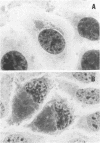
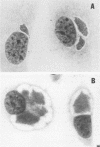
Abstract
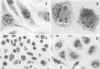
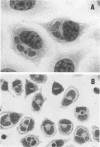
Strains of Chlamydia psittaci from cattle, sheep, pigs, mice, guinea pigs, rabbits, cats, and parrots were subdivided based on their biological characteristics. Chlamydiae grown in the yolk sac of chicken embryos were used to infect L cell monolayers. The host cells were infected without further treatment or treated with diethylaminoethyl-dextran, cycloheximide, or both. The following criteria were used for biotyping the strains: the morphology of the inclusions and time after infection at which they appeared, the effect of chlamydial multiplication on the host cell cytoskeleton, and the change in the number of cells infected in response to diethylaminoethyl-dextran and cycloheximide. These properties were determined for 29 strains of C. psittaci. Based on the results, the strains were placed into eight biotypes.
Full text
PDF


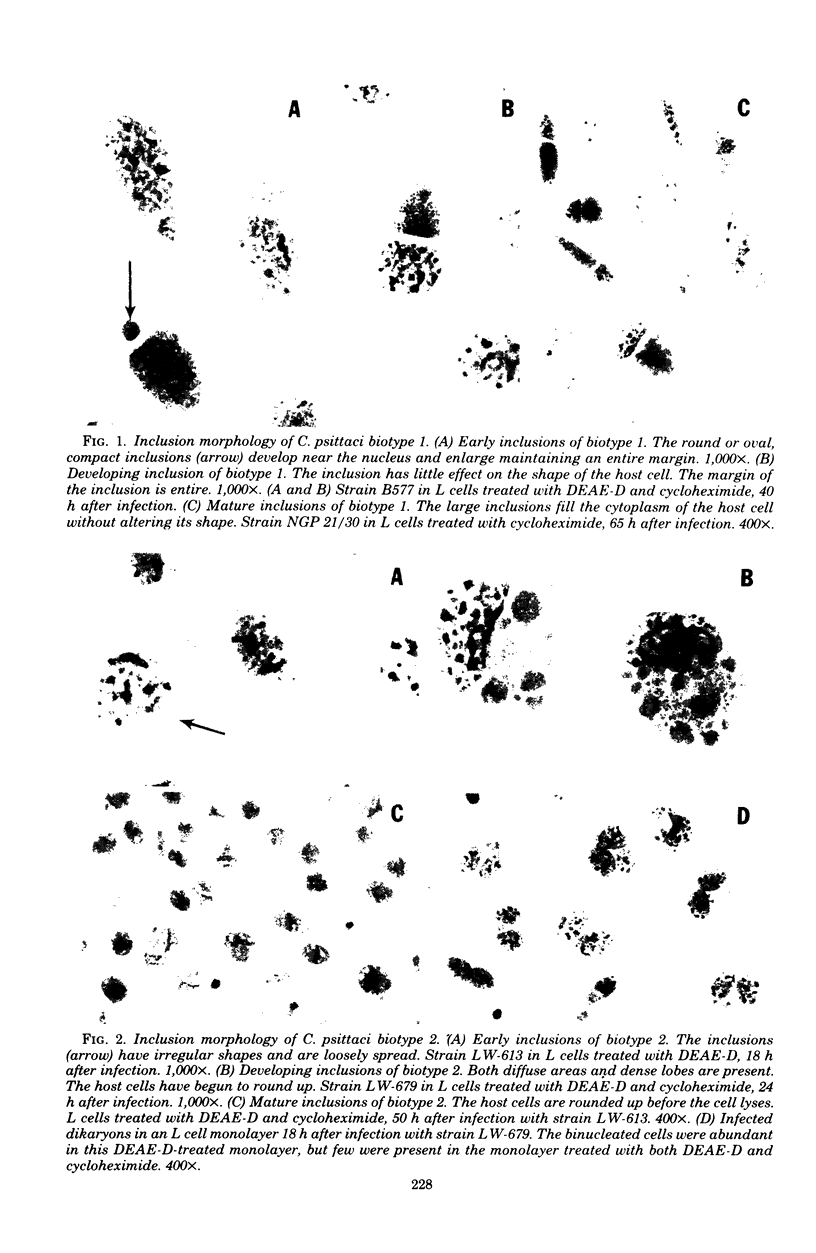



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ata F. A., Stephenson E. H., Storz J. Inapparent respiratory infection of inbred Swiss mice with sulfadiazine-resistant, iodine-negative Chlamydiae. Infect Immun. 1971 Oct;4(4):506–507. doi: 10.1128/iai.4.4.506-507.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker Y. The agent of trachoma. Recent studies of the biology, biochemistry and immunology of a prokaryotic obligate parasite of eukaryocytes. Monogr Virol. 1974;7:1–99. [PubMed] [Google Scholar]
- GORDON F. B., QUAN A. L. OCCURENCE OF GLYCOGEN IN INCLUSIONS OF THE PSITTACOSIS-LYMPHOGRANULOMA VENEREUM-TRACHOMA AGENTS. J Infect Dis. 1965 Apr;115:186–196. doi: 10.1093/infdis/115.2.186. [DOI] [PubMed] [Google Scholar]
- Harrison M. J. Enhancing effect of DEAE-Dextran on inclusion counts of an ovine Chlamydia (Bedsonia) in cell culture. Aust J Exp Biol Med Sci. 1970 Apr;48(2):207–213. doi: 10.1038/icb.1970.20. [DOI] [PubMed] [Google Scholar]
- Hobson D., Johnson F. W., Byng R. E. The growth of the ewe abortion chlamydial agent in McCoy cell cultures. J Comp Pathol. 1977 Jan;87(1):155–159. doi: 10.1016/0021-9975(77)90091-3. [DOI] [PubMed] [Google Scholar]
- Kuo C. C., Wang S. P., Grayston J. T., Alexander E. R. TRIC type K, a new immunologic type of Chlamydia trachomatis. J Immunol. 1974 Aug;113(2):591–596. [PubMed] [Google Scholar]
- MURRAY E. S. GUINEA PIG INCLUSION CONJUNCTIVITIS VIRUS. I. ISOLATION AND IDENTIFICATION AS A MEMBER OF THE PSITTACOSIS-LYMPHOGRANULOMA-TRACHOMA GROUP. J Infect Dis. 1964 Feb;114:1–12. doi: 10.1093/infdis/114.1.1. [DOI] [PubMed] [Google Scholar]
- Moulder J. W., Hatch T. P., Byrne G. I., Kellogg K. R. Immediate toxicity of high multiplicities of Chlamydia psittaci for mouse fibroblasts (L cells). Infect Immun. 1976 Jul;14(1):277–289. doi: 10.1128/iai.14.1.277-289.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton W. L., Storz J. Observations on sheep with polyarthritis produced by an agent of the psittacosis-lymphogranuloma venereum-trachoma group. Arthritis Rheum. 1967 Feb;10(1):1–12. doi: 10.1002/art.1780100102. [DOI] [PubMed] [Google Scholar]
- STORZ J., SHUPE J. L., JAMES L. F., SMART R. A. POLYARTHRITIS OF SHEEP IN THE INTERMOUNTAIN REGION CAUSED BY A PSITTACOSIS-LYMPHOGRANULOMA AGENT. Am J Vet Res. 1963 Nov;24:1201–1206. [PubMed] [Google Scholar]
- STORZ J. SUPERINFECTION OF PREGNANT EWES LATENTLY INFECTED WITH A PSITTACOSIS-LYMPHOGRANULOMA AGENT. Cornell Vet. 1963 Oct;53:469–480. [PubMed] [Google Scholar]
- Schachter J., Banks J., Sugg N., Sung M., Storz J., Meyer K. F. Serotyping of Chlamydia. I. Isolates of ovine origin. Infect Immun. 1974 Jan;9(1):92–94. doi: 10.1128/iai.9.1.92-94.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter J., Banks J., Sugg N., Sung M., Storz J., Meyer K. F. Serotyping of Chlamydia: isolates of bovine origin. Infect Immun. 1975 May;11(5):904–907. doi: 10.1128/iai.11.5.904-907.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storz J., Carroll E. J., Ball L., Faulkner L. C. Isolation of a psittacosis agent (Chlamydia) from semen and epididymis of bulls with seminal vesiculitis syndrome. Am J Vet Res. 1968 Mar;29(3):549–555. [PubMed] [Google Scholar]
- Storz J., Marriott M. E., Winterer B. I. Detection and separation of simultaneous Enterovirus and intestinal Chlamydia infection of calves. Zentralbl Bakteriol Orig. 1969 May;210(1):75–81. [PubMed] [Google Scholar]
- Storz J. Psittacosis-lymphogranuloma infection of sheep. Antigenic structures and interrelations of PL agents associated with polyarthritis, enzootic abortion, intrauterine and latent intestinal infections. J Comp Pathol. 1966 Oct;76(4):351–362. doi: 10.1016/0021-9975(66)90055-7. [DOI] [PubMed] [Google Scholar]
- Storz J., Smart R. A., Marriott M. E., Davis R. V. Polyarthritis of calves: isolation of psittacosis agents from affected joints. Am J Vet Res. 1966 May;27(118):633–641. [PubMed] [Google Scholar]
- Storz J., Spears P. Chlamydiales: properties, cycle of development and effect on eukaryotic host cells. Curr Top Microbiol Immunol. 1977;76:167–214. doi: 10.1007/978-3-642-66653-7_5. [DOI] [PubMed] [Google Scholar]
- Storz J. Uber eine natürliche Infektion eines Meerschweinchenbestandes mit einem Erreger aus der Psittakose-Lymphogranuloma Gruppe. Zentralbl Bakteriol Orig. 1964 Aug;193(4):432–445. [PubMed] [Google Scholar]