Abstract
Background
Optical mammography is a new diagnostic method that uses Near-infrared for detection of functional abnormalities and shows tissue activities by measuring absorption and scattering of Near-infrared light. This study aims to evaluate the safety and effectiveness of this technology.
Methods
Cochrane Library (Issue 10, 2012) and Medline (Nov 2012) weresearched using free text and Mesh. Studies that compared optical mammography with other diagnostic methods and used outcomes such as sensitivity, specificity and safety were included.
Results
Twelve studies were included in this review. A multicenter RCT showed that among 875 biopsied lesions, suspicion index led to 97% sensitivity, 14%specificity, 95% negative predictive value and 24% positive predictive value. In terms of oxygenation index, the included studies found that the process should be used with various wavelengths compared to single wavelength technique (690, 750, 788, 856 nm or 683, 912, 975nm). In terms of sensitivity and specificity, Diffuse Optical Tomography Computer Aided Detection is capable of distinguishing healthy tissues from malignant ones with 89% sensitivity and 94% specificity. Also, this technology could show increased blood flow around the tumor tissue compared to the healthy tissue effectively. Included studies did not report any information about the effects of technology on changing the treatment process or the final health outcomes.
Conclusion
Optical mammography is a safe, noninvasive, non-ionized diagnostic technology that can be used as a diagnostic supplement alongside conventional mammography for differentiating benign and malignant tumors. Women with higher breast density should be screened at younger ages and with more persistence than those who have lower densities.
Keywords: Optical mammography, Diffuse optical imaging, Near-infrared spectroscopy, Breast cancer
Introduction
Breast cancer is the most common type of cancer and the principle cause of cancer related deaths in women worldwide. It is estimated that in western countries, 89% of women diagnosed with breast cancer are still alive 5 years after diagnosis, which is mainly due to the early detection and treatment [1]. A mammogram is used to detect signs of breast cancer by a very low dose of x-ray; 85-90% of breast cancers can be detected by mammograms and they can also identify a tumor up to two years before a lump can be felt. According to the (Food and Drug Administration) FDA, “Mammography is the best method for detecting breast cancer in its earliest stages, when the disease can be most successfully treated and there are more treatment options” [2]. Effectiveness of this method depends on several factors such as age, family history, (Body Mass Index) BMI and hormone therapy [3]. This technique is performed using ionizing radiations, which may sometimes cause problems for the patient in terms of safety; especially in younger women it would have lower effectiveness due to their higher breast density. X-ray mammography is usually associated with false positive and negative results and has low specificity for some types of tumors, which, in turn, leads to increased unnecessary medical procedures (such as biopsy). However, optical mammography using Near Infrared Ray (NIR) diagnoses abnormalities and has become a new diagnostic tool to serve as a supplement for traditional mammography. Unlike the X-ray technique, this type of mammography is noninvasive, non-ionized and cost effective. Using this method, the breast is compressed a bit or there is no need for it at all. This imaging system can diagnose very minor physiologic changes in the tissue and add value to breast imaging [4]. Optical mammography employs near- infrared light (typically in the wavelength range 670-970 nm) to non-invasively probe the female breast. Over this spectral region, hemoglobin is the dominant intrinsic contrast agent. The hemoglobin concentration and its oxygen saturation are two key parameters that may allow not only the detection of cancer, but also the discrimination between malignant and benign breast lesions using optical methods [5]. Optical mammography technology is done in three ways: A) Continuous Wave (CW) in which simply light dilution at breast surface is measured; B) Time Domain (TD), which involves illuminating the breast with short light pulses; and C) Frequency Domain (FD), in which system flows continuous light currents in oscillation fields at higher frequencies (tens to hundreds of MHz) into the tissue [4]. Despite the use of optical mammography technology in many parts of the world, no reliable and comprehensive information is available on its effectiveness, cost effectiveness and safety. This study was conducted by the suggestion of Health Technology Assessment (HTA) office of Iranian Ministry of Health to evaluate the safety and effectiveness of this technology to help appropriate decision making using this technology in Iran.
Materials and Methods
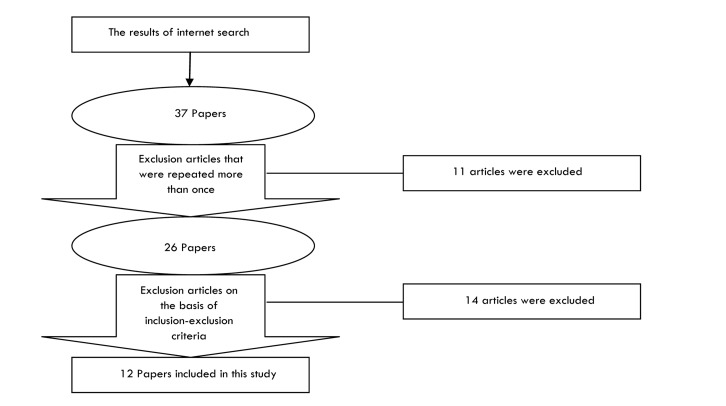
The main electronic medical databases including Cochrane Library (DARE, NHS EEDs, CENTRAL and Cochrane Systematic Reviews) and Ovid Medline were searched for articles published from 1950 to November 2012, with no language restriction. Mesh and free text were used in the search strategy (Table 1 and 2). Retrieved articles were put into an endnote database and the duplicated papers were removed. The titles and abstracts of the remaining papers were checked to exclude non-relevant studies. The full texts of the remaining articles were checked against the inclusion/exclusion criteria to select studies for the review (Figure 1). Papers were checked independently by two reviewers. The risk of biases was examined by one reviewer and checked by a second reviewer using standard criteria suggested for quality appraisal of diagnostic test studies by the Cochrane Collaboration. A structured form was used to collect the data from the included studies. Data were collected by the first reviewer and checked by a second reviewer. Inclusion criteria were patients with suspected breast cancer undergoing optical mammography compared with any other diagnostic technique when the outcomes were sensitivity, specificity and safety. Papers on healthy people or studies related to tissues and chemicals were excluded. Qualitative analysis was done using thematic synthesis.
Table 1.
Search strategy for Cochrane library
| Number of papers | Search strategy | No. |
|---|---|---|
| 1366 | mammogra* | #1 |
| 834 | Mesh descriptor Mammography explode all trees | #2 |
| 1367 | (#1 OR #2) | #3 |
| 3618 | optic* | #4 |
| 2332 | infra* | #5 |
| 5874 | (#4 OR #5) | #6 |
| 15 | (#3 AND #6) | #7 |
Table 2.
Search strategy for Ovid Medline
| Number of papers | Search strategy | No. |
|---|---|---|
| 9409 | mammogra* {No Related Terms} | #1 |
| 14941 | optic* {No Related Terms} | #2 |
| 13365 | infra* {No Related Terms} | #3 |
| 28285 | 2 or 3 | #4 |
| 22 | 1 and 4 | #5 |
Figure 1.
Flow of papers through the study
Results
In this review, 12 articles were included (Figure 1, Table 3 and 4); the majority of them (10 studies) were diagnostic studies [6-16]; one was systematic review [4] and one was multicenter clinical trial [6]. Two papers were published in 2002 [6, 12], four in 2003 [7, 10, 15, 16], three in 2004 [13, 11, 8], one in 2005 [14], one in 2007 [4] and one in 2010 [9]. Six articles were from the U.S. [6, 7, 8, 9, 12, and 13], two from Italy [10, 15], one from the UK [4], one from Netherlands [11], one from Canada [13] and one from Germany [16]. The number of samples in the included studies ranged from 24 to 769. Concerning the safety of technology, two studies used the rate of invasive process of technology and extent of ionizing radiation at the same time [4, 6]. Concerning effectiveness, the included studies used different parameters, two studies used sensitivity and specificity [4, 12], one study used index of suspicion, sensitivity, specificity, positive predictive value and negative predictive value [6], 4 papers used CNR (contrast to noise ratio), resolution and image contrast [7, 9, 10, 11], three studies, oxygenation index [8, 15, 16] and three studies used the physical changes in the breast tissue [13,14,16].
Table 3.
The list of included papers
| No. | Author/publication date | Paper title |
|---|---|---|
| 1 | Leff, D et al./2007[4] | Diffuse optical imaging of the healthy and diseased breast: a systematic review |
| 2 | Parisky YR et al. / 2002[6] | Efficacy of computerized infrared imaging analysis to evaluate mammographically suspicious lesions. |
| 3 | Li A et al. /2003[7] | Tomographic optical breast imaging guided by three-dimensional mammography |
| 4 | Heffer E et al. / 2004[8] | Near-infrared imaging of the human breast: complementing hemoglobin concentration maps with oxygenation images. |
| 5 | DR Busch et al./2010[9] | Computer aided automatic detection of malignant lesions in diffuse optical mammography |
| 6 | Taroni P. /2003[10] | Do shorter wavelengths improve contrast in optical mammography? |
| 7 | van Veen RL et al./ 2004[11] | Intraoperatively assessed optical properties of malignant and healthy breast tissue used to determine the optimum wavelength of contrast for optical mammography. |
| 8 | Cerussi AE et al. /2002[12] | Spectroscopy enhances the information content of optical mammography |
| 9 | Simick M et al. /2004[13] | Non-ionizing near-infrared radiation transillumination spectroscopy for breast tissue density and assessment of breast cancer risk |
| 10 | Turgut Durduran et al./ 2005[14] | Diffuse optical measurement of blood flow in breast tumors |
| 11 | Pifferi A et al. /2003[15] | Four-wavelength time-resolved optical mammography in the 680-980-nm range |
| 12 | Grosenick D et al. /2003[16] | Time-domain optical mammography: initial clinical results on detection and characterization of breast tumors |
Table 4.
The list of excluded paper and exclusion reason
| No. | Author/publication date | Exclusion reason |
|---|---|---|
| 1 | Chon KS et al. /2009[17] | Using digital mammography as an intervention |
| 2 | Grosenick et al. /2007[18] | Using phantom in the study |
| 3 | Benevides LA et al. /2007[19] | Not using optical mammography as an intervention |
| 44 | Krug B et al. /2005[20] | Using digital mammography as an intervention |
| 5 | Bradford et al. / 2002[21] | Using X-ray mammography as an intervention |
| 6 | Ranganathan S et al. /2007[22] | Using digital mammography as an intervention |
| 7 | Dietrich AJ et al. / 2007[23] | Using X-ray mammography as an intervention |
| 8 | Guerrieri-Gonzaga A et al. / 2006[24] | Using X-ray mammography as an intervention |
| 9 | Sardanelli F et al. / 2000[25] | Using X-ray mammography as an intervention |
| 10 | Meeson S et al. /2000[26] | Using X-ray mammography as an intervention |
| 11 | Boyd NF et al. / 1997[27] | Using X-ray mammography as an intervention |
| 12 | Drexler B / 1985[28] | Using Diaphanography for breast cancer diagnosis |
| 13 | Deck W / 2006[29] | Using Vacuum – assisted breast biopsy for breast cancer diagnosis |
| 14 | Atherton Helen et al. /2009[30] | Research protocol |
Safety
Infrared imaging is a safe, noninvasive and non- ionized technique which can be used as a complement technology along with traditional mammography in determining benign or malignant tumors [6]. Unlike X-ray mammography, optical mammography uses near infrared ray which is non- ionized and noninvasive and known as a cost effective technique that does not need breast compression or low breast compression [4].
Performance of the technology
The included studies used different outcomes, such as sensitivity, specificity, index of suspicion, positive predictive value and negative predictive value, CNR (Contrast to Noise Ratio), resolution and image contrast, oxygenation index and the physical changes in the breast tissue as follows (Table 5 and Figure 2).
Table 5.
The indexes of technology performance
| technology performance index Author | Suspicion index | Improving of CNR, image resolution and image contrast | SN | SP | oxygenation index | Physical changes in the breast | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leff et al. [4]*1 | - | - | - | 96% | 93% | - | - | ||||||
| Parisky et al. [6] | 875 biopsied lesions | 479 biopsied lesions and 110 malignancies | - | - | - | - | - | ||||||
| SN | SP | PPV | NPV | SN | SP | PPV | NPV | ||||||
| 97% | 14% | 24% | 95% | 99% | 18% | 27% | 99% | ||||||
| Li et al. [7] | - | P-value<0/05 (by using spatial information) | - | - | - | - | |||||||
| Heffer et al. [8] | - | - | - | - | P-value<0/05 (by 4 combined wavelengths 690, 750, 788, and 856 nm) | - | |||||||
| Cerussi et al [9] | P-value<0/05 (by multi spectral measurement) | - | |||||||||||
| Taroni et al. [10] | - | P-value<0/05 (by wavelengths shorter than 680-780 nm) | - | - | - | - | |||||||
| van Veen et al. [11] | - | P-value<0/05 (by wavelength 640nm) | - | - | - | - | |||||||
| Busch et al [12]*2 | - | - | DOT CAD | DOT CAD | - | - | |||||||
| 89% | 94% | ||||||||||||
| Simick et al. [13] | - | - | - | - | - | P-value<0/05 | |||||||
| Turgut et al. [14] | - | - | - | - | - | P-value<0/05 | |||||||
| Pifferi et al. [15] | - | - | - | - | - | P-value<0/05 (by 3 combined wavelengths 683, 912, 975 nm) | - | ||||||
| Grosenick et al. [16] | - | - | - | - | - | P-value<0/05 (decreased in breast tumors) | P-value<0/05 | ||||||
SN: sensitivity
PPV: positive predictive value
*(1): standard for comparison: X- ray mammography
CNR: contrast to noise ratio
DOT CAD: Diffuse Optical Tomography Computer Aided Detection
SP: specificity
NPV: negative predictive value
*(2): standard for comparison: tissue segmentations
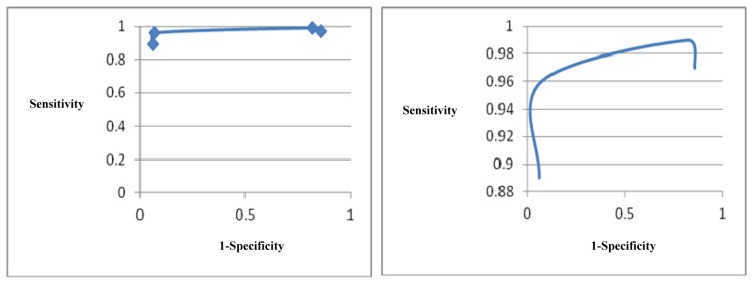
Figure 2.
Receiver Operating Characteristic (ROC) curve of optical mammography
Sensitivity and specificity
Leff et al. suggested that distinguishing between benign and malignant lesions is possible by optical mammography since malignant tissues show higher levels of absorption and scattering compared to healthy tissues and approved that Optical mammography technique is able to discriminate cancer from non-cancer tissues with 96% sensitivity and 93% specificity [4]. The study performed by Parisky et al. found that among 875 biopsied lesions, suspicion index led to 97% sensitivity, 14% specificity, 95% negative predictive value and 24% positive predictive value. Lesions that were considered as false negative by infrared had micro- calcification and an additional evaluation was done among 479 biopsied lesions and 110 malignancies, suspicion index led to 99% sensitivity, 18% specificity, 99% negative predictive value and 27% positive predictive value. Performance analysis of infrared imaging in all 875 biopsied lesions revealed that specificity improved statistically in dense breast tissue compared to fatty breast tissue. This study concluded that infrared imaging is a safe noninvasive technique which can be used as a complement technology along with traditional mammography in determining benign or malignant tumors [6]. Busch et al. found that Diffuse Optical Tomography Computer Aided Detection (DOT CAD) is capable of producing tomographs which distinguish healthy tissues from malignant ones. When this protocol is compared to a gold standard of tissue segmentations, it produces a true positive average rate (sensitivity) of 89% and a true negative rate (specificity) of 94% [12].
CNR (Contrast to Noise Ratio), resolution and image contrast
Li et al. performed a simulation study and indicated that spatial information provided by other imaging modalities such as x-ray tomosynthesis, x- ray CT or MRI may be used as a helping technology before diffuse optical image reconstruction for improving Contrast to Noise Ratio (CNR) and resolution of images [7]. In the study done in California University it was indicated that multi- spectral measurements in comparison with measures limited to one or two wavelengths provide additional information which may be useful in breast disease management [9]. Taroni et al. used 637, 656, 683, and 785 nm wavelengths in 26 patients with 16 tumors and 15 cysts and found that wavelengths shorter than 680-780 nm may be useful in optical mammography and higher imaging contrast for diagnosis of cancer in smaller lesions (diameter 1.5 cm) [10]. In the study conducted by the Dutch Erasmus Medical Center, it was shown that optimal imaging contrast was found in 24 patients in wavelength 640 nm using wavelengths 600-1,100nm [11].
Oxygenation index
Heffer et al. revealed that images of saturation index by oxygenation by 4 combined wavelengths (690, 750, 788, and 856 nm) can help single- wavelength optical mammography through developing better differentiation between benign and malignant breast lesions [8]. Pifferi et al. suggested that 5min imaging with wavelengths 683, 912, and 975nm can differentiate between healthy breast tissues and lesions based on different levels of tissue absorption and optical scattering [15]. The study done by Grosenick et al. found that Hemoglobin concentration increased in all tumors and blood oxygen saturation levels decreased or remained intact [16].
The Physical changes in the breast tissue
In the study conducted by Simick et al. it was shown that Optical Transilumination Spectroscopy is a useful and cost-effective tool to reduce the risk of breast cancer and is capable of indentifying physical changes in a period of time in breast tissue, and it can be used for breasts with tissue density of up to 7 cm [13]. Turgut et al. indicated that this technology using an infrared spectrum can robustly show increased blood flow around tumor tissue compared to healthy tissue [14].
Figure 2 demonstrates a relatively wide variation in interaction between the sensitivity and specificity of optical mammography. According to this figure, a small improvement in sensitivity of optical mammography is associated with a relatively significant reduction in its specificity. In other words, a small improvement in the number of TP patients might lead to a relatively significant increase in the number of FP patients. Based on this figure, it seems that the sensitivity of 96% and specificity of 93% might be considered as the optimal technical performance of this technology.
Discussion
Optical mammography is able to discriminate breast cancer and non-cancerous breast with 96% sensitivity and 93% specificity [4]. In addition, Diffuse Optical Tomography Computer Aided Detection (DOT CAD) is capable of producing tomographs which distinguish healthy tissues from malignant ones. When this protocol is compared to a gold standard of tissue segmentations, it produces a true positive average rate (sensitivity) of 89% and a true negative rate (specificity) of 94% [12]. Optical Transilumination Spectroscopy is a useful and cost- effective tool to reduce the risk of breast cancer and is capable of indentifying physical changes in breast tissue, and it can be used for breasts with tissue density of up to 7 cm [13]. Data suggest that optical imaging may distinguish radiographic density and water content based on hemoglobin concentration.
Women with higher breast radiographic density include a larger proportion of water and hemoglobin. Spectral analysis can predict radiographic density and thus it is able to determine which patients are at higher risk and should be followed. However, further studies involving larger sample sizes are needed before optical imaging can be used as a reliable tool for predicting this disease [4]. Optical mammography is a safe noninvasive, non-ionized and effective (in most cases) diagnostic technology that can be used as a diagnostic supplement alongside conventional mammography for differentiating benign and malignant tumors. It is recommended that younger women with higher breast density should be screened more often by optical mammography than those who have a lower breast density since it is possible that this technology may serve as a viable, cost effective and non- ionized alternative for screening patients who have restrictions in using X-ray mammography with dense breast tissues. In most studies included in this review it has been emphasized that in order to increase the effectiveness of this technology, its process should be used along with various wavelengths since this technique provides better differentiation between malignant versus benign breast lesions compared to single wavelength technique. It seems that optical mammography has the capacity of distinguishing lesions from healthy tissues. It is mostly due to differences in characteristics of scattering and absorption in healthy and unhealthy tissues. This analysis suggests that optical mammography is able to detect 85% of lesions which is relatively low. However, breast lesions may be distinguished from healthy tissues mainly based on increased hemoglobin concentration and less oxygen saturation levels which is consistent with increased vessels around tumors [6]. Despite considerable diversity in the methodology, there is a good consensus among the reviewed studies regarding the absorption and scattering properties of healthy breast tissue which supports the fact that optical evaluation can bring valuable insights on breast tissue composition.
In this review, the search strategy was conducted in electronic databases which is a limitation that might have led to the publication bias, although we tried to minimize the publication bias by using a broad search strategy and broad electronic databases.
Acknowledgments
This paper is derived from the project of Health Technology Assessment of Optical Mammography which was approved by National Institute for Health Research and Iranian Ministry of Health and Medical Education.
Footnotes
Conflicts of Interest
The authors have no conflict of interest in this article.
Authors' Contribution
Ali Akbari Sari designed the study and wrote the manuscript; Mohammadreza Mobinizadeh and Mahdi Azadbakht contributed to the literature review and writing-up process.
REFERENCES
- 1.Anonymous. 2012. [July 2012]. URL: http:// www.worldwidebreastcancer.com/learn/breast-cancerstatistics-worldwide/
- 2.Anonymous. 2012. [July 2012]. URL: http:// http://www.worldwidebreastcancer.com/investigation/breast-cancer-screening/breast-cancer-mammogram/
- 3.Anonymous. 2012. [July 2012]. URL: http://medicalimaging.wikia.com/wiki/Optical_mammography.
- 4.Leff D, Warren O, Enfield A, Gibson A, Athanasiou T, Patten DK, et al. Diffuse optical imaging of the healthy and diseased breast: a systematic review. Breast Cancer Res Treat. 2007;108:9–22. doi: 10.1007/s10549-007-9582-z. [DOI] [PubMed] [Google Scholar]
- 5.Fantini MA, Franceschini G, Gaida E, Gratton H, Jess WW, Mantulin KT, et al. Frequency-Domain Optical Mammography: Edge Effect Corrections. Med Phys. 1996; 23:149. doi: 10.1118/1.597696. [DOI] [PubMed] [Google Scholar]
- 6.Parisky YR, Sardi A, Hamm R. Efficacy of computerized infrared imaging analysis to evaluate mammographically suspicious lesions. AJR. . 2003;180:263–9. doi: 10.2214/ajr.180.1.1800263. [DOI] [PubMed] [Google Scholar]
- 7.Li A, Miller EL, Kilmer ME, Brukilacchio TJ, Chaves T, Stott J, et al. Tomographic optical breast imaging guided by three-dimensional mammography. Appl Opt. 2003;42(25):5181–90. doi: 10.1364/ao.42.005181. [DOI] [PubMed] [Google Scholar]
- 8.Heffer E, Pera V, Schütz O, Siebold H, Fantini S. Near-infrared imaging of the human breast: complementing hemoglobin concentration maps with oxygenation images. Journal of Biomedical Optics. 2004;9(6):1152. doi: 10.1117/1.1805552. [DOI] [PubMed] [Google Scholar]
- 9.Cerussi AE, Jakubowski D, Shah N, Bevilacqua F, Lanning R, Berger AJ, et al. Spectroscopy enhances the information content of optical mammography. Journal of Biomedical Optics. 2002;7(1):60–71. doi: 10.1117/1.1427050. [DOI] [PubMed] [Google Scholar]
- 10.Taroni P, Pifferi A, Torricelli A, Spinelli L, Danesini GM, Cubeddu R. Do shorter wavelengths improve contrast in optical mammography? Phys Med Biol. 2004;49(7):1203–216. doi: 10.1088/0031-9155/49/7/008. [DOI] [PubMed] [Google Scholar]
- 11.Van Veen R, Sterenborg H, Marinelli A, Menke-Pluymers M. Intraoperatively assessed optical properties of malignant and healthy breast tissue used to determine the optimum wavelength of contrast for optical mammography. J Biomed . 2004;9(6):1129–36. doi: 10.1117/1.1803547. [DOI] [PubMed] [Google Scholar]
- 12.Busch DR, Guo W, Choe R, Durduran T, Feldman Carolyn Mies M, Rosen M, et al. Computer aided automatic detection of malignant lesions in diffuse optical mammography. Med Phys. 2010;37(4):1840–9. doi: 10.1118/1.3314075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simick MK, Jong R, Wilson B, Lilge L. Non-ionizing near-infrared radiation transillumination spectroscopy for breast tissue density and assessment of breast cancer risk. Journal of Biomedical Optics. 2004;9(4):794–803. doi: 10.1117/1.1758269. [DOI] [PubMed] [Google Scholar]
- 14.Durduran T, Choe R, Yu G, Zhou C. Diffuse optical measurement of blood how in breast tumors. Opt Lett. 2005;30(21):2915–7. doi: 10.1364/ol.30.002915. [DOI] [PubMed] [Google Scholar]
- 15.Pifferi A, Taroni P, Torricelli A, Messina F, Cubeddu R, Danesini G. Four-wavelength time-resolved optical mammography in the 680-980-nm range. Opt Lett. 2003;28(13):1138–40. doi: 10.1364/ol.28.001138. [DOI] [PubMed] [Google Scholar]
- 16.Grosenick D, Moesta KT, Wabnitz H, Mucke J, Stroszczynski C, Macdonald R, et al. Time-domain optical mammography: initial clinical results on detection and characterization of breast tumors. Appl Opt. 2003;42(16):3170–86. doi: 10.1364/ao.42.003170. [DOI] [PubMed] [Google Scholar]
- 17.Chon KS, Park JG, Son HH, Kang SH, Park SH, Kim HW, et al. Usefulness of a small-field digital mammographic imaging system using parabolic polycapillary optics as a diagnostic imaging tool: a preliminary study. Korean Journal of Radiology. 2009;11(2):214–10. doi: 10.3348/kjr.2009.10.6.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grosenick D, Kummrow A, Macdonald R, Schlag PM, Rinneberg H. Evaluation of higher-order time-domain perturbation theory of photon diffusion on breast- equivalent phantoms and optical mammograms. Physical Review E. Statistical, Nonlinear, & Soft Matter Physics. 2007;76(6):061908. doi: 10.1103/PhysRevE.76.061908. [DOI] [PubMed] [Google Scholar]
- 19.Benevides LA, Huston AL, Justus BL, Falkenstein P, Brateman LF, Hintenlang DE. Characterization of a fiber- optic-coupled radioluminescent detector for application in the mammography energy range. Medical Physics. 2007;74(2):0001. doi: 10.1118/1.2736788. [DOI] [PubMed] [Google Scholar]
- 20.Krug B, Stutzer H, Zahringer M, Morgenroth C, Winnekendonk G, Gossmann A, et al. Digital X-ray mammography: comparison of the image quality achievable with a wet laser imager, a dry infrared laser imager and a dry laser imager using direct thermography. Fortschr Rِntgenstr. 2005;177(7):955–61. doi: 10.1055/s-2005-858287. [DOI] [PubMed] [Google Scholar]
- 21.Bradford CD, Peppler WW, Ross RE. Multi tapered x-ray capillary optics for mammography. Medical Physics. 2002;5(2):1097–108. doi: 10.1118/1.1481517. [DOI] [PubMed] [Google Scholar]
- 22.Ranganathan S, Faridah Y, Ng KH. Moving into the digital era: a novel experience with the first full-field digital mammography system in Malaysia Singapore. Med J. 2007;48(9):804. [PubMed] [Google Scholar]
- 23.Allen J, Dietrich A, Robinson M, Tobin J, Meredith R, Karen A. Translation of an Effcacious Cancer- Screening Intervention to Women Enrolled in a Medicaid Managed Care Organization. Ann Fam Med. 2007;5(4):320–7. doi: 10.1370/afm.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guerrieri-Gonzaga A, Robertson C, Bonanni B, Serrano D, Cazzaniga M, et al. Preliminary Results on Safety and Activity of a Randomized, Double-Blind, 2 _ 2 Trial of Low-Dose Tamoxifen and Fenretinide for Breast Cancer Prevention in Premenopausal Women. JCO. 2006;24(1):129. doi: 10.1200/JCO.2005.02.9934. [DOI] [PubMed] [Google Scholar]
- 25.Sardanelli F, Zandrino F, Imperiale A, Bonaldo E, Quartini M, Cogorno N. Breast Biphasic Compression versus Standard Monophasic Compression in X-ray Mammography. Radiology. 2000;217(2):576–80. doi: 10.1148/radiology.217.2.r00nv07576. [DOI] [PubMed] [Google Scholar]
- 26.Meeson S, Young KC, Cooke J. skin edge perception in mammograms: a comparison of two film-screen combination. The British Journal of Radiology. 2000;73(868):370. doi: 10.1259/bjr.73.868.10844862. [DOI] [PubMed] [Google Scholar]
- 27.Boyd N, Greenberg C, Lockwood G, Little L, Martin L, Byng J, et al. Effects at Two Years of a Low-Fat, High- Carbohydrate Diet on Radiologic Features of the Breast: Results from a Randomized Trial. Journal of the National Cancer Institute. 1997;89(7):488–96. doi: 10.1093/jnci/89.7.488. [DOI] [PubMed] [Google Scholar]
- 28.Drexler B, Davis JL, Schofield G. Diaphanography in the diagnosis of breast cancer. Radiology. 1985;157:41–4. doi: 10.1148/radiology.157.1.4034975. [DOI] [PubMed] [Google Scholar]
- 29.Deck W. Vacuum-assisted breast biopsy. Montreal: Agence d'evaluation des technologies et des modes d'intervention en sante (AETMIS). AETMIS 06-06 RE. 2006 [Google Scholar]
- 30.Sawmynaden P, Atherton H, Majeed A, Car J. Email for the provision of information on disease prevention and health promotion. Cochrane Database Syst Rev. 2012 Nov. 14;11:CD007982. doi: 10.1002/14651858.CD007982.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]