Abstract
The reaction of mercaptoacetic acid esters with pentachloro-2-nitro-1,3-butadiene (1) provides an appropriate precursor for the synthesis of special thiazolidin-4-ones. Applying different anilines as the second constituent for the requisite cyclization step, a series of (Z)-2-allylidenethiazolidin-4-ones was obtained in yields up to 81%. Some subsequent reactions have been examined too, such as the formation of perfunctionalized 1H-pyrazoles upon treatment with hydrazine. Thiazolidinones are as well known for their physiological activities as for their application in optoelectronics.
Keywords: atropisomers, cyclization, 2-nitroperchlorobutadiene, 1H-pyrazoles, thiazolidin-4-ones
Introduction
Preliminary studies in the field of polyhalogenated nitrobutadienes have already shown the enormous potential of pentachloro-2-nitro-1,3-butadiene (1) as a precursor for the “click synthesis” of highly functionalized (hetero)cyclic as well as acyclic compounds [1–15]. The corresponding syntheses that we have developed up to now [2–13], always start with the attack of an appropriate nucleophile at the activated terminal carbon atom of the nitrodichlorovinyl group within 1 to undergo a vinylic substitution. Thus, in case of, e.g., sulfur nucleophiles, the corresponding thioperchlorobutadiene derivatives are easily accessible [2,5,16–17]. In this paper, we describe the formation of uniquely substituted thiazolidin-4-ones, a class of compounds which has proven to exhibit distinctive bioactivity, e.g., antifungal, antibacterial, antitubercular, and anticonvulsant properties [18–28].
Additionally, more recent studies often focus on their anticancer [29–31] and anti-HIV potential [30,32]. Several 4-thiazolidinone derivatives such as ralitoline (anticonvulsant), etozoline (antihypertensive), pioglitazone (hypoglycemic) and thiazolidomycin (activity against streptomyces species) are already in the market [33]. Beyond that, among industrial applications thiazolidinones serve as electron donors, e.g., as ligands in coordination compounds or as vulcanizing agents [31]. Furthermore, it is well known that these heterocycles play a major role in corresponding organic electronics, e.g., they lead to high quantum efficiency as donor compound in solar cells [34]. Very recently, thiazolidinones became a target in the field of photonics and optoelectronics, potentially applicable for reversible optical data storage, photo switching of optical elements, photochromic polymers, and similar applications [35].
The background of thiazolidinone chemistry including classical synthetic strategies has been reviewed comprehensively [18–28,36–39]. Even though among possible synthetic pathways the use of mercaptoacetic acid and its derivatives is well-established [40–44], our approach, starting from a mercaptoacetic acid derivative of perchloro-2-nitro-1,3-butadiene and an aniline, is unprecedented up to now. In this context, it is very interesting that the reaction of a 2-nitro-1-thioperchlorobuta-1,3-diene with N-nucleophiles usually yields a 1-amino-2-nitro-1-thioperchlorobuta-1,3-diene [16,45]. However, our novel ring closing reaction incorporates an arylamine as source for the nitrogen of the thiazolidin-4-one heterocycle.
Results and Discussion
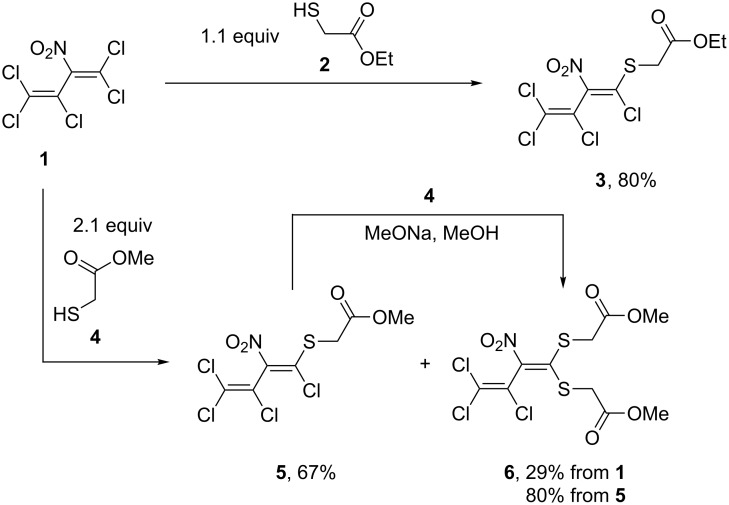
The reaction of pentachloro-2-nitro-1,3-butadiene (1) with 1.1 equivalents of ethyl 2-mercaptoacetate (2) at room temperature without a solvent for 10 days furnished ethyl 2-((1,3,4,4-tetrachloro-2-nitrobuta-1,3-dien-1-yl)thio)acetate (3) as a single isomer in 80% yield (for further details see Supporting Information File 1). In contrast, the conversion of 1 (neat) with 2.1 equivalents of methyl 2-mercaptoacetate (4) results in a mixture of methyl 2-((1,3,4,4-tetrachloro-2-nitrobuta-1,3-dien-1-yl)thio)acetate (5) and 1,1-bis(methoxycarbonylmethylthio)-2-nitro-3,4,4-trichlorobuta-1,3-diene (6) after 10 days at room temperature with 67% and 29% yields, respectively. These nitrodienes 5 and 6 were separated by flash column chromatography and then characterized. Interestingly, the dithio compound 6 was also accessible by treatment of the aforementioned monothio derivative 5 with one equivalent of mercaptoacetate 4. Following this pathway, the disubstituted diene 6 was obtained in 80% yield (Scheme 1).
Scheme 1.
SNVin reactions of pentachloro-2-nitro-1,3-butadiene (1).
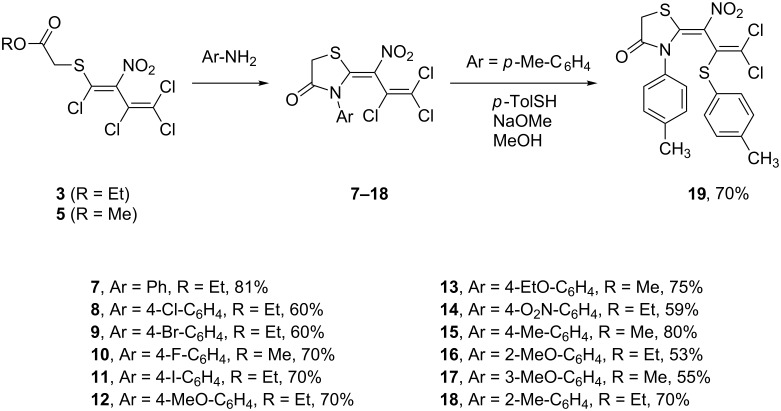
The subsequent reaction of the mercaptoacetates 3 and 5 with 2.1 equivalents of various arylamines is the key step of our thiazolidin-4-one synthesis, that led to the corresponding (Z)-3-aryl-2-(2,3,3-trichloro-1-nitroallylidene)thiazolidin-4-ones 7–18 with up to 81% yield. As an example for the sequential reactivity of the persubstituted allylidene side chain, the thiazolidin-4-one 15 was reacted with p-tolylthiolate. Thereby, the C(2)–Cl position of the allylidene group was selectively substituted by the reactive thiolate nucleophile, again in a SNVin-type process, to give (Z)-3-(4-tolyl)-2-(3,3-dichloro-1-nitro-2-[4-tolylthio]allylidene)thiazolidin-4-one (19) in 70% yield (Scheme 2).
Scheme 2.
Formation of thiazolidin-4-ones 7–19.
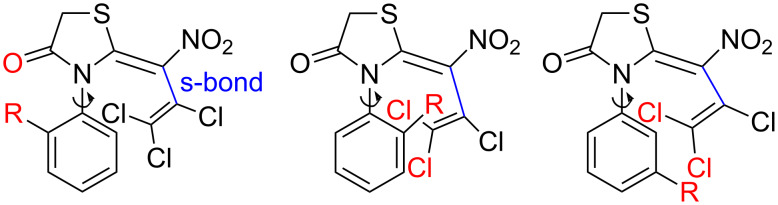
According to proton NMR, the ring-closing step with the parent aniline or with one of its para-substituted derivatives in each case led to a single N-arylthiazolidin-4-one isomer 7–15. In the case of the ortho- or meta-substituted anilines 16–18 two atropisomers were generated. This phenomenon is due to a less hindered rotation of the C–N bond of the former anilines (Figure 1).
Figure 1.
Hindered rotation in the case of ortho- or meta-substituted aniline precursors.
The steric hindrance is additionally forcing by the s-cis conformation of the nitrobutadiene moiety (cf. Figure 2). A DFT calculation of the energy barrier for rotation of the aromatic substituent resulted in extraordinary high values of 169 and 191 kJ/mol for the ortho-methyl compound 18 and the larger, but less rigid ortho-methoxy derivative 16, respectively. As expected, the meta-anisyl derivative 17 showed a lower barrier of 73 kJ/mol (see Supporting Information File 1). For comparison, the well-known atropisomer 1,1’-binaphthalene-2,2’-diol (BINOL) shows a rotational barrier of 158 kJ/mol with calculated data in good accordance with the experimental value [46].
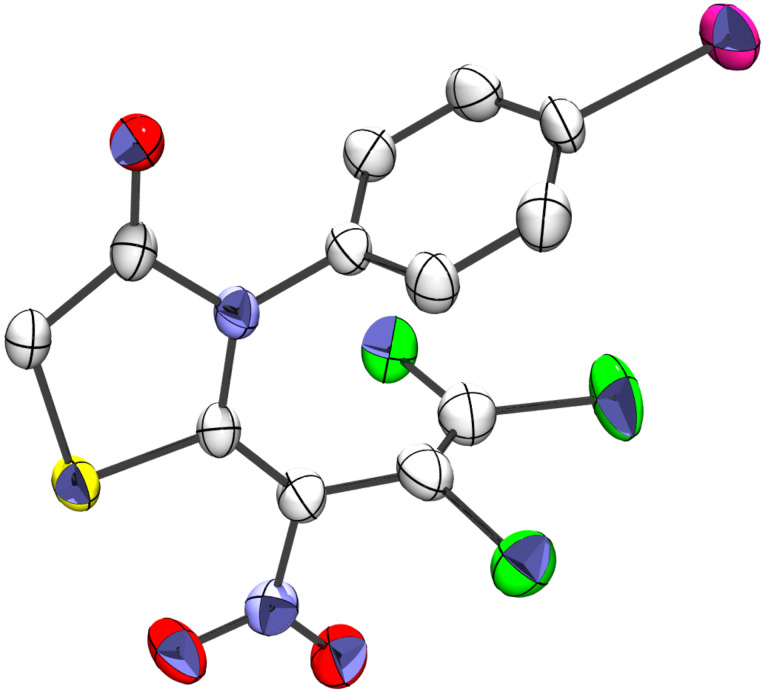
Figure 2.
X-ray analysis of thiazolidin-4-one 11.
In addition to substantial NMR, IR, and mass spectral characterization of the thiazolidinones 7–18, as an example the 4-iodophenyl substituted thiazolidinone 11 was subjected to X-ray analysis (see Supporting Information File 1). This confirmed the Z-configuration of the inner double bond which bears the sulfur atom of the hetero ring as well as the nitro group. The structural plot also gives an idea of the abovementioned bulkiness, even in the case of the depicted para-substitution (Figure 2).
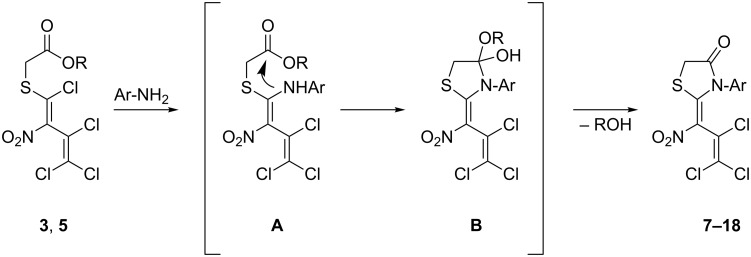
The formation of the thiazolidin-4-ones 7–18 is assumed to consist of three individual steps. In a SNVin type reaction with the aniline derivative intermediate A (Scheme 3) is formed. Subsequently, the thus introduced amino group attacks the carbonyl carbon of the mercaptoacetate moiety. This ring closure affords the temporary imide hemiacetal B which is then stabilized upon elimination of the corresponding alcohol to give the desired thiazolidin-4-ones 7–18 (Scheme 3).
Scheme 3.
Assumed mechanism for the formation of thiazolidin-4-ones 7–18.
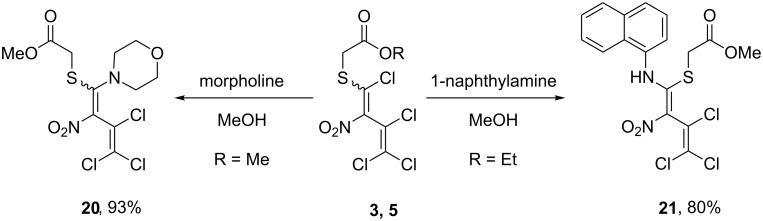
Furthermore, in addition to the anilines described above (Scheme 2), we applied 1-naphthylamine as a bulkier aromatic representative and morpholine as a secondary, more basic aliphatic amine. Conversion of 3 and 5 with morpholine and 1-naphthylamine afforded the open chain products 20 and 21 in 93% and 80% yield, respectively (Scheme 4), without a subsequent cyclization under one-pot conditions. Unexpectedly, even under forcing conditions cyclization of isolated 21 did not take place. Compound 21 was exclusively obtained as E-isomer. This was indicated by a proton NMR signal shifted downfield to about 12 ppm due to a strong hydrogen bond between the amino hydrogen and the nitro group. In contrast, lacking the opportunity to build such a hydrogen bridge, the regiochemistry of compound 20 (also formed as one single isomer) remained unclear.
Scheme 4.
Substitution reactions of the precursors 3 and 5 with additional amines.
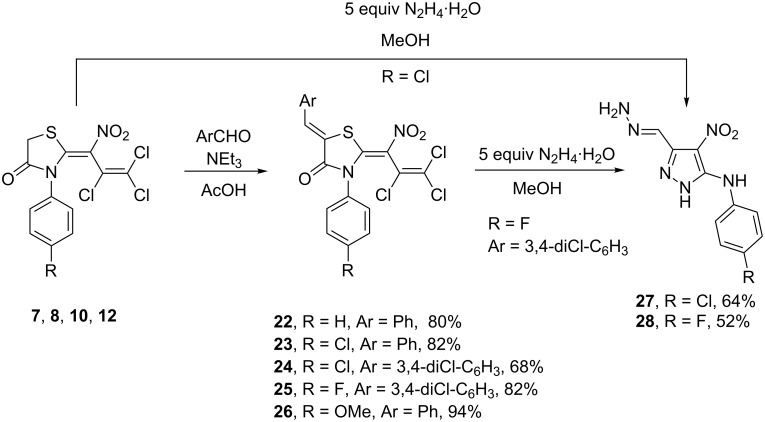
With the thiazolidinones in hand, in the case of 7, 8, 10, and 12 we carried out a Knoevenagel-type condensation with benzaldehyde and 3,4-dichlorobenzaldehyde in acetic acid at 118 °C in the presence of triethylamine. Thus, the 5-arylmethylidenethiazolidin-4-ones 22–26 were obtained in 68–94% yield. The presence of only one signal for the benzylidene proton at 7.83–7.98 ppm in the 1H NMR spectra of 22–26 suggested the formation of a single isomer, which was assigned to the Z-configuration according to the literature for similar compounds [47–48]. Interestingly, close analogues of these structures, i.e., the 5-arylmethylidene rhodanines, possess photosynthesis-inhibiting and antialgal properties [49], show anticancer activity [50–51], and are inhibitors of bacterial enzyme synthetase MurD with E. coli [52].
Additional treatment of thiazolidin-4-one 8 with a fivefold excess of hydrazine hydrate resulted in the formation of a 1H-pyrazole: A total of two hydrazine molecules were incorporated into the final product, whereas two additional equivalents were consumed capturing hydrochloric acid. In this way, the 1H-pyrazole 27 with an exceptional substitution pattern was obtained in 64% yield. The same reaction was applied to 5-arylmethylidene-thiazolidin-4-one 25 which furnished the corresponding 1H-pyrazole 28 in 52% yield (Scheme 5).
Scheme 5.
Synthesis of 5-arylmethylidenethiazolidin-4-ones 22–26 and 1H-pyrazoles 27, 28.
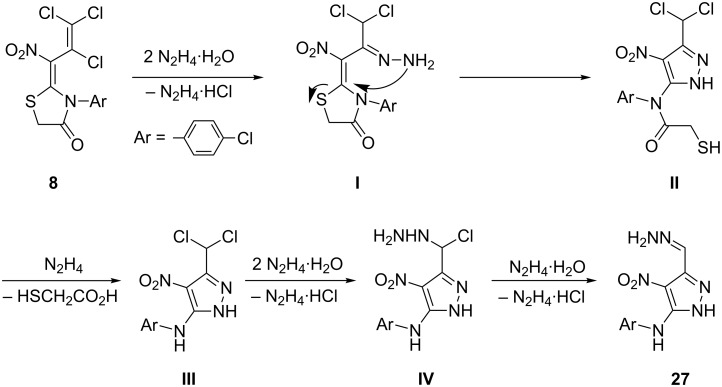
A plausible multistep mechanism of this conversion is shown in Scheme 6: Initially, a first molecule of the strong nucleophile hydrazine is assumed to substitute the single C–Cl group within the trichlorovinyl subunit of 8 to give the intermediate I (SNVin reaction). Thereby, a second equivalent of hydrazine captures hydrochloric acid. The amino group of the hydrazone then attacks the electrophilic C-2 position of the thiazolidinone. This way a threefold substituted 1H-pyrazole is formed (intermediate II) under ring opening of the thiazolidinone. In the course of the reaction, probably at this stage, the N-aryl amide is hydrazinolysed to give the intermediate III, which is converted into the monohydrazino compound IV upon consumption of two further equivalents of hydrazine. Finally, 27 is obtained upon elimination of hydrochloric acid, caught by a fifth equivalent of hydrazine (Scheme 6). An analogous mechanism is also plausible for the formation of pyrazole 28 from thiazolidinone 25. However, instead of the elimination of 2-mercaptoacetic acid from intermediate II, in the latter case 3-(3,4-dichlorophenyl)-2-mercaptoacrylic acid is released.
Scheme 6.
Assumed mechanism for the formation of 1H-pyrazole 27.
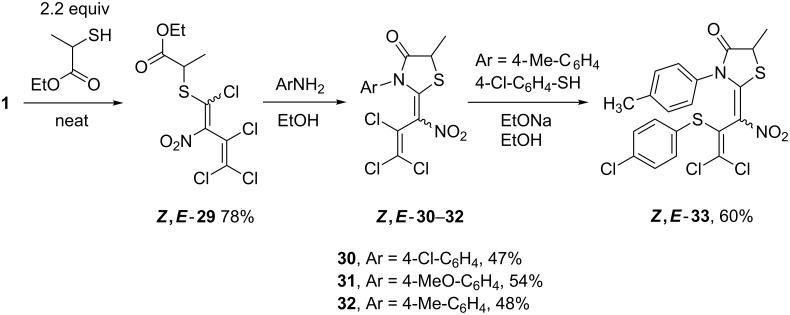
Moreover, nitrodiene 1 was subjected to a vinylic substitution with ethyl 2-mercaptopropanoate to give ethyl 2-[(1,3,4,4-tetrachloro-2-nitrobuta-1,3-dien-1-yl)thio]propanoate (29) as an inseparable mixture of Z- and E-isomers in a 4:1 ratio with 78% total yield. Treatment of dienes (Z,E)-29 with different anilines in ethanol at 0 °C to rt furnished (Z,E)-3-aryl-5-methylthiazolidinones 30–32. As an example, thiazolidinone 32 was reacted with 4-chlorothiophenol in the presence of sodium ethoxide to give 4-chlorophenylthio compound 33 (60%), again as a mixture of Z- and E-isomers, but this time in a 2:1 ratio (Scheme 7).
Scheme 7.
Formation of ethyl propanoate 29 and subsequent reactions.
Conclusion
A two-step synthesis of 2-allylidene-N-arylthiazolidinones 7–18 has been developed, starting from our building block 2-nitroperchlorobutadiene (1), 2-mercaptoacetates 2 and 4, and anilines. Inclusion of o- or m-substituted N-aryl groups leads to the formation of stable thiazolidinone atropisomers, due to hindered rotation between the trichlorovinyl and the arylamino groups. X-ray analysis proved the Z-configuration of the nitrovinylidene group of 11. Subsequent reactions of the thiazolidinones, such as the SNVin thiolation in 2-position of the allylidene backbone, Knoevenagel condensation of the heterocyclic rings with aromatic aldehydes, and an unusual formation of persubstituted pyrazoles demonstrate the chemical versatility of these heterocyclic systems. The newly formed thiazolidinones deserve additional synthetic interest as starting materials, also due to their trichlorovinyl group. Tests on their physiological activities and potential applications as optoelectronic materials are on the way.
Supporting Information
Experimental part.
Acknowledgments
We thank Dr. G. Dräger (Leibniz University, Hannover, Germany) for extensive HRMS–ESI measurements. M. H. H. Drafz is greatfully acknowledged for DFT calculations.
References
- 1.Kaberdin R V, Potkin V I, Zapol’skii V A. Russ Chem Rev. 1997;66:827–842. doi: 10.1070/RC1997v066n10ABEH000310. [DOI] [Google Scholar]
- 2.Zapol’skii V A, Namyslo J C, Adam A E, Kaufmann D E. Heterocycles. 2004;63:1281–1298. doi: 10.3987/COM-04-10020. [DOI] [Google Scholar]
- 3.Zapol’skii V A, Nutz E, Namyslo J C, Adam A E, Kaufmann D E. Synthesis. 2006:2927–2933. doi: 10.1055/s-2006-950187. [DOI] [Google Scholar]
- 4.Zapol’skii V A, Namyslo J C, Blaschkowski B, Kaufmann D E. Synlett. 2006:3464–3468. doi: 10.1055/s-2006-956490. [DOI] [Google Scholar]
- 5.Zapol’skii V A, Namyslo J C, Gjikaj M, Kaufmann D E. ARKIVOC. 2007;(i):76–93. [Google Scholar]
- 6.Zapol’skii V A, Namyslo J C, Gjikaj M, Kaufmann D E. Synlett. 2007:1507–1512. doi: 10.1055/s-2007-982554. [DOI] [Google Scholar]
- 7.Zapol’skii V A, Namyslo J C, Altug C, Gjikaj M, Kaufmann D E. Synthesis. 2008:304–310. doi: 10.1055/s-2007-990948. [DOI] [Google Scholar]
- 8.Nutz E, Zapol’skii V A, Kaufmann D E. Synthesis. 2009:2719–2724. doi: 10.1055/s-0029-1216906. [DOI] [Google Scholar]
- 9.Zapol’skii V A, Fischer R, Namyslo J C, Kaufmann D E. Bioorg Med Chem. 2009;17:4206–4215. doi: 10.1016/j.bmc.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Zapol’skii V A, Namyslo J C, Gjikaj M, Kaufmann D E. Z Naturforsch, B. 2010;65b:843–860. [Google Scholar]
- 11.Zapol’skii V A, Namyslo J C, de Meijere A, Kaufmann D E. Beilstein J Org Chem. 2012;8:621–628. doi: 10.3762/bjoc.8.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vogt E-J, Zapol’skii V A, Nutz E, Kaufmann D E. Z Naturforsch, B. 2012;67:285–294. [Google Scholar]
- 13.Zapol’skii V A, Vogt E-J, Gjikaj M, Kaufmann D E. Heterocycles. 2012;86:1431–1447. doi: 10.3987/COM-12-S(N)108. [DOI] [Google Scholar]
- 14.Ibis C. Bull Soc Chim Belg. 1996;105:317–320. [Google Scholar]
- 15.Ibis C, Sayil M C, Deniz N G. Acta Crystallogr, Sect E: Struct Rep Online. 2006;62:o800–o801. doi: 10.1107/S1600536806001838. [DOI] [Google Scholar]
- 16.Ol'dekop Y A, Kaberdin R V, Potkin V I. J Org Chem USSR. 1980;16:469–472. [Google Scholar]
- 17.Ibiş C, Kirbaşlar F G, Aydinli G. Phosphorus, Sulfur Silicon Relat Elem. 2005;180:365–374. doi: 10.1080/104265090508433. [DOI] [Google Scholar]
- 18.Brown F C. Chem Rev. 1961;61:463–521. doi: 10.1021/cr60213a002. [DOI] [Google Scholar]
- 19.Singh S P, Parmar S S, Raman K, Stenberg V I. Chem Rev. 1981;81:175–203. doi: 10.1021/cr00042a003. [DOI] [Google Scholar]
- 20.Chandrasekhar B. J Sulfur Chem. 2012;33:439–503. doi: 10.1080/17415993.2012.693490. [DOI] [Google Scholar]
- 21.Tian Y, Zhan P, Rai D, Zhang J, De Clercq J, Liu X. Curr Med Chem. 2012;19:2026–2037. doi: 10.2174/092986712800167383. [DOI] [PubMed] [Google Scholar]
- 22.Jain A K, Vaidya A, Ravichandran V, Kashaw S K, Agrawal R K. Bioorg Med Chem. 2012;20:3378–3395. doi: 10.1016/j.bmc.2012.03.069. [DOI] [PubMed] [Google Scholar]
- 23.Verma A, Saraf S K. Eur J Med Chem. 2008;43:897–905. doi: 10.1016/j.ejmech.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Q, Zhou H, Zhai S, Yan B. Curr Pharm Des. 2010;16:1826–1842. doi: 10.2174/138161210791208983. [DOI] [PubMed] [Google Scholar]
- 25.Mehta D P, Sengar N P S, Pathak A K. Orient J Chem. 2008;24:441–454. [Google Scholar]
- 26.Edwards P J. Drug Discovery Today. 2008;13:1107–1108. doi: 10.1016/j.drudis.2008.10.004. [DOI] [Google Scholar]
- 27.Cunico W, Gomes C R B, Vellasco W T., Jr Mini-Rev Org Chem. 2008;5:336–344. doi: 10.2174/157019308786242232. [DOI] [Google Scholar]
- 28.Newkome G R, Nayak A. Adv Heterocycl Chem. 1980;25:83–112. doi: 10.1016/S0065-2725(08)60690-X. [DOI] [Google Scholar]
- 29.Kawakami M, Koya K, Ukai T, Tatsuta N, Ikegawa A, Ogawa K, Shishido T, Chen L B. J Med Chem. 1998;41:130–142. doi: 10.1021/jm970590k. [DOI] [PubMed] [Google Scholar]
- 30.Rida S M, Ashour F A, El-Hawash S A M, ElSemary M M, Badr M H, Shalaby M A. Eur J Med Chem. 2005;40:949–959. doi: 10.1016/j.ejmech.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 31.Hamama W S, Ismail M A, Shaaban S, Zoorob H H. J Heterocycl Chem. 2008;45:939–956. doi: 10.1002/jhet.5570450401. [DOI] [Google Scholar]
- 32.Graciet J C, Faury P, Camplo M, Charvet A S, Mourier N, Trabaud C, Niddam V, Simon V, Kraus J L. Nucleosides Nucleotides. 1995;14:1379–1392. doi: 10.1080/15257779508010698. [DOI] [Google Scholar]
- 33.Tripathi A C, Gupta S J, Fatima G N, Sonar P K, Verma A, Saraf S K. Eur J Med Chem. 2014;72:52–77. doi: 10.1016/j.ejmech.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi K, Nakatani S, Matsuda T, Nanbu H, Komura T, Murata K. Chem Lett. 1994;23:2001–2004. [Google Scholar]
- 35.Smokal V, Derkowska B, Czaplicki R, Krupka O, Kolendo A, Sahraoui B. Opt Mater. 2009;31:554–557. doi: 10.1016/j.optmat.2007.10.019. [DOI] [Google Scholar]
- 36.Singh V P, Upadhyay G S, Singh H. Asian J Chem Rev. 1992;3:12–21. [Google Scholar]
- 37.Abdel-Rahman R M. Boll Chim Farm. 2001;140:401–410. [PubMed] [Google Scholar]
- 38.Lesyk R B, Zimenkovsky B S. Curr Org Chem. 2004;8:1547–1577. doi: 10.2174/1385272043369773. [DOI] [Google Scholar]
- 39.Dudhe R, Imran M, Maurya N P, Sharma P K. Orient J Chem. 2007;23:167–176. [Google Scholar]
- 40.Fahmy H T Y. Boll Chim Farm. 2001;140:422–427. [PubMed] [Google Scholar]
- 41.Vovk M V, Dorokhov V I. Russ J Org Chem. 1993;29:1471–1473. [Google Scholar]
- 42.Matsubara Y, Nakamura T, Yoshihara M, Maeshima T. Chem Pharm Bull. 1985;33:3009–3011. [Google Scholar]
- 43.Satzinger G. Justus Liebigs Ann Chem. 1963;665:150–165. doi: 10.1002/jlac.19636650118. [DOI] [PubMed] [Google Scholar]
- 44.Marković R, Vitnik Ž, Baranac M, Juranic I. J Chem Res, Synop. 2002:485–489. doi: 10.3184/030823402103170673. [DOI] [Google Scholar]
- 45.Ibiş C, Gökmen Z. Phosphorus, Sulfur Silicon Relat Elem. 2004;179:2537–2542. doi: 10.1080/10426500490485615. [DOI] [Google Scholar]
- 46.Meca L, Řeha D, Havlas Z. J Org Chem. 2003;68:5677–5680. doi: 10.1021/jo034344u. [DOI] [PubMed] [Google Scholar]
- 47.Ishida T, In Y, Inoue M, Ueno Y, Tanaka C. Tetrahedron Lett. 1989;30:959–962. doi: 10.1016/S0040-4039(00)95290-0. [DOI] [Google Scholar]
- 48.Delgado P, Quiroga J, Cobo J, Low J N, Glidewell C. Acta Crystallogr, Sect C. 2005;61:o477–o482. doi: 10.1107/S0108270105021888. [DOI] [PubMed] [Google Scholar]
- 49.Opletalova V, Dolezel J, Kralova K, Pesko M, Kunes J, Jampilek J. Molecules. 2011;16:5207–5227. doi: 10.3390/molecules16065207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cutshall N S, O’Day C, Prezhdo M. Bioorg Med Chem Lett. 2005;15:3374–3379. doi: 10.1016/j.bmcl.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 51.Havrylyuk D, Zimenkovsky B, Vasylenko O, Zaprutko L, Gzella A, Lesyk R. Eur J Med Chem. 2009;44:1396–1404. doi: 10.1016/j.ejmech.2008.09.032. [DOI] [PubMed] [Google Scholar]
- 52.Zidar N, Tomašić T, Šink R, Rupnik V, Kovač A, Turk S, Patin D, Blanot D, Martel C C, Dessen A, et al. J Med Chem. 2010;53:6584–6594. doi: 10.1021/jm100285g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental part.