Abstract
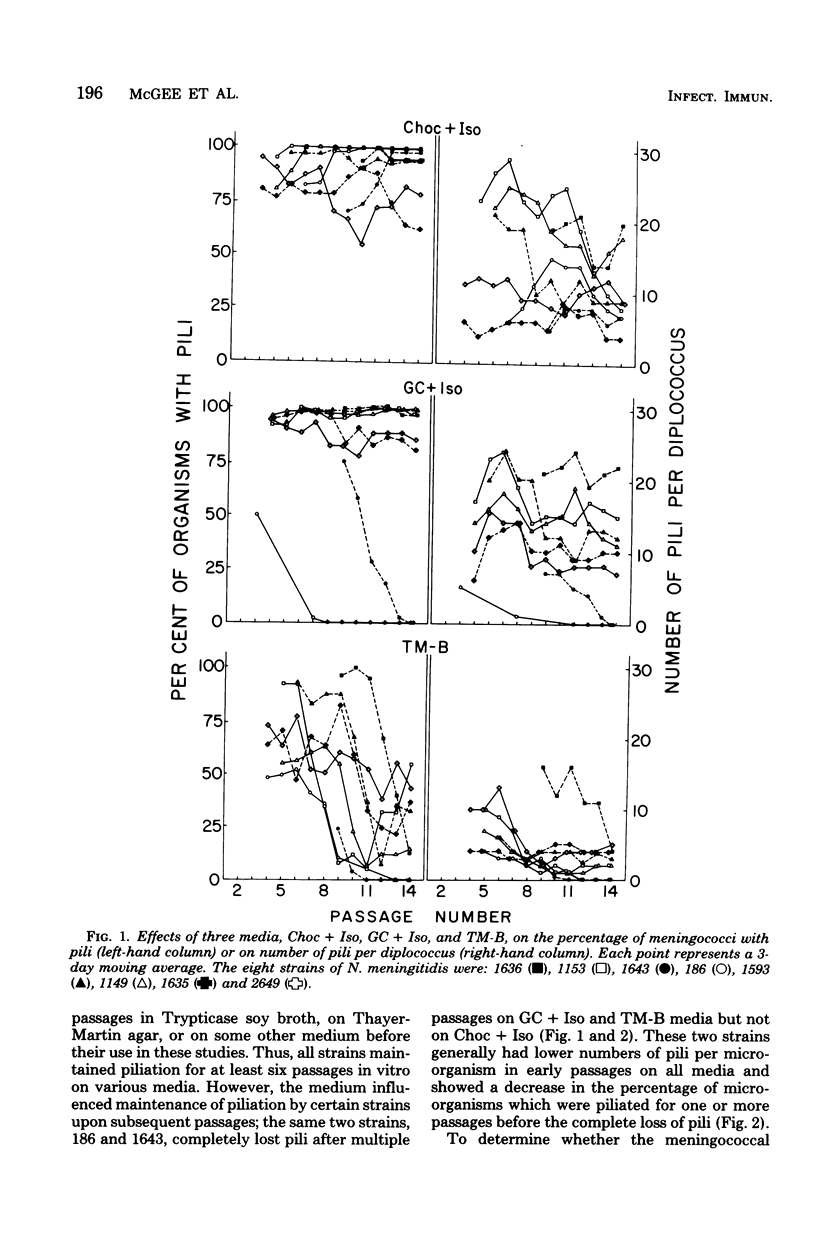
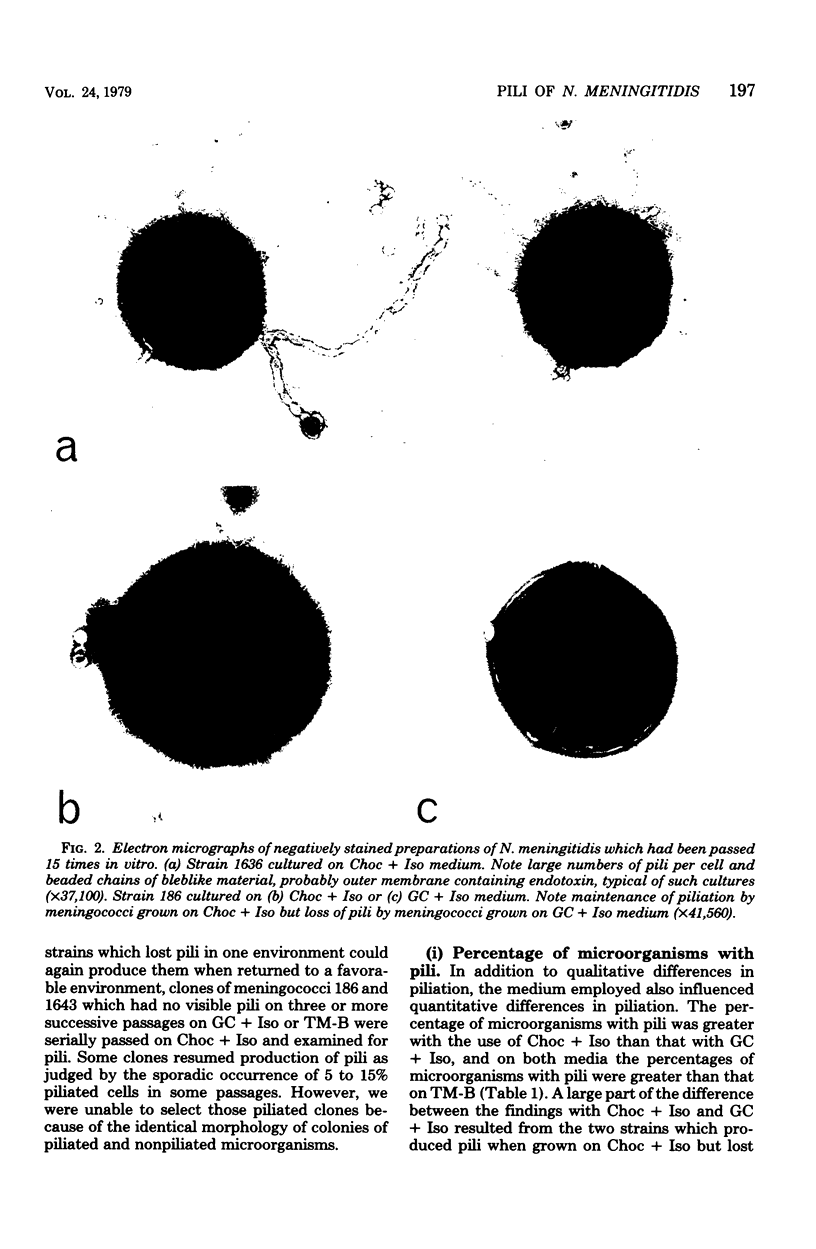
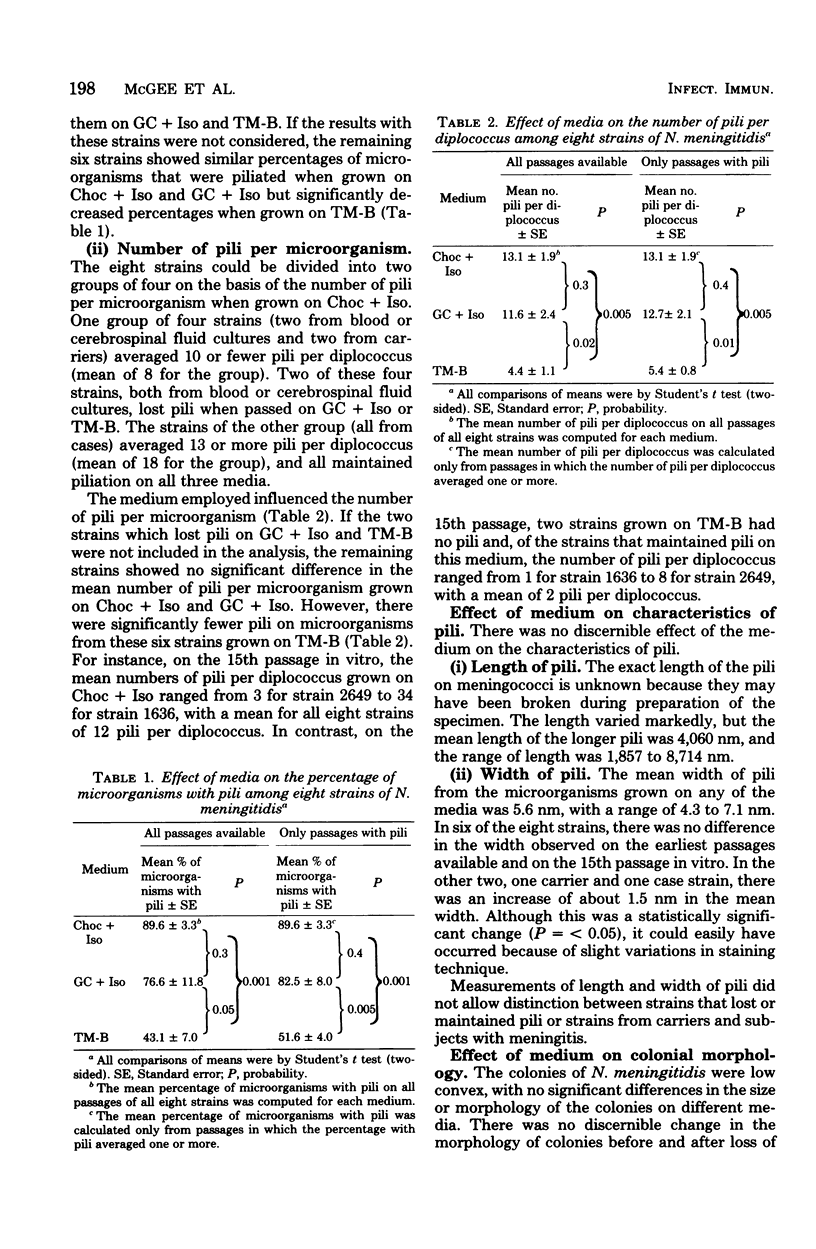
In contrast to information in the literature which indicates that meningococci rapidly lose pili upon cultivation in vitro, we found that piliation of meningococci could be maintained in vitro for 15 or more passages. Pili were present on all eight isolates tested, whether from asymptomatic carriers or from subjects with meningococcal disease. Complete loss of piliation occurred in the same two strains on two of the three media tested. On one medium (Thayer-Martin medium with supplement B), there was partial or complete loss of pili by all strains. The optimal medium for maintaining pili was chocolate agar with 1% IsoVitaleX; 95% or more of the microorganisms of six of the eight strains tested were piliated after 15 passages in vitro, and more than 60% of the microorganisms of the other two strains were piliated. Meningococci passed on this medium generally maintained their initial density of piliation (3 to 34 pili per diplococcus). The ability to predictably cultivate piliated meningococci in vitro and to select piliated and nonpiliated clones of the same strain should allow investigation of the biochemical and immunological properties of meningococcal pili as well as their possible role in the pathogenicity of Neisseria meningitidis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buchanan T. M., Gotschlich E. C. Studies on gonococcus infection. 3. Correlation of gonococcal colony morphology with infectivity for the chick embryo. J Exp Med. 1973 Jan 1;137(1):196–200. doi: 10.1084/jem.137.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan T. M., Pearce W. A. Pili as a mediator of the attachment of gonococci to human erythrocytes. Infect Immun. 1976 May;13(5):1483–1489. doi: 10.1128/iai.13.5.1483-1489.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVoe I. W., Gilchrist J. E. Pili on meningococci from primary cultures of nasopharyngeal carriers and cerebrospinal fluid of patients with acute disease. J Exp Med. 1975 Feb 1;141(2):297–305. doi: 10.1084/jem.141.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVoe I. W., Gilchrist J. E. Piliation and colonial morphology among laboratory strains of meningococci. J Clin Microbiol. 1978 Apr;7(4):379–384. doi: 10.1128/jcm.7.4.379-384.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoe I. W., Gilchrist J. E. Ultrastructure of pili and annular structures on the cell wall surface of Neisseria meningitidis. Infect Immun. 1974 Oct;10(4):872–876. doi: 10.1128/iai.10.4.872-876.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguid J. P., Anderson E. S., Campbell I. Fimbriae and adhesive properties in Salmonellae. J Pathol Bacteriol. 1966 Jul;92(1):107–138. doi: 10.1002/path.1700920113. [DOI] [PubMed] [Google Scholar]
- Evans B. A. Ultrastructural study of cervical gonorrhea. J Infect Dis. 1977 Aug;136(2):248–255. doi: 10.1093/infdis/136.2.248. [DOI] [PubMed] [Google Scholar]
- Froholm L. O., Jyssum K., Bovre K. Electron microscopical and cultural features of Neisseria meningitidis competence variants. Acta Pathol Microbiol Scand B Microbiol Immunol. 1973 Oct;81(5):525–537. doi: 10.1111/j.1699-0463.1973.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Heckels J. E., Blackett B., Everson J. S., Ward M. E. The influence of surface charge on the attachment of Neisseria gonorrhoeae to human cells. J Gen Microbiol. 1976 Oct;96(2):359–364. doi: 10.1099/00221287-96-2-359. [DOI] [PubMed] [Google Scholar]
- Honda E., Yanagawa R. Attachment of Corynebacterium renale to tissue culture cells by the pili. Am J Vet Res. 1975 Nov;36(11):1663–1666. [PubMed] [Google Scholar]
- James-Holmquest A. N., Swanson J., Buchanan T. M., Wende R. D., Williams R. P. Differential attachment by piliated and nonpiliated Neisseria gonorrhoeae to human sperm. Infect Immun. 1974 May;9(5):897–902. doi: 10.1128/iai.9.5.897-902.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James A. N., Knox J. M., Williams R. P. Attachment of gonococci to sperm. Influence of physical and chemical factors. Br J Vener Dis. 1976 Apr;52(2):128–135. doi: 10.1136/sti.52.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James J. F., Swanson J. Piliation of gonococci in vivo. J Infect Dis. 1978 Jan;137(1):94–96. doi: 10.1093/infdis/137.1.94. [DOI] [PubMed] [Google Scholar]
- KELLOGG D. S., Jr, PEACOCK W. L., Jr, DEACON W. E., BROWN L., PIRKLE D. I. NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION. J Bacteriol. 1963 Jun;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg D. S., Jr, Cohen I. R., Norins L. C., Schroeter A. L., Reising G. Neisseria gonorrhoeae. II. Colonial variation and pathogenicity during 35 months in vitro. J Bacteriol. 1968 Sep;96(3):596–605. doi: 10.1128/jb.96.3.596-605.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koransky J. R., Scales R. W., Kraus S. J. Bacterial hemagglutination by Neisseria gonorrhoeae. Infect Immun. 1975 Sep;12(3):495–498. doi: 10.1128/iai.12.3.495-498.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee Z. A., Dourmashkin R. R., Gross J. G., Clark J. B., Taylor-Robinson D. Relationship of pili to colonial morphology among pathogenic and nonpathogenic species of Neisseria. Infect Immun. 1977 Feb;15(2):594–600. doi: 10.1128/iai.15.2.594-600.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee Z. A., Johnson A. P., Taylor-Robinson D. Human fallopian tubes in organ culture: preparation, maintenance, and quantitation of damage by pathogenic microorganisms. Infect Immun. 1976 Feb;13(2):608–618. doi: 10.1128/iai.13.2.608-618.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mårdh P. A., Westtöm L. Adherence of bacterial to vaginal epithelial cells. Infect Immun. 1976 Mar;13(3):661–666. doi: 10.1128/iai.13.3.661-666.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotny P., Short J. A., Walker P. D. An electron-microscope study of naturally occurring and cultured cells of Neisseria Gonorrhoeae. J Med Microbiol. 1975 Aug;8(3):413–427. doi: 10.1099/00222615-8-3-413. [DOI] [PubMed] [Google Scholar]
- Pedersen K. B., Froholm L. O., Bovre K. Fimbriation and colony type of Moraxella bovis in relation to conjunctival colonization and development of keratoconjunctivitis in cattle. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80(6):911–918. doi: 10.1111/j.0365-5563.1973.tb00019.x. [DOI] [PubMed] [Google Scholar]
- Punsalang A. P., Jr, Sawyer W. D. Role of pili in the virulence of Neisseria gonorrhoeae. Infect Immun. 1973 Aug;8(2):255–263. doi: 10.1128/iai.8.2.255-263.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., King G., Zeligs B. Studies on gonococcus infection. VIII. 125Iodine labeling of gonococci and studies on their in vitro interactions with eukaryotic cells. Infect Immun. 1975 Mar;11(3):453–459. doi: 10.1128/iai.11.3.453-459.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Sparks E., Zeligs B., Siam M. A., Parrott C. Studies on gonococcus infection. V. Observations on in vitro interactions of gonococci and human neutrophils. Infect Immun. 1974 Sep;10(3):633–644. doi: 10.1128/iai.10.3.633-644.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. IV. Pili: their role in attachment of gonococci to tissue culture cells. J Exp Med. 1973 Mar 1;137(3):571–589. doi: 10.1084/jem.137.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Surface components affecting interactions between Neisserai gonorrhoeae and eucaryotic cells. J Infect Dis. 1977 Aug;136 (Suppl):S138–S143. doi: 10.1093/infdis/136.supplement.s138. [DOI] [PubMed] [Google Scholar]
- Thongthai C., Sawyer W. D. Studies on the virulence of Neisseria gonorrhoeae. I. Relation of colonial morphology and resistance to phagocytosis by polymorphonuclear leukocytes. Infect Immun. 1973 Mar;7(3):373–379. doi: 10.1128/iai.7.3.373-379.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tramont E. C., Wilson C. Variations in buccal cell adhesion of Neisseria gonorrhoeae. Infect Immun. 1977 May;16(2):709–711. doi: 10.1128/iai.16.2.709-711.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waitkins S. A. Fimbrial haemagglutination by Neisseria gonorrhoeae. Br J Vener Dis. 1974 Aug;50(4):272–278. doi: 10.1136/sti.50.4.272. [DOI] [PMC free article] [PubMed] [Google Scholar]



