Abstract
Background
Wilms' tumor is an emberyonal tumor arising from remnants of immature renal tissue. Her2/neu is an onco-protein which mediates cellular proliferation, differentiation and survival.
Methods
In the current study, we analyzed Her2/neu expression in 40 Wilms' tumors. The clinico-demographic data of 40 patients with Wilms' tumor were retrieved. Immunohistochemical staining for HER2/neu was performed. Her2/neuimmunoreactivity was evaluated by Canadian Consensus 2007 scoring system.
Results
Among the 38 specimens with epithelial component, 68.5% were positive for Her2/neu, whereas there was immunoreactivity in 37% of 38 blastemal, and 12% of 31 stromal components. The Her2/neu expression was significantly higher in early stages (81.5%) than in advanced stages (36.4%) in epithelial component, but not in other components.
Conclusion
This study suggested that Her2/neuexpression is associated with epithelial cell differentiation accompanied by lower stages of tumor. No significant relationship was found between Her2/neu positivity and tumor size and patient's age and gender.
Keywords: Her2/neu, Wilms' tumor, Nephroblastoma, Tumor component
Introduction
Wilms' tumor (nephroblastoma) is a pediatric malignancy and one of the most common solid tumors in children [1]. The prevalence of Wilms' tumor is relatively equal in both genders (male to female ratio: 0.92/1), and the mean age at diagnosis is 3.5 years [2]. Microscopically classic triphasicWilms' tumor is composed of epithelial, blastemal and stromal components, but biphasic and monophasic tumors are not uncommon [3].
C-erb-B2 is a proto-oncogene, located on chromosome 17, and encodes a 185-kd trans-membrane glycoprotein (Her2/neu) which is a member of the epidermal growth factor receptor family with thyrosine kinase activity [4]. Her2/neu overexpression is seen in about 20% of invasive breast cancers and is associated with a worse prognosis [5].
Herceptin is a monoclonal antibody against the Her2/neu receptor and acts through the inhibition of Her/2 mediated signaling, and induces antibody dependent cellular injury [6-8]. Herceptin is mainly used for treatment of breast cancer in women; and compared with other treatments, either alone or in combination with other drugs, causes longer survival of Her2/neu positive breast cancers [9]. The aim of this study is to evaluate Her2/neu expression in Wilms' tumor and to find any significant relationship with histopathologic data. This could be a background for further studies to use Herceptin as a therapeutic agent in Wilms' tumor.
Materials and Methods
The clinico-demographic and pathologic data, including patient's age and gender as well as tumors size, stage and components of the 40 Wilms' tumors were retrieved from the archive of the Pathology Department of the Tehran Ali-Asghar Children Hospital (2001-2011). Then, hematoxylin and eosin-stained slides of each patient were reviewed by 2 pathologists (M.M. and M.B.) to select the best embedded paraffin block for Immunohistochemistry (IHC) study, which contains all components of the tumor, depending on its histological phase.
IHC staining was done according to Envision-based immunohistochemistry method. 3m tissue sections were mounted on Poly-L-Lysin coated slides which were deparaffinized and rehydrated in graded ethanol. The sections were rinsed in H2O2 with 1/10 dilution in methanol to block endogenous peroxidase activity. Slides were incubated by Polyclonal rabbit Anti-Human C-erb-B2 (Dakocytomation Denmark), placed in room temperature for 30 minutes. Then, Envision, labeled with Hoarse-reddish peroxidase, was added to the slides. After washing in Tris Buffered Saline (TBS) buffer, DAB chromogen (diaminobenzidintetrahydrocholoride) and substrate were placed on slides. Finally, the sections were counterstained in hematoxylin. Her2/neuimmunoreactivity was evaluated by Canadian Consensus for Her2/neu testing [10] (Table 1).
Table 1.
Canadian Consensu for Her2/neu test
| Score | Assessment | Staining pattern |
|---|---|---|
| 0 | Negative | No staining is observed in invasive tumor cells: no overexpression |
| 1+ | Negative | Weak, incomplete membrane staining in any proportion of invasive tumor cells, or weak, complete membrane staining in less than 10% of cells: no overexpression |
| 2+ | Weak to moderate positive | Complete membrane staining that is nonuniform or weak but with obvious circumferential distribution in at least 10% of cells, or intense complete membrane staining in 30% or less of tumor cells: equivocal |
| 3+ | Strong positive | Uniform intense membrane staining of more than 30% of invasive tumor cells: overexpression |
Results
Among the 40 cases, male (47.5%) to female (52.5%) ratio was 0.9. The mean age of patients was 3.8±3 years, ranging from 6 months to 13 years. 17 patients (42.5%) were stage I, 12 (30%) stage II, 6 (15%) stage III, 3 (7.5%) stage IV and 2 (5%) were stage V. The tumor size ranged from 3.5-21cm with mean of 10.3±5cm.
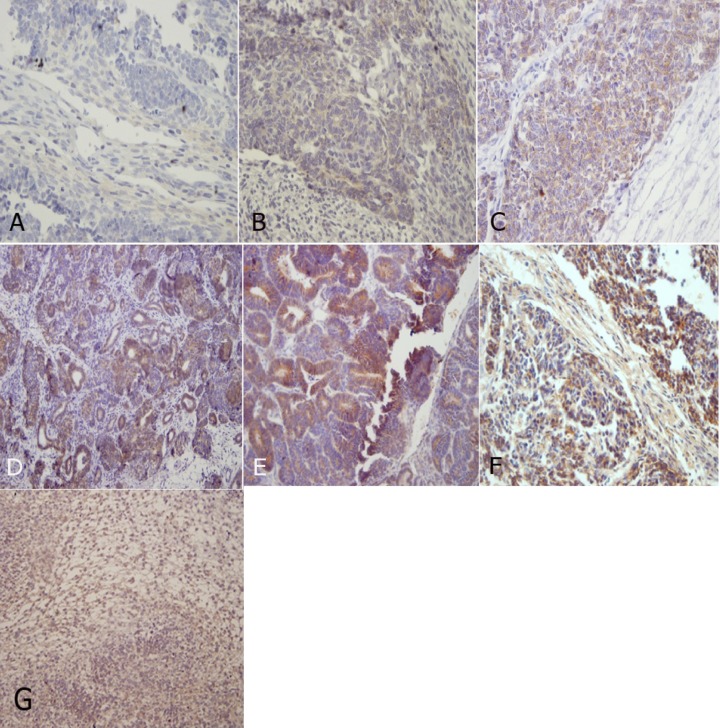
Among the 40 specimens, 33 (82.5%) showed a triphasic pattern and 3 (7.5%) had biphasic pattern, containing epithelial and blastemal elements. The other 4 (10%) cases showed monophasic pattern, containing 2 epithelial and 2 blastemal elements. Figure 1 (A-G) demonstrates Her2/neu staining in different degrees in tumor components.
Figure 1.
Figure 1. It shows Her2/neu staining in different degrees in tumor components: A: Stromal (1+) , B: Blastemal (1+), Epithelial (1+) C: Blastemal (2+), D: Epithelial (2+) , E: Epithelial (3+) F: Blastemal (3+), Stromal (3+) , G: Stromal (2+)
Among the 38 specimen with epithelial component, 26 (68.5%) showed positive reactivity for Her2/neu, whereas among the 38 specimen with blastemal component, 14 (37%) showed Her2/neu positivity. Only 4 (12%) of 31 cases with stromal differentiation showed positive reactivity (P<0.001) (Table 2).
Table 2.
Relation between Her2/neu expression and tumor component
| Tumor component | Her2/neu expression | Percent |
|---|---|---|
| Epithelial | 0 | 4 (10.5%) |
| +1 | 8 (21%) | |
| +2 | 12 (31.5%) | |
| +3 | 14 (37%) | |
| Blastemal | 0 | 11 (29%) |
| +1 | 13 (34%) | |
| +2 | 8 (21%) | |
| +3 | 6 (16%) | |
| Stromal | 0 | 20 (60.7%) |
| +1 | 9 (27.3%) | |
| +2 | 2 (6%) | |
| +3 | 2 (6%) |
The Her2/neu immunoreactivity was significantly higher in early stages (stage I and II) (81.5%) than in advanced stages (stage III, IV and V) (36.4%) in epithelial component (P=0.001), but no difference was found in blastemal (P=0.109) and stromal (P=0.817) components (Table 3).There was no relationship between Her2/neu positivity and tumor size, histological phase, patient's age and gender (P>0.05).
Table 3.
Relation between Her2/neu experssion and stage
| Tumor component | Stage | Her2/neu positive | Her2/neu negative | P value |
|---|---|---|---|---|
| Epithelial | ≤2 | 22 (81.5%) | 5 (18.5%) | 0.001 |
| >2 | 4 (36.4%) | 7 (63.4%) | ||
| Blastemal | ≤2 | 11 (40.7%) | 16 (59.3%) | 0.109 |
| ≥2 | 3 (27.3%) | 8 (72.7%) | ||
| Stromal | ≤2 | 3 (13%) | 20 (87%) | 0.817 |
| >2 | 1 (10%) | 9 (90%) |
Discussion
Wilms' tumor is a typical solitary lesion with no predilection for the left or right kidney or site within the kidney. Approximately, 10% of Wilms' tumors arise multifocally within the single kidney and 7% involve both kidneys either at presentation or subsequently [11]. Recently, some studies evaluated the expression of Her2/neu in several tumors such as breast, salivary gland, prostate, lung, liver and bladder carcinoma that could increase metastatic potential and drug resistance [12], but in Wilms' tumor the expression of Her2/neu has been reported in few studies.
Seham et al. [13] concluded that Her2/neu expression in Wilms' tumor could be a marker for epithelial and homologous differentiation, and its expression could be a good predicator for overall survival and longer recurrence free survival. In this study, there was higher expression of Her2/neu in triphasic tumors than in bi or monophasic ones. No relationship was found between Her2/neupositivity and patient's age, gender and tumor size. Salem et al. [14] suggest that in Wilms' tumor, the extent of Her2/neu receptor expression is associated with epithelial cell differentiation.
Potti et al. [15] found that high expression of Her2/neu causes epithelial differentiation in Wilms' tumor. Akin to Ghanem et al. [16] study, Her2/neu has no prognostic impact on the clinical outcome of patients with Wilms' tumor.
According to Yokoi et al. study [17], blockade of C-er-B2 in an in vivo model appears to inhibit the growth of Wilms' tumor via prevention of angiogenesis.
Finally, Yoram more et al. [18] suggested that in an in vivo model, Herceptin, an approved therapy for breast cancer, could also be employed for the treatment of Wilms' tumor.
In our study, we found that different histological components of Wilms' tumor apparently had different levels of Her2/neu expression. Therefore, there was 68.5% positive reactivity in epithelial component, while there was 37% and 12% positive reactivity in blastemal and stromal components, respectively (P<0.001).
These findings confirm the results of Seham et al. [13], Salem et al. [14] and Potti et al. [15] studies.
All these studies showed that Her2/neuexperssion is high in epithelial component, while it is very low in the stromal component.
We found that in early stages (I and II), there was higher Her2/neu positivity in epithelial component than in advance stages (III, IV and V) (P=0.001), whereas in other components there was no significant relationship between Her2/neuexperssion and tumor stage. The above findings suggested that Her2/neu overexpression favors epithelial differentiation in lower stages. Opposed to our findings, Seham et al. [13] concluded that Her2/neu positive tumors were significantly higher in all components in early stages (I and II) than in advanced stages.
In contrast to Seham et al. study [13] which suggested that Her2/neu is higher in triphasic tumors than bi or monophasic ones, in this study, we found no relationship between tumor histological phase and Her2/neu expression.
In this study, no significant difference was found between Her2/neu positive and negative cases regarding size of the tumors and patient's age and gender. These findings are akin to Seham et al. [13] study results.
In conclusion, this study suggested that Her2/neu expression in Wilms' tumor is associated with epithelial differentiation which is mainly expressed in early stages. Therefore, similar to Yokoi et al. [17] and Yoram more et al. [18] studies, it can be suggested that Herceptin may be used as a therapeutic agent in epithelial predominance or epithelial monophasic Wilms' tumor.
Acknowledgments
I would like to extend my thanks to the technicians of the Pathology Department of Aliasghar Hospital for their help in offering me the resources in running the program. The authors would like to thank Mr. Hematian for his assistance during this project. Finally, I wish to thank my wife for her support and encouragement throughout my study.
Footnotes
Conflicts of Interest
The authors have no conflict of interest in this article.
Authors' Contribution
Mashaallah Babashahi carried out the sampling and participated in the practical work and drafted the manuscript; Mitra Mehrazma performed the practical work; Farid Azizi Jalilian carried out the practical work and participated in the design of the study; Mostafa Rezaei-Tavirani performed the practical work.
REFERENCES
- 1.Beckwith JB. Renal neoplasms of childhood. In: Sternberg SS, editor. Diagnostic surgical pathology. New York: Raven Press.; 1994. pp. 1741–66. [Google Scholar]
- 2.Owens C, Brisse HJ, Olsen OE, Begent J, Smets AM. Bilateral disease and new trend in Wilmstumour. Pediatr Radiol. 2008;38:30–9. doi: 10.1007/s00247-007-0681-0. [DOI] [PubMed] [Google Scholar]
- 3.Cendron M, Wajsman Z. Wilms Tumor excerpt. 2006. Available from: http://WWW.eMedicine.com.
- 4.Cho HS, Mason K, Ramyar KX, Stanley AM, Gabelli SB, Denney DW, et al. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature. 2003;421:756–60. doi: 10.1038/nature01392. [DOI] [PubMed] [Google Scholar]
- 5.Ross JS, Slodkowska EA, Symmans WF, Pusztai L, Ravdin PM, Hortobagyi GN. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2therapy and personalized medicine. The oncologist . 2009;14:320–68. doi: 10.1634/theoncologist.2008-0230. [DOI] [PubMed] [Google Scholar]
- 6.Barok M, lsola J, Palyi-krekk Z, Nagy P, Juhasz I, Vereb G, et al. Trastuzmab causes antibody-dependent cellular cytotoxicity mediated growth inhibitor of submicroscopic JIMT-1 breast cancer xenografts despite intrinsic drug resistance. Mol cancer Ther. 2007;6(7):2065–72. doi: 10.1158/1535-7163.MCT-06-0766. [DOI] [PubMed] [Google Scholar]
- 7.Hudis CA. Trastuzmab -mechanism of action and use in clinical practice. N ENJL J Med. 2007;357(1):39–51. doi: 10.1056/NEJMra043186. [DOI] [PubMed] [Google Scholar]
- 8.Musolino A, Naldi N, Bortesi B, Pezzuolo D, Capelletti M, Missale G, et al. I mmunoglobulin G fragment C receptor polymorphism and clinical efficacy of Trastuzmab-based therapy in patients with HER/2 neu positive metastatic breast cancer. J ClinOncol. 2008;26(11):1789–96. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- 9.Marty M, Cognetti F, Maraninchi D, Snyder R, Mauriac L, Tubiana-Hulin M, et al. Randomized phase II trial of theefficacy and safety of trastuzumab combined with docetaxel in patientswith human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 studygroup. J ClinOncol. 2005;23:4265–74. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 10.Hanna W, O'malley FP, Barnes P, Berendt R, Gaboury L, Magliocco A, et al. Updated recommendations from the Canadian National Consensus Meeting on HER2/neu testing in breast cancer. CurrOncol. 2007;14(4):149–53. doi: 10.3747/co.2007.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breslow N, Olshan A, Beckwith JB, Green DM. Epidemiology of wilms tumor. Med PediatrOncol. 1993;21:172–81. doi: 10.1002/mpo.2950210305. [DOI] [PubMed] [Google Scholar]
- 12.Meert AP, Martin B, Paesmans M, Berghmans T, Mascaux C, Verdebout JM, et al. The role of HER2/ neu expression on the survival of patients with lung cancer: a systemic review of the literature. Br J Cancer. 2003;89:959–65. doi: 10.1038/sj.bjc.6601252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ragab SM, Samaka RM, Shams TM. HER2/neu Expression: A Predictor for Differentiation and Survival in Children WithWilms Tumor. PatholOncol Res. 2010;16(1):61–7. doi: 10.1007/s12253-009-9188-3. [DOI] [PubMed] [Google Scholar]
- 14.Salem M, Kinoshita Y, Tajiri T, Souzaki R, Tatsuta K, Higashi M, et al. Association between the HER2 expression and histological differentiation in Wilms tumor. PediatrSurgInt. 2006;22:891–6. doi: 10.1007/s00383-006-1762-0. [DOI] [PubMed] [Google Scholar]
- 15.Potti A, Forseen S, Koka V, Pervez H, Koch M, Fraimen G, et al. Determination of HER-2/neu overexpression and clinical predictors of survival in a cohortof 347 patients with primary malignant brain tumors. CancerInvestig. 2004;22(4):537–44. doi: 10.1081/cnv-200026523. [DOI] [PubMed] [Google Scholar]
- 16.Ghanem MA, Van Der Kwast TH, Den Hollander JC, Sudaryo MK, Mathoera RB, Van den Heuvel MM, et al. Expression and prognostic value of epidermal growth factor receptor, transforming growth factor-alpha, and c-erbB-2 in nephroblastoma. Cancer. 2001;92(12):3120–9. doi: 10.1002/1097-0142(20011215)92:12<3120::aid-cncr10173>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 17.Yokoi A, Mc Crudden KW, Huang J, Kim ES, Soffer SZ, Frischer JS, et al. Human epidermal growth factor receptor signaling contributes to tumor growth via angiogenesis in her/2neu expressing experimental wilmstumor. jpediatr surg. 2003;38(11):1569–73. doi: 10.1016/s0022-3468(03)00562-1. [DOI] [PubMed] [Google Scholar]
- 18.Mor Y, Harel Y, Pinthus J, Wax T, Kaufman-Francis K, Fridman E, et al. The Effect of Herceptin, an anti-ERBB2 antibody, on wilms tumor.; ESPU Meeting; Thursday 27, April 2006.; 2006. [Google Scholar]