Abstract
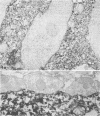
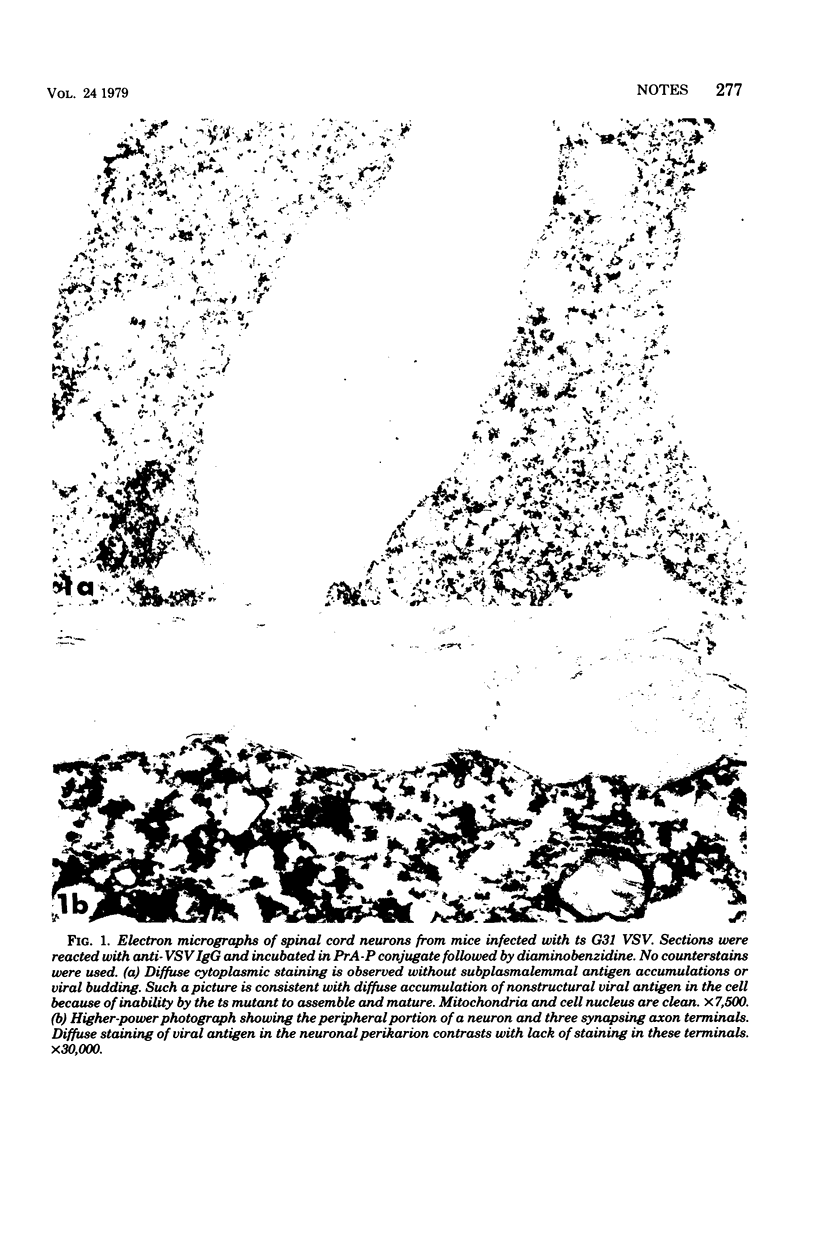
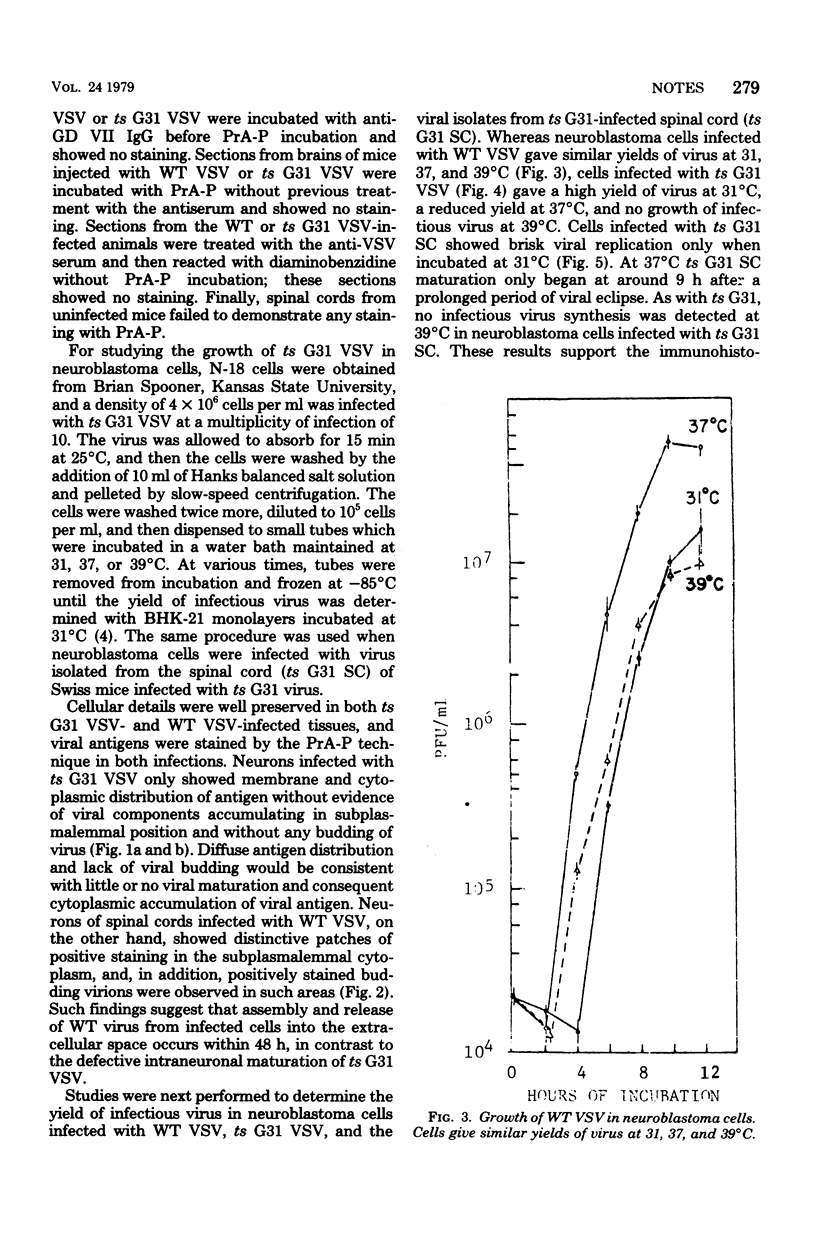
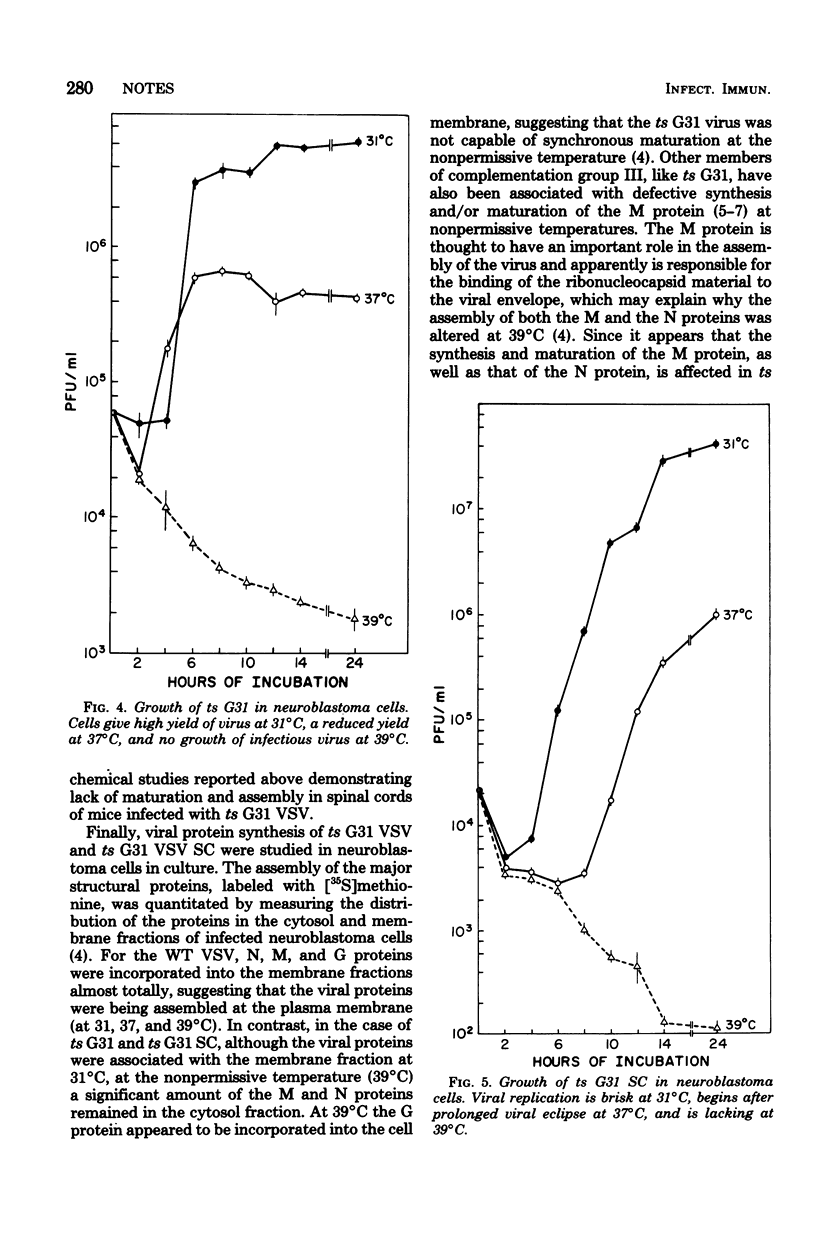
Ultrastructural immunoperoxidase studies were done in spinal cords of mice infected with wild type vesicular stomatitis virus or its temperature-sensitive (ts) mutant G31. Infected neurons showed subplasmalemmal staining of viral antigen and staining of viral particles budding from the neuronal membrane in wild-type vesicular stomatitis virus infection, whereas diffuse membrane and cytoplasmic staining with no budding virus was observed in ts G31 infection. Such findings suggest rapid viral assembly and release of viral particles from cells infected with wild-type virus. In contrast, maturation of ts G31 appears defective, and this would lead to accumulation of viral antigen in the cytoplasm of infected cells. These results correlate with studies in neuroblastoma cells which investigated the growth cycles of wild type, ts G31, and the spinal cord isolate of ts G31 as well as the viral protein-synthetic capacity of these viruses.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dal Canto M. C., Rabinowitz S. G., Johnson T. C. An ultrastructural study of central nervous system disease produced by wild-type and temperature-sensitive mutants of vesicular stomatitis virus. Lab Invest. 1976 Aug;35(2):185–196. [PubMed] [Google Scholar]
- Dal Canto M. C., Rabinowitz S. G., Johnson T. C. In vivo assembly and maturation of vesicular stomatitis virus. Lab Invest. 1976 Dec;35(6):515–524. [PubMed] [Google Scholar]
- Dubois-Dalcq M., McFarland H., McFarlin D. Protein A-peroxidase: a valluable tool for the localization of antigens. J Histochem Cytochem. 1977 Nov;25(11):1201–1206. doi: 10.1177/25.11.199666. [DOI] [PubMed] [Google Scholar]
- Hughes J. V., Johnson T. C., Rabinowitz S. G., Dal Canto M. C. Growth and maturation of a vesicular stomatitis virus temperature-sensitive mutant and its central nervous system isolate. J Virol. 1979 Jan;29(1):312–321. doi: 10.1128/jvi.29.1.312-321.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D. M., Baltimore D., Lodish H. F. Maturation of viral proteins in cells infected with temperature-sensitive mutants of vesicular stomatitis virus. J Virol. 1977 Mar;21(3):1149–1158. doi: 10.1128/jvi.21.3.1149-1158.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D., Lodish H. F., Baltimore D. Analysis of the defects of temperature-sensitive mutants of vesicular stomatitis virus: intracellular degradation of specific viral proteins. J Virol. 1977 Mar;21(3):1140–1148. doi: 10.1128/jvi.21.3.1140-1148.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafay F. Envelope proteins of vesicular stomatitis virus: effect of temperature-sensitive mutations in complementation groups III and V. J Virol. 1974 Nov;14(5):1220–1228. doi: 10.1128/jvi.14.5.1220-1228.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinowitz S. G., Dal Canto M. C., Johnson T. C. Comparison of central nervous system disease produced by wild-type and temperature-sensitive mutants of vesicular stomatitis virus. Infect Immun. 1976 Apr;13(4):1242–1249. doi: 10.1128/iai.13.4.1242-1249.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]