Abstract
Studies in experimental models suggest that n-3 polyunsaturated fatty acids (PUFAs) improve metabolic and anti-inflammatory/antioxidant capacity of the heart, although the mechanisms are unclear and translational evidence is lacking. In this study, patients ingested a moderately high dose of n-3 PUFAs (3.4 g/day eicosapentaenoic (EPA) and doxosahexaenoic acid (DHA) ethyl-esters) for a period of 2–3 weeks before having elective cardiac surgery. Blood was obtained before treatment and at the time of surgery, and myocardial tissue from the right atrium was also dissected during surgery. Blood EPA levels increased and myocardial tissue EPA and DHA levels were significantly higher in n-3 PUFA-treated patients compared with untreated, standard-of-care control patients. Interestingly, n-3 PUFA patients had greater nuclear transactivation of peroxisome proliferator-activated receptor-γ (PPARγ), fatty acid metabolic gene expression, and enhanced mitochondrial respiration supported by palmitoyl-carnitine in the atrial myocardium, despite no difference in mitochondrial content. Myocardial tissue from n-3 PUFA patients also displayed greater expression and activity of key antioxidant/anti-inflammatory enzymes. These findings lead to our hypothesis that PPARγ activation is a mechanism by which fish oil n-3 PUFAs enhance mitochondrial fatty acid oxidation and antioxidant capacity in human atrial myocardium, and that this preoperative therapeutic regimen may be optimal for mitigating oxidative/inflammatory stress associated with cardiac surgery. Antioxid. Redox Signal. 21, 1156–1163.
Introduction
Implementing n-3 polyunsaturated fatty acids (PUFAs) into clinical practice as a therapy for cardiovascular disease has been justified by many, although not all, clinical trials showing an improvement in patient mortality (reviewed in Refs. (2, 7)). In particular, the fish oil n-3 PUFAs eicosapentaenoic (EPA) and doxosahexaenoic (DHA) acids have demonstrated the most promising and consistent cardioprotective effects, including anti-inflammatory, vasodilating, anti-arrhythmic, and antioxidant properties. Recent randomized controlled trials (RCT's) have been disappointing in that n-3 PUFA therapy has had mixed effects on cardiovascular disease outcomes, with some showing benefit and others not (reviewed in Ref. (7)). Reasons for these discrepant findings are multifactorial, although the lack of consensus regarding mechanism, appropriate n-3 PUFA formulation, dose, and duration of treatment necessary to achieve desired effect can be considered major factors underlying these inconsistent findings.
Innovation.
It can be concluded from our study that 2–3 weeks of a moderately high dose n-3 polyunsaturated fatty acid (PUFA) therapy (3.4 g EPA & DHA per day) is associated with PPARγ activation and higher levels of fatty acid metabolic gene expression, mitochondrial fatty acid oxidation, and antioxidant capacity in human atrial myocardium. Based on these findings, we hypothesize that this represents a mechanism by which n-3 PUFA therapy leads to beneficial effects in the human myocardium. Further investigation is needed to explore this potential mechanism and to determine whether this preoperative n-3 PUFA therapeutic regimen can impact postoperative clinical outcomes (e.g., reduce complications).
One clinical indication for n-3 PUFAs that has been recently explored is a prophylactic, preoperative therapy to reduce the incidence of postoperative atrial fibrillation (POAF) with heart surgery. Although some small clinical trials showed early success in mitigating POAF with this therapy, the results of recent RCT's have been disappointing (6, 8, 9). These results may not be surprising, however, when viewed in the context of the large number of unanswered questions with regard to mechanism and appropriate treatment duration. Indeed, optimal n-3 PUFA therapy for heart disease has been prohibited by the absence of information on the cellular and molecular effects of these fatty acids in human heart, due in no small part to obvious challenges with obtaining human heart tissue. The use of n-3 PUFAs as a preoperative therapy in cardiac surgery offers a unique translational research model in this regard, although challenges still exist in this model as cardiac surgery is often performed under emergent circumstances where preoperative n-3 PUFA treatment is simply not feasible. One study reported that 10–15 days of fish oil ingestion in patients before elective cardiac surgery was sufficient to achieve a maximal concentration of EPA/DHA in atrial tissue and erythrocytes, which was concomitant with a maximal decrease in levels of the n-6 PUFA, arachidonic acid (AA) (5). A recent study in our laboratory showed that three weeks of an n-3 PUFA-enriched diet was sufficient to cause increased antioxidant enzyme expression and decreased mitochondrial reactive oxygen species (ROS) generation in mouse heart (1). Whether similar changes in these parameters exist in the human heart following n-3 PUFA therapy within this time frame is completely unknown.
We conducted the present study to examine some of these unanswered questions and to determine whether n-3 PUFA therapy (3.4 g/day EPA/DHA) for 2–3 weeks before cardiac surgery was sufficient to cause changes in antioxidant/anti-inflammatory gene expression in human atrial myocardium, and to identify possible mechanisms by which this occurs. Specific focus was placed on assessing mitochondrial function and redox parameters, as these have been described to become altered in the heart with n-3 PUFA intake. Importantly, the therapeutic regimen used in this study is unique in that the dose of n-3 PUFAs is much higher, and the duration of therapy is longer, than in recent RCTs where n-3 PUFA therapy in the perioperative setting has been explored (6, 8, 9).
Fatty acid composition in whole blood and atrial myocardium is altered with n-3 PUFA treatment
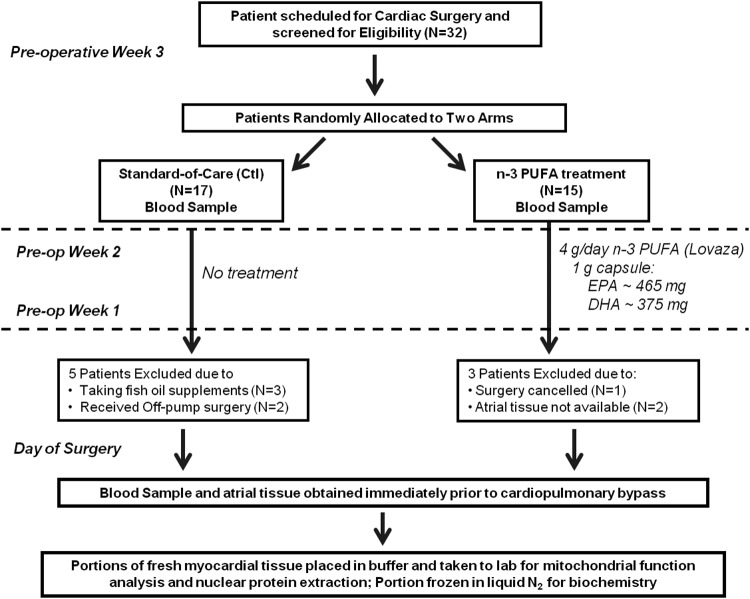
Overall study design and workflow is described in Figure 1. Clinical characteristics were similarly matched between n-3 PUFA-treated and -untreated, standard-of-care control (Ctl) patients, and no statistically significant differences in postoperative events were observed between groups (Table 1). The duration of treatment ranged from 2 to 3 weeks, with a mean duration of 17.5 days. Several patients were excluded from the study at the time of surgery for various reasons (Fig. 1).
FIG. 1.
Flow chart of patient screening and enrollment, and overall experimental design for this study.
Table 1.
Patient Clinical and Demographic Information
| Demographics and clinical variables | Control | n-3 PUFA | p-value |
|---|---|---|---|
| Total n | 12 | 12 | — |
| Age (years) | 63.1±8.4 | 65.8±9.9 | 0.81 |
| Female sex, n (%) | 3 (25) | 5 (42) | 0.41 |
| Race (C/AA) | 9/3 | 11/1 | 0.3 |
| BMI | 32.0±7.8 | 30.2±7.0 | 0.61 |
| HbA1c | 5.9±0.8 | 6.1±1.6 | 0.67 |
| HDL | 40.2±11.8 | 41.0±13.1 | 0.89 |
| LDL | 105.9±38.9 | 88.1±26.2 | 0.22 |
| Triglycerides | 170.8±62.9 | 136.1±43.2 | 0.14 |
| LVEF (%) | 55.8±7.6 | 57.3±6.8 | 0.64 |
| Hypertension, n (%) | 9 (75) | 9 (75) | 1 |
| Diabetes, n (%) | 2 (16) | 3 (25) | 0.63 |
| Intraoperative characteristics | |||
| CABG only, n (%) | 7 (47) | 6 (50) | 0.7 |
| CABG+Valve repair/replace, n (%) | 2 (16) | 3 (25) | 0.63 |
| Valve repair/replace, n (%) | 3 (25) | 3 (25) | 1 |
| CPB Time (min) | 98.5±20.0 | 101.1±17.2 | 0.74 |
| Cross-clamp time (min) | 86.1±18.1 | 87.8±14.1 | 0.8 |
| Preoperative medications–N (%) | |||
| Aspirin | 9 (75) | 10 (83) | 0.78 |
| β-Blocker | 10 (83) | 10 (83) | 1 |
| Anti-depressant | 1 (8) | 2 (16) | 0.56 |
| Statin | 3 (25) | 2 (16) | 0.63 |
| Calcium channel blocker | 1 (8) | 1 (8) | 1 |
| ACE Inhibitor/ARB | 8 (66) | 5 (42) | 0.24 |
| Insulin | 1 (8) | 1 (8) | 1 |
| Biguanides | 0 | 1 (8) | 0.69 |
| Postoperative events | |||
| Postoperative AF | 4 (33) | 2 (16) | 0.37 |
| Hospitalization LOS (days) | 7.4±3.4 | 6.6±2.9 | 0.53 |
| Inotropic Support | 2 (16) | 1 (8) | 0.56 |
| Other Complications, n (%) | 1 (8) | 2 (16) | 0.56 |
Values for continuous variables expressed as mean±SD. Tests of statistical significance were performed using unpaired Student's t-test for continuous variables, or Fisher's exact test for categorical variables. C, Caucasian; AA, African American; BMI, body mass index; HbA1c, glycated hemoglobin; HDL, high-density lipoprotein; LDL, low-density lipoprotein; LVEF, left ventricular ejection fraction; CPB, cardiopulmonary bypass; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; AF, atrial fibrillation; LOS, length of stay.
Treatment with n-3 PUFAs led to increased whole blood levels of EPA and stearic acid, and decreased levels of linoleic acid and AA (Table 2). In the atrial myocardium, n-3 PUFA treatment was associated with greater levels of EPA/DHA and lower levels of AA as compared with Ctl patients (Table 3).
Table 2.
Fatty Acid Composition in Whole Blood
| Fatty acid | Pre | Post | p-value |
|---|---|---|---|
| 14:0 | 0.5±0.4 | 0.4±0.3 | 0.58 |
| 16:0 | 21.7±3.7 | 26.3±7.2 | 0.11 |
| 16:1 | 0.5±0.2 | 0.3±0.2 | 0.07 |
| 18:0 | 18.0±0.6 | 21.8±1.0 | <0.0001 |
| 18:1t (n-9) | 0.02±0.01 | 0.03±0.01 | 0.11 |
| 18:1c (n-9) | 16.6±1.2 | 17.1±1.9 | 0.56 |
| 18:2 (n-6) | 20.1±2.2 | 14.8±1.9 | <0.0001 |
| 20:4 (n-6) | 17.9±1.5 | 12.9±6.4 | 0.03 |
| 20:5 (n-3) | 0.5±0.1 | 1.7±0.6 | <0.0001 |
| 22:5 (n-3) | 2.1±0.3 | 2.0±1.0 | 0.59 |
| 22:6 (n-3) | 3.1±0.7 | 4.1±2.0 | 0.19 |
Fatty acid analysis of whole blood from samples taken before (Pre) and after n-3 PUFA treatment (Post). Values shown are percentage of total fatty acids, mean±SD, and values less than 0.1 are not reported.
Table 3.
Fatty Acid Composition in Atrial Myocardium
| Fatty acid | Ctl | n-3 PUFA | p-value |
|---|---|---|---|
| 14:0 | 0.5±0.6 | 1.2±1.6 | 0.27 |
| 16:0 | 19.7±2.6 | 24.7±7.0 | 0.08 |
| 16:1 | 1.8±1.5 | 1.8±1.9 | 0.9 |
| 18:0 | 13.1±4.2 | 15.1±4.8 | 0.39 |
| 18:1c (n-9) | 25.0±11.4 | 23.9±11.6 | 0.85 |
| 18:2 (n-6) | 21.2±2.6 | 18.3±4.8 | 0.11 |
| 20:4 (n-6) | 16.0±6.5 | 10.8±4.6 | 0.08 |
| 20:5 (n-3) | 0.2±0.1 | 0.6±0.2 | 0.0001 |
| 22:5 (n-3) | 1.0±0.3 | 0.8±0.4 | 0.32 |
| 22:6 (n-3) | 2.3±1.3 | 3.6±1.4 | 0.04 |
Fatty acid analysis of whole myocardial tissue (atrium) obtained from standard-of-care Ctl patients compared with n-3 PUFA-treated patients. Values shown are percentage of total fatty acids, mean±SD, and values less than 0.1 are not reported.
Is n-3 PUFA treatment associated with altered pro-inflammatory and redox signaling pathways in human atrial myocardium?
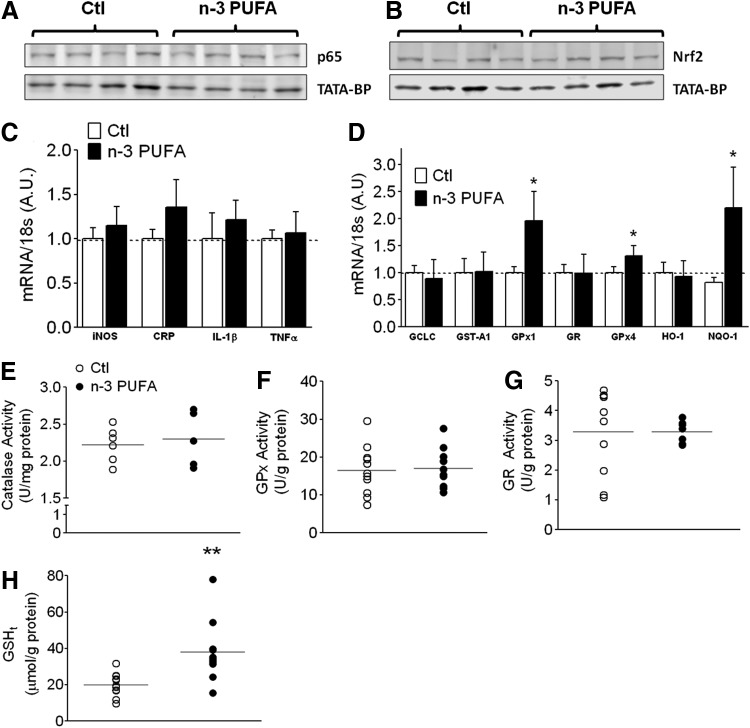
Numerous reports have documented an increase in antioxidant enzymes and a decrease in pro-inflammatory mediators with n-3 PUFA treatment (2, 7). Atrial tissue remodeling is known to be caused by inflammation and oxidative stress. Both of these stressors activate NFκB in the heart, and this has been demonstrated to lead to increased expression of pro-inflammatory cytokines, pro-fibrotic enzymes, and other pathways of cardiomyocyte remodeling (4). An analysis of nuclear extracts prepared from Ctl and n-3 PUFA-treated patients showed no significant differences in nuclear transactivation of pro-inflammatory transcription factor NFκB p65, or its downstream gene targets (Fig. 2A, C). Furthermore, no significant differences in nuclear transactivation of the anti-inflammatory/antioxidant NF E2-related factor 2 (Nrf2) were observed between n-3 PUFA-treated and Ctl groups (Fig. 2B), although the expression of glutathione peroxidase-1 (GPx1), GPx4, and NADPH-quinone oxido-reductase-1 (NQO-1) was significantly higher in the n-3 PUFA-treated atrial myocardium (Fig. 2D). No differences existed between groups in the enzyme activity of catalase, GPx, and glutathione reductase at the level of whole myocardial tissue (Fig. 2E–G). Interestingly, despite no difference in the expression of γ-glutamylcysteine ligase catalytic subunit, the rate-limiting enzyme in glutathione synthesis, total glutathione (GSHt) was ∼2.5-fold higher in the atrial myocardium of n-3 PUFA-treated patients (Fig. 2H).
FIG. 2.
Inflammation, redox-related gene expression, and antioxidant enzymes in atrial myocardium. Representative immunoblots of (A), NFκB-p65, and (B) Nrf2 in nuclear extracts prepared from 4 individual Ctl and n-3 PUFA-treated patients, with TATA-binding protein (TATA-BP) as loading control. Expression of genes known to be activated by NFκB (C), and those activated by Nrf2 (D) are shown for both treatment groups. Activity of antioxidant enzymes in atrial homogenate from both treatment groups is shown for (E), Catalase, (F), glutathione peroxidase (GPx), (G), glutathione reductase (GR), and (H), total GSH (GSHt). Gene expression data are reported as mean±SD. Raw data are shown for enzyme activity and GSHt in both treatment groups, along with the mean (faint horizontal line). N=10–12 for each group. *p<0.05 versus Ctl, **p<0.01 versus Ctl.
PPARγ activation, up-regulation of fatty acid metabolic genes, and enhanced mitochondrial fatty acid oxidation as potential mechanisms underlying effects of n-3 PUFAs in human atrial myocardium
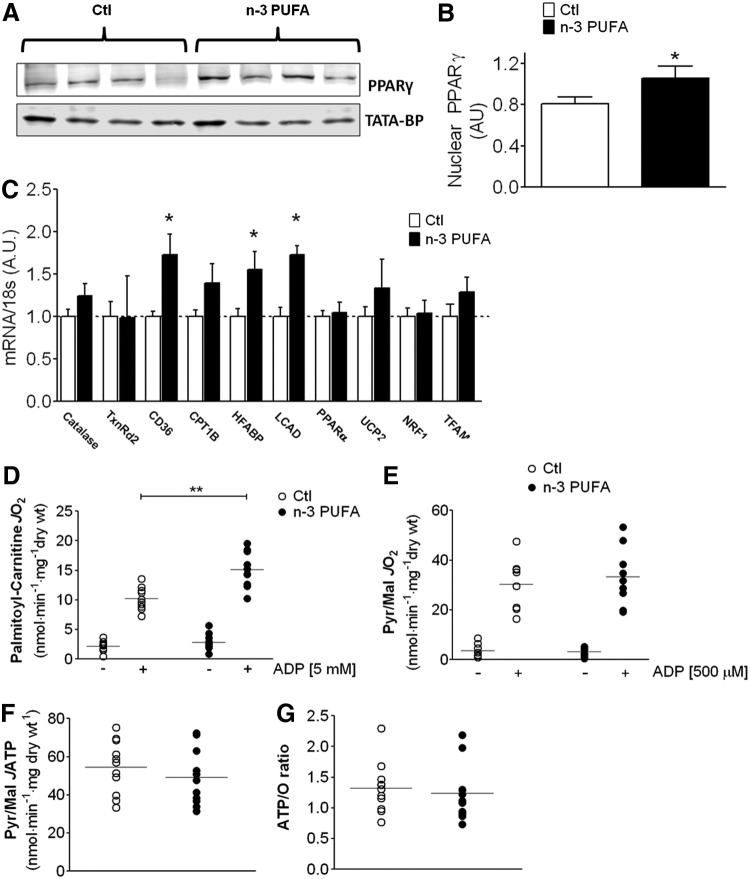
Several studies in experimental models have shown that n-3 PUFAs activate members of the peroxisome proliferator-activated receptor (PPAR) superfamily (2, 7). No differences in nuclear PPARα levels were observed in the atrial myocardium between n-3 PUFA-treated and Ctl patients in our study (not shown). Interestingly, n-3 PUFA treatment was associated with significantly greater nuclear transactivation of PPARγ (Fig. 3A, B). Since PPARγ regulates the expression of genes involved in fatty acid metabolism and mitochondrial biogenesis, we screened a number of these genes and observed greater expression of several genes involved in fatty acid transport and β-oxidation in n-3 PUFA-treated patients (Fig. 3C). To examine whether this increase in gene expression translated to differences in mitochondrial fatty acid metabolism, we measured the rate of O2 consumption (JO2) supported by palmitoyl-carnitine in permeabilized myofibers bundles (PmFBs) prepared from atrial myocardium in these patients. A markedly greater rate of maximal adenosine di-phosphate (ADP)-stimulated JO2 supported by palmitoyl-carnitine was observed in PmFBs from n-3 PUFA patients (Fig. 3D), and this was not due to differences in mitochondrial content as cytochrome oxidase IV (COX IV) and citrate synthase activity in atrial myocardium were similar between groups (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/ars). Moreover, maximal ADP-stimulated JO2 with pyruvate/malate was identical between groups (Fig. 3E). As an additional mitochondrial function parameter, we used a novel method to measure JO2 simultaneously with mitochondrial ATP release (JATP) in the atrial PmFBs. This method enabled us to determine ATP/O ratios in the mitochondria across a range of energetic demand conditions (i.e., range of ADP concentrations). No differences were observed in JATP with n-3 PUFA treatment at submaximal ADP-stimulated conditions (75 μM, not shown), or at maximal (500 μM) ADP-stimulated conditions (Fig. 3F). This translated into similar ATP/O ratios between groups (Fig. 3G), indicating that n-3 PUFA treatment was not associated with mitochondrial “uncoupling.”
FIG. 3.
PPARγ activation and mitochondrial function parameters in atrial myocardium. Shown in (A) are representative immunoblots of PPARγ in nuclear extracts prepared from 4 individual Ctl and n-3 PUFA-treated patients, with TATA-binding protein (TATA-BP) as nuclear protein loading control. In (B) is densitometry analysis of nuclear PPARγ in atrial tissue from both groups (N=10). Shown in (C) is expression of genes known to be activated by PPARγ. Rates of basal and maximal ADP-stimulated (5 mM) mitochondrial O2 consumption supported by (D) palmitoyl-carnitine in both treatment groups. Data shown in (E) are rates of mitochondrial O2 consumption supported by pyruvate+malate (PM) in the absence and presence of ADP (500 μM). In (F) are rates of mitochondrial ATP release with PM+500 μM ADP, and in (G) are ATP/O ratio in the presence of PM+500 μM ADP. Gene expression data are reported as mean±SD. Raw data are shown for mitochondrial experiments in both treatment groups, along with the mean (faint horizontal line). N=10–12 for each group, for all experiments. *p<0.05 versus Ctl, **p<0.01 versus Ctl.
Does n-3 PUFA therapy enhance mitochondrial-specific antioxidant capacity and reduce carbonyl stress as an additional cardioprotective mechanism?
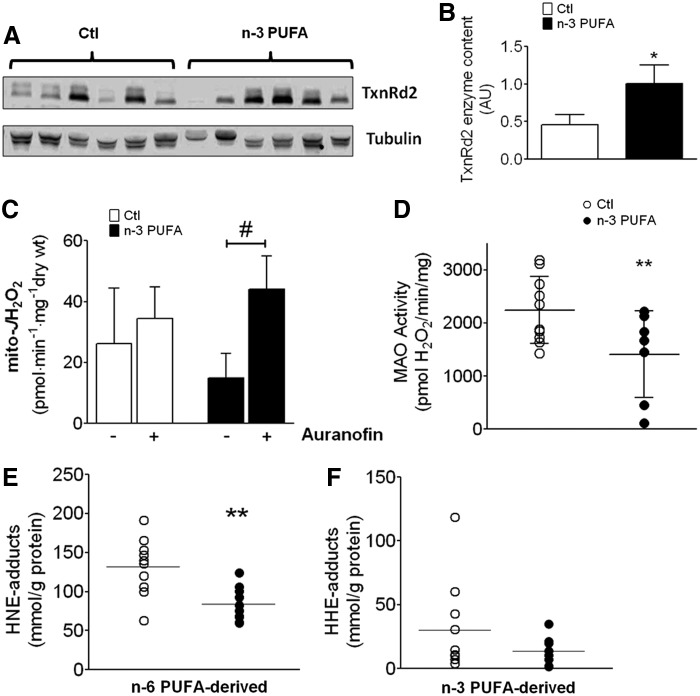
We recently reported that an n-6 PUFA-enriched high-fat diet causes up-regulation of expression and activity of mitochondrial antioxidant enzyme thioredoxin reductase-2 (TxnRd2), along with increased influence of this enzyme in maintaining mitochondrial redox state (3). Although mRNA levels of TxnRd2 were similar between groups (Fig. 3C), immunoblot analysis revealed that n-3 PUFA-treated patients exhibited substantially greater TxnRd2 enzyme content in their myocardial tissue compared with Ctl patients (Fig. 4A, B). On examining the functional consequences of this increased TxnRd2 content, we observed that mitochondrial H2O2 emission rate (mito-JH2O2) in atrial PmFBs from n-3 PUFA-treated, but not Ctl patients, was significantly increased on exposure to auranofin, a TxnRd2 inhibitor (Fig. 4C). The mitochondrial outer membrane-bound enzyme monoamine oxidase, a substantial generator of ROS, was also determined to have significantly lower activity in atrial tissue from n-3 PUFA-treated patients (Fig. 4D).
FIG. 4.
TxnRd2, mitochondrial ROS generation, and PUFA-derived carbonyl stress in atrial myocardium. Shown in (A) are representative immunoblots of TxnRd2 in atrial tissue homogenate from 6 individual Ctl and n-3 PUFA-treated patients, with Tubulin as the loading control. In (B) is densitometry analysis of TxnRd2 in whole atrial tissue from both groups (N=8–10). In (C) are rates of mitochondrial ROS generation (JH2O2) coming from the ETS in permeabilized myofibers supported by PM+100 μM ADP, in the absence and presence of TxnRd2 inhibitor auranofin. In (D) is the rate of MAO activity (with tyramine-supported H2O2 production as index) in atrial tissue homogenate from both treatment groups. Protein carbonyl adducts in atrial tissue formed by (E), n-6 PUFA-derived aldehyde 4-hydroxynonenal (HNE), and (F), n-3 PUFA-derived aldehyde 4-hydroxyhexenal (HHE), are shown for both treatment groups. Raw data are shown for enzyme activity and PUFA-derived carbonyl adducts in both treatment groups, along with the mean (faint horizontal line). N=8–12 for each group, for all experiments. *p<0.05 versus Ctl, **p<0.01 versus Ctl, #p<0.05 versus. no Auranofin.
To determine whether the greater antioxidant capacity observed in the atrial myocardium of n-3 PUFA-treated patients corresponded to decreased protein carbonyl stress, we examined the levels of protein modification from n-6- and n-3 PUFA-derived aldehydes 4-hydroxynonenal (HNE) and 4-hydroxyhexenal (HHE), respectively. We observed a substantially lower concentration of HNE-modified protein adducts in atrial tissue from n-3 PUFA-treated patients (Fig. 4E), although HHE-modified adducts were not significantly different (Fig. 4F).
Based on these novel findings, we hypothesize that PPARγ activation and up-regulation of fatty acid metabolic gene expression, mitochondrial fatty acid oxidation, and antioxidant capacity represent potential mechanisms by which n-3 PUFA therapy confers beneficial effects in the human atrial myocardium.
Limitations and strengths of the study
This study is strengthened by its prospective design, systematic data collection and comprehensive analysis of signaling pathways involved in redox balance and mitochondrial function in human heart tissue. Some limitations to this study exist. First, the patient cohort was too small to derive any significant findings with regard to postoperative clinical outcomes. However, since the objective of this study was to examine whether n-3 PUFA therapy for 2–3 weeks was sufficient to cause changes in gene expression and mitochondrial function in the human atrial myocardium, we believe the sample size used here was sufficient. Nevertheless, we cannot rule out the existence of residual confounders due to the small sample size and mixed etiology of cardiovascular disease in our patient cohort. Since this was an open-label trial with blinded outcome assessment, potential bias could have been introduced if patients receiving fish oil altered other behaviors (e.g., diet, exercise). However, it is not likely that such potential bias would explain the cellular and molecular effects observed in their atrial tissue. Next, the control group used was standard of care, rather than control. The reason for this is that the only placebo made available to us by the sponsor was pure corn oil capsules (concentrated n-6 PUFAs), and based on our work in experimental models (1, 3), we felt that pure corn oil was not a true “placebo” but rather a treatment group. These findings, and others, informed our rationale for using standard of care as the control group. Saturating concentrations of substrate (e.g., palmitoyl-carnitine, pyruvate, NADPH, etc.) were used to measure mitochondrial function, ROS generation, and antioxidant enzyme activities in our assays. This rarely exists in vivo. We believe this limitation is partially off-set by the fact that mitochondrial function was assessed using physiological concentrations of ADP in order to mimic in vivo oxidative phosphorylation conditions. Furthermore, we used a novel method to measure ATP generation and O2 consumption simultaneously in these tissue specimens, which is an additional strength of this study. Finally, since blood samples were only obtained before the intervention and at the termination of the study along with atrial tissue, it was not possible to determine the temporality of adaptations that occurred while n-3 PUFA therapy was ongoing.
Conclusions and Remarks on Future Directions
This is the first study that reports the impact of fish oil n-3 PUFA therapy on metabolic gene regulation, mitochondrial function, and inflammation/oxidative stress parameters in human heart. These findings are important because they (i) establish that a dose of 3.4 g of EPA & DHA/day over a time frame of 2–3 weeks is associated with changes in these parameters in the human atrial myocardium; (ii) implicate PPARγ activation and augmented mitochondrial fatty acid oxidation as possible therapeutic mechanisms of n-3 PUFAs in the human myocardium; and (iii) suggest that n-3 PUFAs exert beneficial effects in the myocardium by enhancing antioxidant capacity. More investigation is needed to delineate the extent to which PPARγ activation mediates the myocardial benefit afforded by n-3 PUFA therapy, much of which can only come from work in experimental systems. Furthermore, the potential role of oxidized n-3 PUFAs and their electrophilic derivatives (e.g., peroxides, α,β-unsaturated aldehydes) in mediating the beneficial and, paradoxically, toxic effects of n-3 PUFAs is an area most worthy of investigation.
Notes
Patient enrollment, inclusion/exclusion criteria, and study design
The overall study plan regarding patient screening and recruitment, along with experimental work flow, is outlined in Figure 1. All aspects of this study were reviewed and approved by the Institutional Review Board of the Brody School of Medicine at East Carolina University. This is a randomized, open-label single-blinded trial registered at www.clinicaltrials.gov under identification number NCT01046604. Adult patients ≤75 years of age scheduled for primary, non-emergent cardiac surgery at East Carolina Heart Institute-Vidant Medical Center (Greenville, NC) were screened for this study. Those with severe hepatic, renal, or pulmonary disease, severely enlarged atria (>4.0 cm diameter), history of arrhythmia, use of anti-arrhythmic drugs other than β-blockers, use of fish oil supplements, and left ventricular ejection fraction <30% were excluded. Patients meeting the inclusion/exclusion criteria were then randomly allocated to Control (Ctl) arm receiving no treatment at all (standard-of-care), or were instructed to take 3.4 g/day of n-3 PUFA ethyl esters (Lovaza, GlaxoSmithKline), equaling 1.86 g EPA/1.5 g DHA per day, beginning at the time of enrollment and ending the night before surgery. This formulation has been successfully used in the clinic and commonly prescribed to mitigate hypertriglyceridemia. The duration of preoperative n-3 PUFA treatment ranged between 2 and 3 weeks for this study, with a median duration of 17.5 days. Blood samples were taken at the time of enrollment and again at the time of surgery, at which time a sample of the right atrial appendage was also taken.
For a detailed description of all experimental procedures and statistical analysis performed, please see Supplementary Data.
Supplementary Material
Abbreviations Used
- AA
arachidonic acid
- ACEI
angiotensin-converting enzyme inhibitor
- ADP
adenosine di-phosphate
- AF
atrial fibrillation
- ARB
angiotensin receptor blocker
- ATP
adenosine tri-phosphate
- BMI
body mass index
- COX IV
cytochrome oxidase IV
- CPB
cardiopulmonary bypass
- CPT1B
carnitine palmitoyl transferase-1B
- CRP
C-reactive protein
- DHA
doxosahexaenoic acid
- EPA
eicosapentaenoic acid
- GCLC
γ-Glutamylcysteine ligase catalytic subunit
- GPx
glutathione peroxidase
- GPx1
glutathione peroxidase 1
- GPx4
glutathione peroxidase 4
- GR
glutathione reductase
- GSHt
total glutathione
- GST-A1
glutathione -s-transferase A-1
- HDL
high-density lipoprotein
- HFABP
human fatty acid binding protein
- HHE
4-hydroxyhexenal
- HNE
4-hydroxynonenal
- HO-1
heme oxygenase-1
- IL1β
interleukin-1β
- iNOS
inducible nitric oxide synthase
- LA
linoleic acid
- LCAD
very long-chain acyl CoA dehydrogenase
- LDL
low-density lipoprotein
- LOS
length of stay
- LVEF
left ventricular ejection fraction
- MAO
monoamine oxidase
- NQO1
NAD(P)H:quinone oxidoreductase 1
- NRF1
nuclear respiratory factor 1
- Nrf2
NF E2-related factor 2
- PmFB
permeabilized myofiber bundles
- POAF
postoperative atrial fibrillation
- PPAR
peroxisome proliferator-activated receptor
- PUFAs
polyunsaturated fatty acids
- RAA
right atrial appendage
- RCTs
randomized controlled trials
- ROS
reactive oxygen species
- TFAM
mitochondrial transcription factor A
- TNFα
tumor necrosis factor α
- TxnRd2
thioredoxin reductase-2
- UCP2
uncoupling protein 2
Acknowledgments
The authors would like to thank all research nurses and clinical staff at ECHI for their assistance with informed consent and study coordination. This research was supported by grant R21HL098780 (E.J.A, A.P.K) from National Institutes of Health, and from an investigator-initiated grant to E.J.A. and S.R.S from GlaxoSmithKline. GlaxoSmithKline supplied the test capsules of Lovaza for the study.
Author Disclosure Statement
The authors have no conflicts of interest to disclose. GlaxoSmithKline had no role in the study design, collection, or analysis of the data, interpretation of the findings, or preparation of this article.
References
- 1.Anderson EJ, Thayne K, Harris M, Carraway K, and Shaikh SR. Aldehyde stress and up-regulation of Nrf2-mediated antioxidant systems accompany functional adaptations in cardiac mitochondria from mice fed n-3 polyunsaturated fatty acids. Biochem J 441: 359–366, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Caterina R. n-3 fatty acids in cardiovascular disease. N Engl J Med 364: 2439–2450, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Fisher-Wellman KH, Mattox TA, Thayne K, Katunga LA, La Favor JD, Neufer PD, Hickner RC, Wingard CJ, and Anderson EJ. Novel role for thioredoxin reductase-2 in mitochondrial redox adaptations to obesogenic diet and exercise in heart and skeletal muscle. J Physiol 591: 3471–3486, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao G. and Dudley SC., Jr Redox regulation, NF-kappaB, and atrial fibrillation. Antioxid Redox Signal 11: 2265–2277, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Metcalf RG, James MJ, Gibson RA, Edwards JR, Stubberfield J, Stuklis R, Roberts-Thomson K, Young GD, and Cleland LG. Effects of fish-oil supplementation on myocardial fatty acids in humans. Am J Clin Nutr 85: 1222–1228, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Mozaffarian D, Marchioli R, Macchia A, Silletta MG, Ferrazzi P, Gardner TJ, Latini R, Libby P, Lombardi F, O'Gara PT, Page RL, Tavazzi L, and Tognoni G. Fish oil and postoperative atrial fibrillation: the Omega-3 Fatty Acids for Prevention of Post-operative Atrial Fibrillation (OPERA) randomized trial. JAMA 308: 2001–2011, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mozaffarian D. and Wu JH. Omega-3 Fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol 58: 2047–2067, 2011 [DOI] [PubMed] [Google Scholar]
- 8.Sandesara CM, Chung MK, Van Wagoner DR, Barringer TA, Allen K, Ismail HM, Zimmerman B, and Olshansky B. A randomized, placebo-controlled trial of omega-3 fatty acids for inhibition of supraventricular arrhythmias after cardiac surgery: the FISH trial. J Am Heart Assoc 1: e000547, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saravanan P, Bridgewater B, West AL, O'Neill SC, Calder PC, and Davidson NC. Omega-3 fatty acid supplementation does not reduce risk of atrial fibrillation after coronary artery bypass surgery: a randomized, double-blind, placebo-controlled clinical trial. Circ Arrhythm Electrophysiol 3: 46–53, 2010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.