Abstract
Background
There are several types of cancer, which cause millions of deaths worldwide every year. Many studies have confirmed that plants are adequate natural sources to be examined as anti-cancer drugs with fewer side effects than chemotherapy and radiotherapy. In this study the anti-cancer properties of Lavender aqueous extract on lymphocytes derived from patients with Hodgkin's lymphoma has been studied.
Methods
In order to determine the cytotoxic effects of the extract on lymphocytes of patients in stages III and IV of Hodgkin's lymphoma and two different cell lines in the presence of different concentrations of aqueous extract of Lavender, MTT colorimetric assay and flow cytometry analysis were used.
Results
Findings indicated that Lavender inhibited cell proliferation in both lymphocytes and cell lines with different effects. The effective concentration of Lavender that decreased viability of Hodgkin's lymphoma cells below Lethal Concentration 50 (LC50) value was 100 µg/ml and this was half of the therapeutic dose. In addition, apoptosis was the main mechanism the Hodgkin's lymphoma cell encountered when exposed to the aqueous extract of Lavender.
Conclusion
This experiment proposes that aqueous Lavender extract can be regarded as a potential anti-cancer agent in future studies.
Keywords: Lavandula, Lymphocytes, Hodgkin's lymphoma, Cytotoxicity, Cell lines
Introduction
Cancer is the third leading cause of death in the world [1-3]. There are many different kinds of cancer. Cancer can develop in almost any organ or tissue such as lung, colon, breast, skin, bone, or nerve tissue [4-7]. Hodgkin's lymphoma is one of the most malignant cancers. The annual prevalence of Hodgkin's lymphoma is about 1 in 25,000 people and the disease accounts for slightly less than 1% of all cancers worldwide [8]. Hodgkin's Lymphoma (HL) is characterized by a minority of abnormal B lymphocyte cells, the Hodgkin and Reed-Sternberg cells (HRS cells), and a general inflammatory background [9, 10]. From ancient times, plants have been considered not only as sources of food, but also as treatments or remedies for many types of diseases. It has been pointed out in many different texts that there are unidentified properties in different parts of plants that can be used as treatments for certain diseases [11-13]. In the mint family, for instance, there is a branch which is called Lavender (of the genus Lavandula officinalis) which has clusters of small purplish flowers and yield soil used in perfumery. There are different species of Lavenderas part of flowering plants around the world which belong to the Lamiaceae family. This genus consists of annuals, herbaceous plants, sub-shrubs, and small shrubs [14, 15]. Lavender has been used in herbal treatments for centuries. In addition, Lavender yields highly effective essential oil with very sweet overtones which can be used in balms, salves, perfume, cosmetics, and topical applications. In alternative medicine, Lavender extraction is believed to be useful in easing or relieving the symptoms of stress, anxiety, exhaustion, irritability, headaches, migraines, insomnia, depression, colds, indigestion, flatulence, upset stomach, liver and gallbladder problems, nervousness and loss of appetite as well as being used as a breath freshener and mouthwash [14-21]. Fragrance compounds and essential oils, such as Lavender oil (Lavandula angustifolia), have a long-standing history as a medical remedy [22]. There has been increasing interest in perillyl alcohol, a monoterpene found in trace amounts in L. angustifolia [23], owing to its chemo-preventative and chemotherapeutic properties [24, 25]. Many studies have shown the ability of chemo-preventive phytochemicals to increase the sensitivity of cancer cells to conventional anticancer drugs [26]. It has been reported that antioxidant properties of Lavender x intermedia are owing to the phenolic component such as Rosmarinic Acid (RA) of the plant [27]. Currently, Perillyl alcohol, a component of the essential oil derived from Lavender, is one of the most important metabolites of d-limonene. Perillyl alcohol is considered a chemo-preventative and chemotherapeutic agent [28]. In view of the fact that the antigrowth activity of aqueous extract of Lavender angustifolia has not yet been evaluated on primary cells, the present study focuses on the anti-cytotoxicity properties of this aqueous extract on in vitro samples of Hodgkin's tumor which were collected from patients in comparison with two different cell lines.
Materials and Methods
Lavender Extraction and Preparation
Lavandula angustifolia was provided by the herbarium of Shahid Beheshti University of Medical Sciences, Tehran, Iran. To prepare the extraction, 250 gr of Lavender flowers were dried and then mixed with 1000 ml boiling water. In the next step, the mixture was stirred for 4 hours in a fully packed container. Finally, the mixture was filtered and concentrated by vaporizing. The plant specimen was determined by the Pharmaceutics Faculty of the University, where voucher specimens (1092) are kept.
Samples Collection
Pelvic bone marrow samples were aspirated from patients in stage III and IV of Hodgkin's lymphoma, the majority of which was provided for diagnostic tests by the BMT Laboratory in Taleghani Hospital, Tehran. 2.5 ml of the samples were prepared for isolating the lymphocytes by Ficoll method.
Samples were kept in heparin tubes (anticoagulant) and diluted with a ratio of 1 to 2 sterile Phosphate Buffered Saline (PBS), followed by Ficoll with a ratio of 1 to 2 and with density gradient method Peripheral Blood Mononuclear Cells (PBMC). Cells were removed from the buffy coat and then transferred to a 15-cc tube and the same volume of sterile PBS was added and centrifuged at 4°C and 1400 rpm for 10 minutes. The supernatant was then discarded and pellet was maintained. Finally, pellet was suspended in 0.5 cc RPMI1640 and then another 5-6 cc of RPMI1640 with FBS was added.
Cell Culture
SW742 and MKN45 carcinoma cells and lymphocytes of Hodgkin's lymphoma patients were cultured in RPMI 1640 supplemented with 10% fetal calf serum, 100U/ml penicillin, and 100 µg/ml streptomycin. They were incubated at 37°C in a humidified CO2 incubator with 5% CO2 and 95% air. Cultures were examined regularly. An inverted microscope (Celti) was used for comparing the cell morphology and pattern of cell spread in the presence of and in the absence of the extract.
Microscopic Study
An inverted microscope (Celti) was used to compare the cell morphology and pattern of cell distribution in various dosages of the extract.
MTT Assay
Antioxidant Activity of Lavender Aqueous Extract:
To evaluate the cytotoxic effects of Lavender aqueous extract on cell lines and lymphocytes of Hodgkin's lymphoma patients, 3-(4, 5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2H-tetrazolium bromide (MTT) colorimetric assay was applied [21]. Etoposide phosphate, an anti-cancer (antineoplastic or cytotoxic) chemotherapy drug, was used as a control sample. Briefly, cells were seeded into 96-well culture plates with 10,000 cells in each well with 200 µl medium. The medium was removed 48 hours after plating and fresh media containing different concentrations of Lavender aqueous extract were added. After incubation for one hour, the medium was discarded, the cells were washed twice with phosphate-buffered saline and 50 µg/ml MTT solution for 4 hours and formazan crystals dissolved by adding 100 µg Dimethyl Sulfoxide (DMSO) to each well. The absorptions were measured in triplicate at 570 nm, with a background correction at 630 nm, using a microplate reader.
A solution without Lavender aqueous extract was used as a blank (control). All the tests were repeated three times. The inhibition percentage was calculated as following:
Inhibition %= [(A0-A1)/ A0] × 100
Where A0 is the test sample or aqueous extract Optical Density (OD), and A1 is the absorption of the blank (OD) at 570 nm.
Flow cytometry Analysis
For flow cytometry purposes, cell lines and lymphocytes of Hodgkin's lymphoma samples were cultured in 6-well plates at a density of 1×106 cells both in the presence and in the absence of the cytotoxic agents for 48 hours. All suspended and adherent cells were harvested and centrifuged at 200 ×g for 10 minutes. Cell pellet was washed with 1X phosphate buffer saline solution and centrifuged at 200 ×g for 10 minutes. The cell pellet was then re-suspended in 100 μL of Annexin V/FLUOS labeling solution (predilute20 μL Annexin V/FLUOS labeling reagent in 1 mL incubation buffer and added 20 μL propidium iodide solution), and incubated at 15-25°C for 10-15 minutes. The samples were assayed by flow cytometer (USA) using 488nm excitation and a 515 nm band pass filter for fluorescein detection and a filter > 600 nm for propidium iodide detection. Analyses were carried out by Cell Quest software supplied with the device.
Statistical Analysis
Results are reported as mean ± SD. Mean difference among groups was calculated by one-way and two-way variance analyses and p < 0.001 was considered statistically significant.
Results
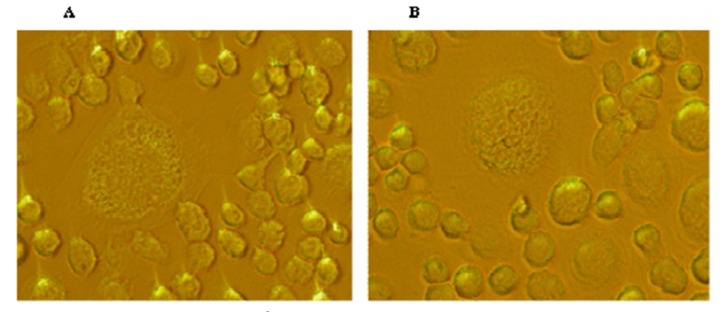
Microscopic evaluations were used to determine morphological changes in Hodgkin's lymphoma cells (Figures 1 and 2).
Figure 1.
It shows two images of Hodgkin's lymphoma cell morphology (original magnification, ×200).
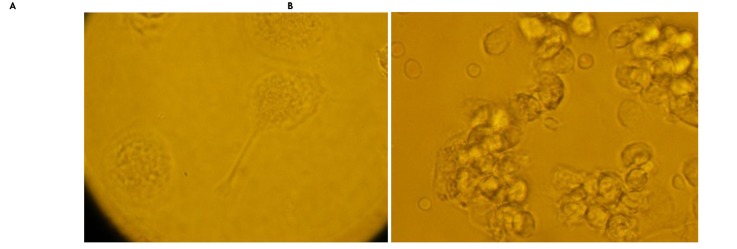
Figure 2.
Hodgkin's lymphocyte cell division characterization is shown.
A: This figure shows the modus of lymphocyte division. B: Lymphocytes are seen cumulatively abnormal while dividing (original magnification, ×200).
Reed-Sternberg cells, which are specific to Hodgkin’s Lymphoma disease [29], are clearly seen with two or three nuclei and are larger than normal lymphocyte cells.
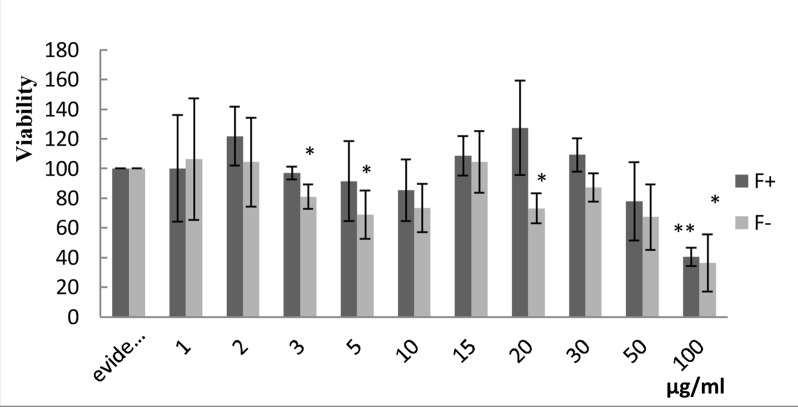
Viability determination of different cell lines (SW742, MKN45 and Hodgkin's lymphoma patient's sample) in the presence of various dosages of Lavender extract after 48 hours have been investigated by MTT assay (Figures 3, 4 and 5).
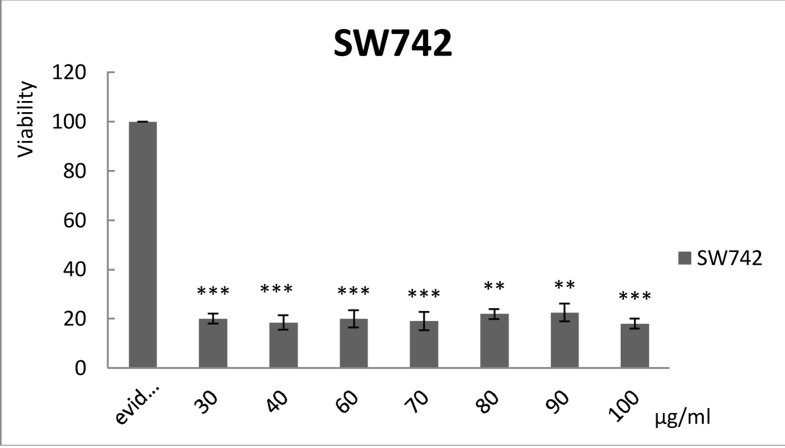
Figure 3.
It shows the effect of various concentrations of filtered aqueous Lavender extract on colon cancer cell line (SW742) (P Value< 0.001). Non-filtered extract shows similar effects (data not shown).
Figure 4.
It shows the effect of various concentrations of filtered aqueous Lavender extract on gastric cancer cell line (MKN45) (P Value< 0.001). Non-filtered extract shows similar effects (data not shown) [30].
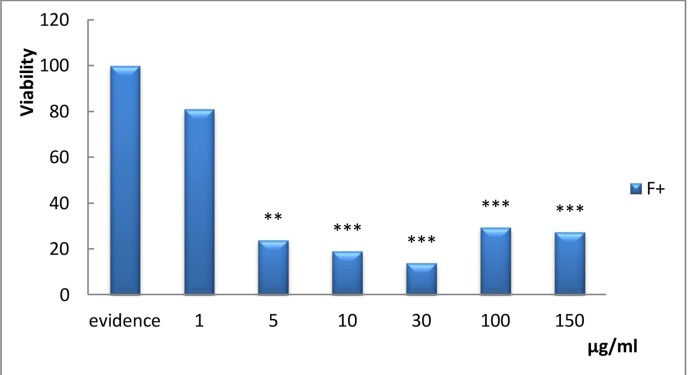
Figure 5.
The effect of various dosages (1, 2, 3, 5, 10, 15, 20, 30, 50, and 100) μg/ml of filtered and non-filtered aqueous extract of Lavender on Hodgkin's lymphoma cells after 48 hours is shown (P Value< 0.001).
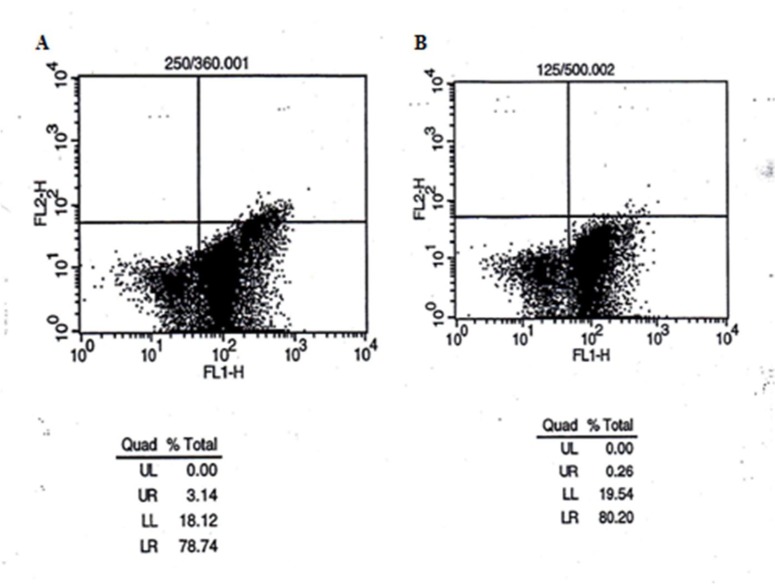
Flow cytometry assay was to determine the cell death mechanism. Four populations of cells are resolved and are shown in Figure 6. Living cells or Annexin- V/FLUOS (-) /PI (-) (LL) are seen in the lower left quadrant. Cells that are Annexin V/FLUOS (+)/PI (-) (LR) are apoptotic (lower right). The cell population with Annexin V/FLUOS (+)/PI (+) (UR) has been described as necrotic or advanced apoptotic (upper right) and Annexin V/FLUOS (-)/PI (+) (UL) may be bare nuclei cells in late necrosis, or cellular debris (upper left) (Figure 6).
Figure 6.
A Flow cytometry scheme in evaluation of: (A and B) cell samples (the cells in the presence of 50 (B) and 100 (A) μg/ml concentration of filtered extraction), after incubation for 48 hours.
Discussion
Hodgkin's lymphoma is one of the main life-threatening disorders throughout the world [8]. Since herbal remedies have been shown promising in cancer studies [31], Lavender extract was used to determine the possible cytotoxic effects on Hodgkin's lymphoma. Microscopic studies are widely used for cell morphology assessment [32]. In this study, the microscopic technique was used for the evaluation of Hodgkin's lymphoma cell characterization and morphology (Figures 1 and 2). As shown in Figures 1 and 2, Hodgkin's lymphoma cells contain two or more nuclei and exhibit abnormal cell growth and cell division. Reed-Sternberg cells are abnormal cells that are typical in Hodgkin's disease; they exist in the cultured cells of the patients. This evidence indicates that the samples correspond to the diagnostic process referring to Hodgkin's lymphoma. As depicted in Figure 2, accumulative cells refer to Hodgkin's lymphoma, providing a real cell line of this disease which can be analyzed by the present study. A viability test is used for determining cytotoxic effects of the treatment on cells [33]. Since some studies suggest that a 200 µg/ml of this extract can be effective as an Alzheimer's disease preventive remedy[34], evaluating other potential properties of the Lavender aqueous extract is essential; therefore, in this assessment a range of 30 to 100 µg/ml of the extract was applied and tested on two different sources including: first, two cancerous cell lines (MKN45 and SW742) and the second, a Hodgkin's lymphoma cell line derived from the patients' blood samples. Figures 3 and 4 show the viability of the two cell lines MKN45 and SW742 in the presence of various concentrations of Lavender aqueous extract. The findings indicate that the SW742 cell line is sensitive to extract, so the viability in the presence of a 30 µg/ml concentration of the extract and also higher concentrations is strongly inhibited in a steady trend. In all the applied dosages of the extract on the SW742 cell line viability were shown equally, the applied range of extract treatment in the MKN45 cell line viability test was altered to a lower degree. The viability study as illustrated in Figure 4 for MKN45 cell line shows that the anti-cell proliferation properties of the extract began at a 1 µg/ml dose. It should be mentioned that these findings (data in Figure 4) had been reported in a previously published study by our group [30]. The cells from different sources have different growth and death rates when exposed to the extract. Comparing Figures 3 and 4 clearly indicates that very low dosages relative to the therapeutic dosage of extract [34] show potent anticancer properties; therefore, aqueous Lavender extract can be considered as an effective antitumor drug.
The in vitro study is precursor for further investigations of patients' cell lines. It was expected that the primary cell line study would confirm the in vitro assessment. In many cases, however, these two processes exhibited dissimilar results [35]. Here, the derived lymphoid cell line from Hodgkin's patients was a candidate for treatment by the extract (Figure 5). The data in Figure 5 clearly shows that cell resistance to the extract is remarkable at high dosage; in fact, only 100 µg/ml inhibits cell survival effectively. There are two interpretations for this occurrence; firstly, the patient-derived cells are more complex and contain a compensative mechanism for response to cytotoxic effects of Lavender. Secondly, this cell line is primarily comparable to SW742 and MKN45 cell lines; more passages in this cell line influence its ability and behavior. On the other hand, the effective concentration of Lavender that decreases viability below LC50 value is 100µg/ml, and this dosage is half the therapeutic dose. Filtered and non-filtered forms of extract were applied in the present study as long as filtration could help to stimulate the dimension of the effective factors of extract. As is shown in Figure 5, however, their results in effective dosages were comparable. It can be concluded that effectual agent of the extract passes through the filter. Therefore, the use of filtered forms of extract did little to influence its efficacy.
Flow cytometry is the technique used to study the mechanism of cell death during exposure to chemical agents [36]. In this study flow cytometry was applied to confirm MTT assay results, and also for appraising the cell death mechanism in Hodgkin's lymphoma samples being treated with aqueous extract of Lavender. The dosages of 100µg/mland 50µg/ml (as a lower dosage) of non-filtered extract were chosen for flow cytometry purposes. Harvested cells were incubated with the extract for 48 hours and then stained with Annexin V/FLUOS (FL-I) and propidium iodide (PI, FL-2), and finally analyzed by the flow cytometry method (Figure 6). According to Figure 6 it can be inferred that survival rate in concentration of 100 µg/mlis lower than that in 50 µg/ml, which verifies previous results obtained from MTT assays. On the other hand, a major portion of the cells that are Annexin V/FLOUS (+)/PI (-) [LR] are to be found in the lower right part of Figure 6. The calculated percentages of apoptotic and necrotic cells following treatment of Hodgkin's lymphoma cells with non-filtered extract indicate that most of the cells underwent apoptosis. In the presence of the 50 µg/ml dosage of extract, apoptosis is the main mechanism, whereas in 100 µg/mlof the concentration, 3.14% of the cell death population is related to a late apoptosis mechanism. In summary, flow cytometry findings reveal that apoptosis is the main mechanism by which the aqueous extract of Lavender brings about Hodgkin's lymphoma cell death.
Conclusion
It can be concluded that Lavender aqueous extract (Lavandula angustifolia) can be a potential candidate for more investigations in the field of cancer treatment. Thus, in vivo studies are vital in order to identify and confirm the exact cytotoxic effect of this extract. Moreover, identifying the effective agent of this extract is necessary for achieving the most successful results.
Acknowledgments
A portion of this research has been derived from the MSc thesis of Ms. Sona Dalilan, and PhD thesis of Ms. Hakimeh Zali. The author would like to thank Ms. Patricia Smolinski, A.A.S., RDH, Ferris State University, for her kind collaboration in the editing of this work.
Footnotes
Conflicts of Interest
The authors have no conflict of interest in this article.
Authors' Contribution
Mostafa Rezaei-Tavirani and Mohammad Nabiuni designed the study, Sona Dalilan and Hakimeh Zali analyzed the data, Saeed Heidari Keshel contributed to the study design and analysis, Mona Zamanian Azodi contributed to the literature review and writing-up the process. All the authors read and approved the final manuscript.
REFERENCES
- 1.Khaghani Razi Abad S, Pooladi M, Hashemi M, Moradi A, Sobhi S, Zali A, et al. Rho GDP Protein Expression Change and Investigation of ItsEffect on GDP/GTP Cycle in Human Brain Oligodendroglioma. Iranian Journal of Cancer Prevention. 2013;6(suppl.):24–9. [Google Scholar]
- 2.Khatib H, Rezaei-Tavirani M, Heidari Keshel S, Zamanian Azodi M, Omidi R, Biglarian M. Flow Cytometry Analysis of Rosa Damascena Effects on Gastric Cancer Cell Line (MKN45). Iranian Journal of Cancer Prevention. 2013;6(suppl.):30–6. [Google Scholar]
- 3.Pooladi M, Sobhi S, Khaghani Razi Abad S, Hashemi M, Moradi A, Zali AR, et al. The Investigation of Heat Shock Protein (HSP70) Expression Change in Human Brain Asterocytoma Tumor. Iranian Journal of Cancer Prevention. 2013;6(suppl.):6–11. [Google Scholar]
- 4.Zali H, Zamanian-Azodi M, Shokrgozar MA, Rezaei-Tavirani M. Cytotoxic effects of human calprotectin on gastric cancer cell line is attenuated by etoposide. Gastroenterology and Hepatology From Bed to Bench. 2012;5(3):132–8. [PMC free article] [PubMed] [Google Scholar]
- 5.Zali H, Rezaei-Tavirani M, Azodi M. Gastric cancer: prevention, risk factors and treatment. Gastroenterology and Hepatology From Bed to Bench. 2011;4(4):175–85. [PMC free article] [PubMed] [Google Scholar]
- 6.Zamanian Azodi M, Azizi Jalilian F. Early Detection of Cancer and Proteomics. HBI_Journals. 2013;21(1):115–26. [Google Scholar]
- 7.Babashah S, Sadeghizadeh M, Tavirani MR, Farivar S, Soleimani M. Aberrant microRNA expression and its implications in the pathogenesis of leukemias. Cellular Oncology. 2012;35(5):317–34. doi: 10.1007/s13402-012-0095-3. [DOI] [PubMed] [Google Scholar]
- 8.Biggar RJ, Jaffe ES, Goedert JJ, Chaturvedi A, Pfeiffer R, Engels EA. Hodgkin lymphoma and immunodeficiency in persons with HIV/AIDS. Blood. 2006;108(12):3786–791. doi: 10.1182/blood-2006-05-024109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma Y, Visser L, Roelofsen H, de Vries M, Diepstra A, van Imhoff G, et al. Proteomics analysis of Hodgkin lymphoma: identification of new players involved in the cross-talk between HRS cells and infiltrating lymphocytes. Blood. 2008;111(4):2339–46. doi: 10.1182/blood-2007-09-112128. [DOI] [PubMed] [Google Scholar]
- 10.Kluk MJ, Ryan KP, Wang B, Zhang G, Rodig SJ, Sanchez T. Sphingosine-1-phosphate receptor 1 in classical Hodgkin lymphoma: assessment of expression and role in cell migration. Laboratory Investigation. 2013:462–71. doi: 10.1038/labinvest.2013.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kosanić M, Ranković B, Dašić M. Mushrooms as Possible Antioxidant and Antimicrobial Agents. Iranian Journal of Pharmaceutical Research. 2012;11(4):1121–6. [PMC free article] [PubMed] [Google Scholar]
- 12.Mohadjerani M. Antioxidant Activity and Total Phenolic Content of Nerium oleander L. Grown in North of Iran. Iranian Journal of Pharmaceutical Research. 2012;11(4):1121–6. [PMC free article] [PubMed] [Google Scholar]
- 13.Aziz Z, Tey NP. Herbal medicines: Prevalence and predictors of use among Malaysian adults. Complementary Therapies in Medicine. 2009;17:44–50. doi: 10.1016/j.ctim.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 14.Piccaglia R, Marotti M, Giovanelli E, Deans S, Eaglesham E. Antibacterial and antioxidant properties of Mediterranean aromatic plants. Industrial crops and Products. 1993;2(1):47–50. [Google Scholar]
- 15.Pedro AS, Cabral-Albuquerque E, Ferreira D, Sarmento B. Chitosan: An option for development of essential oil delivery systems for oral cavity care? Carbohydrate Polymers. 2009;76(4):501–508. [Google Scholar]
- 16.Hamada T, Yamaguch M. Evoked and oscillatory neuromagnetic responses to sniffing odor in human subjects. Behavioural brain research. 2001;123(2):219. doi: 10.1016/s0166-4328(01)00205-4. [DOI] [PubMed] [Google Scholar]
- 17.Kim JT, Ren CJ, Fielding GA, Pitti A, Kasumi T, Wajda M, et al. Treatment with lavender aromatherapy in the post-anesthesia care unit reduces opioid requirements of morbidly obese patients undergoing laparoscopic adjustable gastric banding. Obesity surgery. 2007;17(7):920–5. doi: 10.1007/s11695-007-9170-7. [DOI] [PubMed] [Google Scholar]
- 18.Cassella S, Cassella JP, Smith I. Synergistic antifungal activity of tea tree (Melaleuca alternifolia) and lavender (Lavandula angustifolia) essential oils against dermatophyte infection. International Journal of Aromatherapy. 2002;12(1):2–15. [Google Scholar]
- 19.Tanida M, Niijima A, Shen J, Nakamura T, Nagai K. Olfactory stimulation with scent of lavender oil affects autonomic neurotransmission and blood pressure in rats. Neuroscience letters. 2006;398(1):155–60. doi: 10.1016/j.neulet.2005.12.076. [DOI] [PubMed] [Google Scholar]
- 20.Soden K, Vincent K, Craske S, Lucas C, Ashley S. A randomized controlled trial of aromatherapy massage in a hospice setting. Palliative Medicine. 2004;18(2):87–92. doi: 10.1191/0269216304pm874oa. [DOI] [PubMed] [Google Scholar]
- 21.Katona JM, Sovilj VJ, Petrović LB. Microencapsulation of oil by polymer mixture-ionic surfactant interaction induced coacervation. Carbohydrate Polymers. 2010;79(3):563–70. [Google Scholar]
- 22.Jones WP, Chin YW, Kinghorn A. The role of pharmacognosy in modern medicine and pharmacy. Current drug targets. 2006;7(3):247–64. doi: 10.2174/138945006776054915. [DOI] [PubMed] [Google Scholar]
- 23.Perrucci S, Mancianti F, Cioni P, Flamini G, Morelli I, Macchioni G. In vitro antifungal activity of essential oils against some isolates of Microsporum canis and Microsporum gypseum. Planta medica. 1994;60(2):184–7. doi: 10.1055/s-2006-959448. [DOI] [PubMed] [Google Scholar]
- 24.Schulz S, Bühling F, Ansorge S. Prenylated proteins and lymphocyte proliferation: Inhibition by d‐limonene and related monoterpenes. European journal of immunology. 2005;24(2):301–307. doi: 10.1002/eji.1830240204. [DOI] [PubMed] [Google Scholar]
- 25.Peffley DM, Gayen AK. Plant-derived monoterpenes suppress hamster kidney cell 3-hydroxy-3-methylglutaryl coenzyme a reductase synthesis at the post-transcriptional level. The Journal of nutrition. 2003;133(1):38–44. doi: 10.1093/jn/133.1.38. [DOI] [PubMed] [Google Scholar]
- 26.Gu Y, Ting Z, Qiu X, Zhang X, Gan X, Fang Y, et al. Linalool preferentially induces robust apoptosis of a variety of leukemia cells via upregulating p53 and cyclin-dependent kinase inhibitors. Toxicology. 2010;268(1):19–24. doi: 10.1016/j.tox.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 27.Lopez-Arnaldos T, Zapata J, Calderon A, Barcelo AR. ACS Symposium Series. ACS Publications;; 1997. Antioxidant activity of lavandin (Lavandula x intermedia) cell cultures in relation to their rosmarinic acid content. pp. 206–218. [Google Scholar]
- 28.Zhang Z, Chen H, Chan KK, Budd T, Ganapathi R. Gas chromatographic-mass spectrometric analysis of perillyl alcohol and metabolites in plasma. Journal of Chromatography B: Biomedical Sciences and Applications. 1999;728(1):85–95. doi: 10.1016/s0378-4347(99)00065-1. [DOI] [PubMed] [Google Scholar]
- 29.Hartlapp I, Pallasch C, Weibert G, Kemkers A, Hummel M, Re D. Depsipeptide induces cell death in Hodgkin lymphoma-derived cell lines. Leukemia research. 2009;33(7):929–36. doi: 10.1016/j.leukres.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 30.Zamanian-Azodi M, Rezaie-Tavirani M, Heydari-Kashal S, Kalantari S, Dailian S, Zali H. Proteomics analysis of MKN45 cell line before and after treatment with Lavender aqueous extract. Gastroenterology and Hepatology from bed to bench. 2011;5(1):35–42. [PMC free article] [PubMed] [Google Scholar]
- 31.Rahmati Roodsari M, Zamanian-Azodi M, Salimpour F. Herbal remedies and medicine; introducing some Iranian plants. Journal of Paramedical Sciences. 2013;4(2):127–33. [Google Scholar]
- 32.Ives C, Eskin S, McIntire L. Mechanical effects on endothelial cell morphology: in vitro assessment. In Vitro Cellular & Developmental Biology-Plant. 1986;22(9):500–507. doi: 10.1007/BF02621134. [DOI] [PubMed] [Google Scholar]
- 33.Berridge MV, Herst PM, Tan AS. Tetrazolium dyes as tools in cell biology: new insights into their cellular reduction. Biotechnology annual review. 2005;11:127–52. doi: 10.1016/S1387-2656(05)11004-7. [DOI] [PubMed] [Google Scholar]
- 34.Kashani MS, Tavirani MR, Talaei SA, Salami M. Aqueous extract of lavender (Lavandula angustifolia) improves the spatial performance of a rat model of Alzheimer’s disease. Neuroscience bulletin. 2011;27(2):99–106. doi: 10.1007/s12264-011-1149-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rezaie-Tavirani M, Heydari-Keshel S, Rezaee MB, Zamanian-Azodi M, Rezaei-Tavirani M, Khodarahmi R. Effect of essential oil of Rosa Damascena on human colon cancer cell line SW742. Gastroenterology and Hepatology From Bed to Bench. 2013;6(1):25–31. [PMC free article] [PubMed] [Google Scholar]
- 36.Fulwyler MJ. Electronic separation of biological cells by volume. Science (New York, NY) 1965;150(698):910–11. doi: 10.1126/science.150.3698.910. [DOI] [PubMed] [Google Scholar]