chapter 3. Atmospheric mercury. Canadian Arctic Contaminants Assessment Report III. In review. [Google Scholar]
- Cole AS, Steffen A. Trends in long-term gaseous mercury observations in the Arctic and effects of temperature and other atmospheric conditions. Atmos. Chem. Phys. 2010;10:4661–4672. [Google Scholar]
- Constant P, Poissant L, Villemur R, Yumvihoze E, Lean D. Fate of inorganic mercury and methyl mercury within the snow cover in the low Arctic tundra on the shore of Hudson Bay (Quebec, Canada) J. Geophys. Res. D: Atmos. 2007;112 [Google Scholar]
- Coquery M, Cossa D, Martin JM. The distribution of dissolved and particulate mercury in three Siberian estuaries and adjacent Arctic coastal waters. Water Air Soil Pollut. 1995;80:653–664. [Google Scholar]
- Corbitt ES, Jacob DJ, Holmes CD, Streets DG, Sunderland EM. Global source-receptor relationships for mercury deposition under present-day and 2050 emissions scenarios. Environ. Sci. Technol. 2011;45:10477–10484. doi: 10.1021/es202496y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossa D, Heimburger L-E, Lannuzel D, Rintoul SR, Butler ECV, Bowie AR, Averty B, Watson RJ, Remenyi T. Mercury in the Southern Ocean. Geochim. Cosmochim. Acta. 2011;75:4037–4052. [Google Scholar]
- Cossa D, Averty B, Pirrone N. The origin of methylmercury in open Mediterranean waters. Limnol. Oceanogr. 2009;54:837–844. [Google Scholar]
- Cossa D, Martin JM, Kazufumi T, Sanjuan J. The distribution and cycling of mercury species in the western Mediterranean. Deep Sea Res. II. 1997;44:721–740. [Google Scholar]
- Dastoor A, Davignon D, Theys N, van Roozendael M, Steffen A, Ariya P. Modeling dynamic exchange of gaseous elemental mercury at polar sunrise. Environ. Sci. Technol. 2008;42:5183–5188. doi: 10.1021/es800291w. [DOI] [PubMed] [Google Scholar]
- Department of Health and Human Services. Fourth National Report on Human Exposure to Environmental Chemicals. Department of Health and Human Services, Atlanta. 2012:142. [Google Scholar]
- Deutch B, Dyerberg J, Pedersen HS, Asmund G, Møller P, Hansen JC. Dietary composition and contaminants in north Greenland, in the 1970s and 2004. Sci. Total Environ. 2006;370:372–381. doi: 10.1016/j.scitotenv.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Dietz R, Born EW, Rigét F, Aubail A, Sonne C, Drimmie R. Temporal trends and future predictions of mercury concentrations in Northwest Greenland polar bear (Ursus maritimus) hair. Environ. Sci. Technol. 2011 doi: 10.1021/es1028734. In press. [DOI] [PubMed] [Google Scholar]
- Dietz R, Outridge PM, Hobson KA. Anthropogenic contributions to mercury levels in present-day Arctic animals - a review. Sci. Total Environ. 2009;407:6120–6131. doi: 10.1016/j.scitotenv.2009.08.036. [DOI] [PubMed] [Google Scholar]
- Dietz R, Rigét F, Born EW, Sonne C, Grandjean P, Kirkegaard M, Olsen MT, Asmund G, Renzoni A, Baagøe H, Andreasen C. Trends in mercury in hair from Greenland polar bears (Ursus maritimus) during 1892–2001. Environ. Sci. Technol. 2006a;40:1120–1125. doi: 10.1021/es051636z. [DOI] [PubMed] [Google Scholar]
- Dietz R, Rigét F, Olsen MT, Boertmann D, Sonne C, Kirkegaard M. Time trend of mercury in feathers of west Greenland birds of prey during 1859–2003. Environ. Sci. Technol. 2006b;40:5911–5916. doi: 10.1021/es0609856. [DOI] [PubMed] [Google Scholar]
- Dietz R, Rigét F, Johansen P. Lead, cadmium, mercury and selenium in Greenland marine animals. Sci. Total Environ. 1996;186:67–97. doi: 10.1016/0048-9697(96)05086-3. [DOI] [PubMed] [Google Scholar]
- Dommergue A, Bahlmann E, Ferrara R, Boutron CF. Laboratory simulation of Hg(0) emissions from a snowpack. Anal. Bioanal. Chem. 2007;388:319–327. doi: 10.1007/s00216-007-1186-2. [DOI] [PubMed] [Google Scholar]
- Dommergue A, Ferrari CP, Poissant L, Gauchard PA, Boutron CF. Chemical and photochemical processes at the origin of the diurnal cycle of gaseous mercury within the snowpack at Kuujjuarapik, Quebec, Environ. Sci. Technol. 2003a;37:3289–3297. doi: 10.1021/es026242b. [DOI] [PubMed] [Google Scholar]
- Dommergue A, Ferrari CP, Poissant L, Gauchard,M PA, Boutron CF. Diurnal cycles of gaseous mercury within the snowpack at Kuujjuarapik/Whapmagoostui, Québec (Canada) Environ. Sci. Technol. 2003b;37 doi: 10.1021/es026242b. 10.1021/es026242b., 2003b. [DOI] [PubMed] [Google Scholar]
- Donaldson SG, Van Oostdam J, Tikhonov C, Feeley M, Armstrong B, Ayotte P, Boucher O, Bowers W, Chan HM, Dallaire F, Dallaire R, Dewailly E, Edwards J, Egeland GM, Fontaine J, Furgal C, Leech T, Loring E, Muckle G, Nancarrow T, Pereg D, Plusquellec P, Potyrala M, Receveur O, Shearer RG. Environmental contaminants and human health in the Canadian Arctic. Sci. Total Environ. 2010;408:5165–5234. doi: 10.1016/j.scitotenv.2010.04.059. [DOI] [PubMed] [Google Scholar]
- Douglas TA, Sturm M, Simpson WR, Blum JD, Alvarez-Aviles L, Keeler GJ, Perovich DK, Biswas A, Johnson K. Influence of snow and ice crystal formation and accumulation on mercury deposition to the Arctic. Environ. Sci. Technol. 2008;42:1542–1551. doi: 10.1021/es070502d. [DOI] [PubMed] [Google Scholar]
- Durnford D, Dastoor A. The behavior of mercury in the cryosphere: a review of what we know from observations. J. Geophys. Res. 2011;116:D06305. [Google Scholar]
- Durnford D, Dastoor A, Figueras-Nieto D, Ryjkov A. Long-range transport of mercury to the Arctic and across Canada. Atmos. Chem. Phys. 2010;10:6063–6086. [Google Scholar]
- Eide R, Wesenberg GR, Fosse G. Mercury in primary teeth in preindustrial Norway. Scand. J. Dent. Res. 1993;101:1–4. doi: 10.1111/j.1600-0722.1993.tb01636.x. [DOI] [PubMed] [Google Scholar]
- Egeland GM, Johnson-Down L, Cao ZR, Sheikh N, Weiler H. Food insecurity and nutrition transition combine to affect nutrient intakes in Canadian Arctic communities. J. Nutr. 2011;141:1746–1753. doi: 10.3945/jn.111.139006. [DOI] [PubMed] [Google Scholar]
- Evans MS, Muir D, Lockhart WL, Stern G, Ryan M, Roach P. Persistent organic pollutants and metals in the freshwater biota of the Canadian Subarctic and Arctic: an overview. Sci. Total Environ. 2005;351–352:94–147. doi: 10.1016/j.scitotenv.2005.01.052. [DOI] [PubMed] [Google Scholar]
- Fain X, Ferrari CP, Gauchard P-A, Magand O, Boutron C. Fast depletion of gaseous elemental mercury in the Kongsvegen Glacier snowpack in Svalbard. Geophys. Res. Lett. 2006;33:L06826. Fast depletion of gaseous elemental mercury in the Kongsvegen Glacier snowpack in Svalbard. [Google Scholar]
- Faïn X, Grangeon S, Bahlmann E, Fritsche J, Obrist D, Dommergue A, Ferrari CP, Cairns W, Ebinghaus R, Barbante C, Cescon P, Boutron C. Diurnal production of gaseous mercury in the alpine snowpack before snowmelt. J. Geophys. Res. Atmos. 2007;112:D21311. [Google Scholar]
- Faïn X, Ferrari CP, Dommergue A, Albert M, Battle M, Arnaud L, Barnola J-M, Cairns W, Barbante C, Boutron C. Mercury in the snow and firn at Summit Station, Central Greenland, and implications for the study of past atmospheric mercury levels. Atmos. Chem. Phys. 2008;8:3441–3457. [Google Scholar]
- Ferrari CP, Dommergue A, Boutron CF, Skov H, Goodsite M, Jensen B. Nighttime production of elemental gaseous mercury in interstitial air of snow at Station Nord, Greenland. Atmos. Environ. 2004;38:2727–2735. [Google Scholar]
- Ferrari CP, Gauchard PA, Dommergue A, Magand O, Nagorski S, Boutron CF, Temme C, Bahlmann E, Ebinghaus R, Steffen A, Banic C, Aspmo K, Berg T, Planchon F, Barbante C. Snow to air exchange of mercury in an Arctic seasonal snow pack in Ny-Alesund, Svalbard. Atmos. Environ. 2005;39:7633–7645. [Google Scholar]
- Fisher JA, Jacob DJ, Soerensen AL, Amos HM, Steffen A, Sunderland EM. Riverine source of Arctic Ocean mercury inferred from atmospheric observations. Nat. Geosci. 2012;5:499–504. [Google Scholar]
- Fisher JA, Jacob DJ, Wang Q, Bahreini R, Carouge CC, Cubison MJ, Dibb JE, Diehl T, Jimenez JL, Leibensperger EM, Lu Z, Meinders MBJ, Pye HOT, Quinn PK, Sharma S, Streets DG, van Donkelaar A, Yantosca RM. Sources, distribution, and acidity of sulphate-ammonium aerosol in the Arctic in winter-spring. Atmos. Environ. 2011;45:7301–7318. [Google Scholar]
- Fontaine J, Dewailly E, Benedetti JL, Pereg D, Ayotte P, Déry S. Re-evaluation of blood mercury, lead and cadmium concentrations in the Inuit population of Nunavik (Québec): a cross-sectional study. Environ. Health. 2008;2:7–25. doi: 10.1186/1476-069X-7-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford J, Borg H, Dam M, Rigét F. AMAP Assessment 2002: Heavy Metals in the Arctic. Oslo, Norway: Arctic Monitoring and Assessment Programme (AMAP); 2005. Chapter 4: Spatial Patterns; pp. 42–83. [Google Scholar]
- Furgal C, Powell S, Myers G. Digesting the message about contaminants and country foods in the Canadian North: a review and recommendations for future research and action. Arctic. 2005;56:103–114. [Google Scholar]
- Gaden A, Ferguson SH, Harwood L, Melling H, Stern GA. Mercury trends in ringed seals (Phoca hispida) from the western Canadian Arctic since 1973: Associations with length of ice-free season. Environ. Sci. Technol. 2009;43:3646–3651. doi: 10.1021/es803293z. [DOI] [PubMed] [Google Scholar]
- Gamberg M, Braune B, Davey E, Elkin B, Hoekstra PF, Kennedy D, Macdonald DC, Muir D, Nirwal A, Wayland M, Zeeb B. Spatial and temporal trends of contaminants in terrestrial biota from the Canadian Arctic. Sci. Total Environ. 2005;351–352:148–164. doi: 10.1016/j.scitotenv.2004.10.032. [DOI] [PubMed] [Google Scholar]
- Gantner N, Power M, Babaluk JA, Reist JD, Köck G, Lockhart LW, Solomon KR, Muir DCG. Temporal trends of mercury, cesium, potassium, selenium, and thallium in Arctic char (Salvelinus alpinus) from lake Hazen, Nunavut, Canada: Effects of trophic position, size and age. Environ. Toxicol. Chem. 2009;28:254–263. doi: 10.1897/08-054.1. [DOI] [PubMed] [Google Scholar]
- Garbarino JR, Snyder-Conn E, Leiker TJ, Hoffman GL. Contaminants in Arctic snow collected over northwesr Alaskan sea ice. Water Air Soil Pollut. 2002;139:183–214. [Google Scholar]
- Gårdfeldt K, Jonsson M. Is bimolecular reduction of Hg(II) complexes possible in aqueous systems of environmental importance. J. Phys. Chem. 2003;107:4478–4482. [Google Scholar]
- Geynrikh AK. Mass species of oceanic phytophagous copepods and their ecology. Oceanology. 1986;26:213–217. [Google Scholar]
- Gobeil C, Macdonald RW, Smith JN. Mercury profiles in sediments of the Arctic Ocean basins. Environ. Sci. Technol. 1999;33:4194–4198. [Google Scholar]
- Graydon JA, Emmerton CA, Lesack LFW, Kelly EN. Mercury in the Mackenzie River delta and estuary: concentrations and fluxes during open-water conditions. Sci. Total Environ. 2009;407:2980–2988. doi: 10.1016/j.scitotenv.2008.12.060. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt CR, Fitzgerald WF, Lamborg CH, Balcom PH, Mao Tseng C. Biogeochemical cycling of methylmercury in lakes and Tundra watersheds of Arctic Alaska. Environ. Sci. Technol. 2006;40:1204–1211. doi: 10.1021/es051322b. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt CR, Fitzgerald WF. Methylmercury cycling in sediments on the continental shelf of southern New England. Geochimica Et Cosmochimica Acta. 2006;70:918–930. [Google Scholar]
- Hammerschmidt CR, Fitzgerald WF, Lamborg CH, Balcom PH, Vissher PT. Biogeochemistry of methylmercury in sediments of Long Island Sound. Mar. Chem. 2004;90:31–52. [Google Scholar]
- Hansen J. Environmental contaminants and human health in the Arctic. Toxicology Letters. 2000;112–113:119–135. doi: 10.1016/s0378-4274(99)00203-9. [DOI] [PubMed] [Google Scholar]
- Hansen JC, Toribara TY, Muhs AG. Hansen JP, Gulløv H, editors. Trace metals in humans and animal hair from the 15th century graves in Qilakitsoq, compared with recent samples. The mummies from Qilakitsoq Meddr Groenl. Man. Soc. 1989;12:161–167. [Google Scholar]
- Hare A, Stern GA, Macdonald RW, Kuzyk ZZ, Wang F. Contemporary and perindustrial mass budgets of mercury in the Hudson Bay marine system: the role of sediment recycling. Sci. Total Environ. 2008;406:190–204. doi: 10.1016/j.scitotenv.2008.07.033. [DOI] [PubMed] [Google Scholar]
- Hare AA, Stern GA, Kuzyk ZZA, MacDonald RW, Johannessen SC, Wang F. Natural and anthropogenic mercury distribution in marine sediments from Hudson Bay, Canada. Environ. Sci. Technol. 2010;44:5805–5811. doi: 10.1021/es100724y. [DOI] [PubMed] [Google Scholar]
- Harwood LA, Norton P, Day B, Hall PA. The harvest of beluga whales in Canada's western Arctic: hunter-based monitoring of the size and composition of the catch. Arctic. 2002;55:10–20. [Google Scholar]
- Heimburger LE, Cossa D, Marty JC, Migon C, Averty B, Dufour A, Ras J. Methyl mercury distributions in relation to presence of nano- and picophytoplankton in an oceanic water column (Ligurian Sea, North-western Mediterranean) Geochim. Cosmochim. Acta. 2010;74:5549–5559. [Google Scholar]
- Helgason LB, Barrett R, Lie E, Polder A, Skaare J, Gabrielsen GW. Levels and temporal trends (1983–2003) of persistent organic pollutants (POPs) and mercury (Hg) in seabird eggs from northern Norway. Environ. Pollut. 2008;155:190–198. doi: 10.1016/j.envpol.2007.10.022. [DOI] [PubMed] [Google Scholar]
- Hollweg TA, Gilmour CC, Mason RP. Methylmercury production in sediments of Chesapeake Bay and the mid-Atlantic continental margin. Mar. Chem. 2009;114:86–101. [Google Scholar]
- Holmes CD, Jacob DJ, Corbitt ES, Mao J, Yang X, Talbot R, Slemr F. Global atmospheric model for mercury including oxidation by bromine atoms. Atmos. Chem. Phys. Discuss. 2010;10:19845–19900. [Google Scholar]
- Hoydal K, Dam M. AMAP Faroe Islands heavy metals and POPs programme 2005–2008, Report no. 2009:1. Faroe Islands: Environment Agency; 2009. p. 101. [Google Scholar]
- ICC. Welcome to the Inuit Circumpolar Council. Inuitcircumpolar.com. 2008-12-05. Retrieved 2011-09-19. [Google Scholar]
- Jaeger I, Hop H, Gabrielsen GW. Biomagnification of mercury in selected species from an Arctic marine food web in Svalbard. Sci. Total Environ. 2009;407:4744–4751. doi: 10.1016/j.scitotenv.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Kannan K, Agusa T, Evans TJ, Tanabe S. Trace element concentrations in livers of polar bears from two populations in northern and western Alaska. Archives of Environ. Contam. Toxicol. 2007;53:473–482. doi: 10.1007/s00244-007-0018-x. [DOI] [PubMed] [Google Scholar]
- Karimi R, Chen CY, Pickhardt PC, Fisher NS, Folt CL. Stoichiometric controls of mercury dilution by growth. PNAS. 2007;104:7477–7482. doi: 10.1073/pnas.0611261104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd K, Paterson MJ, Hesslein RH, Muir DCG, Hecky RE. Effects of northern pike (Esox lucius) additions on pollutant bioaccumulation and food web structure, as determined by δ13C and δ15N, in a eutrophic and an oligotrophic lake. Can. J. Fish. Aquat. Sci. 1999;56:2193–2202. [Google Scholar]
- Kirk JL, St. Louis VL. Multiyear total and methyl mercury exports from two major sub-Arctic rivers draining into Hudson Bay, Canada. Environ. Sci. Technol. 2009;43:2254–2261. doi: 10.1021/es803138z. [DOI] [PubMed] [Google Scholar]
- Kirk JL, St. Louis VL, Hintelmann H, Lehnherr I, Else B, Poissant L. Methylated mercury species in marine waters of the Canadian High and Sub-Arctic. Environ. Sci. Technol. 2008;42:8367–8373. doi: 10.1021/es801635m. [DOI] [PubMed] [Google Scholar]
- Kirk JL, St. Louis VL, Sharp MJ. Rapid reduction and reemission of mercury deposited into snowpacks during atmospheric mercury depletion events at Churchill, Manitoba, Canada. Environ. Sci. Technol. 2006;40:7590–7596. doi: 10.1021/es061299+. [DOI] [PubMed] [Google Scholar]
- Kotnik J, Horvat M, Tessier E, Ogrinc N, Monperrus M, Amouroux D, Fajon V, Gibičar D, Žižek S, Sprovieri F, Pirrone N. Mercury speciation in the surface and deep waters of the Mediterranean Sea. Mar. Chem. 2007;107:13–30. [Google Scholar]
- Kuhn M. The nutrient cycle through snow and ice, a review. Aquat. Sci. 2001;63:150–167. [Google Scholar]
- Kuhnlein H, Receveur O, Soueida R, Egeland G. Arctic indigenous peoples experience the nutrition transition with changing dietary patterns and obesity. J. Nutr. 2004;124:1447–1453. doi: 10.1093/jn/134.6.1447. [DOI] [PubMed] [Google Scholar]
- Kuhnlein H, Chan H. Environment and contaminants in traditional food systems of northern indigenous peoples. Ann. Rev. Nutr. 2000;20:595–626. doi: 10.1146/annurev.nutr.20.1.595. [DOI] [PubMed] [Google Scholar]
- Kuhnlein HV, Receveur O, Chan HM, Loring E. Assessment of dietary benefit/risk in Inuit communities. Technical report (ISBN # 0-7717-0558-1) Centre for Indigenous Peoples’ Nutrition and Environment (CINE), McGill. 2000 [Google Scholar]
- Kuss J, Schneider B. Variability of the gaseous elemental mercury sea-air flux of the Baltic Sea. Environ. Sci. Technol. 2007;41:8018–8023. doi: 10.1021/es0716251. [DOI] [PubMed] [Google Scholar]
- Lahoutifard N, Poissant L, Scott SL. Scavenging of gaseous mercury by acidic snow at Kuujjuarapik, northern Quebec. Sci. Total Environ. 2006;355:118–126. doi: 10.1016/j.scitotenv.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Lahoutifard N, Sparling M, Lean D. Total and methyl mercury patterns in Arctic snow during springtime at Resolute, Nunavut, Canada. Atmos. Environ. 2005;39:7597–7606. [Google Scholar]
- Laird BD, Shade C, Gantner N, Chan HM, Siciliano SD. Bioaccessibility of mercury from traditional northern country foods measured using an in vitro gastrointestinal model is independent of mercury concentration. Sci. Total Environ. 2009;407:6003–6008. doi: 10.1016/j.scitotenv.2009.08.014. [DOI] [PubMed] [Google Scholar]
- Lalonde JD, Amyot M, Doyon MR, Auclair JC. Photo-induced Hg(II) reduction in snow from the remote and temperate Experimental Lakes Area (Ontario, Canada) J. Geophys. Res. D: Atmos. 2003;108:11–11. 11–18. [Google Scholar]
- Lalonde JD, Poulain AJ, Amyot M. The role of mercury redox reactions in snow on snow to air mercury transfer. Environ. Sci. Technol. 2002;36:174–178. doi: 10.1021/es010786g. [DOI] [PubMed] [Google Scholar]
- Larose C, Dommergue A, De Angelis M, Cossa D, Averty B, Marusczak N, Soumis N, Schneider D, Ferrari C. Springtime changes in snow chemistry lead to new insights into mercury methylation in the Arctic. Geochim. Cosmochim. Acta. 2010;74:6263–6275. [Google Scholar]
- Laurier FJG, Mason RP, Gill GA, Whalin L. Mercury distributions in the north Pacific Ocean-20 years of observations. Mar. Chem. 2004;90:3–19. [Google Scholar]
- Lehnherr I, St. Louis VL, Emmerton CA, Barker JD, Kirk JL. Methylmercury cycling in High Arctic wetland ponds: sources and sinks. Environ. Sci. Technol. 2012 doi: 10.1021/es300576p. http://dx.doi.org/10.1021/es300576p. [DOI] [PubMed] [Google Scholar]
- Lehnherr I, St. Louis VL, Hintelmann H, Kirk JL. Methylation of inorganic mercury in polar marine waters. Nat. Geosci. 2011;3:298–302. [Google Scholar]
- Lehnherr I, St. Louis VL. Importance of ultraviolet radiation in the photodemethylation of methylmercury in freshwater ecosystems. Environ. Sci. Technol. 2009;43:5692–5698. doi: 10.1021/es9002923. [DOI] [PubMed] [Google Scholar]
- Leitch DR, Carrie J, Lean D, Macdonald RW, Stern GA, Wang FY. The delivery of mercury to the Beaufort Sea of the Arctic Ocean by the Mackenzie River. Sci. Total Environ. 2007;373:178–195. doi: 10.1016/j.scitotenv.2006.10.041. [DOI] [PubMed] [Google Scholar]
- Lin CJ, Pongprueksa P, Lindberg SE, Pehkonen SO, Byun D, Jang C. Scientific uncertainties in atmospheric mercury models I : Model science evaluation. Atmos. Environ. 2006;40:2911–2928. [Google Scholar]
- Lockhart L, Stern GA, Wagemann R, Hunt RV, Metner DA, DeLaronde J, Dunn B, Stewart REA, Hyatt CK, Harwood LA, Mount K. Concentrations of mercury in tissues of beluga whales (Delphinapterus leucas) from several communities in the Canadian Arctic from 1981–2002. Sci. Total Environ. 2005:351–352. 391–412. doi: 10.1016/j.scitotenv.2005.01.050. [DOI] [PubMed] [Google Scholar]
- Loseto LL, Stern GA, Connelly TL, Deibel D, Gemmill B, Prokopowicz A, Fortier L, Ferguson SH. Summer diet of beluga whales inferred by fatty acid analysis of the eastern Beaufort Sea food web. J. Exp. Mar. Bio. Ecol. 2009;374:12–18. [Google Scholar]
- Loseto LL, Stern GA, Deibel D, Connelly TL, Prokopowicz A, Lean DRS, Fortier L, Ferguson SH. Linking mercury exposure to habitat and feeding behaviour in Beaufort Sea beluga whales. J. Mar. Sys. 2008a;74:1012–1024. [Google Scholar]
- Loseto LL, Stern GA, Ferguson SH. Size and biomagnification: how habitat selection explains beluga mercury levels. J. Environ. Sci. Technol. 2008b;42:3982–3988. doi: 10.1021/es7024388. [DOI] [PubMed] [Google Scholar]
- Loseto LL. Beaufort Sea beluga whales: an ecological approach to examining diet and dietary sources of mercury. University of Manitoba, Winnipeg; 2007. [Google Scholar]
- Loseto LL, Richard P, Stern GA, Orr J, Ferguson SH. Segregation of Beaufort Sea beluga whales during the open water season. Can. J. Zool. 2006;84:1743–1751. [Google Scholar]
- Loseto LL, Lean DRS, Siciliano SD. Snowmelt sources of methylmercury to High Arctic ecosystems. Environ. Sci. Technol. 2004;38:3004–3010. doi: 10.1021/es035146n. [DOI] [PubMed] [Google Scholar]
- MDN (Mercury Deposition Network) [Retrieved 2007 01-17]; http://nadp.sws.uiuc.edu/mdn/maps/2004/04MDNdpo.pdf. [Google Scholar]
- MacDonald RW, Loseto L. Are Arctic ecosystems exceptionally vulnerable to global emissions of mercury? a call for emphasized research on methylation and the consequences of climate change. Environ. Chem. 2010;7:133–138. [Google Scholar]
- Macdonald RW, Thomas DJ. Chemical interactions and sediments of the western Canadian Arctic shelf. Cont. Shelf Res. 1991;11:843–863. [Google Scholar]
- Manizza M, Follows MJ, Dutkiewicz S, McClelland JW, Menemenlis D, Hill CN, Townsend-Small A, Peterson BJ. Modeling transport and fate of riverine dissolved organic carbon in the Arctic Ocean. Global Biogeochem. Cycles. 2009;23:GB4006. [Google Scholar]
- Mann JL, Long SE, Shuman CA, Kelly WR. Determination of mercury content in a shallow firn core from Greenland by isotope dilution inductively coupled plasma mass spectrometry. Water Air Soil Pollut. 2005;163:19–32. [Google Scholar]
- Mason R, Choi A, Fitzgerald W, Hammerschmidt C, Lamborg C, Sunderland EM. Mercury biogeochemical cycling in the ocean and policy implications. Environ. Res. 2012 doi: 10.1016/j.envres.2012.03.013. http://dx.doi.org/10.1016/j.envres.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason RP, Lawson NM, Sheu GR. Mercury in the Atlantic Ocean: factors controlling air-sea exchange of mercury and its distribution in the upper waters. Deep Sea Res II. 2001;48:2829–2823. [Google Scholar]
- Mason RP, Sullivan KA. The distribution and speciation of mercury in the South and equatorial Atlantic. Deep Sea Res. II. 1999;46:937–956. [Google Scholar]
- Mason RP, Rolfhus KR, Fitzgerald WF. Mercury in the north Atlantic. Mar. Chem. 1998;61:37–53. [Google Scholar]
- Mason RP, Rolfhus KR, Fitzgerald WF. Methylated and elemental mercury cycling in surface and deep ocean waters of the north Atlantic. Water Air Soil Pollut. 1995;80:665–677. [Google Scholar]
- Mason RP, Fitzgerald WF, Morel FMM. The biogeochemical cycling of elemental mercury-anthropogenic influences. Geochimica et Cosmochimica Acta. 1994;58:3191–3198. [Google Scholar]
- Mason RP, Fitzgerald WF. The distribution and biogeochemical cycling of mercury in the equatorial Pacific Ocean. Deep Sea Res. I. 1993;40:1897–1924. [Google Scholar]
- Mason RP, Fitzgerald WF. Alkylmercury species in the equatorial Pacific. Nature. 1990;347:457–459. [Google Scholar]
- Mason RP, Reinfelder JR, Morel FMM. Uptake, toxicity, and trophic transfer of mercury in a coastal diatom. Environ. Sci. Technol. 30:1835–1845. [Google Scholar]
- McMeans BC, Svavarsson J, Dennard S, Fisk A. Diet and resource use among Greenland sharks (Somniosus microcephalus) and teleosts sampled in Icelandic waters, using del13C, del15N, and mercury. Can. J. Fish. Aquat. Sci. 2010;67:1428–1438. [Google Scholar]
- Michel C, Ingram RG, Harris LR. Variability in oceanographic and ecological processes in the Canadian Arctic Archipelago. Prog. Oceanogr. 2006;71:379–401. [Google Scholar]
- Miljeteig C, Strøm H, Gavrilo MV, Volkov A, Jenssen BM, Gabrielsen GW. High levels of contaminants in ivory gull (Pagophila eburnea) eggs from the Russian and Norwegian Arctic. Environ. Sci. Technol. 2009;43:5521–5528. doi: 10.1021/es900490n. [DOI] [PubMed] [Google Scholar]
- Muir D, Wang W, Bright D, Lockhart L, Köck G. Spatial and temporal trends of mercury and other metals in landlocked char from lakes in the Canadian Arctic archipelago. Sci. Total Environ. 2005:351–352. 464–478. doi: 10.1016/j.scitotenv.2004.07.036. [DOI] [PubMed] [Google Scholar]
- Muir DCG, Wang X, Yang F, Nguyen N, Jackson TA, Evans MS, Douglas M, Köck G, Lamoureux S, Pienitz R, Smol JP, Vincent WF, Dastoor A. Spatial trends and historical deposition of mercury in Eastern and Northern Canada inferred from lake sediment cores. Environ. Sci. Technol. 2009;43:4802–4809. doi: 10.1021/es8035412. [DOI] [PubMed] [Google Scholar]
- Mumm N, Auel H, Hanssen H, Hagen W, Richter C, Hirche HJ. Breaking the ice: large scale distribution of mesozooplankton after a decade of Arctic and transpolar cruises. Polar Biology. 1998;20:189–197. [Google Scholar]
- Munthe JZF, Xiao, Lindqvist O. The aqueous reduction of divalent mercury by sulphite. Water Air Soil Pollut. 1991;56:621–630. [Google Scholar]
- Niki H, Maker PS, Savage CM, Breitenbach LP. A fourier transform infrared study of the kinetics and mechanism for the reaction of Cl + CH3HgCH3. J. Phys. Chem. 1983;87:3722–3724. [Google Scholar]
- NCP. Canadian Arctic Contaminants Assessment Report III: Mercury in Canada’s North. Ottawa: Northern Contaminants Program (NCP), Aboriginal Affairs and Northern Development Canada; 2012. [Google Scholar]
- Odsjö T, Räikkönen J, Bignert A. Time trends of metals in liver and muscle of reindeer (Rangifer tarandus) from northern and central Lapland, Sweden, 1983–2005. Swedish monitoring programme in terrestrial biota, Rapport till Naturvårdsverket. 2007:33. 2007. [Google Scholar]
- Ogrinc N, Monperrus M, Kotnik J, Fajon V, Vidimova K, Amouroux D, Kocman D, Tessier E, Zizek S, Horvat M. Distribution of mercury and methylmercury in deep-sea surficial sediments of the Mediterranean Sea. Mar. Chem. 2007;107:31–48. [Google Scholar]
- Oken E, Wright RO, Kleinman KP, Bellinger D, Amarasiriwardena CJ, Hu H, Rich-Edwards JW, Gillman MW. Maternal fish consumption, hair mercury, and infant cognition in a US cohort. Environ. Health Perspect. 2005;113:1376–1380. doi: 10.1289/ehp.8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outridge PM, Hobson KA, Savelle JM. Long term changes of mercury levels in ringed seal (Phoca hispida) from Amundsen Gulf, and beluga (Delphinapterus leucas) from the Beaufort Sea, western Canadian Arctic. Sci. Total Environ. 2009;407:6044–6051. doi: 10.1016/j.scitotenv.2009.08.018. [DOI] [PubMed] [Google Scholar]
- Outridge PM, Macdonald RW, Wang F, Stern GA, Dastoor AP. A mass balance inventory of mercury in the Arctic Ocean. Environ. Chem. 2008;5:89–111. [Google Scholar]
- Outridge PM, Hobson KA, Savelle JM. Changes in mercury and cadmium concentrations and the feeding behaviour of beluga (Delphinapterus leucas) near Somerset Island, Canada, during the 20th Century. Sci. Total Environ. 2005;350:106–118. doi: 10.1016/j.scitotenv.2004.12.081. [DOI] [PubMed] [Google Scholar]
- Outridge PM, McNeely R, Hobson KA, Dyke A. A comparison of modern and preindustrial levels of mercury in the teeth of beluga in the Mackenzie Delta, Northwest Territories, and walrus at Igloolik, Nunavut, Canada. Arctic. 2002;55:123–132. [Google Scholar]
- Pirrone N, Keating T, editors. Hemispheric transport of air pollution 2010 Part B: Mercury. New York and Geneva: United Nations; 2010. [Google Scholar]
- Point D, Sonke JE, Day RD, Roseneau DG, Hobson KA, Vander Pol SS, Moors AJ, Pugh RS, Donard OFX, Becker PR. Methylmercury photodegradation influenced by sea ice cover in Arctic marine ecosystems. Nat. Geosci. 2011;4:188–194. [Google Scholar]
- Pongratz R, Heumann KG. Production of methylated mercury, lead and cadmium by marine bacteria as a significant natural source for atmospheric heavy metals in polar regions. Chemosphere. 1999;39:89–102. [Google Scholar]
- Pongratz R, Heumann KG. Determination of concentration profiles of methyl mercury compounds in surface waters of polar and other remote oceans by GC AFD. Environ. Anal. Chem. 1998a;71:41–56. [Google Scholar]
- Pongratz R, Heumann KG. Production of methylated mercury and lead by polar macroalgae - a significant natural source for atmospheric heavy metals in clean room compartments. Chemosphere. 1998b;38:1935–1946. [Google Scholar]
- Poulain AJ, Chadhain SMN, Ariaya P, Amyot M, Garcia E, Campbell PGC, Zylstra GJ, Barkay T. Potential for mercury reduction by microbes in the high Arctic. Appl. Environ. Microb. 2007a;73:2230–2238. doi: 10.1128/AEM.02701-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulain AJ, Garcia E, Campbell PGC, Amyot M, Ariya PA. Mercury distribution, partitioning and speciation in coastal vs. inland high Arctic snow. Geochimica et Cosmochimica Acta. 2007b;71:3419–3431. [Google Scholar]
- Poulain AJ, Lalonde JD, Amyot JD, Shead JA, Raofie F, Ariya PA. Redox transformations of mercury in an Arctic snowpack at springtime. Atmos. Environ. 2004;38:6763–6774. [Google Scholar]
- Rigét F, Braune B, Bignert A, Wilson S, Aars J, Born E, Dam M, Dietz R, Evans M, Evans T, Gamberg M, Gantner N, Green N, Gunnlaugsdóttir H, Kannan K, Letcher R, Muir D, Roach P, Sonne C, Stern G, Wiig O. Temporal trends of Hg in Arctic Biota, an update. Sci. Total Enivron. 2011;409:3520–3526. doi: 10.1016/j.scitotenv.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Rigét F, Dietz R, Born EW, Sonne C, Hobson KA. Temporal trends of mercury in marine biota of west and northwest Greenland. Marine Pollution Bulletin. 2007;54:72–80. doi: 10.1016/j.marpolbul.2006.08.046. [DOI] [PubMed] [Google Scholar]
- Rigét F, Muir D, Kwan M, Savinova T, Nyman M, Woshner V, O’Hara T. Circumpolar pattern of mercury and cadmium in ringed seals. Sci. Total Environ. 2005:351–352. 312–322. doi: 10.1016/j.scitotenv.2004.05.032. [DOI] [PubMed] [Google Scholar]
- Rigét F, Dietz R, Vorkamp K, Johansen P, Muir D. Levels and spatial and temporal trends of contaminants in Greenland biota: an updated review. Sci. Total Environ. 2004;331:29–52. doi: 10.1016/j.scitotenv.2004.03.022. [DOI] [PubMed] [Google Scholar]
- Rush SA, Borga K, Dietz R, Born EW, Sonne C, Evans T, Muir DCG, Letcher RJ, Norstrom RJ, Fisk AT. Geographic distribution of selected elements in the livers of polar bears from Greenland, Canada and the United States. Environ. Pollut. 2008;153:618–626. doi: 10.1016/j.envpol.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Saint Amour D, Roy MS, Bastien C, Ayotte P, Dewailly E, Després C, Gingras S, Muckle G. Alterations of visual evoked potentials in preschool Inuit children exposed to methylmercury and polychlorinated biphenyls from a marine diet. Neurotoxicology. 2006;27:567–578. doi: 10.1016/j.neuro.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Sanei H, Outridge PM, Goodarzi F, Wang F, Armstrong D, Warren K, Fishback L. Wet deposition mercury fluxes in the Canadian sub-Arctic and southern Alberta, measured using an automated precipitation collector adapted to cold regions. Atmos. Environ. 2010;44:1672–1681. [Google Scholar]
- Savinov VM, Gabrielsen GW, Savinova TN. Cadmium, zinc, copper, arsenic, selenium and mercury in seabirds from the Barents Sea: levels, inter specific and geographical differences. Sci. Total Environ. 2003;306:133–159. doi: 10.1016/S0048-9697(02)00489-8. [DOI] [PubMed] [Google Scholar]
- Schroeder WH, Steffen A, Scott K, Bender T, Prestbo E, Ebinghaus R, Lu J, Lindberg S. Summary report: first international Arctic atmospheric mercury research workshop. Atmos. Environ. 2003;37:2551–2555. [Google Scholar]
- Schuster PF, Striegl RG, Aiken GR, Krabbenhoft DP, Dewild JF, Butler K, Kamark B, Dornblaster M. Mercury export from the Yukon River basin and potential repponse to a changing climate. Environ. Sci. Technol. 45:9262–9267. doi: 10.1021/es202068b. [DOI] [PubMed] [Google Scholar]
- Selin NE. Global biogeochemical cycling of mercury: a review. Annu. Rev. Environ. Resour. 2009;34:43–63. [Google Scholar]
- Sellers P, Kelly CA, Rudd JWW, MacHutchon RA. Photodegredation of methylmercury in lakes. Nature. 1996;380:694–697. [Google Scholar]
- Senn DB. Stable isotope (N, C, Hg) study of methylmercury sources and trophic transfer in the northern gulf of Mexico. Department of Environmental Health, Harvard School of Public Health, Boston, Massachusetts 02115, USA. Environ. Sci. Technol. 2010;44:1630–1637. doi: 10.1021/es902361j. [DOI] [PubMed] [Google Scholar]
- Sherman LS, Blum JD, Johnson KP, Keeler GJ, Barres JA, Douglas TA. Mass independent fractionation of mercury isotopes in Arctic snow driven by sunlight. Nat. Geosci. 2010;3:173–177. [Google Scholar]
- Simpson WR, Carlson D, Honninger G, Douglas TA, Sturm M, Perovich D, Platt U. First-year sea-ice contact predicts bromine monoxide (BrO) levels at Barrow, Alaska better than potential frost flower contact. Atmos. Chem. Phys. 2007a;7:621–627. [Google Scholar]
- Simpson WR, von Glasow R, Riedel K, Anderson P, Ariya P, Bottenheim J, Burrows J, Carpenter LJ, Friesβ U, Goodsite ME, Heard D, Hutterli M, Jacobi HW, Kaleschke L, Neff B, Plane J, Platt U, Richter A, Roscoe H, Sander R, Shepson P, Sodeau J, Steffen A, Wagner T, Wolf E. Halogens and their role in polar boundary layer ozone depletion. Atmos. Chem. Phys. 2007b;7:4375–4418. [Google Scholar]
- Sommar J, Wängberg I, Berg T, Gårdfeldt K, Munthe J, Richter A, Urba A, Wittrock F, Schroeder WH. Circumpolar transport and air surface exchange of atmospheric mercury at Ny-Ålesund (79° N), Svalbard. Atmos. Chem. Phys. 2002;7:151–166. [Google Scholar]
- Sommar JM, Hallquist M, Ljungstrom E. Rate of reaction between the nitrate radical and dimethyl mercury in the gas phase. Chem. Phys. Let. 1996;257:434–438. [Google Scholar]
- Soerensen AL, Sunderland EM, Holmes CD, Jacob DJ, Yantosca R, Skov H, Christensen JH, Strode SA, Mason RB. An Improved Global Model for Air sea exchange of Mercury: High concentrations over the North Atlantic. Environ. Sci. Technol. 2010;44:8574–8580. doi: 10.1021/es102032g. [DOI] [PubMed] [Google Scholar]
- Springer AM, McRoy CP, Flint MV. The Bering Sea green belt: shelf edge process and ecosystem production. Fish Oceanography. 1996;5:205–223. [Google Scholar]
- St. Louis VL, Derocher AE, Stirling I, Graydon JA, Lee C, Jocksch E, Richardson E, Ghorpade S, Kwan AK, Kirk JL, Lehnherr I, Swanson HK. Differences in mercury bioaccumulation between polar bears (Ursus maritimus) from the Canadian high and sub Arctic. Environ. Sci. Technol. 2011;45:5922–5928. doi: 10.1021/es2000672. [DOI] [PubMed] [Google Scholar]
- St. Louis V, Hintlemann H, Graydon JA, Kirk JL, Barkar J, Dimock B, Sharp MJ, Lehnherr I. Methylated species in the Canadian high Arctic marine surface waters and snowpacks. Environ. Sci. Technol. 2007;41:6433–6441. doi: 10.1021/es070692s. [DOI] [PubMed] [Google Scholar]
- St. Louis VL, Sharp MJ, Steffen A, May A, Barker J, Kirk JL, Kelly DJA, Arnott SE, Keatley BE, Smol JP. Some sources and sinks of monomethyl and inorganic mercury on Ellesmere Island in the Canadian High Arctic. Environ. Sci. Technol. 2005;39:2686–2701. doi: 10.1021/es049326o. [DOI] [PubMed] [Google Scholar]
- Steen AO, Berg T, Dastoor AP, Durnford DA, Hole LR, Pfaffhuber KA. Dynamic exchange of gaseous elemental mercury during polar night and day. Atmos. Environ. 2009;43:5604–5610. [Google Scholar]
- Steffen A, Douglas T, Amyot M, Ariya P, Aspmo K, Berg T, Bottenheim J, Brooks S, Cobbett FD, Dastoor A, Dommergue A, Ebinghaus R, Ferrari C, Gardfeldt K, Goodsite ME, Lean D, Poulain AJ, Scherz C, Skov H, Sommar J, Temme C. A synthesis of atmospheric mercury depletion event chemistry in the atmosphere and snow. Atmos. Chem. Phys. 2008;8:1445–1482. [Google Scholar]
- Steffen A, Schroeder WH, Bottenheim J, Narayan J, Fuentes JD. Atmospheric mercury concentrations: measurements and profiles near snow and ice surfaces in the Canadian Arctic during Alert 2002. Atmos. Environ. 2000;36:2653–2661. [Google Scholar]
- Stern GA. Synopsis of research conducted under the 2009–2010 Northern Contaminants Program. Indian and Northern Affairs Canada; 2010. Mercury in beluga, narwhal and walrus from the Canadian Arctic: status 2010; pp. 126–134. [Google Scholar]
- Stern GA, Macdonald RW. Biogeographical provinces of total and methyl mercury in zooplankton and fish from the Beaufort and Chukchi Seas: results from the SHEBA drift. Environ. Sci. Technol. 2005;39:4707–4713. doi: 10.1021/es0482278. [DOI] [PubMed] [Google Scholar]
- Stevick PT, McConnell BJ, Hammond PS. Patterns of movement. In: Hoelzel AR, editor. Marine mammal biology: an evolutionary approach. Oxford: Blackwell Science; 2002. pp. 185–216. [Google Scholar]
- Sunderland EM, Amirbahman A, Burgess NM, Dalziel J, Harding G, Jones SH, Kamai E, Karagas MR, Shi X, Chen CY. Mercury sources and fate in the Gulf of Maine. Environ. Res. 2012 doi: 10.1016/j.envres.2012.03.011. http://dx.doi.org/10.1016/j.envres.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderland EM, Dalziel J, Heyes A, Branfireun BA, Krabbenhoft DP, Gobas FAPC. Response of a macrotidal estuary to changes in anthropogenic mercury loading between 1850 and 2000. Environ. Sci. Technol. 2010;44:1698–1704. doi: 10.1021/es9032524. [DOI] [PubMed] [Google Scholar]
- Sunderland EM, Krabbenhoft DP, Moreau JW, Strode SA, Landing WM. Mercury sources, distribution, and bioavailability in the north Pacific Ocean: Insights from data and models. Global Biogeochem. Cycles. 2009;23:GB2010. [Google Scholar]
- Tian W, Egeland GM, Sobol I, Chan HM. Mercury hair concentrations and dietary exposure among Inuit preschool children in Nunavut, Canada. Environ Int. 2010;37:42–48. doi: 10.1016/j.envint.2010.05.017. [DOI] [PubMed] [Google Scholar]
- Travnikov O. Contribution of the intercontinental atmospheric transport to mercury pollution in the Northern Hemisphere. Atmos. Environ. 2005;39:7541–7548. [Google Scholar]
- Travnikov O, Ilyin I. The EMEP/MSC-E mercury modeling system. In: Pirrone N, Mason RP, editors. Mercury fate and transport in the global atmosphere. Dordecht: Springer; 2009. pp. 571–587. [Google Scholar]
- Tvinnereim HM, Eide R, Fosse GR. Heavy metals in human primary teeth: some factors influencing the metal concentrations. Sci. Total Environ. 2000;255:21–27. doi: 10.1016/s0048-9697(00)00436-8. [DOI] [PubMed] [Google Scholar]
- Valera B, Dewailly E, Poirier P. Environmental mercury exposure and blood pressure among Nunavik Inuit adults. Hypertension. 2009;54:981–986. doi: 10.1161/HYPERTENSIONAHA.109.135046. [DOI] [PubMed] [Google Scholar]
- Van Loon LE, Mader, Scott SL. Reduction of the aqueous mercuric ion by sulfite: UV spectrum of HgSO3 and its intramolecular redox reaction. J. Phys. Chem. 2000;104:1621–1626. [Google Scholar]
- Van Oostdam J, Donaldson SG, Feeley M, Arnold D, Ayotte P, Bondy G, Chan L, Dewaily E, Furgal CM, Kuhnlein H, Loring E, Muckle G, Myles E, Receveur O, Tracy B, Gill U, Kalhok S. Human health implications of environmental contaminants in Arctic Canada: a review. Sci. Total Environ. 2005:351–352. 165–246. doi: 10.1016/j.scitotenv.2005.03.034. [DOI] [PubMed] [Google Scholar]
- Van Oostdam J, Gilman A, Dewailly E, Usher P, Wheatley B, Kuhnlein H, Neve S, Walker J, Tracy B, Feeley M, Jerome V, Kwavnick B. Human health implications of environmental contaminants in Arctic Canada: a review. Sci. Total Environ. 1999;230:1–82. doi: 10.1016/s0048-9697(99)00036-4. [DOI] [PubMed] [Google Scholar]
- Wagemann R, Innes S, Richard PR. Overview and regional and temporal differences of heavy metals in Arctic whales and ringed seals in the Canadian Arctic. Sci. Total Environ. 1996;186:41–66. doi: 10.1016/0048-9697(96)05085-1. [DOI] [PubMed] [Google Scholar]
- Whalin L, Kim EH, Mason R. Factors influencing the oxidation, reduction, methylation, and demethylation of mercury species in coastal waters. Mar. Chem. 2007;107:278–294. [Google Scholar]
- Wheatley B, Wheatley M. Methylmercury and the health of indigenous peoples: a risk management challenge for physical and social science and for public health policy. Sci. Total Environ. 2000;259:23–29. doi: 10.1016/s0048-9697(00)00546-5. [DOI] [PubMed] [Google Scholar]
- Wheatley B, Wheatley MA. Methylmercury in the Canadian Arctic environment past and present natural or industrial? Arctic Med. Res. 1988;47:163–167. [PubMed] [Google Scholar]
- Zhang T, Hsu-Kim H. Photolytic degradation of methylmercury enhanced by binding to natural organic ligands. Nat. Geosci. 2010;3:473–476. doi: 10.1038/ngeo892. [DOI] [PMC free article] [PubMed] [Google Scholar]