Abstract
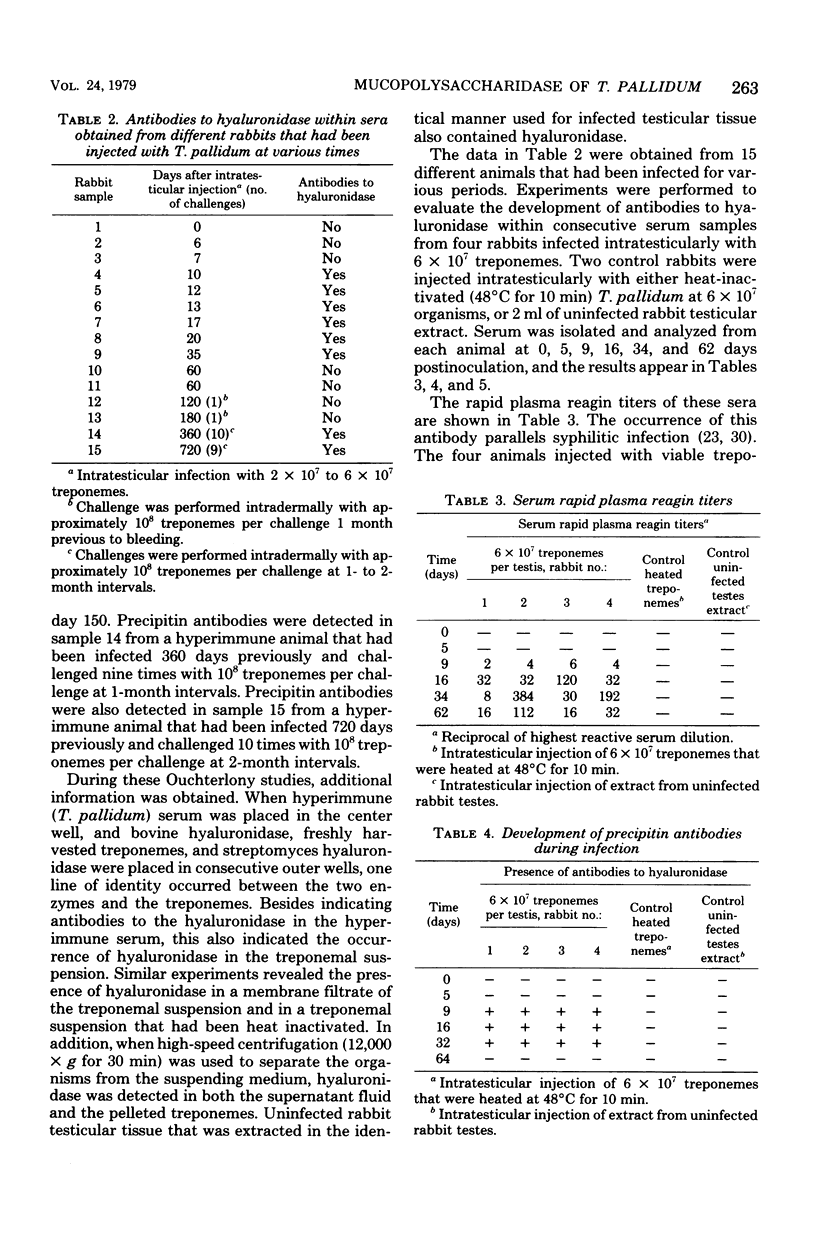
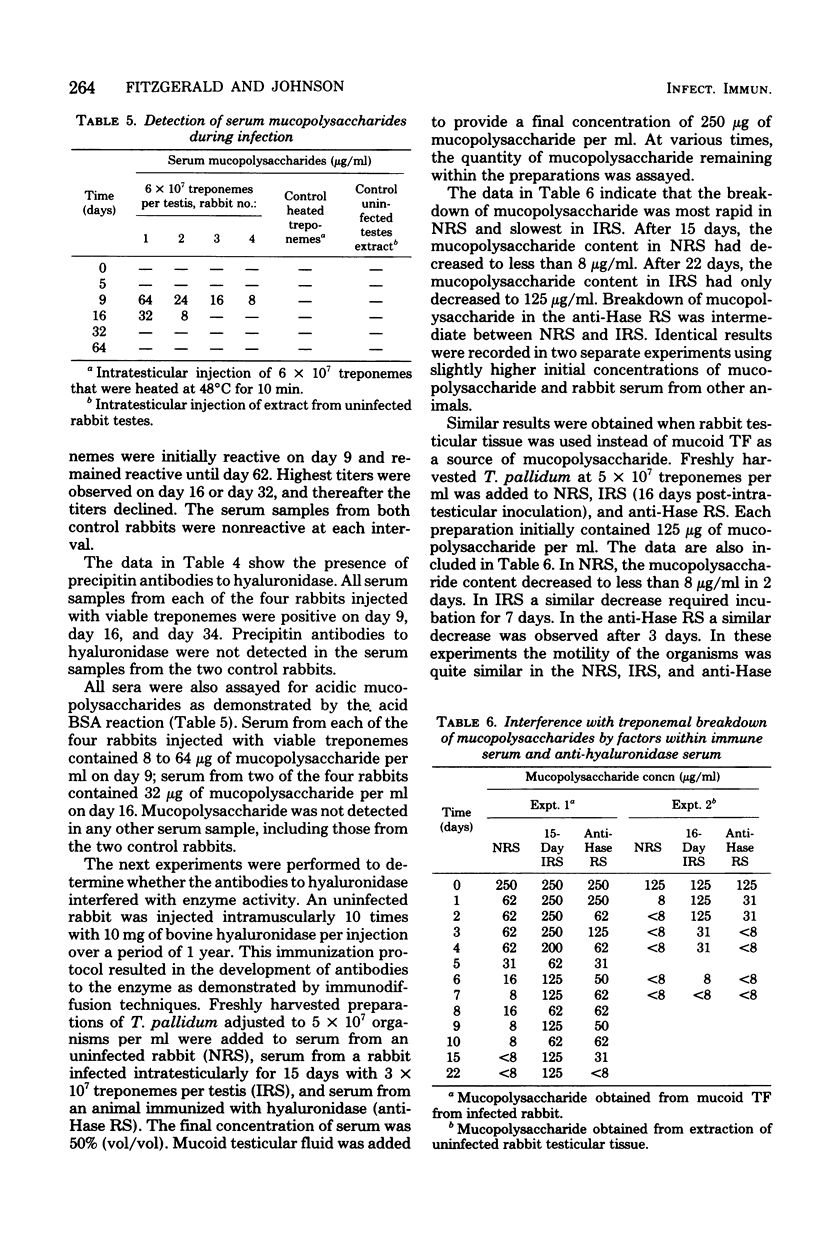
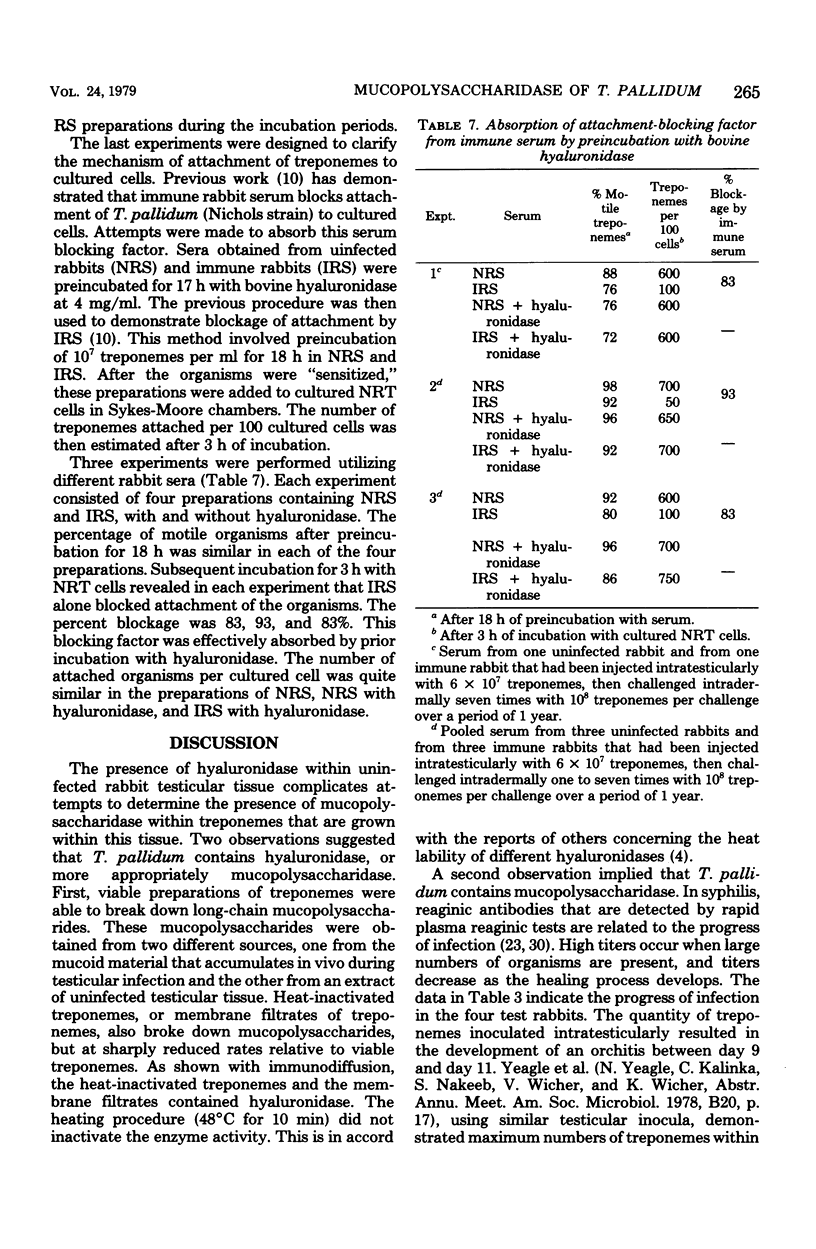
Treponema pallidum (Nichols strain) exhibited mucopolysaccharidase activity. Acidic mucopolysaccharides were broken down more rapidly by viable treponemes than by heat-inactivated treponemes or membrane filtrates of treponemal suspensions. Ouchterlony immunodiffusion demonstrated the occurrence of antibodies to the hyaluronidase-like enzyme within syphilitic sera. After intratesticular inoculation of 2 × 107 to 6 × 107 treponemes, these anti-mucopolysaccharidase antibodies were detected between 9 and 35 days postinoculation. In addition, acidic mucopolysaccharides were present in the serum of infected animals 9 and 16 days postinoculation. Immune serum that contained antibodies to the mucopolysaccharidase restricted treponemal breakdown of acidic mucopolysaccharides. It has been previously demonstrated that immune rabbit serum contains a factor that blocks attachment of T. pallidum (Nichols strain) to cultured mammalian cells. This factor was effectively absorbed by prior incubation with bovine hyaluronidase. It is postulated that T. pallidum attaches to acidic mucopolysaccharides on the surface of cultured cells through the mucopolysaccharidase enzyme at the surface of the organisms. These findings are discussed in terms of the histopathogenesis of T. pallidum with applications to the healing immune response.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azar H. A., Pham T. D., Kurban A. K. An electron microscopic study of a syphilitic chancre. Engulfment of Treponema pallidum by plasma cells. Arch Pathol. 1970 Aug;90(2):143–150. [PubMed] [Google Scholar]
- CLARK J. W., Jr Loss of virulence in vitro of motile Treponema pallidum. Br J Vener Dis. 1962 Jun;38:78–81. doi: 10.1136/sti.38.2.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canale-Parola E. Physiology and evolution of spirochetes. Bacteriol Rev. 1977 Mar;41(1):181–204. doi: 10.1128/br.41.1.181-204.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLAMATER E. D., SAURINO V. R., URBACH F. Studies on the immunology of spirochetoses. I. Effect of cortisone and experimental spirochetosis. Am J Syph Gonorrhea Vener Dis. 1952 Mar;36(2):127–139. [PubMed] [Google Scholar]
- Fitzgerald T. J., Cleveland P., Johnson R. C., Miller J. N., Sykes J. A. Scanning electron microscopy of Treponema pallidum (Nichols strain) attached to cultured mammalian cells. J Bacteriol. 1977 Jun;130(3):1333–1344. doi: 10.1128/jb.130.3.1333-1344.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Johnson R. C., Miller J. N., Sykes J. A. Characterization of the attachment of Treponema pallidum (Nichols strain) to cultured mammalian cells and the potential relationship of attachment to pathogenicity. Infect Immun. 1977 Nov;18(2):467–478. doi: 10.1128/iai.18.2.467-478.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Johnson R. C., Ritzi D. M. Relationship of Treponema pallidum to acidic mucopolysaccharides. Infect Immun. 1979 Apr;24(1):252–260. doi: 10.1128/iai.24.1.252-260.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Johnson R. C. Surface mucopolysaccharides of Treponema pallidum. Infect Immun. 1979 Apr;24(1):244–251. doi: 10.1128/iai.24.1.244-251.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Johnson R. C., Sykes J. A., Miller J. N. Interaction of Treponema pallidum (Nichols strain) with cultured mammalian cells: effects of oxygen, reducing agents, serum supplements, and different cell types. Infect Immun. 1977 Feb;15(2):444–452. doi: 10.1128/iai.15.2.444-452.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald T. J., Miller J. N., Sykes J. A. Treponema pallidum (Nichols strain) in tissue cultures: cellular attachment, entry, and survival. Infect Immun. 1975 May;11(5):1133–1140. doi: 10.1128/iai.11.5.1133-1140.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSSFELD H. Studies on production of hyaluronic acid in tissue culture; the presence of hyaluronidase in embryo extract. Exp Cell Res. 1958 Feb;14(1):213–216. doi: 10.1016/0014-4827(58)90231-3. [DOI] [PubMed] [Google Scholar]
- Germaine G. R., Schachtele C. F. Streptococcus mutans dextransucrase: mode of interaction with high-molecular-weight dextran and role in cellular aggregation. Infect Immun. 1976 Feb;13(2):365–372. doi: 10.1128/iai.13.2.365-372.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim A. N., Streitfeld M. M. The microassay of hyaluronic acid concentration and hyaluronidase activity by capillary turbidity (CT) and capillary turbidity reduction (CTR) tests. Anal Biochem. 1973 Dec;56(2):428–434. doi: 10.1016/0003-2697(73)90208-x. [DOI] [PubMed] [Google Scholar]
- Kantor F. S. Infection, anergy and cell-mediated immunity. N Engl J Med. 1975 Mar 20;292(12):629–634. doi: 10.1056/NEJM197503202921210. [DOI] [PubMed] [Google Scholar]
- Levene G. M., Turk J. L., Wright D. J., Grimble A. G. Reduced lymphocyte transformation due to a plasma factor in patients with active syphilis. Lancet. 1969 Aug 2;2(7614):246–247. doi: 10.1016/s0140-6736(69)90010-5. [DOI] [PubMed] [Google Scholar]
- Logan L. C. Rabbit globulin and antiglobulin factors associated with Treponema pallidum growth in rabbits. Br J Vener Dis. 1974 Dec;50(6):421–427. doi: 10.1136/sti.50.6.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER J. N. THE APPEARANCE AND PERSISTENCE OF VDRL, RPCF, AND TPI ANTIBODY DURING THE COURSE AND TREATMENT OF EXPERIMENTAL SYPHILIS IN THE RABBIT. J Invest Dermatol. 1964 May;42:367–371. doi: 10.1038/jid.1964.80. [DOI] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of adherence of Streptococcus mutans to smooth surfaces. I. Roles of insoluble dextran-levan synthetase enzymes and cell wall polysaccharide antigen in plaque formation. Infect Immun. 1973 Oct;8(4):555–562. doi: 10.1128/iai.8.4.555-562.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCOTT V., DAMMIN G. J. Hyaluronidase and experimental syphilis. III. Metachromasia in syphilitic orchitis and its relationship to hyaluronic acid. Am J Syph Gonorrhea Vener Dis. 1950 Nov;34(6):501–514. [PubMed] [Google Scholar]
- SCOTT V., DAMMIN G. J. Morphologic and histochemical sequences in syphilitic and in tuberculous orchitis in the rabbit. Am J Syph Gonorrhea Vener Dis. 1954 May;38(3):189–202. [PubMed] [Google Scholar]
- Sykes J. A., Miller J. N. Intracellular location of Treponema pallidum (Nichols strain) in the rabbit testis. Infect Immun. 1971 Sep;4(3):307–314. doi: 10.1128/iai.4.3.307-314.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes J. A., Miller J. N., Kalan A. J. Treponema pallidum within cells of a primary chancre from a human female. Br J Vener Dis. 1974 Feb;50(1):40–44. doi: 10.1136/sti.50.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TURNER T. B., HOLLANDER D. H. Cortisone in experimental syphilis; a preliminary note. Bull Johns Hopkins Hosp. 1950 Nov;87(5):505–509. [PubMed] [Google Scholar]
- TURNER T. B., HOLLANDER D. H. Studies on the mechanism of action of cortisone in experimental syphilis. Am J Syph Gonorrhea Vener Dis. 1954 Sep;38(5):371–387. [PubMed] [Google Scholar]
- Wicher V., Wicher K. In vitro cell response of Treponema pallidum-infected rabbits. II. Inhibition of lymphocyte response to phytohaemagglutinin by serum of T. pallidum-infected rabbits. Clin Exp Immunol. 1977 Sep;29(3):487–495. [PMC free article] [PubMed] [Google Scholar]
- Wilkinson A. E., Rayner C. F. Studies on the fluorescent treponemal antibody (FTA) test. Br J Vener Dis. 1966 Mar;42(1):8–15. doi: 10.1136/sti.42.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeigler J. A., Jones A. M., Jones R. H., Kubica K. M. Demonstration of extracellular material at the surface of pathogenic T. pallidum cells. Br J Vener Dis. 1976 Feb;52(1):1–8. doi: 10.1136/sti.52.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]



