Abstract
New cancer therapies with novel mechanisms and functions are needed to treatpatients with different cancers. Virotherapy is a good scenario for such treatment. The advantages of virotherapy include the potential lack of cross resistance with standard therapies and the ability to cause tumor destruction by numerous mechanisms. Oncolytic virus not only possesses unique mechanisms of action that are distinct from other treatment modalities, its self-perpetuating nature provides an ideal platform for therapeutic transgenic insertion. In this review article, a variety of oncolytic viruses in cancer gene therapy will be described.
Keywords: Cancer, Oncolytic viruses, Gene therapy
Introduction
New ideas and methods are needed to kill cancer cells selectively. In the last 40 years, using virus to treat different cancers seemed interesting; however, due to multiple genes involved to progress and metastasize cancer cells we are still looking to find a gene as a main target if we believe cancer is a genetic disease. In my opinion, tumors are as same as black holes. The light can not escape the black hole but it is still inside it, so it looks dark. Interestingly, immune antigens (immunogens) cannot escape the tumor (same light). The main question is: How oncolytic viruses can disrupt and escape from the tumor mass after intratumoral injection? On the other hand, the concept of oncolytic virotherapy has been around for a sufficiently long period of time and now there is increasing pressure for developers of this technology to deliver clinical trials that give rise to at least some suggestion of this therapeutic potential [1]. Direct infection of tumor cells with viruses transferring protective or therapeutic genes, is a frequently used procedure for production of tumor vaccines in human gene therapy [2]. There has been active interest in the potential use of replication competent oncolytic viruses as therapeutic agents in the treatment of cancer [3]. Recently, Motalleb et al. (2009) showed proliferation of NDV-AF2240 in breast tumor tissue in mice [2] (Figure 1). Oncolytic Viruses (OVs) kill cancer cells while sparing normal cells. Often they utilize sophisticated gene products to facilitate immune evasion, allow recognition and penetration of cells, co-opt cellular biosynthetic machinery and ultimately manipulate cell death programs. Interestingly, many of the biological pathways that viruses manipulate are the same ones that tumor cells deregulate during their malignant evolution; and as a consequence, these same pathways have become the targets for anticancer drug development [4]. This review will attempt to provide some insight about the types of viruses that could be selected for development in virotherapy of cancer.
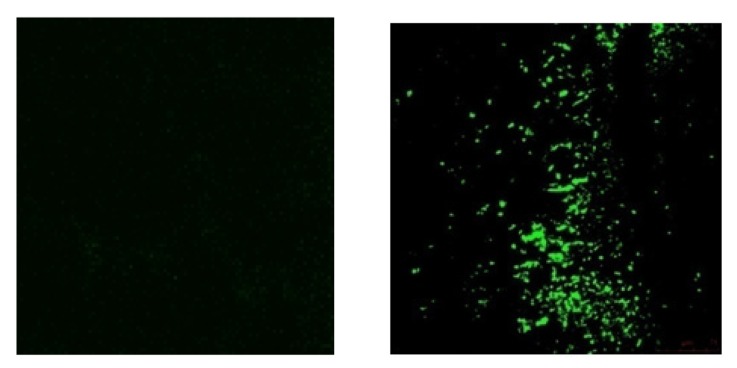
Figure 1.
Confocal laser scanning micrographs of NDV-AF2240 in breast tumor tissue (B). No signal was observed in negative control (A), X200. [2]
Gene Therapy
Gene therapy has the potential to significantly impact human healthcare in the twenty-first century. The idea behind gene therapy is simple: to deliver genetic material to cells that will slow down or halt the progression of disease, or to help repair or regenerate damaged or lost tissues [5]. The field of gene therapy is rapidly advancing in molecular biology techniques. Gene therapy involves insertion of genes into malignant or normal cells in order to modify gene expression for therapeutic benefits. Genes are transfected using either viral or non-viral vectors. When viruses are used, they must be attenuated, so they no longer could be harmful to the patient [6]. Defined targets in cancer can also be exploited for gene therapy. In theory, gene therapy is a more straightforward approach than drug or immune therapy. However, the development of new drugs and vaccines can be pursued on a strong fundament of established procedures and long term experience, whereas in gene therapy almost everything has to be developed from scratch [7].
Oncolytic Viruses
All growth of viruses is favored in actively proliferating cells. However, some viruses are particularly oncotropic by nature. These viruses include human reovirus, the parvoviruses H-1 and minute virus of mice, Vesicular Stomatitis Virus (VSV) and Newcastle Disease Virus (NDV). These naturally occurring oncolytic viruses usually have no or very mild clinical symptoms under normal conditions. Their oncolytic nature appears to result from a tumor associated deficiency in the interferon response pathway [8]. In recent years, there has been active interest in the potential use of replication competent oncolytic viruses as therapeutic agents in the treatment of cancer [9]. The earliest report on suppression of human tumors is cervical carcinoma that regressed after inoculation with attenuated rabies vaccine [2]. Replication selective oncolytic viruses have been introduced as a new method for cancer therapy. Using live viruses for cancer patients dates back to 20th century, and the advances of molecular biology and virology have fostered the development of genetically engineered viruses. The list of oncolytic wild viruses is briefly described in Table 1. Numerous reports exist about replication of selective viruses in clinical trials for cancer therapy (Table 2). These viruses are engineered or non- engineered. Adenovirus, herpes simplex and vaccinia are engineered viruses, and newcastle disease virus, autonomous parvovirus and reovirus are non- engineered viruses. Interestingly, many viruses preferentially grow in tumor cells. Recent studies of leukaemia and solid tumors showed significant heterogeneity within a population of tumor cells, suggesting that only a subpopulation of cells is responsible for tumorigenesis. These cells have been named Cancer Stem Cells (CSCs) or Cancer Initiating Cells (CICs). CICs have been shown to be relatively resistant to conventional anticancer therapies and could be responsible for relapse of the disease, and therefore they represent a potentially critical therapeutic target. Oncolytic viruses kill cancer cells by mechanisms that are different from conventional therapeutics. Therefore, they are not susceptible to the same pathways of drug or radiation resistance, so it is important to know that CICs are susceptible to oncolytic virus infection or not [10].
Table 1.
Viruses with oncolytic selective activities
| Virus | Tumor target | Reference |
|---|---|---|
| Reovirus | Pancreatic cancer | [11-14] |
| Myxomavirus | Glioma | [15-16] |
| Parvovirus H-1 | Breast and hepatocellular carcinoma | [17-20] |
| Human adenoviruses | Cervical cancer | [21] |
| Newcastle disease virus | Diverse | [22] |
| Vesicular stomatotis viruse | Hepatocellular carcinoma and breast cancer | [22] |
| Bovine herpes virus 4 | Lung carcinoma | [23-24] |
| Coxsackie virus A21 | Melanoma | [25-26] |
Table 2.
Example of replication selective viruses in clinical trials for cancer [27]
| Strain | Clinical phase | Tumor targets | Genetic alteration | Cell phenotype |
|---|---|---|---|---|
| ************************Engineered*********************** | ||||
| Adenovirus (2/5 chimera) | I-III | SCCHN | E1B-55kD gene deletion | Controversial cells lacking p53 function |
| Colorectal | ||||
| Ovarian | E3-10.4/14.5 deletion | |||
| Pancreatic | ||||
| Adenovirus (serotype 5) | I | Prostate | E1A expression driven by SPE element | Prostate cells |
| Adenovirus (2/5 chimera) | I | Prostate | E1B-55kD gene deletion | Controversial cells lacking p53 function |
| Herpes simplex (virus-1) | I-II | GBM | Ribonucleotide reductase distruption | Proliferating cells |
| Herpes simplex | I | Colorectal | Neuropathogenesis gene mutation | Proliferating cells |
| Vaccinia virus | I | Melanoma | None or tk deletion | Unknown |
| *********************Non-engineered********************** | ||||
| NDV | I | Bladder | Unknown | Loss of IFN response in tumor cells |
| SCCHN | ||||
| Autonomous parvovirus | I | Ovarian | None | Transformed cells |
| ↑proliferation | ||||
| ↑differentiation | ||||
| Ras, p53 mutation | ||||
| Reovirus | SCCHN | None | Ras pathway activation | |
Tumor Selective Replication Mechanisms
Some non engineered oncolytic viruses that could be used to destroy cancer cells will be described.
Newcastle Disease Virus (NDV)
NDV is a no segmented, single stranded, negative sense, enveloped RNA virus [2] that is not harmful for human health and the first report of its oncolytic activity was introduced in mid-1950s [28]. NDV are categorized as velogenic (highly virulent), mesogenic (intermediate), or lentogenic (no virulent), depending on severity of the disease [2]. The heat stable, viscerotropic NDV (AF2240) isolated in 1960s has anti neoplastic properties and was tested as an anticancer agent in vivo and in vitro (Figure 2) in Malaysia [29]. It was showed that NDV is dependent on activated ras-pathway to replicate efficiently. Due to defects in IFN-pathway, which are often found in tumor cells, cancer cells are sensitive to NDV [30]. Other naturally attenuated strains of NDV, named PV701 and 73-T have been shown to exhibit tumors selectivity against human tumors [31] and induction regression of tumors in different cancer cell lines and fibrosarcoma, neuroblastoma, colon, prostate and breast carcinoma xenografts in mouse model [30] respectively. Further clinical trial phase studies are now being conducted.
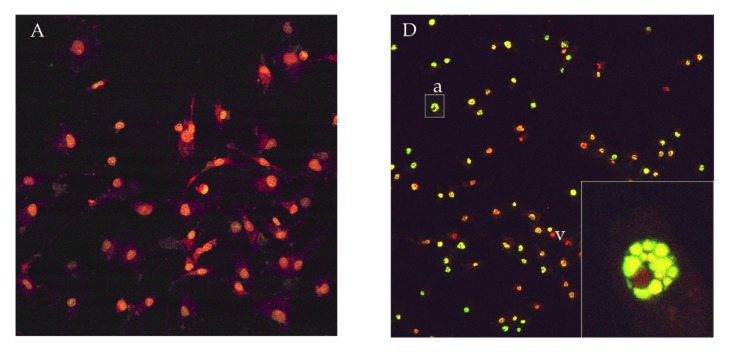
Figure 2.
Confocal micrographs of MCF-7 cells treated with AF2240 strain of NDV and stained by TUNEL technique [9]. (A) Untreated (D) treated for 72 hours respectively. Note the fragmented nucleus (D). Magnification: (A-D): 40x, inserted picture:120x.
Autonomous Parvovirus
Autonomous parvovirus is a non-enveloped, ssDNA that is unable to push the cells to S phase. Thus, parvoviruses can replicate in transformed cell lines probably due to the high cell cycle control by transformed cells [32]. Parvoviral vectors could carry cytotoxic agents to tumor cells; therefore, it is able to increase the antitumor activity of autonomous parvovirus in human cancer cell cultures [33].
Reovirus
Reovirus is a non-enveloped dsRNA virus with low pathogenicity in humans [34]. Tropism and cytotoxicity of reovirus in neoplastic cell lines has been shown but its mechanism has just recently been explained. Mouse fibroblasts are resistant to reovirus infection but after activation of ras pathway in tumorigenesis become susceptible to infection [35]. In addition, gene deletion of activated Protein Kinase (PKR) which is an important factor in host defence system against viruses permits reovirus infection and replication [36](Figure 3). As most tumors have an activating mutation in ras-pathway [37], reovirus could be an ideal oncolytic agent. Reovirus has been shown to selectively destroy neoplastic cells; for example, ovaries, lymphoid malignancies, breast, and colon, both in vitro and xenograft in mice model (intratumoral and intravenously) [38,35]. Reovirus therapy combined with cyclosporine A or anti-CD4/anti- CD8 antibodies will increase the oncolytic effect of the virus [39].
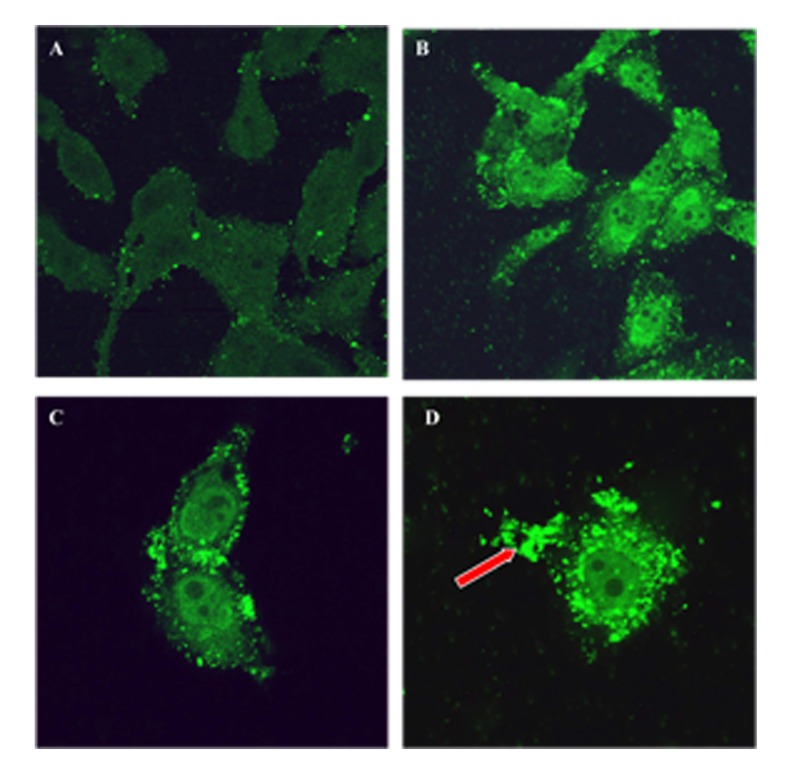
Figure 3.
Confocal laser scanning micrograph of MCF-7 cells treated with NDV-AF2240 and labelled with polyclonal antibody and anti-chicken FITC for untreated (A) and treated for 24, 48 and 72 h (B, C and D) respectively. Note the fluorescence staining in the cytoplasm at 24 and 48 h post-treatment (B, C) and budding-off of the virus (arrow) at 72 h post-treatment (D). Magnification: (A, B) 60X, (C,D) 120X[9]
Increasing the Antitumor Effect of Oncolytic Virotherapy
Tumor growth contains complex and flexible pathways and for this reason increasing resistance and progression of tumor still is the rule for patients with metastatic disease. However, new agents with independent therapeutic pathways represent a central alternative. Among them, oncolytic viruses are unique since they can be amplified by infected cells, armed to selectively infect and kill cancer cells and induce an immune response against the tumor [40]. In order to increase the antitumor effect of oncolytic viruses, we need new and multimodal method to maximize the function and action of viruses inside the tumor mass without virus dissemination out of the tumor. Dissemination of virus will decrease the antitumor activity due to decrease of dose and concentration of the virus [2]. On the other hand, pathogenesis of virus should be considered after dissemination into different organs. Nowadays, evaluation of oncolytic virus's application in combination with chemotherapy, radiotherapy, or suicide gene therapy is under investigation (Table 3).
Table 3.
Oncolytic virotherapy with standard therapies [41]
| Strain of virus | Factor | Drug | Effect | Tumor |
|---|---|---|---|---|
| VSV | VSV-C:U | Cytosine deaminase | Enhanced | Mammary carcinoma |
| HSV-1 | rRp450 | Cytochrome P450 oxidase | Enhanced | Hepatocellular carcinoma |
| Vaccinia virus | VVCD | Cytosine deaminase | Enhanced | Colon adenocarcinoma |
| Suicide gene therapy treatment Adenovirus | Ad-TK | Thymidine kinase | Enhanced | Glioma |
| HSV-1 | NV1020 | Synergistic | Glioma | |
| Suicide gene therapy-radiotherapy treatment Adenovirus | Ad5-CD/TKrep | Cytosine deaminase/thymidine kinase fusion | Enhanced | Prostate cancer, Glioma, cervical carcinoma |
| HSV-1 | G207 | Enhanced | Cervical cancer | |
| Radiotherapy treatment Adenovirus | ONYX-015 | ---- | Additive | Colon cancer |
| Chemotherapy treatment Adenovirus | CV890 | Doxorubicin | Synergistic | Liver cancer |
| Chemotherapy treatment Adenovirus | CV787 | Paclitaxel | Synergistic | Prostate cancer |
| Chemotherapy treatment Adenovirus | ONYX-015 | Doxorubicin | Synergistic | Thyroid cancer |
| Chemotherapy treatment Adenovirus | ONYX-015 | Paclitaxel | Synergistic | Thyroid cancer |
| Adeno-associated virus | AAVtk | Thymidine kinase | Enhanced | Laryngeal cancer |
| Radiotherapy treatment Adenovirus | CV706 | ---- | Synergistic | Prostate cancer |
| HSV-1 | G207 | Vincristine | Enhanced | Rhabdomyosarcoma |
HSV-1: herpes simplex virus 1; VSV: vesicular stomatitis virus; rRp450: Oncolytic herpes virus mutant rRp450; Ad5-CD/TKrep: replication-competent Ad5-CD/TKrep adenovirus containing a cytosine deaminase (CD)/herpes simplex virus thymidine kinase (HSV-1 TK) fusion gene; AAVtk: adenoassociated virus thymidine kinase
Conclusion
Combining therapies may increase antitumor effects than either of these therapies alone therefore the balance of combination strategy is needed. Interestingly, no overlapping resistance between oncolytic viruses and the other therapies was observed. Finally, it may be possible to use lower doses of the virus, and decrease virus toxicity in normal tissues after dissemination of the virus. It can be concluded that further studies should be conducted on oncolytic viruses as therapeutic agents in this setting which may result in the extension of anticancer armamentarium in the future.
Acknowledgments
This manuscript just cited selected number of articles. Therefore, I appreciate all researchers in this field.
Footnotes
Conflicts of Interest
The author have no conflict of interest.
Authors' Contribution
The subject selection and article structure made and wrote by Gholamreza Motalleb.
REFERENCES
- 1.Pandha H, Melcher A, Harrington K, Vile R. Oncolytic Viruses: Time to Compare, Contrast, and Combine? Mol Ther. 2009;17(6):934–5. doi: 10.1038/mt.2009.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Motalleb G, Fauziah O, Aini I, Asmah R. Dissemination of Newcastle Disease Virus (NDV-AF2240) in Liver during Intratumoral Injection of 4T1 Xenotransplant Breast Cancer in BALB/c Mice. The Cell. 2009;11(3):303–310. [Google Scholar]
- 3.Motalleb G, Fauziah O, Aini I, Asmah R. Transmission Electron Microscopy and Confocal laser Scanning Microscopy of Newcastle Disease Virus (NDV2240) in Lung during Intratumoral Injection in 4T1 Breast Cancer in BALB/c Mice. Malaysian J of Microscopy. 2009;5:42–53. [Google Scholar]
- 4.Ottolino-Perry K, Diallo JS, Lichty BD, Bell JC, McCart JA. Intelligent Design: Combination Therapy with Oncolytic Viruses. Mol Ther. 2010;18(2):251–63. doi: 10.1038/mt.2009.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le Doux JM. Methods in Molecular Biology, Vol 433 (1). Production and In Vivo Applications of Gene Transfer Vectors. Totowa: Humana Press; 2008. pp. 243–4. [PubMed] [Google Scholar]
- 6.King RJB, Robins MW. Cancer biology. England: Pearson Education Limited.; 2006. pp. 259–60. [Google Scholar]
- 7.Schulz WA. Molecular Biology of Human Cancers. Springer; 2005. p. 264. [Google Scholar]
- 8.William J, Zhou Q. Viral Vectors for Cancer Gene Therapy: Viral dissemination and tumor targeting. Curr Gene Ther. 2005;5:133–42. doi: 10.2174/1566523052997460. [DOI] [PubMed] [Google Scholar]
- 9.Othman F, Ideris A, Motalleb Gh, Eshak ZB, Rahmat A. Oncolytic effect of Newcastle disease virus AF2240 strain on the MCF-7 breast cancer cell line . Cell J. 2012;12(1):17–24. [Google Scholar]
- 10.Cripe TP, Wang PY, Marcato P, Mahller YY, Lee PWK. Targeting Cancer-initiating Cells With Oncolytic Viruses. Mol Ther. 2009;17(10):1677–82. doi: 10.1038/mt.2009.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norman KL, Hirasawa K, Yang AD, Shields MA, Lee PW. Reovirus oncolysis: the Ras/RaiGEF/p38 pathway dicates host cell permissiveness to reovirus infection. Proc natl Acad sci. 2004;101(30):11099–104. doi: 10.1073/pnas.0404310101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marcato P, Schmulevitz M, Lee PW. Connecting reovirus on-colysis and Ras signaling. Cell Cycle. 2005;4(4):556–9. doi: 10.4161/cc.4.4.1600. [DOI] [PubMed] [Google Scholar]
- 13.Hirasawa K, Nishikawa SG, Norman KL, Coffey MC, Thomp-son BG, Yoon CS, et al. Systemic reovirus therapy of metastatic cancer in immune-competent mice. Cancer Res. 2003;63(2):348–53. [PubMed] [Google Scholar]
- 14.Etoh T, Himeno Y, Matsumoto T, Aramaki M, Kawano K, Nishizono A, et al. Oncolytic viral therapy for human pan-creatic cancer cells by reovirus. Clin Cancer Res. 2003;9(3):1218–23. [PubMed] [Google Scholar]
- 15.Stanford MM, Barrett JW, Nazarian SH, Werden S, McFadden G. Oncolytic virotherapy synergism with signaling inhibitors:Rapamycin increases myxoma virus tropism for human tumor cells. J Virol. 2007;81(3):1251. doi: 10.1128/JVI.01408-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lun X, Yang W, Alain T, Shi ZQ, Muzik H, Barret JW, et al. Myxoma virus is a novel oncolytic virus with significant antitu-mor activity against experimental human gliomas. Cancer Res. 2005;65(21):9982–90. doi: 10.1158/0008-5472.CAN-05-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brandenburger A, Legendre D, Avalosse B, Rommelaere J. NS-1 and NS-2 proteins may act synergistically in the cyto-pathogenicity of parvovirus MVMp. Virology. 1990;174(2):576–84. doi: 10.1016/0042-6822(90)90110-d. [DOI] [PubMed] [Google Scholar]
- 18.Di Piazza M, Mader C, Geletneky K, Herrero y, Calle M, Weber E, et al. Cytosolic activation of cathepsins mediates parvovirus H-1-induced killing of cisplatin and TRAIL-resistant glioma cells. J Virol. 2007;81(8):4186. doi: 10.1128/JVI.02601-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herrero YCM, Cornelis JJ, Herold-Mende C, Rommelaere J, Schlehofer JR, Geletneky K. Parvovirus H-1 infection of human glioma cells leads to complete viral replication and effi-cient cell killing. Int J Cancer. 2004;109(1):76–84. doi: 10.1002/ijc.11626. [DOI] [PubMed] [Google Scholar]
- 20.Geletneky K, Herrero YCM, Rommelaere J, Schlehofer JR. Oncolytic potential of rodent parvoviruses for cancer therapy in humans: a brief review. J Vet Med. 2005;52(7-8):327–30. doi: 10.1111/j.1439-0450.2005.00877.x. [DOI] [PubMed] [Google Scholar]
- 21.Smith RR, Huebner RJ, Rowe WP, Schatten WE, Thomas LB. Studies on the use of viruses in the treatment of carcinoma of the cervix. Cancer. 1956;9(6):1211–18. doi: 10.1002/1097-0142(195611/12)9:6<1211::aid-cncr2820090624>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 22.Cervantes-Garcia D, Ortiz-Lopez R, Mayek-Pérez N, Rojas-Martinez A. Oncolytic virotherapy. Annals of Hepatol. 2008;7(1):34–45. [PubMed] [Google Scholar]
- 23.Donofrio G, Caviran S, van Santen V, Flammini CF. Potential secondary pathogenic role for bovine herpesvirus 4. J Clin Microbiol. 2005;43(7):3421–6. doi: 10.1128/JCM.43.7.3421-3426.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gillet L, Dewals B, Farnir F, de Leval L, Vanderplasschen A. Bovine herpesvirus 4 induces apoptosis of human carcinoma cell lines in vitro and in vivo . Cancer Res. 2005;65(20):9463–72. doi: 10.1158/0008-5472.CAN-05-1076. [DOI] [PubMed] [Google Scholar]
- 25.Shafren DR, Dorahy DJ, Ingham RA, Burns GF, Barry RD. Coxsackievirus A21 binds to decay-accelerating factor but requires intercellular adhesion molecule 1 for cell entry. J Virol. 1997;71(6):4736–43. doi: 10.1128/jvi.71.6.4736-4743.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shafren DR, Au GG, Nguyen T, Newcombe NG, Haley ES, Beagley L, et al. Systemic therapy of malignant human melanoma tumors by a common cold-producing enterovirus, Coxsackievirus A21. Clin Cancer Res. 2004;10(1):53–60. doi: 10.1158/1078-0432.ccr-0690-3. [DOI] [PubMed] [Google Scholar]
- 27.David K, Robert LM, Zwiebel J. Replication-selective virotherapy for cancer: Biological principles, risk management and future directions. Nature Med. 2001;7(7):781. doi: 10.1038/89901. [DOI] [PubMed] [Google Scholar]
- 28.Flanagan AD, Love R, Tesar W. Propagation of Newcastle disease virus in Ehrlich ascites cells in vitro and in vivo. Proc Soc Exp Biol Med. 1995;90:82–6. doi: 10.3181/00379727-90-21945. [DOI] [PubMed] [Google Scholar]
- 29.Fauziah O, Aini I, Asmah R, Omar AR, Abdul-Manaf A, Jafri M. Proceedings Yemeni Scietific Conference: Oct 11-13 2004; Sanaa. Yemen.: Replication of Newcastle disease virus in the breast cancer cell lines.; 2004. [Google Scholar]
- 30.Lorence RM, Katubig BB, Reichard KW. Complete regression of human fibrosarcoma xenografts after local Newcastle disease virus therapy. Cancer Res. 1994;54:6017–21. [PubMed] [Google Scholar]
- 31.Mullen JT, Tanabe KK. Viral oncolysis. Oncologist. 2002;7:106–119. doi: 10.1634/theoncologist.7-2-106. [DOI] [PubMed] [Google Scholar]
- 32.Rommelaere J, Cornelis JJ. Antineoplastic activity of parvoviruses. J Virol Methods. 1991;33:233–51. doi: 10.1016/0166-0934(91)90024-t. [DOI] [PubMed] [Google Scholar]
- 33.Olijslagers S, Dege AY, Dinsart C. Potentiation of a recombinant oncolytic parvovirus by expression of apoptin. Cancer Gene Ther. 2001;8:958–65. doi: 10.1038/sj.cgt.7700392. [DOI] [PubMed] [Google Scholar]
- 34.Madigan MT, Martinko JM, Parker J. Brock Biology of Microorganisms. 9th. Upper Saddal River,NJ: Prentice-Hall, Inc.; 2000. [Google Scholar]
- 35.Coffey MC, Strong JE, Forsyth PA, Lee PW. Reovirus therapy of tumors with activated Ras pathway. Science. 1998;282:1332–4. doi: 10.1126/science.282.5392.1332. [DOI] [PubMed] [Google Scholar]
- 36.Strong JE, Coffey MC, Tang D, Sabinin P, Lee PW. The molecular basis of viral oncolysis: usurpation of the Ras signaling pathway by reovirus. EMBO J. 1998;17:3351–62. doi: 10.1093/emboj/17.12.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–70. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 38.Norman KL, Coffey MC, Hirasawa K. Reovirus oncolysis of human breast cancer. Hum Gene Ther. 2002;13:641–52. doi: 10.1089/10430340252837233. [DOI] [PubMed] [Google Scholar]
- 39.Hirasawa K, Nishikawa SG, Norman KL. Systemic reovirus therapy of metastatic cancer in immune-compe- tent mice. Cancer Res. 2003;63:348–53. [PubMed] [Google Scholar]
- 40.Paiva LR, Silva HS, Ferreira HS, Martins ML. Multiscale model for the effects of adaptive immunity suppression on the viral therapy of cancer. Phys Biol. 2013;10(2):5005. doi: 10.1088/1478-3975/10/2/025005. [DOI] [PubMed] [Google Scholar]
- 41.Everts B, Van der Poel HG. Replication-selective oncolytic viruses in the treatment of cancer. Cancer Gene Ther. 2005;12(2):141–61. doi: 10.1038/sj.cgt.7700771. [DOI] [PubMed] [Google Scholar]