Abstract
Significance: Chronic hypoxia can drive maladaptive responses in numerous organ systems, leading to a multitude of chronic mammalian diseases. Oxygen homeostasis is intimately linked with mitochondrial metabolism, and dysfunction in these systems can combine to form the backbone of hypoxic-ischemic injury in multiple tissue beds. Increased appreciation of the crucial roles of hypoxia-associated miRNA (hypoxamirs) in metabolism adds a new dimension to our understanding of the regulation of hypoxia-induced disease. Recent Advances: Myriad factors related to glycolysis (e.g., aldolase A and hexokinase II), tricarboxylic acid cycle function (e.g., glutaminase and iron-sulfur cluster assembly protein 1/2), and apoptosis (e.g., p53) have been recently implicated as targets of hypoxamirs. In addition, several hypoxamirs have been implicated in the regulation of the master transcription factor of hypoxia, hypoxia-inducible factor-1α, clarifying how the cellular program of hypoxia is sustained and resolved. Critical Issues: Central to the discussion of metabolic change in hypoxia is the Warburg effect, a shift toward anaerobic metabolism that persists after normal oxygen levels have been restored. Many newly discovered targets of hypoxia-driven microRNA converge on pathways known to be involved in this pathological phenomenon and the apoptosis-resistant phenotype associated with it. Future Directions: The often synergistic functions of miRNA may make them ideal therapeutic targets. The use of antisense inhibitors is currently being considered in diseases in which hypoxia and metabolic dysregulation predominate. In addition, exploration of pleiotripic miRNA functions will likely continue to offer unique insights into the mechanistic relationships of their downstream target pathways and associated hypoxic phenotypes. Antioxid. Redox Signal. 21, 1189–1201.
Introduction
Hypoxia presents a unique form of stress to the aerobic metazoan cell. Under normal oxygen conditions, adenosine triphosphate (ATP) is generated by means of oxidative phosphorylation and a sequence of redox reactions, culminating in the reduction of oxygen that serves to generate a proton gradient across the inner mitochondrial membrane. The potential energy of this gradient is harvested to fuel the synthesis of ATP. While the majority of oxygen molecules are reduced to water at Complex IV of the electron transport chain (ETC), a minority are reduced earlier in the chain, resulting in the generation of toxic superoxide radicals (83). These radicals, also termed reactive oxygen species (ROS), are minimized during normoxia by the superoxide dismutase (SOD) family of proteins, which further reduce superoxide molecules to H2O2. Under hypoxic conditions, however, the production of ROS is dramatically increased at Complex III of the ETC (83). The resulting high levels of ROS, a condition globally referred to as oxidative stress, obligate the cell to rely on anaerobic metabolic pathways until normal oxygen levels are restored.
The metabolic response to hypoxia is characterized by a shift in ATP production to glycolysis and lactic acid fermentation at the expense of oxidative phosphorylation. This shift is associated with the suppression of apoptosis, as well as a reduction in oxygen-sensing potassium channels (70) and quenching of cytosolic ROS (62). Since anaerobic metabolism is inherently less efficient than glucose oxidation, such cells also show an associated increase in glucose transport and processing to compensate for the loss of ATP (95). All metazoan cells display this so-called “glycolytic shift” when exposed to low levels of oxygen (1) (known as the “Pasteur effect”), and on short time scales, such adaptations serve to improve cell survival and function by striking an optimal balance between cellular energy production and oxidative stress. During chronic or prolonged hypoxia, however, this phenomenon can result in persistent changes in cellular energy metabolism that do not resolve when oxygen supplies are restored. This “Warburg effect” is considered a major component of many chronic pathologies, including cancer (95), pulmonary hypertension (91), and others. Moreover, even when anaerobic metabolism does not persist, the long-term effects of mitochondrial ROS production during hypoxia can be seen in cases of stroke (85), hypoxic-ischemic injury (7), and diabetes mellitus (24, 68). In all such cases, hypoxia has a profound effect on cellular metabolism, and these changes have clinical relevance to a wide range of seemingly disparate diseases.
At the heart of the hypoxic response is hypoxia-inducible factor (HIF), often referred to as the master regulator of the hypoxic response (45). HIF is a heterodimeric transcription factor that is composed of either HIF-1α or HIF-2α and HIF-1β. Under normoxic conditions, HIF-α is targeted by the prolyl hydroxylase (PHD) family of enzymes, which add post-translational modifications to HIF-α for recognition by the von Hippel-Lindau tumor suppressor protein (VHL) (80). After its association with VHL, HIF-α is ubiquitinated and rapidly degraded by the 26S proteasome. This process is oxygen dependent, and in hypoxic conditions, prolyl-hydroxylation of HIF-α is suppressed, allowing for the dimerization of HIF-α and HIF-β (80). A third HIF-α isoform, HIF-3α, lacks the transactivation domain that is common to both HIF-1α and HIF-1β (35). Though its function remains largely unknown, it is thought to serve as a negative regulator of the other HIF-α isoforms (39). Once assembled, HIF selectively targets genes carrying cis-recognition sites, termed hypoxia response elements (HREs), within their promoter regions (96). It is estimated that HIF has upward of 100 distinct transcriptional targets, and is responsible for the vast majority of hypoxia-driven transcriptional changes in the cell (13). However, while the direct effects of HIF are well characterized, the downstream consequences of its stabilization are not fully understood.
Recently, the dynamic regulation of a specific set of endogenous microRNA (miRNA) has been described under low oxygen conditions. Termed “hypoxamirs,” these miRNA are thought to play an essential role in the phenotypic changes that occur after the stabilization of HIF, at both acute and chronic time scales. A handful of these miRNA, such as miR-210, have been shown to carry functional HREs within their promoters (13), and many more have been shown to interact with known HIF-targets and to participate in the regulation of HIF itself (13, 36, 52). At the same time, recent studies have also revealed a role for miRNA in the regulation of mitochondrial metabolism (15, 76). This article will focus on the nexus of these two sets of findings, examining the roles that hypoxamirs play in the hypoxia-induced metabolic response and their implications for the treatment of clinical pathologies driven by hypoxia ischemia.
Hypoxamirs: HIF's Inner Circle of miRNA
miRNA are short, noncoding RNA strands that are 19–23 nucleotides in length. Most have been highly conserved throughout evolution, emphasizing their essential role in cellular function. As a component of the RNA-induced silencing complex (RISC), the primary function of a given miRNA is the down-regulation of its target genes. The miRNA transcript binds to a complementary sequence within the 3′ untranslated region of its mRNA targets, thereby either blocking protein translation or inducing mRNA degradation (13). The site of interaction on the mRNA target is often referred to as the “seed sequence.” It is estimated that upward of 1000 miRNA genes are encoded in the human genome, and between 30% and 60% of all mRNA transcripts are thought to be under some form of miRNA regulation (6).
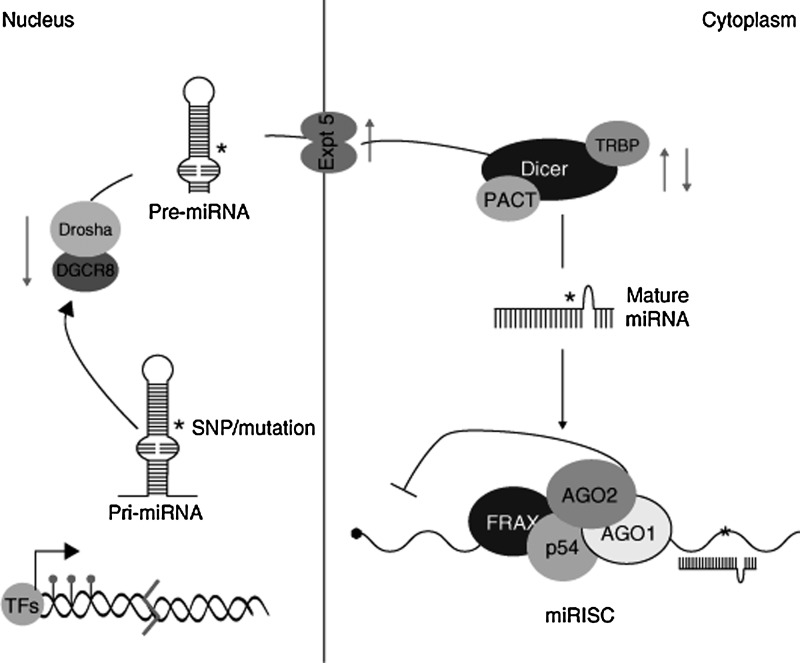
After transcription of the miRNA gene, a hairpin-looped primary miRNA molecule is formed. This structure is processed in the nucleus, resulting in the production of a smaller miRNA precursor, termed pre-miRNA, which is then exported to the cytoplasm (22, 57). Once there, the RNA endonuclease Dicer removes the pre-miRNA hairpin loop, producing a double-stranded miRNA duplex that can then be incorporated into the RISC. Once the RISC has formed, the active strand of the duplex is fully functional and sheds its antisense (miRNA*) strand. The miRNA* molecule proceeds to degradation or, in some cases, binds its own set of target mRNA molecules (13). These interactions are summarized in Figure 1.
FIG. 1.
After transcription, a hairpin loop primary-microRNA (pri-miRNA) molecule is formed. This structure is processed in the nucleus by a microprocessor complex consisting of Drosha and DGCR8. The resulting pre-miRNA molecule is then exported to the cytoplasm by exportin 5 (Expt.5). Dicer and its cofactors, PACT and TRBP, cleave the hairpin loop, producing a functional miRNA strand that associates with the RISC (components of which include AGO1, AGO2, p54, and FRAX). TRBP, TAR RNA-binding protein; AGO2, argonaute 2; FRAX, fragile X protein; RISC, RNA-induced silencing complex. Adapted with permission from Soifer et al. (86).
Expression profiling has demonstrated a wide range of miRNA whose expression is altered under hypoxia, in both primary (14, 27) and transformed (11, 33, 36, 52) cell types, although the results can be quite tissue specific. To date, nearly 100 miRNA have been found to show differential expression during hypoxia in some cellular context (13). Though the bulk of miRNA research has focused on cellular miRNA, miRNA levels in the blood have also been shown to correlate with hypoxia and tissue damage in a variety of diseases, including myocardial infarction (42), chronic heart failure (92), and cancer (74, 94). Notably, levels of the hypoxia-induced miRNA, miR-21, and miR-210 are elevated in the serum of patients with ovarian and pancreatic cancer, respectively (74, 94), and levels of miR-21, along with miR-146a and miR-221, become elevated during sustained aerobic exercise in healthy human subjects (4).
It is likely that many of these hypoxia-induced miRNA are indirect targets of HIF, or are otherwise up-regulated by secondary hypoxia-associated conditions such as inflammation and oxidative stress (59). Nonetheless, all of these are potential contributors to the hypoxic program, and several have been shown to participate in metabolic regulation (Table 1). Furthermore, miRNA activity has been uncovered at nearly every level of the mitochondrial response to hypoxia, from HIF stabilization (9, 32, 46, 88), to the induction of anaerobic glycolysis (44, 53, 55, 82, 101), to the suppression of oxidative phosphorylation (13, 28, 56, 71). Thus, hypoxamirs represent key intermediaries between hypoxia, HIF, and the mitochondrial phenotype.
Table 1.
List of Hypoxamirs with Known Functions in Metabolism
| microRNA | Cell type | HIF-dependence | Metabolic targets |
|---|---|---|---|
| miR-15a (47, 50) | SCC | Unknown | TPI (84) |
| AlDOA (84) | |||
| ALDO6A1 (84) | |||
| miR-17/92 (98) | OV2008 | Unknown | HIF-1α (92) |
| HUVEC | |||
| SCC | |||
| miR-21 (13) | HPAEC | HIF-1α | PTEN (12) |
| HUVEC | SOD2 (9) | ||
| HT-29 | SOD3 (9) | ||
| MCF-7 | |||
| PANC1 | |||
| miR-29b (50) | OV2008 | Unknown | PI3K (38) |
| CDC42 (38) | |||
| miR-30d (47) | HT-29 | Unknown | P53 (94) |
| MCF-7 | |||
| CNE | |||
| SCC | |||
| OV2008 | |||
| HUVEC | |||
| miR-122 (50) | OV2008 | Unknown | Cyclin G1 (10) |
| miR-125b (13) | HT-29 | Unknown | P53 (4) |
| MCF-7 | |||
| HUVEC | |||
| miR-155 (47) | OV2008 | Unknown | C/EBPβ (74) |
| SCOV3 | HIF-1α (11) | ||
| HUVEC | |||
| miR-204 (43) | HUVEC | Unknown | Src/STAT3/NFAT (3) |
| miR-210 (13, 47, 50) | Primary: | HIF-1α HIF-2α |
ISCU1/2 (22) TfR (89) GPD1L (42) |
| HUVEC | |||
| HPAEC | |||
| PASMC | |||
| HMEC | |||
| VHL (−/−) | |||
| Transformed: | |||
| HT-29 | |||
| HCT116 | |||
| DLD1 | |||
| RKO | |||
| MCF-7 | |||
| SCC | |||
| CNE | |||
| HeLa | |||
| ME-180 | |||
| U-251 | |||
| RCC4 | |||
| 786-O | |||
| HFF | |||
| MDA-MD231 | |||
| OV2008 | |||
| SKOV3 | |||
| Jurkat | |||
| 293 | |||
| MRC-5 | |||
| SU86.86 | |||
| PANC1 | |||
| miR-328 (63) | HUVEC | Unknown | L-type calcium channel (63) |
| HPAEC | |||
| HeLa | |||
| miR-424 (23, 50) | OV2008 | Unknown | CUL2 (33) |
Targeting the Warburg Effect
HIF has long been known to play a central role in Pasteur and Warburg physiology. This section will focus primarily on the role of HIF in the glycolytic shift (as displayed in Fig. 2), in both healthy and pathological tissues, as well as on the set of miRNA that directly target glycolytic enzymes and components of the tricarboxylic acid (TCA) cycle.
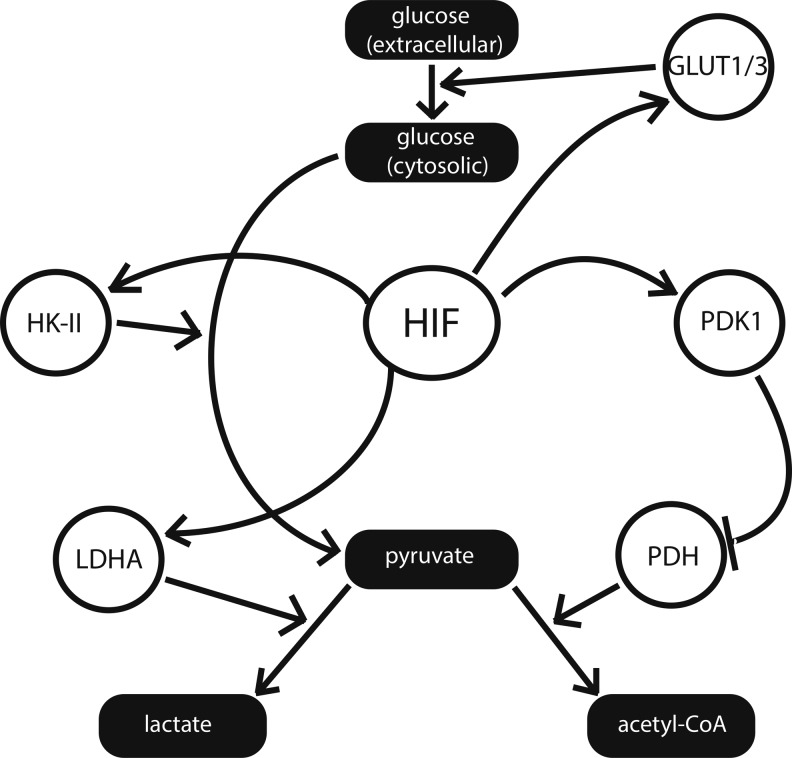
FIG. 2.
Hypoxia-inducible factor (HIF) promotes anaerobic glycolysis during hypoxia. HIF up-regulates a variety of glycolytic factors, including the glycolytic enzyme hexokinase II (HK-II) and the lactic acid fermentation enzyme lactate dehydrogenase A (LDHA). Simultaneously, HIF suppresses pyruvate entry into the mitochondria via inhibition of pyruvate dehydrogenase (PDH) by induction of pyruvate dehydrogenase kinase 1 (PDK1), suppressing tricarboxylic acid cycle function. Various miRNA influence these pathways, as shown in Figures 3–5.
Among the prominent mitochondrial enzymes that display differential expression in hypoxia is pyruvate dehydrogenase (PDH). Often referred to as the “mitochondrial gate-keeping enzyme,” PDH is responsible for the fate of glucose after its initial conversion into pyruvate during glycolysis (8). Pyruvate that remains in the mitochondria is converted by PDH into acetyl-CoA, which is used as a substrate for the TCA cycle and the ETC. Pyruvate that is exported into the cytoplasm is instead converted into lactate, a process which marks the second stage of anaerobic respiration. While PDH is not itself a direct target of HIF, HIF modulates its expression via up-regulation of pyruvate dehydrogenase kinase 1 (PDK1) (47), whose phosphorylation of PDH decreases its activity. The importance of this relationship is reflected by the reversion to oxidative phosphorylation in models in which HIF-1α is absent (47). Mouse embryo fibroblasts lacking HIF-1α display suppression of PDK1 expression and persistent accumulation of ROS during hypoxia, thus indicating the lack of normal suppression of glucose oxidation. ROS production is rescued when such cells are transfected with an expression vector encoding PDK1 (47).
HIF is also responsible for the induction of lactate dehydrogenase A (84), a cytoplasmic enzyme that is responsible for the conversion of pyruvate into lactate, as well as for the up-regulation of glucose transporters GLUT1 and GLUT3 (50) and the glycolytic enzyme hexokinase II (HK-II) (1). Positron emission tomography imaging has confirmed that the most malignant tumor cells—often subject to hypoxic microenvironments—show increased glucose uptake and metabolism when compared with their healthy counterparts (8). A similar result has been observed in the pulmonary vasculature of human patients who are diagnosed with pulmonary hypertension, another condition that is strongly associated with hypoxia at the cellular level (97).
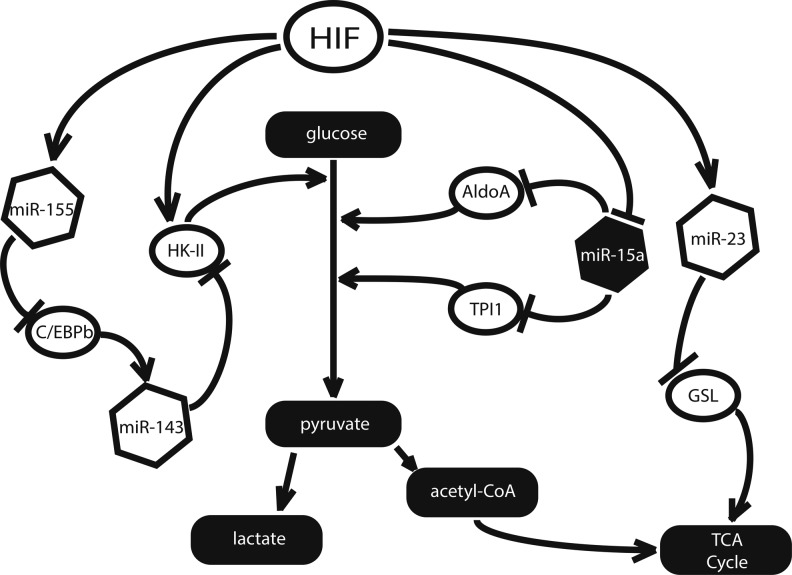
While HIF is primarily responsible for the induction of the glycolytic shift, many of its transcriptional targets will continue to maintain this cellular phenotype even in the absence of further stimulation from HIF itself (Fig. 3). MiR-155 and the miR-23a/b cluster are two such examples—both acting directly on enzymes of the TCA and glycolytic pathways (31, 44). Expressed in a variety of tissues, miR-23 is suppressed by the proto-oncoprotein Myc and up-regulated by hypoxia (31, 52). Recently, Gao et al. demonstrated that Myc expression is correlated with high levels of glutaminase (GLS) in human prostate cancer cells (31). By analyzing endogenous target gene transcript and reporter gene construct expression in both gain- and loss-of-function assays, these authors demonstrated that GLS is a direct target of miR-23 and can be rescued by overexpression of either Myc or anti-miR-23 agents (31). Furthermore, GLS catalyzes the hydrolysis of glutamine to glutamate, and in the process, GLS generates α-ketoglutarate, which is used as a source of fuel in the TCA cycle (Fig. 3). In the context of hypoxia, where miR-23a/b is overexpressed, the authors found that this activity results in the suppression of oxidative metabolism by reducing α-ketoglutarate entry into the TCA cycle (40).
FIG. 3.
Several miRNA participate in the induction of the Warburg effect. MiR-155 supports HK-II up-regulation by suppressing C/EBPb, which activates the transcription of the HK-II suppressor miR-143. MiR-15a suppresses the Warburg effect by targeting the glycolytic enzymes aldolase A (AldoA) and triosephosphate isomerase I (TPI1).
A separate hypoxamir, miR-155, has been directly linked to the up-regulation of glycolysis through its suppression of the anti-glycolytic miR-143 (26, 64). While not itself a hypoxamir, miR-143 targets HK-II, the enzyme that is responsible for catalyzing a key transformation early in the glycolysis pathway. This relationship was demonstrated by Fang et al. in small cell lung cancer cells, and may extend to other cell types as well (26). Best known for its induction by inflammatory enzymes such as C-Jun N-terminal kinase (JNK), nuclear factor-κB, and activator protein 1, miR-155 is a widely expressed miRNA that is up-regulated by hypoxia in a variety of cell types (41). Working with human breast cancer cells, Jiang et al. demonstrated that miR-155 suppresses miR-143 expression via degradation of its transcriptional activator, C/EBPβ, thus increasing HK-II expression at the post-transcriptional level (Fig. 3) (44). Supporting the relevance of this relationship, miR-155 has been shown to up-regulate HK-II in this same cell type via indirect activation of STAT3, a factor known to promote the transcription of HK-II (44).
Finally, miR-15a—a hematopoietic miRNA whose deletion is commonly associated with chronic lymphocytic leukemia—has been shown to serve a protective role via down-regulation of the glycolytic enzymes aldolase A and triosephosphate isomerase I (10). miR-15a impairs the rapid glycolysis which is necessary to sustain cells that have suppressed glucose oxidation, and it is concordantly down-regulated under hypoxic conditions (38).
In general, miRNA are reasonable candidates for prolonged regulation of the glycolytic shift, as they possess a remarkably long half life. By examining miRNA decay rates in mouse embryonic fibroblasts after the silencing of Dicer, Gantier et al. demonstrated that miRNA molecules may be approximately 10 times as stable as mRNA, with an average half life of 5 days (30). This finding implicates hypoxamirs as potential contributors to the persistence of Warburg physiology that is characteristic of tumorigenic and hypertrophic cells in cancer and heart disease, respectively, particularly in cases in which HIF stabilization has been nominally resolved.
Targeting Glycolysis Via the p53 Signaling Pathway
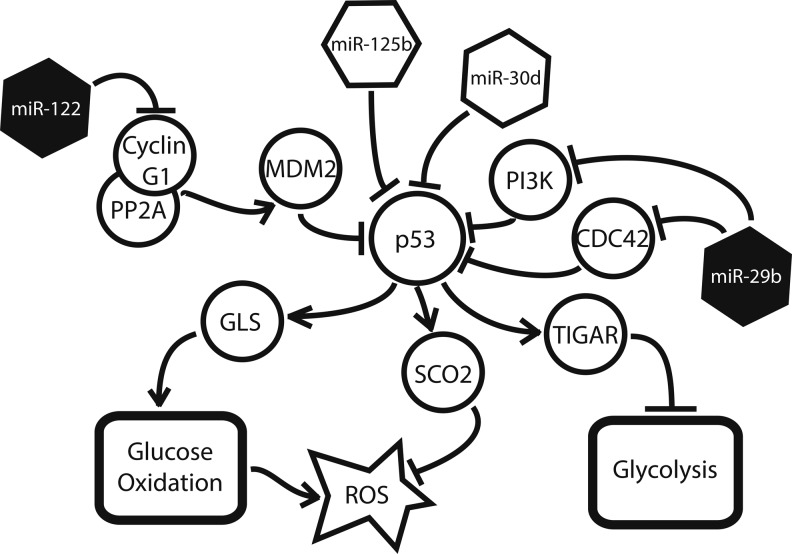
The tumor suppressor p53 maintains an antagonistic relationship with HIF during hypoxia—both directly through up-regulation of GLS, and indirectly through its transcriptional target, Tp53-induced glycolysis and apoptosis regulator (TIGAR), a known inhibitor of glycolysis (5). Correspondingly, miRNA that regulate this signaling network are also affected by the induction of hypoxia (Fig. 4). The two most dramatic examples of such miRNA are miR-125b and miR-30d. MiR-125b, a hypoxamir (52) with particular enrichment in the brain and eye, is up-regulated during neurogenesis and thought to play a role in neuronal differentiation (55). Studying both human and zebrafish cells, Le et al. recently demonstrated that miR-125b binds directly to p53, reducing its expression in a dose-dependent manner (55). They were also able to identify a matching miR-125b seed sequence in two transactivational targets—p21 and Bax—as well as seven upstream regulators of p53, demonstrating that the involvement of miR-125b occurs at multiple points in the pathway and is likely to involve synergistic regulatory actions (55). Similarly, Kumar et al. have uncovered an additional hypoxamir, miR-30d, which suppresses p53 and several of its downstream targets, including p21, Bad, Puma, and Gadd45α, in human multiple myeloma cells (53). Since many of these targets are potent regulators of apoptosis, their suppression by miR-30d results in an apoptosis-resistant phenotype (53). Interestingly, miR-30d is expressed in a variety of tissues, most notably pancreatic β cells, and is known to be up-regulated by the presence of glucose (89), a fact that may help in explaining its induction during hypoxia.
FIG. 4.
Several miRNA play a role in the p53 signaling network. miR-29 up-regulates p53 by suppressing two of its inhibitors, CDC42 and PI3K. Likewise, miR-122 indirectly suppresses the p53 inhibitor, MDM2. MiR-125b and miR-30d suppress p53 expression directly. Filled miRNA are down-regulated by hypoxia, whereas unfilled miRNA are up-regulated.
This suppression of p53 has a variety of downstream consequences for the hypoxic cell (Fig. 4). p53 is responsible for the up-regulation of GLS2, which indirectly generates α-ketoglutarate for the TCA cycle as previously described (36). In addition, the p53-induced factor, TIGAR, is a key inhibitor of glycolysis (48). Structurally, it resembles fructose-2,6-bisphosphatase phosphofructokinase-2 (FBPase-2), the bisphosphatase domain of the bifunctional glycolytic enzyme, PFK-2/FBPase-2; accordingly, TIGAR has been shown to suppress FBPase-2, leading to a marked decrease in glycolytic flux (5). As demonstrated by Kimata et al., the inhibition of TIGAR induced glycolysis in hypoxic cardiomyocytes, while its overexpression preserved glucose oxidation, regardless of environmental oxygen tension (48).
Several miRNA known to induce or protect p53 expression have also been shown to be down-regulated in hypoxia (38), further emphasizing the antagonistic relationship of HIF and p53. Both MiR-122, a liver-specific miRNA involved in lipid metabolism and liver homeostasis (25), and miR-29b, a widely expressed miRNA with particular enrichment in pancreatic β cells (73), have been shown to induce p53 under certain cellular conditions. Working with hepatocarcinoma cells, Fornari et al. demonstrated that miR-122 stabilizes p53 expression via suppression of cyclin G1, a protein that is known to enhance the p53 inhibitor MDM2 (29). Similarly, Park et al. found that miR-29b up-regulates p53 levels—both in mouse embryo fibroblasts and in human cervical and colon carcinoma cells—via the suppression of p53 suppressors, CDC42 and PI3 kinase (66).
The relationship between HIF and p53 is further complicated by the role that p53 plays in the quenching of intracellular ROS. TIGAR-mediated suppression of glycolytic flux enables shunting of glucose derivatives into the pentose phosphate pathway, resulting in the production of NADPH, an electron donor that is capable of reducing ROS (specifically H2O2) to H2O (via glutathione peroxidase-dependent glutathione regeneration from glutathione disulfide) and decreasing cytotoxicity (5). p53 is itself responsible for the up-regulation of the factor SCO2, a protein that is responsible for the transfer of copper to the cytochrome c oxidase complex, which, in turn, mediates the reduction of oxygen to water at the final stage of the ETC (60). Excess ROS also induces p53 reciprocally, resulting in a protective feedback mechanism that has been demonstrated in tumorigenic cells (16), as well as cardiomyocytes in this setting of congestive heart failure (48). Thus, the hypoxamir-mediated suppression of p53 expression and activity can result in an increase in intracellular ROS. While this is not itself advantageous to the survival of the hypoxic cell, increased ROS production is known to induce HIF stabilization (91), which, in turn, suppresses ROS by a variety of other means. This functional “bypassing” of the usual mechanisms of oxidative homeostasis is mirrored in the regulation of iron homeostasis by HIF-induced miR-210 (99), as discussed next.
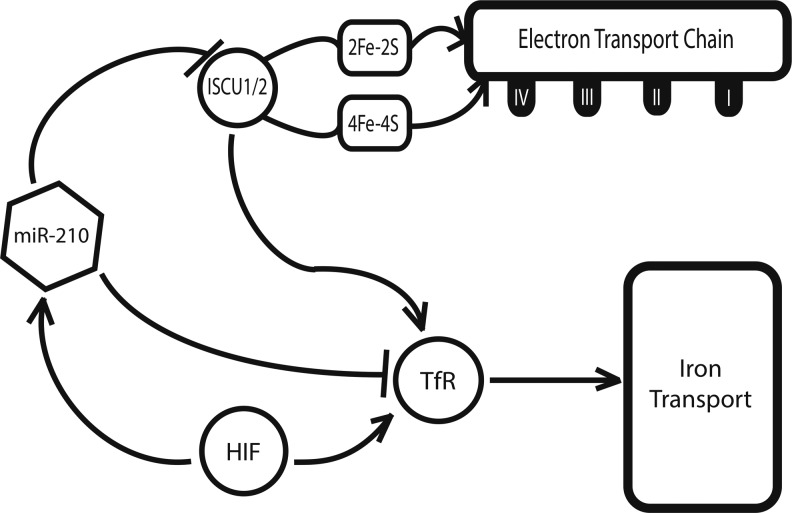
Targeting the ETC and Iron Homeostasis Via miR-210
While the majority of the hypoxamirs described thus far are known to be differentially expressed during hypoxia, most are not direct targets of HIF-dependent transcriptional activation (13). A notable exception to this point is the hypoxamir miR-210, a ubiquitous factor that is reliably and robustly induced by HIF across a wide range of primary and transformed cell types (11, 33, 36, 52). miR-210 is also known to be induced in a variety of clinical pathologies that are associated with hypoxia, including preeclampsia (69, 102), ischemia (72), and pulmonary hypertension (14). At a metabolic level, our group found that miR-210 directly targets the iron-sulfur cluster assembly proteins ISCU1 and ISCU2 (14), members of a family of proteins that play a key role in the assembly of [4Fe-4S] and [2Fe-2S] iron-sulfur clusters (58).
Serving essential roles in electron transport and cellular redox state (54), iron-sulfur clusters are an important component of the mitochondrial respiratory complexes in the ETC, and they are incorporated into TCA cycle enzymes, such as aconitase and succinate dehydrogenase (78). Corresponding with a down-regulation of ISCU1 and ISCU2, there is a disruption of intact iron-sulfur clusters, as assessed by electron paramagnetic resonance spectroscopy. In turn, through repressing ISCU1/2 during hypoxia, miR-210 decreases the activity of prototypical iron-sulfur enzymes controlling mitochondrial metabolism, including Complex I and aconitase; decreases mitochondrial respiration; and consequently, improves cell survival in the acute setting (14). These results are consistent across pulmonary arterial endothelial cells (14), tumorigenic cells of breast cancer (28), and trophoblasts in human placental tissue (56), reflecting the potential relevance of this finding to pathologies as disparate as pulmonary hypertension, preeclampsia, and cancer. In addition, miR-210 has been found to down-regulate other related mitochondrial targets, including the NDUFA4 subunit of ETC Complex I and the subunit D of the succinate dehydrogenase complex (ETC Complex II) (17, 71), thereby potentially producing a synergistic effect on inhibiting electron transport and mitochondrial respiration.
The role of miR-210 in the regulation of iron-sulfur clusters in hypoxia is complicated by the need for strict control of iron levels within the cell (Fig. 5). Excess iron promotes the generation of free radicals and is ultimately toxic (17), while iron insufficiency induces hypoferric anemia (87). Specifically, genetic deficiencies of iron-sulfur biogenesis can lead to toxic increases in mitochondrial iron, such as that seen in Friedreich's ataxia (75). In part, such overload is mediated by an increase in the expression of the transferrin receptor 1 (TfR). Recently, Yoshioka et al. have shown that TfR is an additional direct target of miR-210 in hypoxia (99), enabling greater control by this miRNA over the amount of iron that fluxes through the cellular membrane at a given time. Thus, miR-210 is able to induce a suppression of ETC function via ISCU without an associated toxic increase in intracellular iron.
FIG. 5.
miR-210 in the iron homeostasis pathway. miR-210 suppresses the iron-sulfur cluster assembly proteins, ISCU1 and ISCU2, inhibiting electron transport chain activity. Inhibition of ISCU1/2 indirectly induces the iron transport protein transferrin receptor (TfR) and increases the uptake of iron. MiR-210 directly suppresses TfR, inhibiting this side effect of ISCU1/2 regulation.
Targeting Mitochondrial Apoptosis Via the AKT/HK-II Signaling Pathway
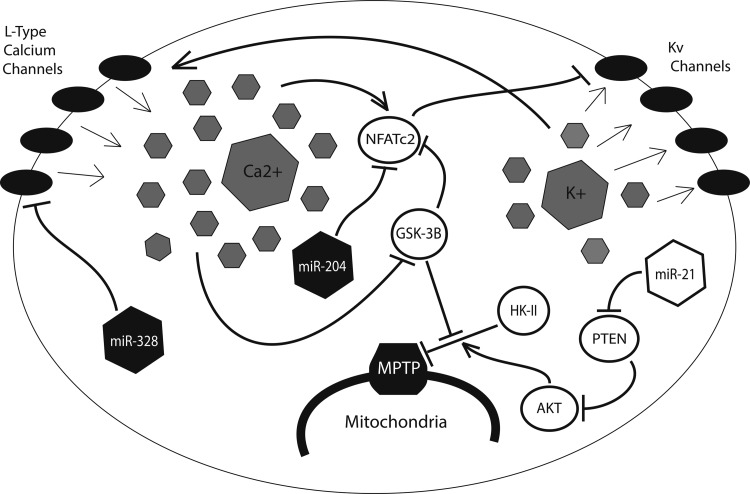
Persistent resistance to apoptosis, another hallmark of Warburg physiology, is a direct result of the HIF-mediated glycolytic shift (Fig. 6). Kv1.5—a member of the redox-sensitive voltage-gated family of K+ ion channels (Kv)—is known to be activated by H2O2, the byproduct of superoxide reduction by SOD (12). When glucose oxidation is suppressed, ROS production is low and relatively little H2O2 is present in the mitochondria and the cytosol. Referred to by Michelakis and colleagues as the “mitochondria-ROS-Kv channel axis,” this system is thought to serve as an important oxygen sensor for the cell, with low levels of H2O2 promoting closure of Kv1.5 channels and retention of intracellular potassium (63). Potassium, which can serve as an anti-apoptotic mediator via inhibition of caspase activity, is, thus, retained in the cell, promoting a pro-survival phenotype during periods of hypoxic stress (77).
FIG. 6.
Prolonged hypoxia results in the closure of redox-sensitive Kv channels, increasing intracellular potassium. The resulting cellular depolarization triggers the opening of voltage-sensitive L-type calcium channels and increased intracellular calcium. In this cellular context, calcium inhibits opening of the mitochondrial permeability transition pore (MPTP), a key determinant of apoptosis, and suppresses Kv potassium channel expression. Hypoxamir miR-21 also inhibits the MPTP through suppression of phosphate and tensin homolog (PTEN), thereby increasing the activity of AKT, which, in turn, activates HK-II. Protective miRNA, miR-328, inhibits the opening of L-type calcium channels, while miR-204 indirectly up-regulates Kv channel expression through suppression of NFATc2. Filled miRNA are down-regulated by hypoxia, whereas unfilled miRNA are up-regulated. Adapted with permission from Cottrill and Chan (20).
The resulting cellular depolarization triggers the opening of voltage-gated L-type calcium channels, enabling an influx of Ca2+ into the cell (62). High intracellular calcium, in turn, promotes the transcription of nuclear factor of activated T cells (NFAT), which is responsible for the further down-regulation of Kv1.5 potassium channels (8). A direct consequence of this electrical remodeling is the hyperpolarization of the mitochondrial membrane, and the associated dysregulation of the mitochondrial permeability transition pore (MPTP), the large nonselective channel that allows for the passage of proapoptotic mediators from the mitochondria into the cytoplasm (63). HIF-mediated up-regulation of HK-II exacerbates this effect by binding to and inhibiting the voltage-dependent anion channel (VDAC), a key component of the MPTP (63). The proto-oncoprotein Akt contributes as well, by inducing the transport of HK-II across the mitochondrial membrane (8).
Several miRNA have been shown to play a role in this process. In addition to the miR-155/miR-143/HK-II signaling pathway previously described (44), miR-21, a widely expressed hypoxamir known to be up-regulated in a variety of cancers (3, 67), as well as in tissue ischemia (61), pulmonary hypertension (93), and cardiac fibrosis (79), has been shown to up-regulate Akt through suppression of its negative regulator phosphate and tensin homolog (81). The resulting inhibition of VDAC reduces ion flux between the mitochondria and the cytosol, resulting in mitochondrial hyperpolarization and an increased threshold for the opening of the MPTP. Intriguingly, Zhang et al. have demonstrated that miR-21 also modulates cellular ROS levels by targeting SOD2 and SOD3, the proteins which are responsible for reduction of more reactive free radical anion superoxide to the less reactive hydrogen peroxide (101). By promoting the accumulation of ROS, miR-21 up-regulation induces a cytosolic environment that mimics that which would occur after a period of hypoxia, while the additional role of miR-21 in MPTP regulation ensures that the cell is prepared to withstand such cytotoxic effects without succumbing to apoptosis.
Two additional miRNA, miR-204 and miR-328, have been shown to be protective against the induction of Warburg physiology. Both miR-328 and miR-204 are down-regulated in the hypoxic cellular environment (2, 90). Furthermore, miR-328, a widely expressed miRNA with particular enrichment in hematopoeitic and vascular cells, is known to be ectopically suppressed in hypoxia-induced pulmonary hypertension (90). As a result, miR-328 can directly inhibit the expression of L-type calcium channels, thereby preventing some of the electrical remodeling that is associated with the hypoxic mitochondria (90). Similarly, miR-204, a miRNA primarily expressed in the nervous system, targets NFAT for degradation, preventing its inhibition of redox-sensitive Kv1.5 channels (2).
Feedback Control of HIF Stabilization
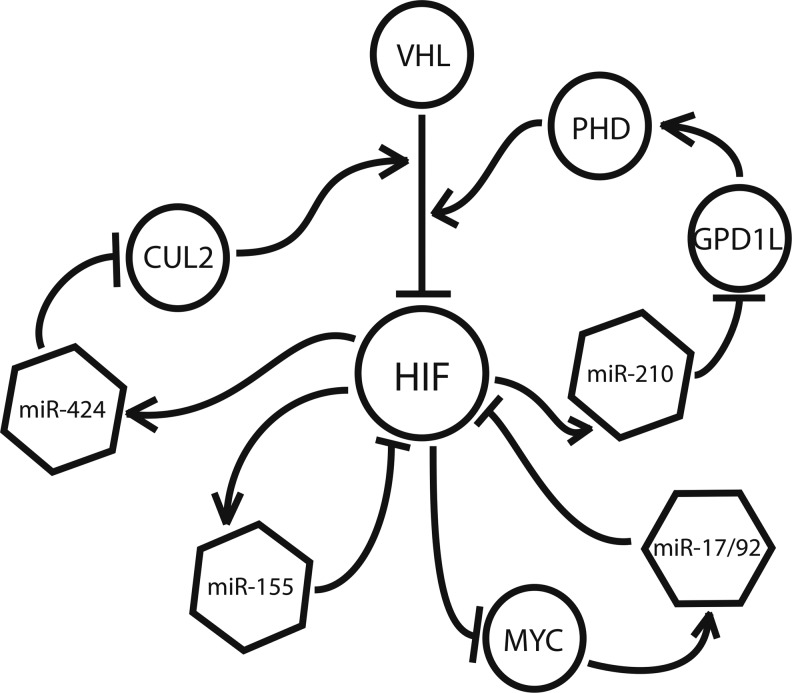
In addition to their role as downstream effectors of the hypoxic response, many hypoxamirs regulate HIF itself, creating a series of regulatory feedback loops that serve to modulate HIF up-regulation during and after periods of oxidative stress (Fig. 7). Kelly et al. have recently uncovered an additional function of miR-210, demonstrating that it down-regulates the factor, glycerol-3-phosphate dehydrogenase 1-like (GPD1L), a novel protein which has been shown to modulate HIF stabilization (46). GPD1L suppresses cytosolic HIF by increasing the activity of the PHDs responsible for prolyl hydroxylation of HIF-1α. As previously mentioned, prolyl hydroxylation by PHDs tags HIF-1α for recognition by VHL, which, in turn, leads to its ubiquitination and proteasomal degradation. By targeting GPD1L, miR-210 allows for cytosolic HIF to remain high, resulting in a positive feedback mechanism between HIF and HIF-induced miR-210.
FIG. 7.
Several hypoxia-induced miRNA engage in regulatory feedback loops with HIF. MiR-155 and miR-17/92 suppress HIF directly, while miR-210 and miR-424 support its stabilization by suppressing prolyl hydroxylases (PHDs), which tag HIF for recognition by the von Hippel Lindau (VHL) tumor suppressor, and the scaffolding protein CUL2, a component of the E3 ubiquitin ligase complex that marks HIF for degradation in the proteasome.
Following a similar principle, hypoxia-induced miR-424 has been shown to target the protein cullin 2 (CUL2), a member of the scaffolding complex that links prolyl hydroxylated HIF-α to the E3 ubiquitin ligase complex, triggering its recognition by the 26S proteasome (37). miR-424 is expressed throughout the vasculature, and it is implicated in the induction of angiogenesis (32). By targeting CUL2, miR-424 destabilizes the E2-ligase assembly, resulting in the up-regulation of HIF (32). Conversely, a negative feedback mechanism is seen in the case of miR-155, which promotes the resolution of HIF during transient hypoxia by targeting HIF-α directly, bypassing the ubiquitin-proteasome pathway altogether (9). Recently, Bruning et al. showed that, when hypoxia exposure is brief, intestinal epithelial cells demonstrate a transient up-regulation of HIF-α, followed by its rapid degradation at the hands of a number of factors, including miR-155 (9).
Finally, the miR-17/92 cluster, a ubiquitously expressed set of miRNA known to be induced by the proto-oncoprotein Myc, has been shown to engage in an antagonistic relationship with HIF (88). Working with human lung cancer cells, Taguchi et al. found that members of the miR-17/92 cluster target HIF-1α directly, and decrease its expression in a dose-dependent manner (88). Conversely, HIF has been shown to suppress miR-17/92 expression via inhibition of Myc (19, 21), a function that is likely responsible for the marked down-regulation of this miRNA cluster under hypoxic conditions (15). Taken together, these mechanisms illustrate that the role of miRNA in hypoxia is not restricted to that of downstream effectors, as many hypoxamirs are actively involved in modulating HIF expression and activity throughout the hypoxic program.
Innovation
Hypoxia and consequent metabolic dysfunction underlie a vast array of cellular pathologies. Hypoxia-relevant microRNA (hypoxamirs) offer insights into the metabolic phenotypes of myriad disorders in which hypoxia is known to play a causative role. Their pleiotropic nature makes them intriguing therapeutic targets, and with the aid of new tools such as microRNA mimics and antagomiRs, the role of hypoxamirs in hypoxia-induced metabolic disease may be interrogated and exploited for therapeutic effect. In addition, next-generation computational algorithms may be used to predict new gene targets of validated hypoxamirs and will continue to provide a context for known microRNA-target relationships in a metabolic setting.
Conclusions and Future Directions
Ongoing discoveries involving the often surprising biology of miRNA represent a new dimension of our understanding of metabolic regulation. As a result, these findings may offer miRNA as potential therapeutic targets in the treatment of human diseases. In particular, the discovery of hypoxamirs in metabolic regulation provides new insights into the persistent metabolic dysfunction which is seen in a wide variety of pathologies that share hypoxia as a common causative feature. With the advent of a variety of antisense miRNA inhibitors to suppress miRNA as well as miRNA replacement therapy to restore deficient miRNA function, these factors may represent new opportunities for the treatment of cancer, pulmonary hypertension, hypoxic-ischemic injury, and many others.
In addition, the pleiotropic nature of many hypoxamirs may help pave the way for a better understanding of the function of HIF and its metabolic actions in contexts other than hypoxia. Several hypoxamirs are already known to have robust functions in related cellular contexts, such as inflammation, infection, and tumorigenesis. In this way, hypoxamirs may provide a link between hypoxia and metabolism, and their sibling pathobiologies. Similarly, given the wide “network effect” of each miRNA that can target several genes in a single pathway or related network of genes (100), we predict that many hypoxamirs may harbor multiple, but as yet undiscovered, metabolic targets which affect more robustly the hypoxic cellular response.
Finally, the conserved nature of the miRNA seed sequence has made possible the development of several computational algorithms, such as TargetScan and DIANA (51, 65), that match mRNA transcripts to targeting miRNA (34, 49). With the use of such algorithms, it is possible to generate a network of predicted target interactions for a given miRNA. These types of in silico analyses can help in lending context to those targets that have been validated in vivo and in vitro. Perhaps more importantly, they may themselves point to targets that are not considered canonical components of the pathways under investigation, thus expanding our understanding of the cellular and systemic contexts in which they occur.
Abbreviations Used
- AGO2
argonaute 2
- ALDOA
aldolase A/fructose bisphosphate aldolase
- CUL2
cullin 2
- ETC
electron transport chain
- FBPase-2
fructose-2,6-bisphosphatase
- FRAX
fragile X
- GLS
glutaminase
- GLUT
glucose transporter
- GPD1L
glycerol-3-phosphate dehydrogenase 1-like
- HIF
hypoxia inducible factor
- HK-II
hexokinase II
- HRE
hypoxia response element
- IRP1
iron regulatory protein
- ISCU
iron-sulfur cluster assembly protein
- LDHA
lactate dehydrogenase A
- miRNA
microRNA
- MPTP
mitochondrial permeability transition pore
- PDH
pyruvate dehydrogenase
- PDK
pyruvate dehydrogenase kinase
- PHD
prolyl hydroxylase
- RISC
RNA-induced silencing complex
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- TIGAR
Tp53 induced glycolysis and apoptosis regulator
- TfR
transferrin receptor
- TPI
trios phosphate isomerase
- VDAC
voltage-dependent anion channel
- VHL
von Hippel-Lindau protein
Acknowledgments
This work was supported by NIH grants HL61795, HL48743, HL070819, HL108630 (to J.L.), and HL096834, the Lerner, Harris, and Watkins Funds, Gilead Research Scholars Fund, and the Pulmonary Hypertension Association (to S.Y.C.). The authors thank Stephanie Tribuna for assistance with the preparation of this article.
References
- 1.Aisenberg AC, Reinafarje B, and Potter VR. Studies on the Pasteur effect. I. General observations. J Biol Chem 224: 1099–1113, 1957 [PubMed] [Google Scholar]
- 2.Ali S, Ahmad A, Banerjee S, Padhye S, Dominiak K, Schaffert JM, Wang Z, Philip PA, and Sarkar FH. Gemcitabine sensitivity can be induced in pancreatic cancer cells through modulation of miR-200 and miR-21 expression by curcumin or its analogue CDF. Cancer Res 70: 3606–3617, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Andersson B, Janson V, Behnam-Motlagh P, Henriksson R, and Grankvist K. Induction of apoptosis by intracellular potassium ion depletion: using the fluorescent dye PBFI in a 96-well plate method in cultured lung cancer cells. Toxicol In Vitro 20: 986–994, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Baggish AL, Hale A, Weiner RB, Lewis GD, Systrom D, Wang F, Wang TJ, and Chan SY. Dynamic regulation of circulating microRNA during acute exhaustive exercise and sustained aerobic exercise training. J Physiol 589: 3983–3994, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bensaad K, Tsuruta A, Selak MA, Vidal MN, Nakano K, Bartrons R, Gottlieb E, and Vousden KH. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell 126: 107–120, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Berezikov E, Guryev V, van de Belt J, Wienholds E, Plasterk RH, and Cuppen E. Phylogenetic shadowing and computational identification of human microRNA genes. Cell 120: 21–24, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Blomgren K, Zhu C, Hallin U, and Hagberg H. Mitochondria and ischemic reperfusion damage in the adult and in the developing brain. Biochem Biophys Res Commun 304: 551–559, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Bonnet S, Archer SL, Allalunis-Turner J, Haromy A, Beaulieu C, Thompson R, Lee CT, Lopaschuk GD, Puttagunta L, Harry G, Hashimoto K, Porter CJ, Andrade MA, Thebaud B, and Michelakis ED. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell 11: 37–51, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Bruning U, Cerone L, Neufeld Z, Fitzpatrick SF, Cheong A, Scholz CC, Simpson DA, Leonard MO, Tambuwala MM, Cummins EP, and Taylor CT. MicroRNA-155 promotes resolution of hypoxia-inducible factor 1alpha activity during prolonged hypoxia. Mol Cell Biol 31: 4087–4096, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calin GA, Cimmino A, Fabbri M, Ferracin M, Wojcik SE, Shimizu M, Taccioli C, Zanesi N, Garzon R, Aqeilan RI, Alder H, Volinia S, Rassenti L, Liu X, Liu CG, Kipps TJ, Negrini M, and Croce CM. MiR-15a and miR-16-1 cluster functions in human leukemia. Proc Natl Acad Sci U S A 105: 5166–5171, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camps C, Buffa FM, Colella S, Moore J, Sotiriou C, Sheldon H, Harris AL, Gleadle JM, and Ragoussis J. hsa-miR-210 Is induced by hypoxia and is an independent prognostic factor in breast cancer. Clin Cancer Res 14: 1340–1348, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Caouette D, Dongmo C, Berube J, Fournier D, and Daleau P. Hydrogen peroxide modulates the Kv1.5 channel expressed in a mammalian cell line. Naunyn Schmiedebergs Arch Pharmacol 368: 479–486, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Chan SY. and Loscalzo J. MicroRNA-210: a unique and pleiotropic hypoxamir. Cell Cycle 9: 1072–1083, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan SY, Zhang YY, Hemann C, Mahoney CE, Zweier JL, and Loscalzo J. MicroRNA-210 controls mitochondrial metabolism during hypoxia by repressing the iron-sulfur cluster assembly proteins ISCU1/2. Cell Metab 10: 273–284, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen B, Li H, Zeng X, Yang P, Liu X, Zhao X, and Liang S. Roles of microRNA on cancer cell metabolism. J Transl Med 10: 228, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen K, Albano A, Ho A, and Keaney JF, Jr., Activation of p53 by oxidative stress involves platelet-derived growth factor-beta receptor-mediated ataxia telangiectasia mutated (ATM) kinase activation. J Biol Chem 278: 39527–39533, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Chen Z, Li Y, Zhang H, Huang P, and Luthra R. Hypoxia-regulated microRNA-210 modulates mitochondrial function and decreases ISCU and COX10 expression. Oncogene 29: 4362–4368, 2010 [DOI] [PubMed] [Google Scholar]
- 18.This reference has been deleted.
- 19.Clifford SC, Astuti D, Hooper L, Maxwell PH, Ratcliffe PJ, and Maher ER. The pVHL-associated SCF ubiquitin ligase complex: molecular genetic analysis of elongin B and C, Rbx1 and HIF-1alpha in renal cell carcinoma. Oncogene 20: 5067–5074, 2001 [DOI] [PubMed] [Google Scholar]
- 20.Cottrill KA. and Chan SY. Metabolic dysfunction in pulmonary hypertension and the expanding influence of Otto Warburg. Eur J Clin Invest 43: 855–865, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Courboulin A, Paulin R, Giguere NJ, Saksouk N, Perreault T, Meloche J, Paquet ER, Biardel S, Provencher S, Cote J, Simard MJ, and Bonnet S. Role for miR-204 in human pulmonary arterial hypertension. J Exp Med 208: 535–548, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Denli AM, Tops BB, Plasterk RH, Ketting RF, and Hannon GJ. Processing of primary microRNAs by the microprocessor complex. Nature 432: 231–235, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Donker RB, Mouillet JF, Nelson DM, and Sadovsky Y. The expression of Argonaute2 and related microRNA biogenesis proteins in normal and hypoxic trophoblasts. Mol Hum Reprod 13: 273–279, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Duchen MR. Roles of mitochondria in health and disease. Diabetes 53Suppl 1: S96–S102, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Fabani MM. and Gait MJ. miR-122 targeting with LNA/2'-O-methyl oligonucleotide mixmers, peptide nucleic acids (PNA), and PNA-peptide conjugates. RNA 14: 336–346, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fang R, Xiao T, Fang Z, Sun Y, Li F, Gao Y, Feng Y, Li L, Wang Y, Liu X, Chen H, Liu XY, and Ji H. MicroRNA-143 (miR-143) regulates cancer glycolysis via targeting hexokinase 2 gene. J Biol Chem 287: 23227–23235, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fasanaro P, D'Alessandra Y, Di Stefano V, Melchionna R, Romani S, Pompilio G, Capogrossi MC, and Martelli F. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol Chem 283: 15878–15883, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Favaro E, Ramachandran A, McCormick R, Gee H, Blancher C, Crosby M, Devlin C, Blick C, Buffa F, Li JL, Vojnovic B, Pires das Neves R, Glazer P, Iborra F, Ivan M, Ragoussis J, and Harris AL. MicroRNA-210 regulates mitochondrial free radical response to hypoxia and krebs cycle in cancer cells by targeting iron sulfur cluster protein ISCU. PLoS One 5: e10345, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fornari F, Gramantieri L, Giovannini C, Veronese A, Ferracin M, Sabbioni S, Calin GA, Grazi GL, Croce CM, Tavolari S, Chieco P, Negrini M, and Bolondi L. MiR-122/cyclin G1 interaction modulates p53 activity and affects doxorubicin sensitivity of human hepatocarcinoma cells. Cancer Res 69: 5761–5767, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Gantier MP, McCoy CE, Rusinova I, Saulep D, Wang D, Xu D, Irving AT, Behlke MA, Hertzog PJ, Mackay F, and Williams BR. Analysis of microRNA turnover in mammalian cells following Dicer1 ablation. Nucleic Acids Res 39: 5692–5703, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao P, Tchernyshyov I, Chang TC, Lee YS, Kita K, Ochi T, Zeller KI, De Marzo AM, Van Eyk JE, Mendell JT, and Dang CV. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature 458: 762–765, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghosh G, Subramanian IV, Adhikari N, Zhang X, Joshi HP, Basi D, Chandrashekhar YS, Hall JL, Roy S, Zeng Y, and Ramakrishnan S. Hypoxia-induced microRNA-424 expression in human endothelial cells regulates HIF-alpha isoforms and promotes angiogenesis. J Clin Invest 120: 4141–4154, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giannakakis A, Sandaltzopoulos R, Greshock J, Liang S, Huang J, Hasegawa K, Li C, O'Brien-Jenkins A, Katsaros D, Weber BL, Simon C, Coukos G, and Zhang L. miR-210 links hypoxia with cell cycle regulation and is deleted in human epithelial ovarian cancer. Cancer Biol Ther 7: 255–264, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grimson A, Farh KK, Johnston WK, Garrett-Engele P, Lim LP, and Bartel DP. MicroRNA targeting specificity in mammals: determinants beyond seed pairing. Mol Cell 27: 91–105, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gu YZ, Moran SM, Hogenesch JB, Wartman L, and Bradfield CA. Molecular characterization and chromosomal localization of a third alpha-class hypoxia inducible factor subunit, HIF3alpha. Gene Expr 7: 205–213, 1998 [PMC free article] [PubMed] [Google Scholar]
- 36.Guimbellot JS, Erickson SW, Mehta T, Wen H, Page GP, Sorscher EJ, and Hong JS. Correlation of microRNA levels during hypoxia with predicted target mRNAs through genome-wide microarray analysis. BMC Med Genomics 2: 15, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo L, Qiu Z, Wei L, Yu X, Gao X, Jiang S, Tian H, Jiang C, and Zhu D. The microRNA-328 regulates hypoxic pulmonary hypertension by targeting at insulin growth factor 1 receptor and L-type calcium channel-alpha1C. Hypertension 59: 1006–1013, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Hebert C, Norris K, Scheper MA, Nikitakis N, and Sauk JJ. High mobility group A2 is a target for miRNA-98 in head and neck squamous cell carcinoma. Mol Cancer 6: 5, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heikkila M, Pasanen A, Kivirikko KI, and Myllyharju J. Roles of the human hypoxia-inducible factor (HIF)-3alpha variants in the hypoxia response. Cell Mol Life Sci 68: 3885–3901, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu W, Zhang C, Wu R, Sun Y, Levine A, and Feng Z. Glutaminase 2, a novel p53 target gene regulating energy metabolism and antioxidant function. Proc Natl Acad Sci U S A 107: 7455–7460, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hua Z, Lv Q, Ye W, Wong CK, Cai G, Gu D, Ji Y, Zhao C, Wang J, Yang BB, and Zhang Y. MiRNA-directed regulation of VEGF and other angiogenic factors under hypoxia. PLoS One 1: e116, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ji X, Takahashi R, Hiura Y, Hirokawa G, Fukushima Y, and Iwai N. Plasma miR-208 as a biomarker of myocardial injury. Clin Chem 55: 1944–1949, 2009 [DOI] [PubMed] [Google Scholar]
- 43.Jian X, Xiao-yan Z, Bin H, Yu-feng Z, Bo K, Zhi-nong W, and Xin N. MiR-204 regulate cardiomyocyte autophagy induced by hypoxia-reoxygenation through LC3-II. Int J Cardiol 148: 110–112, 2011 [DOI] [PubMed] [Google Scholar]
- 44.Jiang S, Zhang LF, Zhang HW, Hu S, Lu MH, Liang S, Li B, Li Y, Li D, Wang ED, and Liu MF. A novel miR-155/miR-143 cascade controls glycolysis by regulating hexokinase 2 in breast cancer cells. EMBO J 31: 1985–1998, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaelin WG, Jr., and Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell 30: 393–402, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Kelly TJ, Souza AL, Clish CB, and Puigserver P. A hypoxia-induced positive feedback loop promotes hypoxia-inducible factor 1alpha stability through miR-210 suppression of glycerol-3-phosphate dehydrogenase 1-like. Mol Cell Biol 31: 2696–2706, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim JW. and Dang CV. Multifaceted roles of glycolytic enzymes. Trends Biochem Sci 30: 142–150, 2005 [DOI] [PubMed] [Google Scholar]
- 48.Kimata M, Matoba S, Iwai-Kanai E, Nakamura H, Hoshino A, Nakaoka M, Katamura M, Okawa Y, Mita Y, Okigaki M, Ikeda K, Tatsumi T, and Matsubara H. p53 and TIGAR regulate cardiac myocyte energy homeostasis under hypoxic stress. Am J Physiol Heart Circ Physiol 299: H1908–H1916, 2010 [DOI] [PubMed] [Google Scholar]
- 49.Kiriakidou M, Nelson PT, Kouranov A, Fitziev P, Bouyioukos C, Mourelatos Z, and Hatzigeorgiou A. A combined computational-experimental approach predicts human microRNA targets. Genes Dev 18: 1165–1178, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koppenol WH, Bounds PL, and Dang CV. Otto Warburg's contributions to current concepts of cancer metabolism. Nat Rev Cancer 11: 325–337, 2011 [DOI] [PubMed] [Google Scholar]
- 51.Koshiji M, Kageyama Y, Pete EA, Horikawa I, Barrett JC, and Huang LE. HIF-1alpha induces cell cycle arrest by functionally counteracting Myc. EMBO J 23: 1949–1956, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kulshreshtha R, Davuluri RV, Calin GA, and Ivan M. A microRNA component of the hypoxic response. Cell Death Differ 15: 667–671, 2008 [DOI] [PubMed] [Google Scholar]
- 53.Kumar M, Lu Z, Takwi AA, Chen W, Callander NS, Ramos KS, Young KH, and Li Y. Negative regulation of the tumor suppressor p53 gene by microRNAs. Oncogene 30: 843–853, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lazazzera BA, Beinert H, Khoroshilova N, Kennedy MC, and Kiley PJ. DNA binding and dimerization of the Fe-S-containing FNR protein from Escherichia coli are regulated by oxygen. J Biol Chem 271: 2762–2768, 1996 [DOI] [PubMed] [Google Scholar]
- 55.Le MT, Xie H, Zhou B, Chia PH, Rizk P, Um M, Udolph G, Yang H, Lim B, and Lodish HF. MicroRNA-125b promotes neuronal differentiation in human cells by repressing multiple targets. Mol Cell Biol 29: 5290–5305, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee DC, Romero R, Kim JS, Tarca AL, Montenegro D, Pineles BL, Kim E, Lee J, Kim SY, Draghici S, Mittal P, Kusanovic JP, Chaiworapongsa T, Hassan SS, and Kim CJ. miR-210 targets iron-sulfur cluster scaffold homologue in human trophoblast cell lines: siderosis of interstitial trophoblasts as a novel pathology of preterm preeclampsia and small-for-gestational-age pregnancies. Am J Pathol 179: 590–602, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, Lee J, Provost P, Radmark O, Kim S, and Kim VN. The nuclear RNase III Drosha initiates microRNA processing. Nature 425: 415–419, 2003 [DOI] [PubMed] [Google Scholar]
- 58.Lill R. Function and biogenesis of iron-sulphur proteins. Nature 460: 831–838, 2009 [DOI] [PubMed] [Google Scholar]
- 59.Loscalzo J. The cellular response to hypoxia: tuning the system with microRNAs. J Clin Invest 120: 3815–3817, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matoba S, Kang JG, Patino WD, Wragg A, Boehm M, Gavrilova O, Hurley PJ, Bunz F, and Hwang PM. p53 regulates mitochondrial respiration. Science 312: 1650–1653, 2006 [DOI] [PubMed] [Google Scholar]
- 61.Meng F, Henson R, Wehbe-Janek H, Ghoshal K, Jacob ST, and Patel T. MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology 133: 647–658, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Michelakis ED, Hampl V, Nsair A, Wu X, Harry G, Haromy A, Gurtu R, and Archer SL. Diversity in mitochondrial function explains differences in vascular oxygen sensing. Circ Res 90: 1307–1315, 2002 [DOI] [PubMed] [Google Scholar]
- 63.Michelakis ED, Thebaud B, Weir EK, and Archer SL. Hypoxic pulmonary vasoconstriction: redox regulation of O2-sensitive K+ channels by a mitochondrial O2-sensor in resistance artery smooth muscle cells. J Mol Cell Cardiol 37: 1119–1136, 2004 [DOI] [PubMed] [Google Scholar]
- 64.O'Neill LA, Sheedy FJ, and McCoy CE. MicroRNAs: the fine-tuners of toll-like receptor signalling. Nat Rev Immunol 11: 163–175, 2011 [DOI] [PubMed] [Google Scholar]
- 65.Parikh VN, Jin RC, Rabello S, Gulbahce N, White K, Hale A, Cottrill KA, Shaik RS, Waxman AB, Zhang YY, Maron BA, Hartner JC, Fujiwara Y, Orkin SH, Haley KJ, Barabasi AL, Loscalzo J, and Chan SY. MicroRNA-21 integrates pathogenic signaling to control pulmonary hypertension: results of a network bioinformatics approach. Circulation 125: 1520–1532, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Park SY, Lee JH, Ha M, Nam JW, and Kim VN. miR-29 miRNAs activate p53 by targeting p85 alpha and CDC42. Nat Struct Mol Biol 16: 23–29, 2009 [DOI] [PubMed] [Google Scholar]
- 67.Pastorino JG, Hoek JB, and Shulga N. Activation of glycogen synthase kinase 3beta disrupts the binding of hexokinase II to mitochondria by phosphorylating voltage-dependent anion channel and potentiates chemotherapy-induced cytotoxicity. Cancer Res 65: 10545–10554, 2005 [DOI] [PubMed] [Google Scholar]
- 68.Petersen KF, Dufour S, Befroy D, Garcia R, and Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med 350: 664–671, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pineles BL, Romero R, Montenegro D, Tarca AL, Han YM, Kim YM, Draghici S, Espinoza J, Kusanovic JP, Mittal P, Hassan SS, and Kim CJ. Distinct subsets of microRNAs are expressed differentially in the human placentas of patients with preeclampsia. Am J Obstet Gynecol 196: 261.e1–e6, 2007 [DOI] [PubMed] [Google Scholar]
- 70.Pozeg ZI, Michelakis ED, McMurtry MS, Thebaud B, Wu XC, Dyck JR, Hashimoto K, Wang S, Moudgil R, Harry G, Sultanian R, Koshal A, and Archer SL. In vivo gene transfer of the O2-sensitive potassium channel Kv1.5 reduces pulmonary hypertension and restores hypoxic pulmonary vasoconstriction in chronically hypoxic rats. Circulation 107: 2037–2044, 2003 [DOI] [PubMed] [Google Scholar]
- 71.Puissegur MP, Mazure NM, Bertero T, Pradelli L, Grosso S, Robbe-Sermesant K, Maurin T, Lebrigand K, Cardinaud B, Hofman V, Fourre S, Magnone V, Ricci JE, Pouyssegur J, Gounon P, Hofman P, Barbry P, and Mari B. miR-210 is overexpressed in late stages of lung cancer and mediates mitochondrial alterations associated with modulation of HIF-1 activity. Cell Death Differ 18: 465–478, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pulkkinen K, Malm T, Turunen M, Koistinaho J, and Yla-Herttuala S. Hypoxia induces microRNA miR-210 in vitro and in vivo ephrin-A3 and neuronal pentraxin 1 are potentially regulated by miR-210. FEBS Lett 582: 2397–2401, 2008 [DOI] [PubMed] [Google Scholar]
- 73.Pullen TJ, da Silva Xavier G, Kelsey G, and Rutter GA. miR-29a and miR-29b contribute to pancreatic beta-cell-specific silencing of monocarboxylate transporter 1 (Mct1). Mol Cell Biol 31: 3182–3194, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Resnick KE, Alder H, Hagan JP, Richardson DL, Croce CM, and Cohn DE. The detection of differentially expressed microRNAs from the serum of ovarian cancer patients using a novel real-time PCR platform. Gynecol Oncol 112: 55–59, 2009 [DOI] [PubMed] [Google Scholar]
- 75.Rotig A, de Lonlay P, Chretien D, Foury F, Koenig M, Sidi D, Munnich A, and Rustin P. Aconitase and mitochondrial iron-sulphur protein deficiency in Friedreich ataxia. Nat Genet 17: 215–217, 1997 [DOI] [PubMed] [Google Scholar]
- 76.Rottiers V. and Naar AM. MicroRNAs in metabolism and metabolic disorders. Nat Rev Mol Cell Biol 13: 239–250, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rouault TA. Biogenesis of iron-sulfur clusters in mammalian cells: new insights and relevance to human disease. Dis Model Mech 5: 155–164, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rouault TA. and Tong WH. Iron-sulfur cluster biogenesis and human disease. Trends Genet 24: 398–407, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Roy S, Khanna S, Hussain SR, Biswas S, Azad A, Rink C, Gnyawali S, Shilo S, Nuovo GJ, and Sen CK. MicroRNA expression in response to murine myocardial infarction: miR-21 regulates fibroblast metalloprotease-2 via phosphatase and tensin homologue. Cardiovasc Res 82: 21–29, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Salceda S. and Caro J. Hypoxia-inducible factor 1alpha (HIF-1alpha) protein is rapidly degraded by the ubiquitin-proteasome system under normoxic conditions. Its stabilization by hypoxia depends on redox-induced changes. J Biol Chem 272: 22642–22647, 1997 [DOI] [PubMed] [Google Scholar]
- 81.Sarkar J, Gou D, Turaka P, Viktorova E, Ramchandran R, and Raj JU. MicroRNA-21 plays a role in hypoxia-mediated pulmonary artery smooth muscle cell proliferation and migration. Am J Physiol Lung Cell Mol Physiol 299: L861–L871, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sayed D, He M, Hong C, Gao S, Rane S, Yang Z, and Abdellatif M. MicroRNA-21 is a downstream effector of AKT that mediates its antiapoptotic effects via suppression of Fas ligand. J Biol Chem 285: 20281–20290, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Semenza GL. Oxygen-dependent regulation of mitochondrial respiration by hypoxia-inducible factor 1. Biochem J 405: 1–9, 2007 [DOI] [PubMed] [Google Scholar]
- 84.Semenza GL, Jiang BH, Leung SW, Passantino R, Concordet JP, Maire P, and Giallongo A. Hypoxia response elements in the aldolase A, enolase 1, and lactate dehydrogenase A gene promoters contain essential binding sites for hypoxia-inducible factor 1. J Biol Chem 271: 32529–32537, 1996 [DOI] [PubMed] [Google Scholar]
- 85.Sims NR. and Anderson MF. Mitochondrial contributions to tissue damage in stroke. Neurochem Int 40: 511–526, 2002 [DOI] [PubMed] [Google Scholar]
- 86.Soifer HS, Rossi JJ, and Saetrom P. MicroRNAs in disease and potential therapeutic applications. Mol Ther 15: 2070–2079, 2007 [DOI] [PubMed] [Google Scholar]
- 87.Stadtman ER. Metal ion-catalyzed oxidation of proteins: biochemical mechanism and biological consequences. Free Radic Biol Med 9: 315–325, 1990 [DOI] [PubMed] [Google Scholar]
- 88.Taguchi A, Yanagisawa K, Tanaka M, Cao K, Matsuyama Y, Goto H, and Takahashi T. Identification of hypoxia-inducible factor-1 alpha as a novel target for miR-17-92 microRNA cluster. Cancer Res 68: 5540–5545, 2008 [DOI] [PubMed] [Google Scholar]
- 89.Tang X, Muniappan L, Tang G, and Ozcan S. Identification of glucose-regulated miRNAs from pancreatic {beta} cells reveals a role for miR-30d in insulin transcription. RNA 15: 287–293, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thum T, Gross C, Fiedler J, Fischer T, Kissler S, Bussen M, Galuppo P, Just S, Rottbauer W, Frantz S, Castoldi M, Soutschek J, Koteliansky V, Rosenwald A, Basson MA, Licht JD, Pena JT, Rouhanifard SH, Muckenthaler MU, Tuschl T, Martin GR, Bauersachs J, and Engelhardt S. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 456: 980–984, 2008 [DOI] [PubMed] [Google Scholar]
- 91.Tuder RM, Davis LA, and Graham BB. Targeting energetic metabolism: a new frontier in the pathogenesis and treatment of pulmonary hypertension. Am J Respir Crit Care Med 185: 260–266, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Voellenkle C, van Rooij J, Cappuzzello C, Greco S, Arcelli D, Di Vito L, Melillo G, Rigolini R, Costa E, Crea F, Capogrossi MC, Napolitano M, and Martelli F. MicroRNA signatures in peripheral blood mononuclear cells of chronic heart failure patients. Physiol Genomics 42: 420–426, 2010 [DOI] [PubMed] [Google Scholar]
- 93.Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, Prueitt RL, Yanaihara N, Lanza G, Scarpa A, Vecchione A, Negrini M, Harris CC, and Croce CM. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A 103: 2257–2261, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang J, Chen J, Chang P, LeBlanc A, Li D, Abbruzzesse JL, Frazier ML, Killary AM, and Sen S. MicroRNAs in plasma of pancreatic ductal adenocarcinoma patients as novel blood-based biomarkers of disease. Cancer Prev Res (Phila) 2: 807–813, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Warburg O, Posener K, and Negelein E. Ueber den stoffwechsel der carcinomzelle. Biochem Z 152: 319–344, 1924 [Google Scholar]
- 96.Weidemann A. and Johnson RS. Biology of HIF-1alpha. Cell Death Differ 15: 621–627, 2008 [DOI] [PubMed] [Google Scholar]
- 97.Xu W, Koeck T, Lara AR, Neumann D, DiFilippo FP, Koo M, Janocha AJ, Masri FA, Arroliga AC, Jennings C, Dweik RA, Tuder RM, Stuehr DJ, and Erzurum SC. Alterations of cellular bioenergetics in pulmonary artery endothelial cells. Proc Natl Acad Sci U S A 104: 1342–1347, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yan HL, Xue G, Mei Q, Wang YZ, Ding FX, Liu MF, Lu MH, Tang Y, Yu HY, and Sun SH. Repression of the miR-17–92 cluster by p53 has an important function in hypoxia-induced apoptosis. EMBO J 28: 2719–2732, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yoshioka Y, Kosaka N, Ochiya T, and Kato T. Micromanaging iron homeostasis: hypoxia-inducible micro-RNA-210 suppresses iron homeostasis-related proteins. J Biol Chem 287: 34110–34119, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang H, Gao P, Fukuda R, Kumar G, Krishnamachary B, Zeller KI, Dang CV, and Semenza GL. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell 11: 407–420, 2007 [DOI] [PubMed] [Google Scholar]
- 101.Zhang X, Ng WL, Wang P, Tian L, Werner E, Wang H, Doetsch P, and Wang Y. MicroRNA-21 modulates the levels of reactive oxygen species by targeting SOD3 and TNFalpha. Cancer Res 72: 4707–4713, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 102.Zhu XM, Han T, Sargent IL, Yin GW, and Yao YQ. Differential expression profile of microRNAs in human placentas from preeclamptic pregnancies vs normal pregnancies. Am J Obstet Gynecol 200: 661.e1–e7, 2009 [DOI] [PubMed] [Google Scholar]