Abstract
Background
Current anti-cancer drug therapy results in systemic side effects due to non-specific uptake by normal healthy noncancerous tissues. To alleviate this difficulty, many attempts have been devoted to the development of new delivery systems such as polymeric Nanoparticles (NPs). In this study, we prepared ICD-85 NPs based on sodium alginate and analyzed the cytotoxic activity of ICD-85 NPs relative to free ICD-85 on primary lamb kidney cells.
Methods
ICD-85 loaded sodium alginate nanoparticles were prepared by ionic gelation method and were characterized by the particle size, size distribution and Fourier Transform Infrared (FT-IR) spectroscopy. The in vitro cytotoxicity was evaluated by MTT assay and membrane integrity was evaluated by measuring Lactate Dehydrogenase (LDH) activity. The morphological alterations of untreated and treated cells were assessed by light inverted microscope.
Results
MTT assay showed that ICD-85 NPs could significantly decrease the in vitro cytotoxicity on primary lamb kidney cells compared to the free ICD-85. The IC10 value at 72 hours was increased from 9±2.7 μg/ml for free ICD-85 to 52±4.3 μg/ml for ICD-85 NPs. LDH assay demonstrated that free ICD-85 had dose-dependent cytotoxicity on primary lamb kidney cells while ICD-85 NPs exhibited significantly decreased cytotoxicity at equivalent concentrations. Moreover, morphological analysis showed no significant difference between control and treated cells with ICD-85 NPs.
Conclusion
Based on the results obtained in the present study it can be concluded that encapsulation of ICD-85 with sodium alginate nanoparticles can reduce its necrotic effect on primary lamb kidney cells.
Keywords: ICD-85 peptide, Primary cell culture, Sodium alginate, IC10, Lactate dehydrogenase
Introduction
Most current anti-cancer agents do not greatly differentiate between cancerous and normal cells, leading to systemic toxicity and adverse effects [1]. With the aim of avoiding cancer therapy failure, several approaches such as design of new anti-cancer drugs, chemical engineering of conventional drugs and development of drug delivery systems have been proposed [2]. Recently, nano-particulate drug delivery systems containing anti-cancer agents have received much attention [3]. Polymeric nanoparticles among other systems such as drug-polymer conjugates, drug-loaded liposomes and polymeric micelles are promising solutions to the problem as within such formulations cytotoxic drug molecules are encapsulated and their release can be concentrated at tumor sites [4]. The currently approved nanoparticle systems have in some cases improved the therapeutic index of drugs by reducing drug toxicity or enhancing drug efficacy [5]. Paclitaxel (TaxolTM) in a new formulation was conjugated to albumin nanoparticles (AbraxaneTM) has been approved by the FDA to treat metastatic breast cancer and side effects were fewer for new formulation than the conventional formulation [6]. Among the different carriers for controlled drug delivery, there has been rising interest in nano-sized self-aggregates composed of natural polysaccharides such as alginate [7] and chitosan [8]. Alginate is well-known anionic polysaccharide and colloidal biocompatible macromolecules, which are inert in biological systems and do not affect cell viability [9, 10].
Our previous studies revealed an inhibitory effect of ICD-85 (venom derived peptides) on breast cancer cell line MDA-MB231 [11]. ICD-85 was also confirmed by in vivo studies to suppress the breast tumor in mice [12]. However since encapsulation of ICD-85 as nanoparticles may reduce its toxicity towards normal cells, hence the present study was undertaken to evaluate and compare the cytotoxic effect of ICD-85 versus ICD-85 encapsulated in sodium alginate nanoparticles on primary lamb kidney cells.
Materials and Methods
The active fraction of ICD-85 is a combination of three peptides, ranging from 10,000 to 30,000 Da, derived from the venoms of snake (Agkistrodon halys) and scorpion (Hemiscorpius lepturus) was obtained from Razi Vaccine and Serum Research Institute (Karaj, Iran). The cell culture medium (DMEM/F12), Fetal Bovine Serum (FBS), trypsin-EDTA, penicillin and streptomycin were provided by Gibco (USA). Sodium alginate and poly-L-lysine were purchased from Sigma-Aldrich Chemical (Germany). Calcium chloride, 3-(4, 5-dimethyl-thiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) and Dimethyl Sulfoxide (DMSO) were purchased from Merck (Darmstadt, Germany). Other chemicals used were of analytical grades.
Preparation of ICD-85 NPs
ICD-85 NPs was prepared by the ionic-gelation method [9]. Briefly, 2ml aqueous calcium chloride (0.2mg/ml) was added drop-wise to 8ml aqueous sodium alginate (1.0mg/ml) while stirring for 30 min, and then 4 ml poly-L-lysine solution (0.3mg/ml) was added and stirred for an additional 1 hour. Nanoparticles were separated by centrifuging (Ependorf, Germany) at 13,000rpm at 14°C for 30 min, freeze-dried, and stored at 4°-8°C. The weights of freeze-dried nanoparticles were also measured. The ICD-85 loading nanoparticles were prepared with incorporation of sodium alginate solution, into calcium chloride solution containing 750μg/ml of ICD-85.
Characterization of ICD-85 NPs
The mean particle size and size distributions (Polydispersity Index (PDI)) of ICD-85 NPs were determined by Dynamic Light Scattering (DLS) technique using Zetasizer (Malvern Instruments, UK). The intensity of scattered light was performed at 25°C and scattering angle of 90°C.
The interactions between the different components of the nanoparticulate systems were analyzed by Fourier Transform Infrared (FT-IR). ICD-85 NPs were separated from the suspension by centrifugation (Ependorf, Germany) at 13,000 rpm and 14°C for 30 min and lyophilized. These dried nanoparticles were mixed with Potassium Bromide (KBr) and pressed to the plate for measurements. FT-IR spectra were recorded on FT-IR spectrometer (FTIR- 410®Jasco Colchester, UK).
Encapsulation Efficiency and Loading Capacity
The protein concentration was estimated by Bradford method at 595nm [13]. The amount of ICD-85 encapsulated in the nanoparticles was measured by calculating the difference between the total amounts of the ICD-85 added in the nanoparticle preparation solution and the amount of nonentrapped ICD-85 remaining in the clear supernatant after the centrifugation of samples at 13000 rpm for 30 min at 5±3°C. The amount of free ICD-85 in the supernatant was estimated by the above mentioned method. The ICD-85 Encapsulation Efficiency (EE) and Loading Capacity (LC) were calculated according to the following equations:
%EE= [(A-B)/A] ×100
%LC= [(A-B)/C] ×100
A = total amount of ICD-85 in added solution; B = total amount of ICD-85 in supernatant after centrifugation; and C = weight of the nanoparticles measured after freeze-drying [14].
Isolation and Culture of Primary Cells
For the isolation of kidney cells, the excised kidney was minced with a scalpel, and tissue fragments were then incubated and agitated at 37°C in a collagenase-trypsin solution. Cells were washed three times in growth medium and recovered by centrifugation at 500rpm for 5 minutes, after each wash. Freshly isolated kidney cells were plated in 25 cm2 culture flasks (Nunc, Denmark) containing 5 ml growth medium (Basal medium DMEM/F12: 50:50 mixture of DMEM and Ham's F12) supplemented with Penicillin (100 Units/ml), Streptomycin (100 μg/ml) and 10% (v/v) heat inactivated fetal bovine serum for 48 hours. The cells were grown in CO2 incubator (Memmert, Germany) at 37°C with 90% humidity and 5% CO2. During subculture, cells were detached by trypsinization when they reached 80% confluency and split (1:3). Growth medium was changed every 3 days. All the experiments were carried out between passage nos. 1 to 3 [15].
Cell Viability Assay
Cytotoxicity was assessed using a standard MTT colorimetric method described by Mosmann [16]. Briefly, cells were seeded at a density of 1 × 104 cells/well in 96-well tissue culture plates (Nunc, Denmark). After 24 hours, the cells were treated with different concentrations of free ICD-85 and ICD-85 NPs for 72 hours. The concentrations of ICD-85 NPs treatments were calculated in such a way to include equivalent amounts as free ICD-85 treatments. After the treatment, 20 μl of MTT dye solution (5 mg/ml in PBS, pH=7.4) was added to each well. After 4 hours of incubation at 37°C, the medium was removed and formazan crystals were solubilised with 200 μl of DMSO and the solution was vigorously mixed to dissolve the reacted dye. After 15 minutes, Optical Density (OD) values for each well were measured at 570 nm using an ELISA plate reader (Dynex MRX II, USA). Cell viability was expressed relative to untreated cultures. The percentage of growth inhibition was calculated according to the formula: [1- (OD of treated/OD of control)] ×100. Each experiment was performed using six replicates for each concentration and repeated in triplicate. Free ICD-85 and ICD-85 NPs IC10 was determined as a concentration showing 10% cell growth inhibition as compared with control cell growth.
LDH Leakage
Lactate Dehydrogenase (LDH), an indicator of cell injury, was detected with an assay kit (CytoTox 96®, Promega, USA) according to the manufacturer's protocol. Samples from clarified medium of treated and untreated control wells were taken after 24 hours of incubation and the LDH activity was measured associated with a fully automated microplate reader photometer (Bio-Tek, USA).
Analysis of Morphological Changes
For these experiments the primary lamb kidney cells were treated with 60 μg/ml of free ICD-85 and ICD-85 NPs in six-well transparent plates (Nunc, Denmark). After 24 hours exposure, the cell morphology was examined under an inverted light microscope (Olympus CK2, Japan).
Statistical Analysis
Experiments were carried out at least in triplicate and results were expressed as mean ± SD. Computer program (Graph Pad Prism) was used to calculate the IC10 (10% inhibition of cell proliferation) values. In all cases, P<0.05 was considered statistically significant.
Results
Characterization of ICD-85 NPs
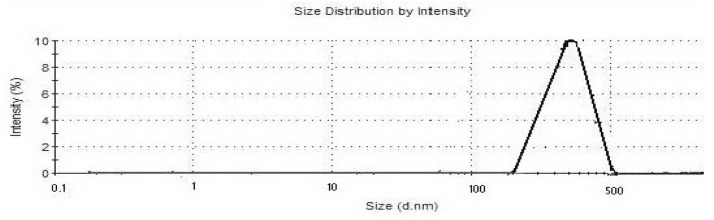
The hydrodynamic diameter of the ICD-85 NPs and its distribution measured by DLS are shown in Figure 1. The mean hydrodynamic diameter of particle was approximately 320 nm. The polydispersity index of ICD-85 NPs was estimated to be 0.475.
Figure 1.
It shows size distribution of ICD-85 NPs.
Loading Capacity (LC) and Encapsulation Efficiency (EE) were 86.24% (w/w) and 86.12% (w/w), respectively.
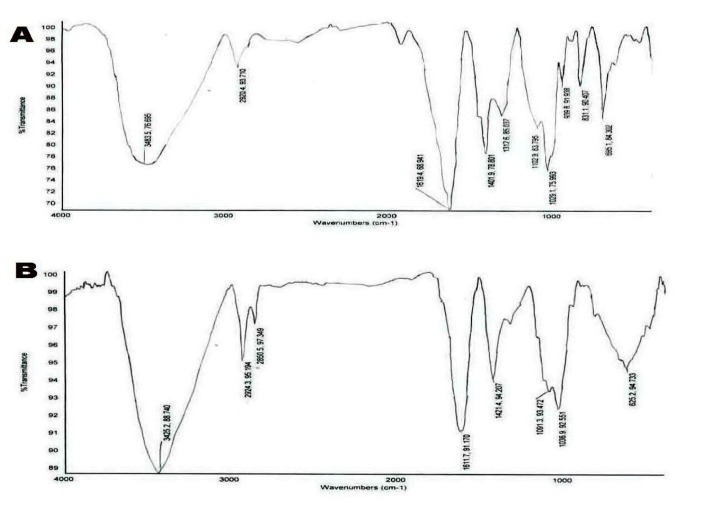
FT-IR spectra of sodium alginate NPs and ICD-85 NPs are shown in Figures 2A and 2B.
Figure 2.
It shows FT-IR spectra of sodium alginate NPs (curve A) and ICD-85 NPs (curve B).
The band around 3300-3400 cm-1 range in the spectrum contributed O-H stretching and intermolecular hydrogen bonding. The peaks observed at 1400-1700 cm-1 in the spectra represented peaks belong to the C=O stretching (amide). The characteristic peak observed around 1600 cm-1 (salt of carboxyl group) in the FT-IR spectrum of nanoparticles was attributed to the ionic interaction between these two reactive groups.
In Vitro Cytotoxicity Studies
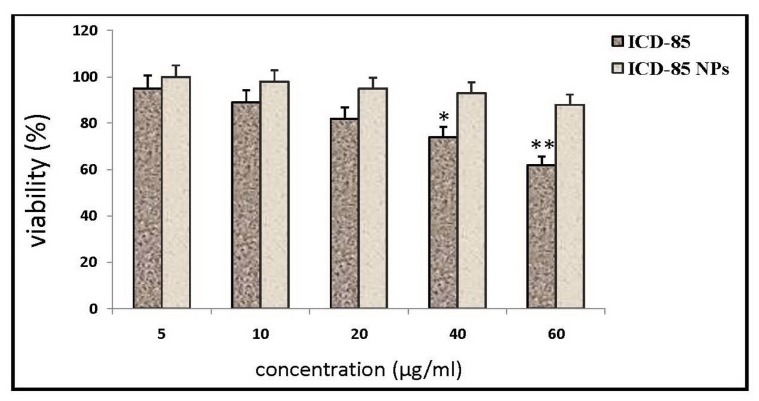
The cell viability was determined by MTT assay. The results of the cytotoxicity of free ICD-85 versus ICD-85 NPs on primary lamb kidney cells are presented in Figure 3. Treatment of cells with free ICD-85 concentrations less than 40μg/ml did not have significant effect on cell proliferation activity. In contrast, treatment of cells with ICD-85 NPs concentrations even at 60 μg/ml did not affect the cell proliferation activity. Data obtained from this assay indicated a dose response relationship with regard to the cytotoxic property of free ICD-85. There was a gradual decrease in the viability of primary lamb kidney cells, with increasing concentrations of free ICD-85 whereas ICD-85 NPs showed a slight decrease in cell viability between 40 µg/ml to 60 µg/ml (Figure 3). The inhibition effect of free ICD-85 on primary lamb kidney cells was about 35% at concentration 60µg/ml. ICD-85 NPs were found to be less toxic to primary lamb kidney cells than free ICD-85. The IC10 value at 72 hours was increased from 9±2.7 μg/ml for free ICD-85 to 52±4.3 μg/ml for ICD-85 NPs.
Figure 3.
It shows viability of primary lamb kidney cells exposed to free ICD-85 and ICD-85 NPs at 72 hours and measured by MTT assay. Viability of untreated cells (0 μg) was taken as 100%. The measurements of the treated cells were normalized to the control measurement (100%). Results are expressed as mean±SD of three independent experiments. *P<0.05 and **P<0.01 were considered to be statistically significant.
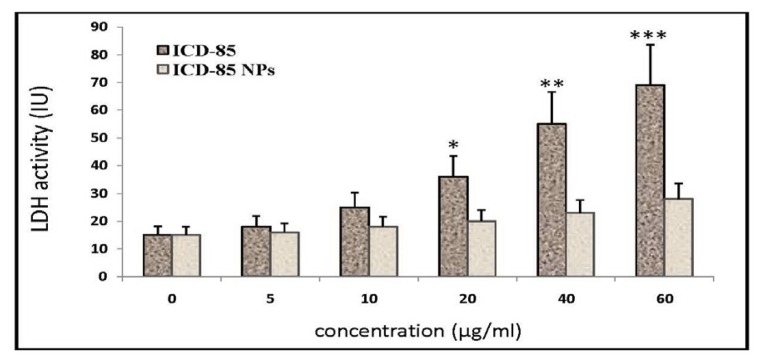
Cells treated with free ICD-85 resulted in a significant increase in LDH release as compared with untreated cells. Although treatment of primary lamb kidney cells with free ICD-85 at concentrations of 5 and10 µg/ml did not significantly increase LDH release but when it's concentration increased to 20 μg/ml and above, the LDH activity in the cultured media increased significantly (P<0.05). In contrast, the evaluation of the necrotic effect of ICD-85 NPs showed surprising results. LDH determination of cultured media of primary lamb kidney cells exposed to various concentrations of ICD-85 NPs revealed no significant difference between control and exposed cells.
Morphological Alterations
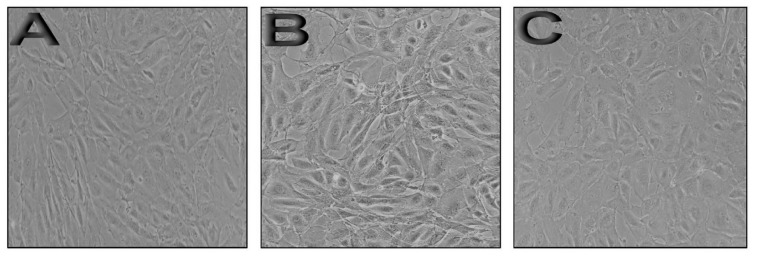
Morphological analysis of primary lamb kidney cells treated and untreated with free ICD-85 and ICD-85 NPs are presented in Figure 5. Control cells exhibited a typical morphology after 24 hours in culture (Figure 5A). In contrast, treated cells with 60 µg/ml of free ICD-85 showed morphological changes, including irregular cell membranes and slight cytoplasmic granulations (Figure 5B). The microscopic examination of treated cells with 60 µg/ml ICD-85 NPs showed no significant alterations compared to control cells (Figure 5C).
Figure 5.
Morphological alterations of primary lamb kidney cells exposed to free ICD-85 and ICD-85 NPs in cell culture medium for 24 hours are presented: A) Micrograph of control cells; B) Primary lamb kidney cells treated with 60 μg/ml of free ICD-85; C) Primary lamb kidney cells treated with 60 μg/ml of ICD-85 NPs.
Discussion
The utility of cancer chemotherapy is limited by undesirable toxic side effects to normal cells and tissues. These limitations are the result of a lack of selectivity to malignant cells [1]. Many clinically successful anti-cancer drugs were themselves either naturally occurring molecules or have been developed from their synthetic analogues [17]. Great interest is currently being paid to natural products for their interesting anti-cancer activities [17-19].
ICD-85 used in the present study was a combination of 3 peptides isolated partially from two different venoms [11, 12]. The combination of these peptides used because they work together synergistically having anti-proliferative activity along with anti-angiogenic activity. Previous studies in our laboratory had evaluated the potential cytotoxicity of free and NPs forms of ICD-85 on the human cervical carcinoma HeLa cells and we found that ICD-85 NPs was significantly more potent than free ICD-85 [20]. In this study, primary lamb kidney cells were selected for in vitro cytotoxic activity of ICD-85 because the primary cultured cells represented a useful tool for studying toxicity. Moreover, primary cells isolated from target tissues are desirable for cytotoxicity testing to simulate the in vivo situation more closely [21].
Natural polysaccharide polymers such as sodium alginate have been extensively studied in the recent years as a primary material in synthesizing carriers for therapeutic protein molecules [22]. In addition, sodium alginate was used in drug delivery system (producing microsphere, beads, microcapsule and tablets) and it showed long time effects and reduced side-effects of the drug [23]. Hence, in this study, we designed to prepare ICD-85 NPs based on the desirable properties of sodium alginate as a carrier for ICD-85 delivery system. ICD-85 NPs was prepared using ionic gelation method. This method offered many advantages such as simple and mild preparation without the use of organic solvent or ambient temperature [24]. The relatively mild gelation process of alginate helped in encapsulation of proteins, cells and DNA with retention of full biological activity [25]. In this case, carboxylic groups on molecular chains of alginate structure can be cross-linked by bivalent calcium ions to form nanoparticles [26]. The ability of the ionic gelation process to form ICD-85 loaded NPs was assessed by employing FT-IR to determine ICD-85 and sodium alginate NPs interactions. In the sodium alginate NPs spectra, the strong and broad peaks in the 3300-3450 cm−1 range correspond to O-H stretching and intermolecular hydrogen bonding. We can see carboxyl peaks near 1619 cm-1 (symmetric -COO- stretching vibration) and 1401 cm-1 (asymmetric COO- stretching vibration) that are relatively broad after interaction with ICD-85. In ICD-85 NPs spectra, the peak in the 1619 cm־1 shifted to 1611 cm־1 and the relative intensity of this peak was reduced. In addition, the peak at 831 cm־1 disappeared and a new broad peak appeared at 625 cm־1.
It has been shown that particle size and size distribution are the most important characteristics of nanoparticle systems [27]. Currently, the fastest and most routine method of determining particle size is by DLS [27]. The mean diameter of ICD-85 NPs was approximately 320 nm with PDI 0.475, which suggested a relatively suitable size distribution (PDI<0.5).
The results of MTT assay gave us an indication of cytotoxicity of free ICD-85 on primary lamb kidney cells with a dose-dependent decrease in the cell viability. The IC10 value at 72 hours was increased from 9±2.7 μg/ml for free ICD-85 to 52±4.3 μg/ml for ICD-85 NPs. It is noteworthy that the IC10 of ICD-85 NPs was about 6-fold higher than free ICD-85. The cytotoxic sensitivity of primary lamb kidney cells to free ICD-85 and ICD-85 NPs were also evaluated using the LDH assay. Lactate dehydrogenase assay was used in order to study whether the initial cytotoxicity could be due to the cell membrane damaging effects of free ICD-85 and ICD-85 NPs or not? The release of LDH to culture medium in the treated cells indicates the loss of cell membrane integrity and therefore it is an indirect method to assess the drug-induced cytotoxicity [28]. LDH assay showed a dose-dependent manner increase in the LDH levels following treatment with increasing concentrations of free ICD-85. The rise in LDH level following exposure free ICD-85 indicating the possibility of increased cell membrane damage at concentrations above 20 μg/ml (Figure 4). Previous studies showed no significant cell damage in normal MRC-5 cells treated with ICD-85 at low concentrations (5-15 μg/ml). However, when concentration increased above 20 μg/ml, the cytotoxic effect of ICD-85 on normal MRC-5 cells was evident [11]. This is in accordance with LDH assay analysis in the present study that demonstrated the necrotic effect of free form of ICD-85 at high concentrations.
Figure 4.
It shows activity of Lactate Dehydrogenase (LDH) after treatment of primary lamb kidney cells in the presence and absence (control) of free ICD-85 and ICD-85 NPs for 24 hours. Results are expressed as mean±SD of three independent experiments. All the values are compared with values from control (0 μg). *P<0.05, **P<0.01 and ***P<0.001 were considered to be statistically significant.
Biodegradable polymeric nanoparticles have been intensively studied as a possible way to reduce drug toxicity and degradation [29]. Lactate dehydrogenase determination in cultured media of primary lamb kidney cells, treated with various concentrations of ICD-85 NPs showed no significant rise as compared to untreated cells (Figure 4). These results confirmed that ICD-85 NPs had less toxicity to primary lamb kidney cells than free ICD-85. Morphological studies showed that, primary lamb kidney cells did not alter morphologically in the presence of ICD-85 NPs, which was an indication of safety of ICD-85 NPs (Figure 5C). Morphological studies confirmed data of MTT assay obtained by comparison of cytotoxic effect induced by free ICD-85 and ICD-85 NPs on primary lamb kidney cells.
When considering the chemotherapy side effects, it is very important to verify whether the drug shows a harmful effect against normal cells [30]. Clinicians know that cytotoxic drugs kill cancer cells, but unfortunately, most of the currently used drugs are highly toxic to a diversity of normal tissues [31]. For example, docetaxel has been shown to be one of the chemotherapy drugs for the treatment of a variety of cancer. However, the use of docetaxel has been limited because of its toxicity to non-malignant cells [32]. Thus, a novel formulation of docetaxel with less toxicity and better targeting to tumor sites is desirable in clinical application and the sustained release of formulated docetaxel would be less likely to cause systemic toxicity than the continuous systemic administration of conventionally formulated docetaxel during similar periods of time [33]. Numerous investigations have shown that nanoparticulate drug delivery systems can increase anti-tumor efficacy while reducing systemic side effects [34, 35]. Park et al. demonstrated that encapsulation of doxorubicin within Poly Lactic-co-Glycolic Acid (PLGA) nanoparticles resulted in improved safety of the drug and cardiotoxicity was significantly reduced by encapsulation in nanoparticles [36]. It has been demonstrated in other studies that the in vivo toxicity in the various drug delivery systems was found to be significantly lower than that of a conventional formulation of paclitaxel [37, 38]. Hence the present in vitro studies proved less cytotoxic effects of ICD-85 NPs compared with free ICD-85 on primary lamb kidney cells.
Conclusion
Results of this investigation clearly showed that sodium alginate can be considered as alternative and promising biocompatible polymer to be used on development of ICD-85 for least toxicity on normal cells. However additional studies are now underway to evaluate efficacy and safety of ICD-85 NPs in cancer and normal cells.
Acknowledgments
This research work was supported by Razi Vaccine and Serum Research Institute of Iran.
Footnotes
Conflicts of Interest
The authors have no conflict of interest in this article.
Authors' Contribution
Abbas Zare Mirakabadi designed the study and reviewed the manuscript. Saeed Moradhaseli wrote the manuscript and analyzed the data. Both authors read and approved the final revision of the manuscript.
REFERENCES
- 1.Nie S, Xing Y, Kim GJ, Simons JW. Nanotechnology Applications in Cancer. Annu Rev Biomed Eng. 2007;9:257–88. doi: 10.1146/annurev.bioeng.9.060906.152025. [DOI] [PubMed] [Google Scholar]
- 2.Saez-Fernandez E, Ruiz MA, Arias JL. Drug delivery systems based on poly (ε-caprolactone) for cancer treatment. Ars Pharm. . 2009;50(2):83–96. [Google Scholar]
- 3.Van Vlerken LE, Amiji MM. Multi-functional polymeric nanoparticles for tumour-targeted drug delivery. Expert Opin Drug Deliv. 2006;3(2):205–16. doi: 10.1517/17425247.3.2.205. [DOI] [PubMed] [Google Scholar]
- 4.McNeil SE. Nanotechnology for the biologists. J Leukocyte Biol. 2005;78:585–94. doi: 10.1189/jlb.0205074. [DOI] [PubMed] [Google Scholar]
- 5.Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. Nanoparticles in Medicine: Therapeutic Applications and Developments. Clin pharm ther. 2008;83(5):761–9. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]
- 6.Sparreboom A, Scripture CD, Trieu V, Williams PJ, De T, Yang A, et al. Comparative, preclinical and clinical pharmacokinetics of a cremophor-free, nanoparticle albumin-bound paclitacel (ABI-007) and paclitaxel formulated in cremophor (taxol). Clin Cancer Res. 2005;11:4136–43. doi: 10.1158/1078-0432.CCR-04-2291. [DOI] [PubMed] [Google Scholar]
- 7.Leonard M, Boisseson MRD, Hubert P, Dalenccon F, Dellacherie E. Hydrophobically modified alginate hydrogels as protein carriers with specific controlled release properties. J Control Release. 2004;98:395–405. doi: 10.1016/j.jconrel.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Park JH, Kwona S, Nam JO, Park RW, Chung H, Seo SB, et al. Self-assembled nanoparticles based on glycol chitosan bearing 5h-cholanic acid for RGD peptide delivery. J Control Release. 2004;95:579–88. doi: 10.1016/j.jconrel.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 9.Rajaonarivony M, Vauthier C, Couarraze G, Puisieux F, Couvreur P. Development of a new drug carrier made from alginate. J Pharm Sci. 1993;82(9):912–7. doi: 10.1002/jps.2600820909. [DOI] [PubMed] [Google Scholar]
- 10.Cafaggi S, Russo E, Stefani R, Leardi R, Caviglioli G, Parodi B, et al. Preparation and evaluation of nanoparticles made of chitosan or N-trimethyl chitosan and a cisplatin-alginate complex. J Control Release. 2007;121:110–23. doi: 10.1016/j.jconrel.2007.05.037. [DOI] [PubMed] [Google Scholar]
- 11.Zare Mirakabadi A, Mahdavi S, Koohi MK, Taghavian M. Cytotoxic effect of ICD-85 (venom-derived peptides) on MDA-MB-231 cell line. J Venom Anim Toxins incl Trop Dis. 2008;14(4):619–27. [Google Scholar]
- 12.Koohi MK, Zare Mirakabadi A, Moharrami M, Hablolvarid MH. Anticancer effect of ICD-85 (venom derived peptides) on MDA-MB231 cell line (in vitro) and experimental mice with breast cancer (in vivo). Int J Vet Res. 2009;3(1):49–54. [Google Scholar]
- 13.Bradford M. A Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1-2):248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 14.Mohammadpour dounighi N, Behfar A, Ezabadi A, Zolfagharian H, Heydari M. Preparation of Chitosan nanoparticles containing naja-naja oxiana snake venom. Nanomedicine: NBM. 2010;6:137–43. doi: 10.1016/j.nano.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 15.Freshney RI. Culture of animal cells: a manual of basic technique. 5th. New York: Wiley-Liss; 2005. pp. 389–91. [Google Scholar]
- 16.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assay. J Immunol Method. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 17.Srivastava V, Negi AS, Kumar JK, Gupta MM, Suman PS, Khanuja SPS. Plant-based anticancer molecules: A chemical and biological profile of some important leads. Bioorg Med Chem. 2005;13:5892–908. doi: 10.1016/j.bmc.2005.05.066. [DOI] [PubMed] [Google Scholar]
- 18.Pilatova M, Varinska L, Perjesi P, Sarissky M, Mirossay L, Solar P, et al. In vitro antiproliferative and antiangiogenic effects of synthetic chalcone analogues. Toxicology in Vitro. 2010;24:1347–55. doi: 10.1016/j.tiv.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 19.Wang WX, Ji YH. Scorpion venom induces glioma cell apoptosis in vitro and inhibits glioma tumor growth in vivo. J Neurooncol. 2005;73:1–7. doi: 10.1007/s11060-004-4205-6. [DOI] [PubMed] [Google Scholar]
- 20.Moradhaseli S, Zare Mirakabadi A, Sarzaeem A, Kamalzadeh M, Haji hosseini R. Cytotoxicity of ICD-85 NPs on human cervical carcinoma HeLa cells through caspase-8 mediated pathway. Iran J Pharm Res. 2013;12(1):155–63. [PMC free article] [PubMed] [Google Scholar]
- 21.Zurlo J, Arterburn LM. Characterization of a primary hepatocyte culture system for toxicological studies. In Vitro Cell Dev Biol. 1996;32:211–20. doi: 10.1007/BF02722948. [DOI] [PubMed] [Google Scholar]
- 22.Gombotz WR, Wee SF. Protein release from alginate matrices. Adv Drug Deliver Rev. 1998;31(3):267–85. doi: 10.1016/s0169-409x(97)00124-5. [DOI] [PubMed] [Google Scholar]
- 23.Fana L, Jiang L, Xu Y, Zhou Y, Shen Y, Xie W, et al. Synthesis and anticoagulant activity of sodium alginate sulfates. Carbohyd Polym. 2011;83:1797–803. [Google Scholar]
- 24.Tiyaboonchai W. Chitosan Nanoparticles: A Promising System for Drug Delivery. Naresuan University Journal. 2003;11(3):51–66. [Google Scholar]
- 25.Sahoo SK, Prusty AK. Two Important Biodegradable Polymers and Their Role in Nanoparticle Preparation by Complex Coacervation Method- A Review. Int J Pharm Appl Sci. 2010;1(2):1–8. [Google Scholar]
- 26.De S, Robinson D. Polymer relationships during preparation of chitosan-alginate and poly-l-lysine-alginate nanospheres. J Control Release. 2003;89(1):101–12. doi: 10.1016/s0168-3659(03)00098-1. [DOI] [PubMed] [Google Scholar]
- 27.Jahanshahi M, Babaei Z. Protein nanoparticle A unique system as drug delivery vehicles. Afr J Biotechnol. 2008;7(25):4926–34. [Google Scholar]
- 28.Lu YH, Su MY, Huang HY, Li L, Yuan CG. Protective effects of the citrus flavanones to PC12 cells against cytotoxicity induced by hydrogen peroxide. Neuroscience Letters. 2010;484:6–11. doi: 10.1016/j.neulet.2010.07.078. [DOI] [PubMed] [Google Scholar]
- 29.Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B: Biointerfaces. 2010;75(1):1–18. doi: 10.1016/j.colsurfb.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Zuco V, Supino R, Righetti SC, Cleris L, Marchesi E, Passerine-Gambacorti C, et al. Selective cytotoxicity of betulinic acid on tumor cell lines, but not on normal cells. Cancer Letters. 2002;175:17–25. doi: 10.1016/s0304-3835(01)00718-2. [DOI] [PubMed] [Google Scholar]
- 31.Sporn MB, Suh N. Chemoprevention of cancer. Carcinogenesis. 2000;21:525–30. doi: 10.1093/carcin/21.3.525. [DOI] [PubMed] [Google Scholar]
- 32.Baker J, Ajani J, Scotte F, Winther D, Martin M, Aapro MS, et al. Docetaxel-related side effects and their management. Eur J Oncol Nurs. 2008;12:253–68. doi: 10.1016/j.ejon.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 33.Zheng D, Li X, Xu H, Lu X, Hu Y, Fan W. Study on docetaxel-loaded nanoparticles with high antitumor efficacy against malignant melanoma. Acta Biochim Biophys Sin. 2009;41(7):578–87. doi: 10.1093/abbs/gmp045. [DOI] [PubMed] [Google Scholar]
- 34.Brigger I, Dubernet C, Couvreur P. Nanoparticles in cancer therapy and diagnosis. Adv Drug Deliv Rev. 2002;54:631–51. doi: 10.1016/s0169-409x(02)00044-3. [DOI] [PubMed] [Google Scholar]
- 35.Mattheolabakis G, Taoufik E, Haralambous S, Roberts ML, Avgoustakis K. In vivo investigation of tolerance and antitumor activity of cisplatin-loaded PLGA-mPEG nanoparticles. Eur J Pharm Biopharm. 2009;71:190–5. doi: 10.1016/j.ejpb.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 36.Park J, Fong PM, Lu J, Russell KS, Booth CJ, Saltzman WM, et al. PEGylated PLGA nanoparticles for the improved delivery of doxorubicin. Nanomed: Nanotechnol Biol Med. 2009;5:410–8. doi: 10.1016/j.nano.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang X, Burt HM, Hoff DV, Dexter D, Mangold G, Degen D, et al. An investigation of the antitumour activity and biodistribution of polymeric micellar paclitaxel. Cancer Chemother Pharmacol. 1997;40:81–6. doi: 10.1007/s002800050630. [DOI] [PubMed] [Google Scholar]
- 38.Li C, Price JE, Milas L, Hunter NR, Ke S, Yu DF, et al. Antitumor activity of poly(L-glutamic acid)-paclitaxel on syngeneic and xenografted tumors. Clin Cancer Res. 1999;5:891–7. [PubMed] [Google Scholar]