Abstract
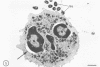
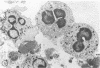
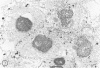
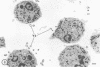
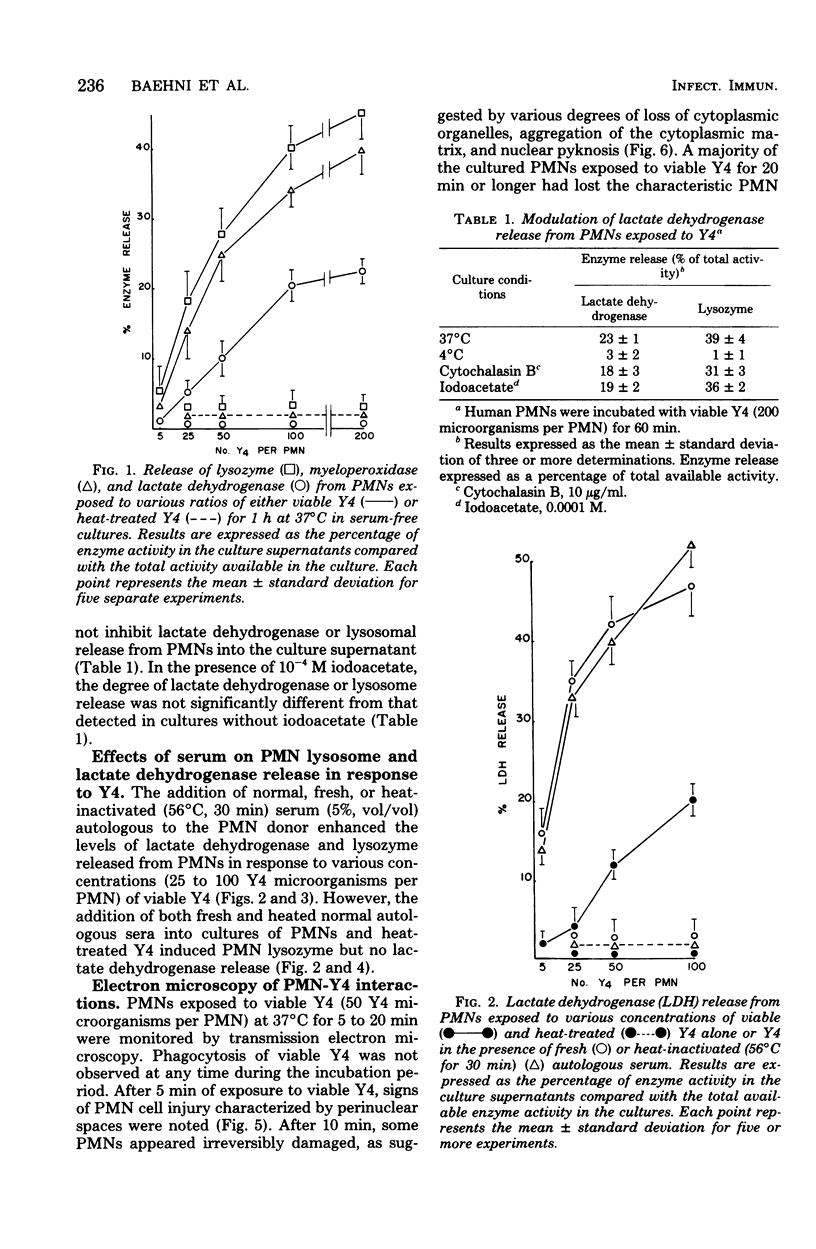
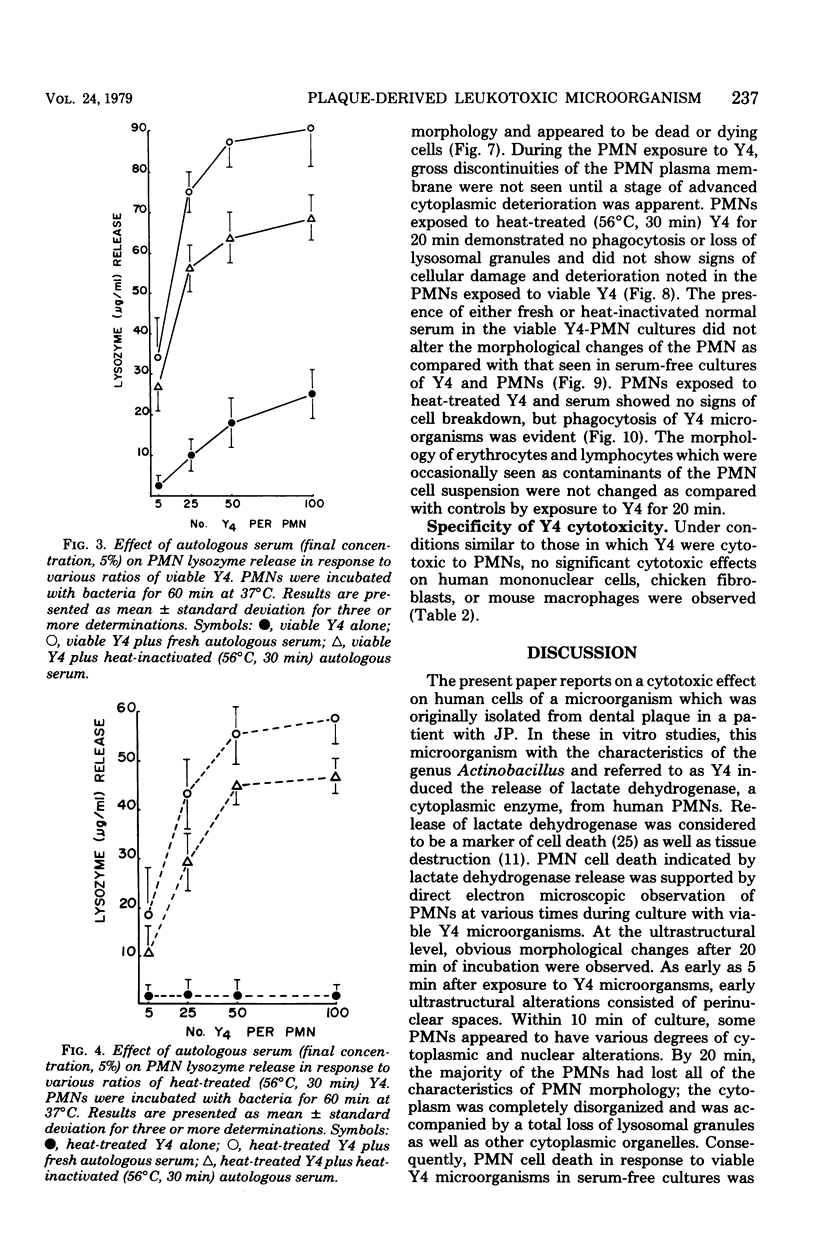
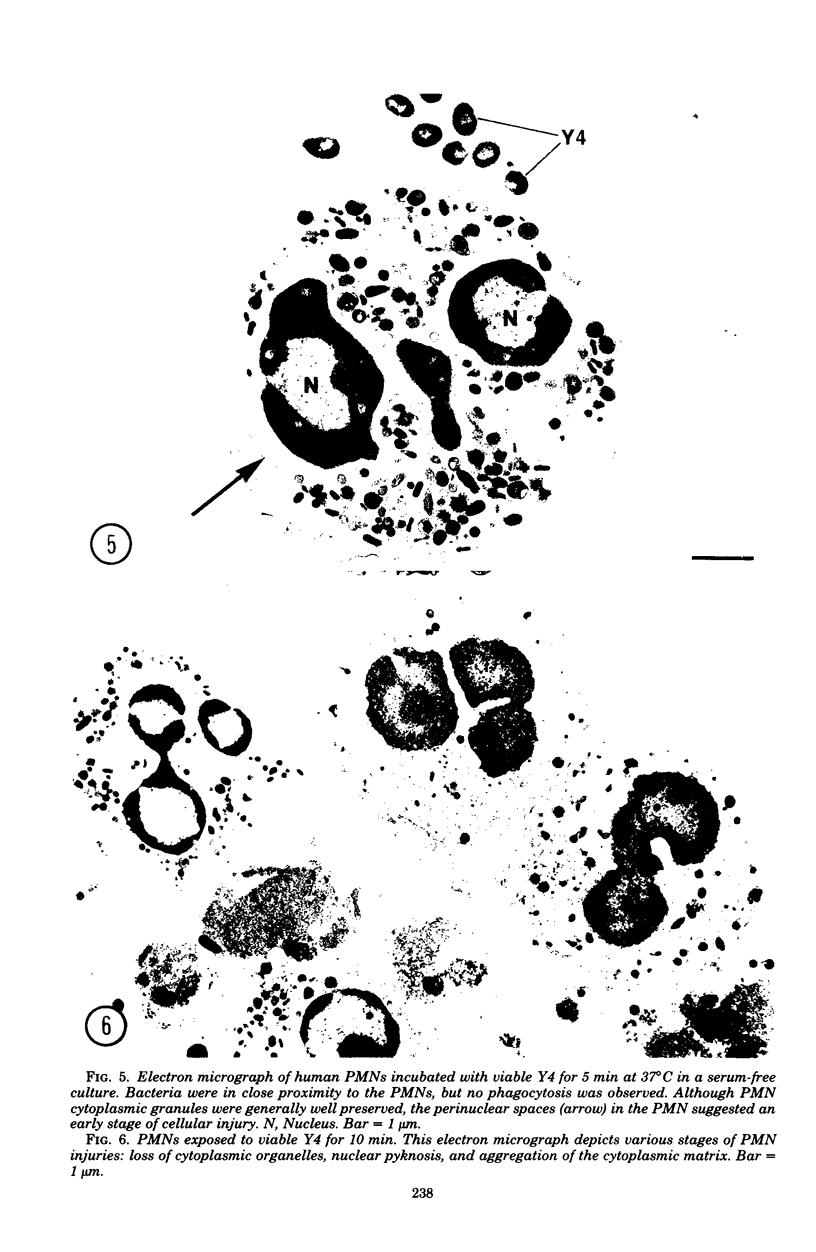
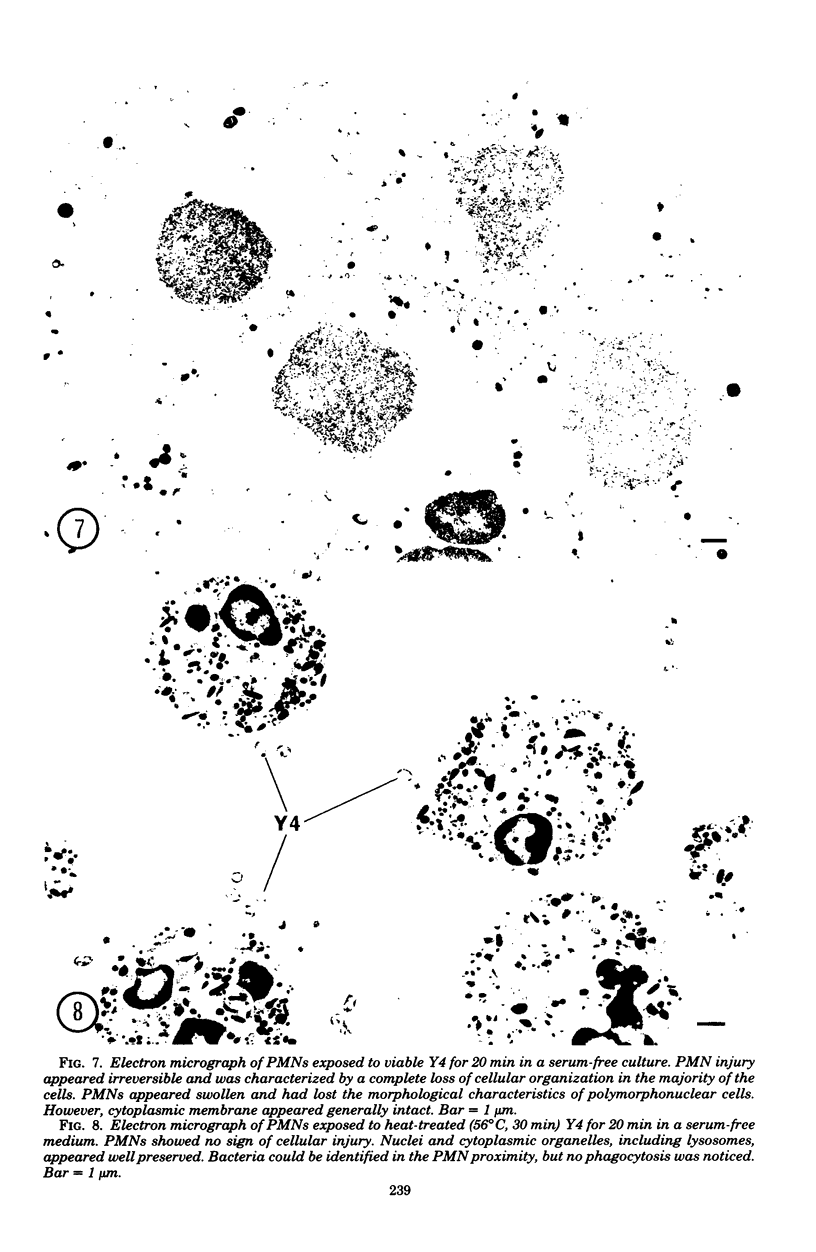
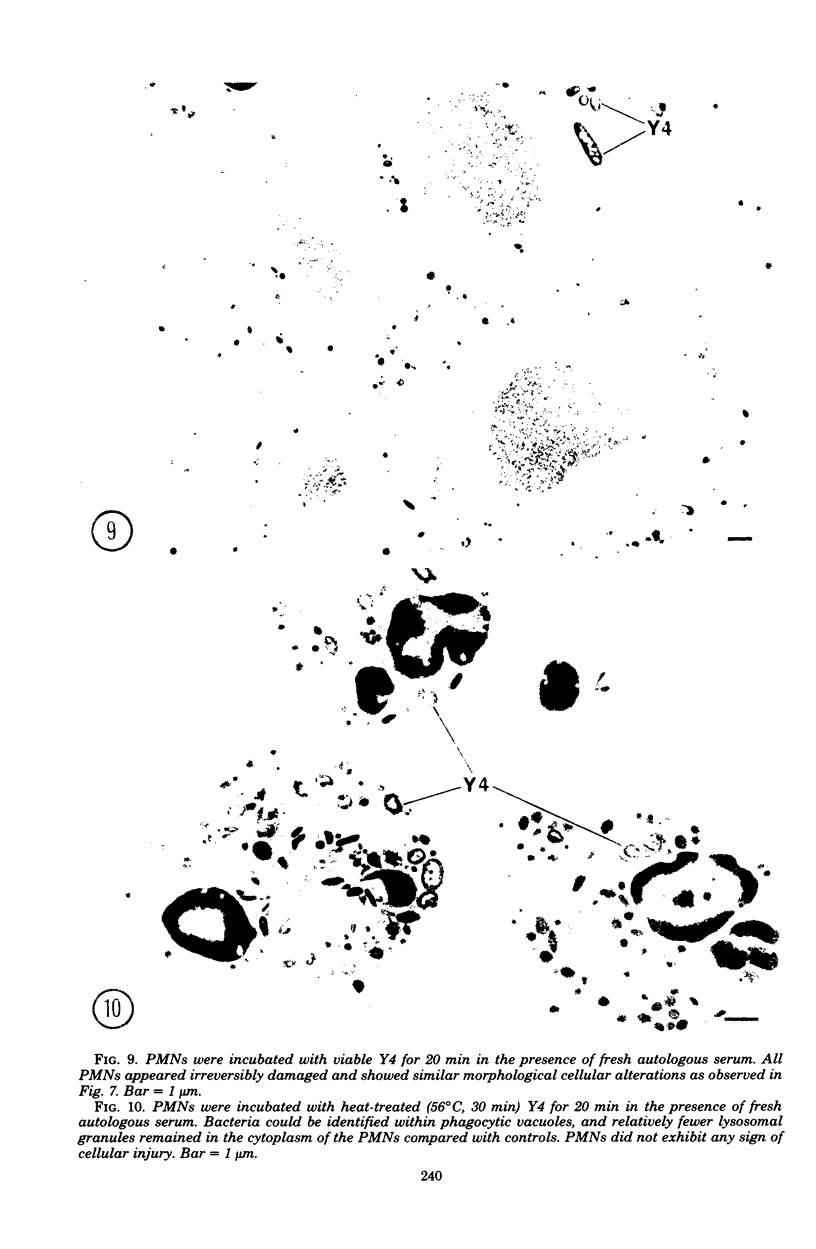
In the present study we identified a gram-negative anaerobic rod referred to as Y4 which was cytotoxic for human polymorphonuclear leukocytes. Y4 was isolated from dental plaque of a patient with juvenile periodontitis and presented most of the taxonomic characteristics of Actinobacillus species. Under experimental conditions, viable Y4 were cytotoxic for human peripheral blood polymorphonuclear leukocytes in serum-free cultures. Cytotoxicity was dependent on bacterial concentrations and was enhanced in the presence of a fresh or heat-inactivated (56 degrees C, 30 min) autologous serum. Leukotoxicity was independent of phagocytosis. Y4 leukotoxic effect was abolished when bacteria were heat treated (56 degrees C, 30 min) or when incubations were carried out at 4 degrees C instead of at 37 degrees C. The leukotoxicity was monitored by electron microscopy and biochemically by measuring lactate dehydrogenase indicator of cell viability. No cytotoxic effects of Y4 on human mononuclear cells, chicken fibroblasts, or mouse macrophages were detected under the conditions studied. Polymorphonuclear leukocytes may play an important role in the host defense against bacteria in periodontal disease. The cytotoxic effect of Y4 for polymorphonuclear leukocytes presented in this study is the first report of a direct offensive microbial vector in a plaque-derived microorganism and may prove to be relevant in the pathogenesis of juvenile periodontitis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Goldman R. The effect of cytochalasin B and colchicine on concanavalin A induced vacuolation in mouse peritoneal macrophages. Exp Cell Res. 1976 May;99(2):385–394. doi: 10.1016/0014-4827(76)90596-6. [DOI] [PubMed] [Google Scholar]
- Griffin F. M., Jr, Silverstein S. C. Segmental response of the macrophage plasma membrane to a phagocytic stimulus. J Exp Med. 1974 Feb 1;139(2):323–336. doi: 10.1084/jem.139.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther G. R., Wang J. L., Yahara I., Cunningham B. A., Edelman G. M. Concanavalin A derivatives with altered biological activities. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1012–1016. doi: 10.1073/pnas.70.4.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRSCH J. G., STRAUSS B. STUDIES ON HEAT-LABILE OPSONIN IN RABBIT SERUM. J Immunol. 1964 Jan;92:145–154. [PubMed] [Google Scholar]
- Ofek I., Beachey E. H., Bisno A. L. Resistance of Neisseria gonorrhoeae to phagocytosis: relationship to colonial morphology and surface pili. J Infect Dis. 1974 Mar;129(3):310–316. doi: 10.1093/infdis/129.3.310. [DOI] [PubMed] [Google Scholar]
- Ofek I., Mirelman D., Sharon N. Adherence of Escherichia coli to human mucosal cells mediated by mannose receptors. Nature. 1977 Feb 17;265(5595):623–625. doi: 10.1038/265623a0. [DOI] [PubMed] [Google Scholar]
- Old D. C. Inhibition of the interaction between fimbrial haemagglutinins and erythrocytes by D-mannose and other carbohydrates. J Gen Microbiol. 1972 Jun;71(1):149–157. doi: 10.1099/00221287-71-1-149. [DOI] [PubMed] [Google Scholar]
- Oliver J. M., Ukena T. E., Berlin R. D. Effects of phagocytosis and colchicine on the distribution of lectin-binding sites on cell surfaces. Proc Natl Acad Sci U S A. 1974 Feb;71(2):394–398. doi: 10.1073/pnas.71.2.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovitch M. Phagocytosis: the engulfment stage. Semin Hematol. 1968 Apr;5(2):134–155. [PubMed] [Google Scholar]
- SHEDDEN W. I. Fimbriae and haemagglutinating activity in strains of Proteus hauseri. J Gen Microbiol. 1962 Apr;28:1–7. doi: 10.1099/00221287-28-1-1. [DOI] [PubMed] [Google Scholar]
- Salit I. E., Gotschlich E. C. Type I Escherichia coli pili: characterization of binding to monkey kidney cells. J Exp Med. 1977 Nov 1;146(5):1182–1194. doi: 10.1084/jem.146.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon N., Lis H. Lectins: cell-agglutinating and sugar-specific proteins. Science. 1972 Sep 15;177(4053):949–959. doi: 10.1126/science.177.4053.949. [DOI] [PubMed] [Google Scholar]
- Silverblatt F. J. Host-parasite interaction in the rat renal pelvis: a possible role for pili in the pathogenesis of pyelonephritis. J Exp Med. 1974 Dec 1;140(6):1696–1711. doi: 10.1084/jem.140.6.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverblatt F. J., Ofek I. Influence of pili on the virulence of Proteus mirabilis in experimental hematogenous pyelonephritis. J Infect Dis. 1978 Nov;138(5):664–667. doi: 10.1093/infdis/138.5.664. [DOI] [PubMed] [Google Scholar]
- Silverstein S. C., Steinman R. M., Cohn Z. A. Endocytosis. Annu Rev Biochem. 1977;46:669–722. doi: 10.1146/annurev.bi.46.070177.003321. [DOI] [PubMed] [Google Scholar]
- Stossel T. P. How do phagocytes eat? Ann Intern Med. 1978 Sep;89(3):398–402. doi: 10.7326/0003-4819-89-3-398. [DOI] [PubMed] [Google Scholar]
- Stossel T. P., Mason R. J., Pollard T. D., Vaughan M. Isolation and properties of phagocytic vesicles. II. Alveolar macrophages. J Clin Invest. 1972 Mar;51(3):604–614. doi: 10.1172/JCI106850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stossel T. P., Pollard T. D., Mason R. J., Vaughan M. Isolation and properties of phagocytic vesicles from polymorphonuclear leukocytes. J Clin Invest. 1971 Aug;50(8):1745–1747. doi: 10.1172/JCI106664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Sparks E., Young D., King G. Studies on Gonococcus infection. X. Pili and leukocyte association factor as mediators of interactions between gonococci and eukaryotic cells in vitro. Infect Immun. 1975 Jun;11(6):1352–1361. doi: 10.1128/iai.11.6.1352-1361.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R., Karnovsky M. J. Ligand-induced movement of lymphocyte membrane macromolecules. V. Capping, cell movement, and microtubular function in normal and lectin-treated lymphocytes. J Exp Med. 1974 Nov 1;140(5):1207–1220. doi: 10.1084/jem.140.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unanue E. R., Karnovsky M. J. Redistribution and fate of Ig complexes on surface of B lymphocytes: functional implications and mechanisms. Transplant Rev. 1973;14:184–210. doi: 10.1111/j.1600-065x.1973.tb00107.x. [DOI] [PubMed] [Google Scholar]
- Van Oss C. J., Gillman C. F. Phagocytosis as a surface phenomenon. Contact angles and phagocytosis of non-opsonized bacteria. J Reticuloendothel Soc. 1972 Sep;12(3):283–292. [PubMed] [Google Scholar]
- White J. G. Fine structural alterations induced in platelets by adenosine diphosphate. Blood. 1968 May;31(5):604–622. [PubMed] [Google Scholar]
- Wilkinson P. C. Recognition and response in mononuclear and granular phagocytes. Clin Exp Immunol. 1976 Sep;25(3):355–366. [PMC free article] [PubMed] [Google Scholar]
- van Oss C. J., Gillman C. F., Good R. J. The influence of the shape of phagocytes on their adhesiveness. Immunol Commun. 1972;1(6):627–636. doi: 10.3109/08820137209022969. [DOI] [PubMed] [Google Scholar]