Abstract
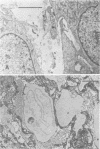
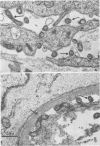
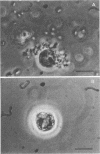
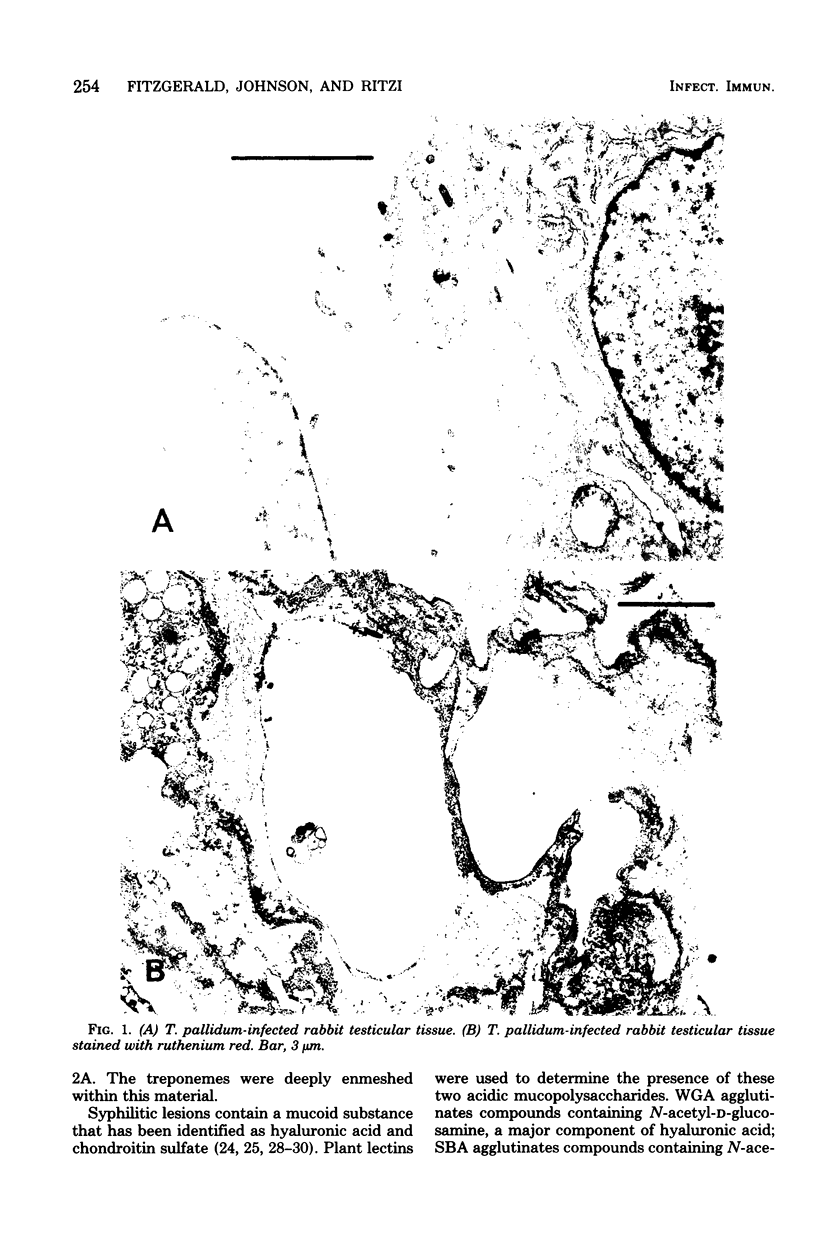
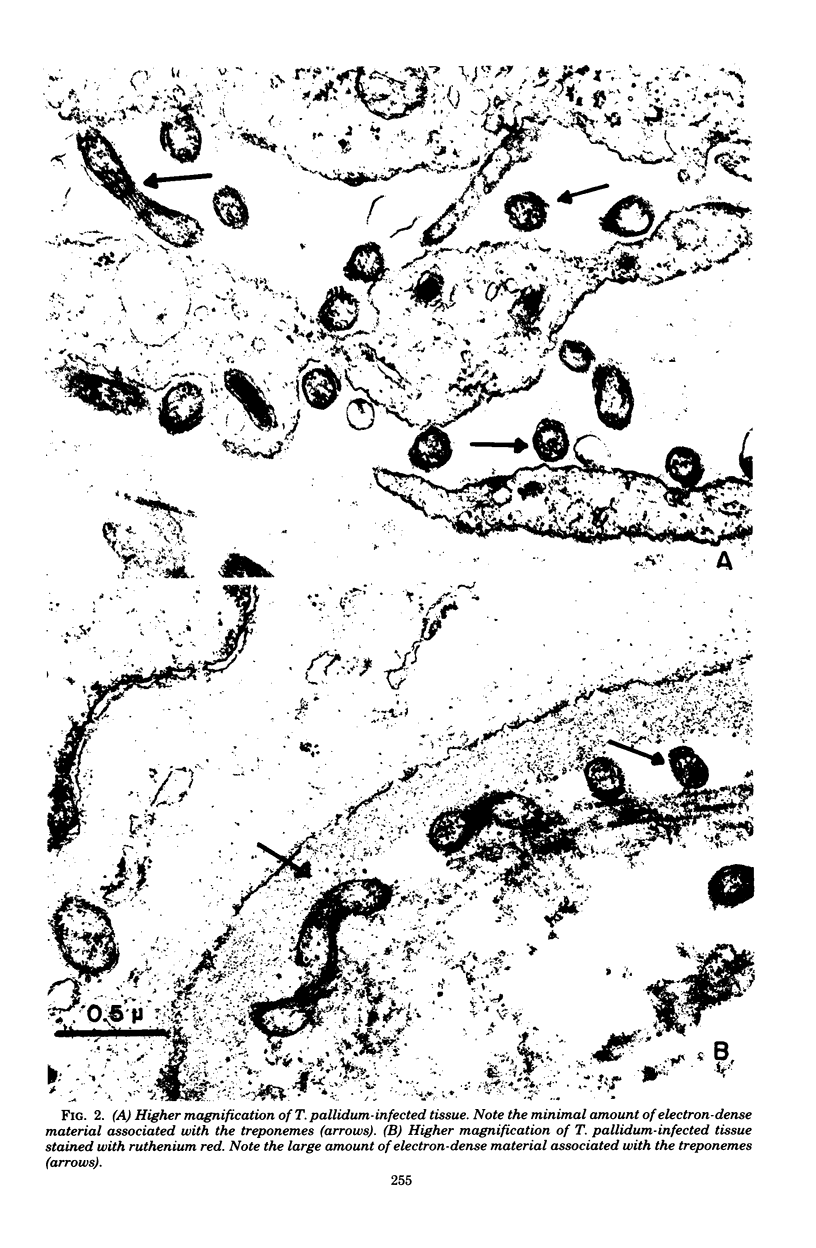
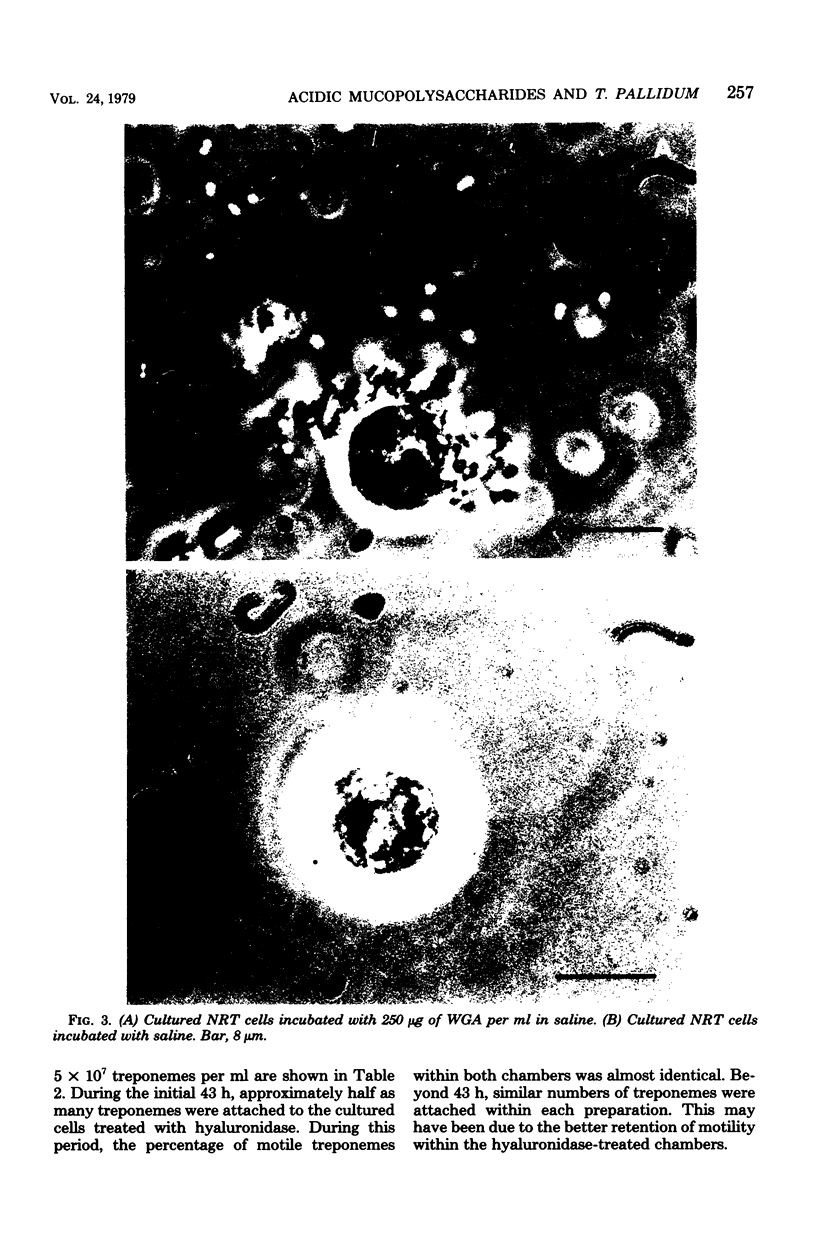
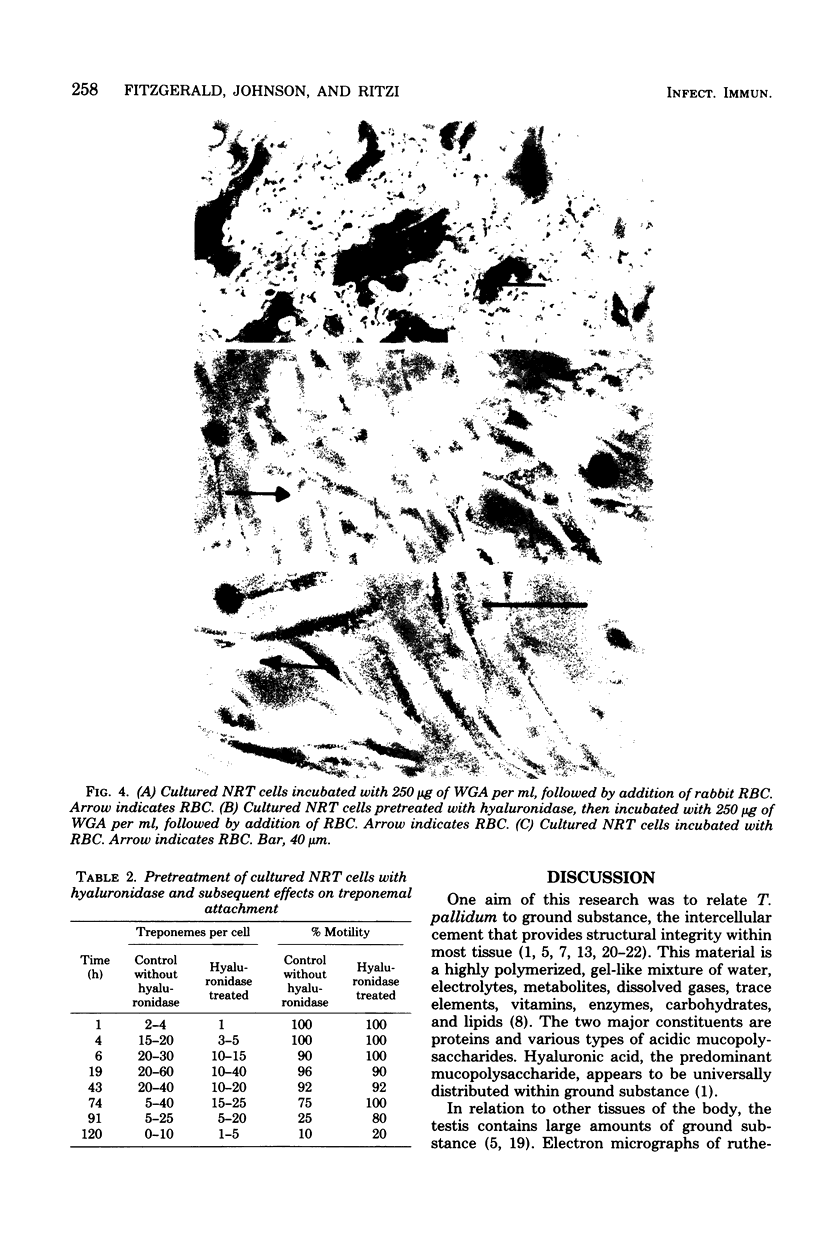
Attempts were made to relate Treponema pallidum to the acidic mucopolysaccharides that occur in vivo within host ground substance and in vitro on the surface of cultured testicular cells. Infected testicular tissue was fixed and processed for transmission electron microscopy in the presence of ruthenium red. The use of this inorganic dye demonstrated the large quantity of mucopolysaccharide within testicular tissue and the intimate association of treponemes with this material. Wheat germ agglutinin and soybean agglutinin agglutinated freshly harvested trypsinized testicular cells and trypsinized cultured cells derived from normal rabbit testes (NRT). When stained with toluidine blue, both cell preparations were metachromatic. Prior treatment of cultured NRT cells with hyaluronidase slightly decreased their sensitivity to agglutination by wheat germ agglutinin and soybean agglutinin. Lectin agglutination, metachromasia, and hyaluronidase susceptibility indicated that freshly harvested testicular cells and NRT cells have surface-associated acidic mucopolysaccharides that are probably hyaluronic acid and chondroitin sulfate. A rabbit erythrocyte “sandwich” technique was devised to show that hyaluronidase removed wheat germ agglutinin receptors from the cultured NRT cells. Prior incubation of NRT cells with hyaluronidase, followed by the addition of T. pallidum, resulted in a reduction in numbers of treponemes attached to the NRT cells. The attachment of T. pallidum appears to be mediated through the acidic mucopolysaccharides on the surface of NRT cells. The findings are discussed in terms of the importance of host ground substance mucopolysaccharide to the syphilitic infective process.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BELANGER L. F., HARTNETT A. Persistent toluidine blue metachromasia. J Histochem Cytochem. 1960 Jan;8:75–75. doi: 10.1177/8.1.75. [DOI] [PubMed] [Google Scholar]
- BELANGER L. F., MIGICOVSKY B. B. A comparison between different mucopolysacchwride stains as applied to chick epiphyseal cartilage. J Histochem Cytochem. 1961 Jan;9:73–78. doi: 10.1177/9.1.73. [DOI] [PubMed] [Google Scholar]
- BLUMENKRANTZ N. Microtest for mucopolysaccharides by means of toluidine blue: with special reference to hyaluronic acid. Clin Chem. 1957 Dec;3(6):696–702. [PubMed] [Google Scholar]
- Burger M. M., Martin G. S. Agglutination of cells transformed by Rous sarcoma virus by wheat germ agglutinin and concanavalin A. Nat New Biol. 1972 May 3;237(70):9–12. doi: 10.1038/newbio237009a0. [DOI] [PubMed] [Google Scholar]
- Cameron E., Pauling L. Ascorbic acid and the glycosaminoglycans. An orthomolecular approach to cancer and other diseases. Oncology. 1973;27(2):181–192. doi: 10.1159/000224733. [DOI] [PubMed] [Google Scholar]
- DeLAMATER E. D., SAURINO V. R., URBACH F. Studies on the immunology of spirochetoses. I. Effect of cortisone and experimental spirochetosis. Am J Syph Gonorrhea Vener Dis. 1952 Mar;36(2):127–139. [PubMed] [Google Scholar]
- Fitzgerald T. J., Johnson R. C., Miller J. N., Sykes J. A. Characterization of the attachment of Treponema pallidum (Nichols strain) to cultured mammalian cells and the potential relationship of attachment to pathogenicity. Infect Immun. 1977 Nov;18(2):467–478. doi: 10.1128/iai.18.2.467-478.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Johnson R. C. Surface mucopolysaccharides of Treponema pallidum. Infect Immun. 1979 Apr;24(1):244–251. doi: 10.1128/iai.24.1.244-251.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Miller J. N., Sykes J. A. Treponema pallidum (Nichols strain) in tissue cultures: cellular attachment, entry, and survival. Infect Immun. 1975 May;11(5):1133–1140. doi: 10.1128/iai.11.5.1133-1140.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERSH I., CATCHPOLE H. R. The organization of ground substance and basement membrane and its significance in tissue injury disease and growth. Am J Anat. 1949 Nov;85(3):457-521, incl 7 pl. doi: 10.1002/aja.1000850304. [DOI] [PubMed] [Google Scholar]
- GROSSFELD H., MEYER K., GODMAN G., LINKER A. Mucopolysaccharides produced in tissue culture. J Biophys Biochem Cytol. 1957 May 25;3(3):391–396. doi: 10.1083/jcb.3.3.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSSFELD H. Positive mucin clot test in supernates of cultures of avian embryonic brain. Proc Soc Exp Biol Med. 1957 Dec;96(3):844–846. doi: 10.3181/00379727-96-23627. [DOI] [PubMed] [Google Scholar]
- GROSSFELD H. Studies on production of hyaluronic acid in tissue culture; the presence of hyaluronidase in embryo extract. Exp Cell Res. 1958 Feb;14(1):213–216. doi: 10.1016/0014-4827(58)90231-3. [DOI] [PubMed] [Google Scholar]
- HOLLANDER D. H., TURNER T. B. The role of temperature in experimental treponemal infection. Am J Syph Gonorrhea Vener Dis. 1954 Nov;38(6):489–505. [PubMed] [Google Scholar]
- Luft J. H. Fine structures of capillary and endocapillary layer as revealed by ruthenium red. Fed Proc. 1966 Nov-Dec;25(6):1773–1783. [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. II. Fine structural localization in animal tissues. Anat Rec. 1971 Nov;171(3):369–415. doi: 10.1002/ar.1091710303. [DOI] [PubMed] [Google Scholar]
- MOORE R. D., SCHOENBERG M. D. Studies on connective tissue. I. The polysaccharides of the human umbilical cord. AMA Arch Pathol. 1957 Jul;64(1):39–45. [PubMed] [Google Scholar]
- MORRIS C. C. Quantitative studies on the production of acid mucopolysaccharides by replicate cell cultures of rat fibroblasts. Ann N Y Acad Sci. 1960 Jun 30;86:878–915. doi: 10.1111/j.1749-6632.1960.tb42848.x. [DOI] [PubMed] [Google Scholar]
- SCOTT V., DAMMIN G. J. Hyaluronidase and experimental syphilis. III. Metachromasia in syphilitic orchitis and its relationship to hyaluronic acid. Am J Syph Gonorrhea Vener Dis. 1950 Nov;34(6):501–514. [PubMed] [Google Scholar]
- SCOTT V., DAMMIN G. J. Morphologic and histochemical sequences in syphilitic and in tuberculous orchitis in the rabbit. Am J Syph Gonorrhea Vener Dis. 1954 May;38(3):189–202. [PubMed] [Google Scholar]
- SYKES J. A., MOORE E. B. A simple tissue culture chamber. Tex Rep Biol Med. 1960;18:288–297. [PubMed] [Google Scholar]
- TURNER T. B., HOLLANDER D. H. Cortisone in experimental syphilis; a preliminary note. Bull Johns Hopkins Hosp. 1950 Nov;87(5):505–509. [PubMed] [Google Scholar]
- TURNER T. B., HOLLANDER D. H. Studies on the mechanism of action of cortisone in experimental syphilis. Am J Syph Gonorrhea Vener Dis. 1954 Sep;38(5):371–387. [PubMed] [Google Scholar]
- Wight T. N., Ross R. Proteoglycans in primate arteries. I. Ultrastructural localization and distribution in the intima. J Cell Biol. 1975 Dec;67(3):660–674. doi: 10.1083/jcb.67.3.660. [DOI] [PMC free article] [PubMed] [Google Scholar]