Abstract
The macrophages role within the tumor microenvironment has amended by a variety of factors, thus serves a vital role in tissue morphogenesis. The role of macrophages in health and disease differs enormously as the macrophage has shown dual functions. Macrophage has a basic role in antigen presentation serving as the first line of defense in diseases. However the presence of cytokines and growth factors, both together have regulated the macrophage to become negative effectors promoting tumor activity. Hence macrophages are a double edged weapon, and any imbalance in the regulatory mechanisms caused a shift from tumoricidal to tumorigenic activities. TAMs would be the main reason of the invasion in tumor microenvironment enhancing as well as tumor invasion, angiogenesis and metastasis promoting tumor genesis. Macrophages are the multifunctional cells which have conducted by the tumor cells to produce tumor promoting factors that enable the stimulation of angiogenesis, and tumor cell invasion. This fact has resulted initiation or promotion of tumor genesis, where the tumor has progressed to an upper malignant stage. The present review has focused on the tumor associated macrophages and their roles in tumor genesis.
Keywords: Angiogenesis, Cytokines, Immunology, Macrophage, Tumor
Introduction
Tumor microenvironment has not constituted just the malignant cells, but also other resident cell types and migratory hematopoietic cells, predominantly the macrophages, neutrophils and mast cells which would produce a unique environment modifying the neoplastic properties of tumor cells [1]. These cells have a crucial role in progression and metastasis of tumors [2]. Hematopoietic cells have recruited to almost all of the tumors, which the largest proportions of them have the Tumor Associated Macrophages (TAMs) [1]. Macrophages have played a fundamental role in mediating the inflammatory response, and this fact that chronic inflammation is a significant cause of cancer have also perceived by the line of investigations in the correlations between macrophages and poor prognosis [1].
The relation between the roles of macrophages in tumor genesis has been a query for a long period. However, the role of macrophage in tumor progression could be validated by this fact that there has been a positive correlation between chronic inflammation and tumor initiation and progression [3]. Secondly there has been poor prognosis in tumors with high density of TAMs [4]. At the earlier stages of carcinogenesis, the activated macrophages have played a role by production of free radicals which have led to DNA damage resulting in mutation [5]. As the growth of the tumor has progressed, significant changes have occurred within the microenvironment, which has resulted formation of the tumor related macrophages. TAMs have undergone phenotypic changes with a series of factors like prostaglandins, extracellular nucleotides, high molecular hyaluran fragments, hypoxia, apoptotic cells, and IgG which has a synergistic effect. These regulatory macrophages have inhibited the immune response to the antigens expressed by the tumors and also deactivated the neighboring macrophages [6].
Phenotypes of Macrophages
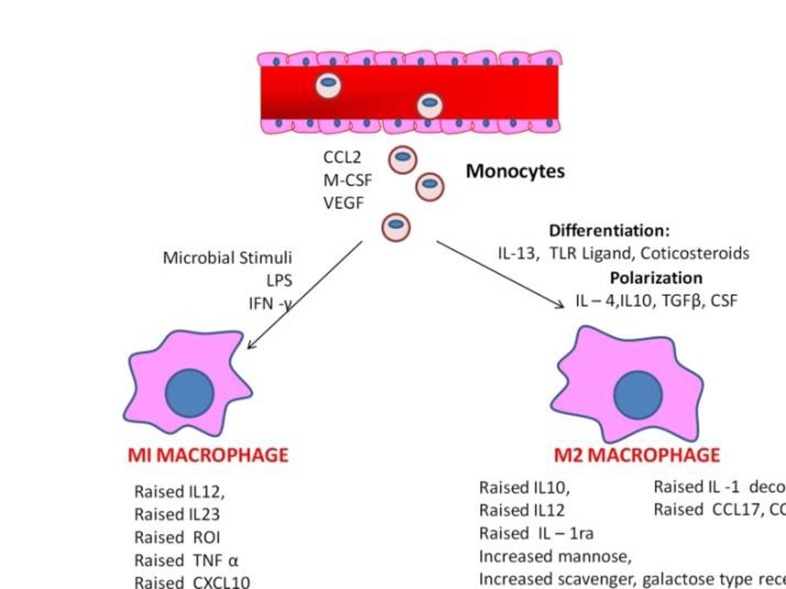
Macrophages are the multifaceted differentiated cells of the mononuclear phagocytic lineage with expression of particular markers which renders specific phenotypic characteristics [7]. Macrophages are classified into subsets participating in a particular immunological process based on the origin, lineage and the growth factors expressed by them (Figure 1). They are plastic cells and the phenotype of these cells that is determined by the anatomic location and the physiologic/pathological context. The first subset is the “activated macrophage” (M1) which are activated by interferon gamma and engagement of Toll-Like Receptors (TLRs) with expression of cytokines. The production of pro-inflammatory cytokines such as TNF, IL12, IL23 and IL6 in large amounts have resulted in generation of reactive oxygen species and nitric oxide stimulating the helper T cells (Th 1 type) to destroy the pathogens [8].
Figure 1.
It shows the phenotypes and function of the subsets of Macrophage.
The alternatively activated macrophages (M2) constitute another subset which has frequently involved in Th2 a type response that differentiates IL4 and IL13. Tumor associated macrophages resemble this group of alternatively activated macrophage, that produce high amount of IL-10 and exhibit properties like anti-inflammatory function and tissue repair functions [9]. The polarization states of M1 and M2 shows distinct metabolic features associated with glucose, amino acids, iron and lipid metabolism [10]. The third subset is the antigen presenting migratory dendritic cell involved in tissue development and homeostasis [7]. They are regulated by CSF 1 and they are not classified under immunological categories. In the tumor environment the macrophages shift from activated type to alternatively activated type which is an immune-regulatory type. However, the both populations of macrophages are present in tumor microenvironment with greater predilection of macrophages involved in developmental process [11].
Tumor Associated Macrophages
In normal physiologic conditions, the recruitment of macrophage in either wounding or a pathogenic challenge has known by local expressions of a diversity of growth factors including Colony stimulating factor 1 (CSF-1), granulocyte macrophage CSF (GM CSF), Transforming growth factor β1 (TGF β1), Macrophage Stimulating Protein (MSP) and various chemokine like CCL2, CCL7, CCL8 (monocyte chemo attractant protein family-1-3), CCL3 (macrophage inflammatory protein-1α (MIP-1α)), CCL4/(MIP-1β) and macrophage Migration Inhibitory Factor (MIF). These factors have recruited circulating monocytes, then stimulate them to differentiate into macrophages which in turn mediate immune response, and remove the pathogens, to result in tissue repair [12, 13]. Thus, macrophages would be the sentinel cells which have been performed a multitude of functions which provided support for developing tissues, and organized immune defenses against cancer through their remodeling capacities, presence of angiogenesis factors, and apoptotic cell engulfment [4].
In a tumor microenvironment, the TAMs have originated from the monocyte lineage by the factors that have expressed by stromal cells and the neoplastic cells. The chemokine CCL2 is the most important among these, as it has previously referred to as tumor derived chemotactic factor [14]. Other Various factors like fibronectin, fibrinogen, cleavage products of extracellular matrix proteins, a variety of chemokine and cytokines like VEGF, PDGF and M-CSF have also involved in the recruitment of monocytes [15]. Recent studies have demonstrated that angiotensin II has amplified the production of TAMs, which fostered the growth of cancer [16]. TAMs in the tumor microenvironment have resembled alternatively activated macrophages, thus had an M2 phenotype with pre tumor functions, thereby promoting tumor cell proliferation, survival and dissemination [1].
However when macrophages have recruited to the tumor microenvironment, the immune functions of macrophages have suppressed and the tumor cells lose their “off switches” due to intrinsic mutations resulting in lack of response to positional data. This has helped the growth of the tumor and survival of malignant cells promoting inflammation and angiogenesis which has subverted the adaptive immune responses resulting in tumor cell migration and metastasis [1, 17].
Macrophages as a Double Edged Sword
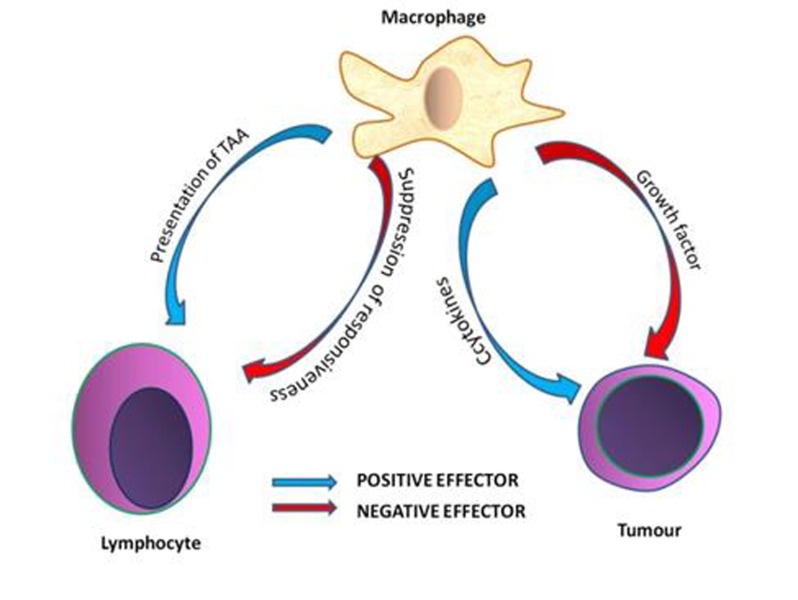
Macrophages have a dual effect on the immune system, either positive or negative mediators [18]. The positive effector macrophages have acted as antigen presenting cells which would be tumor associated, but on the other hand has acted direct antitumor cytotoxicity which could not be an effective method in eradication of the cancer [19]. However macrophage also have an effect on the immune system negatively by the tumor cell growth promoting abilities that aids in tumor growth. Macrophages have also resulted in suppression of T cell response and Natural killer cells, and antitumor response (Figure 2) [20]. A balance between health and disease has existed through the regulatory mechanisms that have controlled the macrophage functions during tumor growth. The role of cytokines and pro-inflammatory substances that could play role in modifying the macrophage functions subverting the macrophage antitumor functions, favored the growth of tumor [21].
Figure 2.
It shows macrophage has dual pathways serving as positive and negative effectors.
Traits Exhibited by a Tumor Associated Macrophage
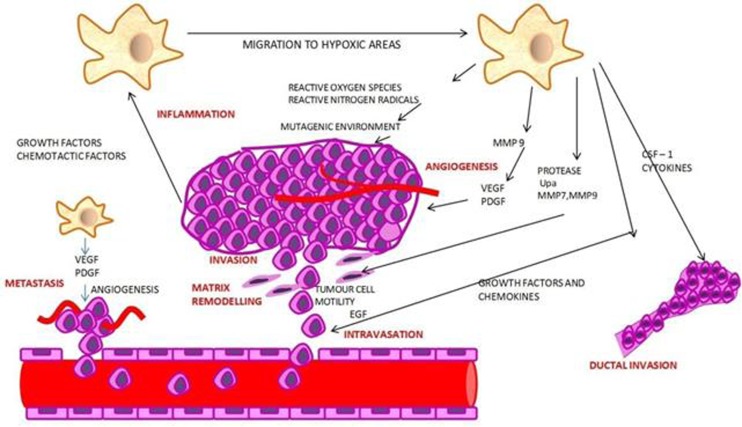
Macrophages act as centers of invasion and facilitate angiogenesis and extracellular matrix breakdown and remodeling thereby promoting tumor cell motility. The interaction between the macrophage and malignant cells leads to invasion of the tumor cells into the blood vessels [1]. The traits have required for a cell to promote tumor genesis includes self-sufficiency from external growth signals, insensitivity to negative growth signals, sustained angiogenesis, resistance to apoptosis, limitless replicative potential and acquisition of tissue invasiveness [22]. The enhancement of tumor initiation, progression and metastasis has facilitated by six factors. These have included chronic inflammation, intravasation, matrix remodeling, tumor cell invasion, angiogenesis and seeding at distant places (Figure3) [1].
Figure 3.
It shows the functional traits exhibited by a macrophage.
Inflammation and Matrix Remodeling as a Mediator to Tumor Genesis
The role of chronic inflammation in tumor microenvironment is through the ROS and reactive nitrogen species that renders the macrophage mutagenic. The recruitment of growth factors and cytokines have also stimulated the proliferation and survival of mutated premalignant cells thereby initiating malignant changes [2, 3, 7]. This kind of low grade inflammation without obvious clinical consequences which have resulted in an increased risk of cancer due to chronic infection or persistent irritation, that has called “smoldering inflammation” [23].
Role of Macrophage in Intravasation and Metastasis
Macrophages have initially found at the invasive front of the tumor and at the points of basement membrane breakdown during the malignant transition suggesting the exploitation of normal matrix remodeling activities enabling them to egress into the stroma [24, 25]. The capacity of the tumor to invade the surrounding matrix, the blood and lymphatic vessels, and its ability to settle down at a distant site represents the crucial phase of metastasis. The formation of extracellular matrix and its remodeling, invasion and metastasis involves a series of cross talk events between the macrophages and the tumor cells .Tumor associated macrophages have enhanced intravasation through the signaling of CSF-1 and EGF receptors on macrophage and tumor cells respectively. Furthermore, an increased macrophage density is associated with increased migration of tumor cells into the blood vessels have resulted in invasion of the primary tumor [25, 26]. The TNF dependent MMP induction in macrophages has played a significant role in enhancing the invasiveness of the tumor cells. The production of IL-β by the TAMs has also induced the process of metastasis [27, 28].
Role of Macrophage in Angiogenesis
Macrophages have played a crucial role in angiogenesis and thereby created an environment which not only sustained the tumor, but also promoted metastasis by promoting tumor cell motility. This process has precisely regulated by a variety of local factors including angiogenic factors such as Interleukin 8 (IL 8), Basic Fibroblast Growth Factor (bFGF), Vascular Endothelial Growth Factor (VEGF), and Transforming Growth Factor Alpha (TGF-α) [29-31]. The stroma rich in growth factors, cytokines and angiogenic factors have directly interacted with tumor cells and express angiogenic factors .This interaction has promoted the expression of IL8 in tumor cells as well as in the stroma [32, 33]. Upon activation, the tumor has associated macrophages which have released a wide variety of growth factors, proteolytic enzymes, cytokines and inflammatory mediators which acted as key factors in angiogenesis [34]. TAMs have localized in the hypoxic regions into the tumor microenvironment which promoted the expression of HIF-1 and HIF-2. This resulted in activation and over-expression of a specific proangiogenic program. This could be correlated with the increase in the vascularity of the tumors [35]. These clusters of events have placed macrophage at the core of an invasion microenvironment.
A Key for Tumor Migration
Gene-expression profiling of tumor cells that has migrated with macrophages indicates that macrophages might help to elicit a particular “invasion signature” of gene expression in these tumor cells [36]. This identified the tumor cells as neither proliferating nor apoptotic. The genes in the invasion signature fall into coordinately regulated pathways that suggested that these tumor cells could communicate with then follow macrophages during invasion. Based on this signature, a tumor microenvironment invasion model has proposed in which tumorigenesis led to the development of microenvironments within the tumor, which presumably resulted from the stable gene-expression patterns seen by whole-tumor profiling. These stable expression patterns might lead, for example, to increase inflammation and macrophage involvement in tumor progression and to the invasion microenvironment, which in turn would elicit transient gene-expression patterns in tumor cells that support invasion. An interesting prediction of the tumor microenvironment model would be this point that: the early and uniform expression of certain genes could lead to the random appearance, in time and location, of an invasion microenvironment. This would result in repeated episodes of invasion and micro metastasis that increase the frequency as the tumor progresses [37].
Tumor Associated Macrophages Serve as Markers for Poor Prognosis
The tumor microenvironment with abundance of tumor associated macrophages density often has a correlation with poor prognosis [4]. The TAMs recruit chemokine and CSF-1 in excess which is proportional to the prognosis [38, 39]. More than about 80% of tumors have shown a positive correlation between the poor prognosis and TAMs, then only tumors less than 10% of TAMs density demonstrated good prognosis [4]. Hence, increasing infiltration of TAMs has directly linked with advanced tumor prognosis and metastasis [17].
Tumor Associated Macrophages in an Irradiated Tumor Microenvironment
Radiotherapy has remained the front line treatment modality for more than 50% of tumors. The method has caused damages in the DNA and enhanced the tumor hypoxia in a targeted region. In spite of these advanced radio curative methodologies, a percentage of tumor tends to recur [40]. Radiation has exposed to tumors have a perturbed cellular environment with variations in the number and phenotypes. Early experimental studies in 1990’s suggested that macrophages would be innately radio-resistant, then the cellular balance within the tumor re-establishes itself within a short span of time though there is an initial disturbance following irradiation [41]. However recent reports have led to the hypothesis that the irradiation largely affects the phenotype and the function of TAMs creating a microenvironment favoring the growth of the tumor [42]. Jenkins et al. has suggested that the radiation exposed tumor environment had an activated M2 polarized macrophage population, in contrast to the quiescent M1 macrophages, as they are sensitive to radiation induced DNA damage [43]. The recruitment of TAMs in the radiation has induced environment occurs in a similar manner as that of tumor hypoxia. The increased level of HIF1, a transcription factor appears to be the primary mechanism for the influx of TAMs in the microenvironment. Increased tumor hypoxia following radiation has also resulted in recruitment of TAMs [40]. The CXCR4 and CFR signaling pathways have promoted the polarization of the macrophage towards the M2 phenotype. Thus increased radiation levels have resulted in increased density of TAMs eventually resulting in increased production of proangiogenic cytokines and vascular recruitment within the tumor and consequently re-growth of the tumor [40, 44].
Immune Suppression
Unlike macrophages from healthy tissues, which are capable of presenting tumor-associated antigens, lysing tumor cells, and stimulating the antitumor functions of T cells and NK cells, TAMs in the tumor microenvironment lack these activities, leaving the host without the ability to build an effective anti-tumor immune response [45] .tumor associated macrophages lack the basic metabolic activity whenever they could be present in the tumor microenvironment leaving the host incapable of mounting the immune response. A number of studies have shown that tumor-derived molecules, like cytokine, growth factors, chemotactic molecules, and proteases, influence TAMs functions [8]. Tumors that have undergone a process called immune edition, wherein the functions of the macrophages which are potentially dangerous to the tumor, have down regulated, even when tumor antigens have been presented. The antitumor activity of TAMs has likely suppressed by hypoxia in the tumor microenvironment as it stimulates the production of potent immunosuppressive factors like IL-10 and PGE2 [46]. These factors impair the immune cell development and inhibit the antitumor activity of the cells, that being formed by acting on the primitive stages of development from the embryonic pluripotent stem cells. Besides, they also have an effect on TAMs wherein the cytotoxicity activity has reduced towards the tumor cells. This has involved the formation of reduced oxygen radicals due to a decrease of the substrate availability. Hypoxia has also played a major role in inhibiting the ability of the macrophages with relation to phagocytosis and antigen presentation.
In the hypoxic regions within the tumor microenvironment, TAMs up regulate the expression of MMP7 which has cleaved the Fas ligand from the adjacent cells. This made the tumor cells not just less responsive to the chemotherapeutic agents, but also promoted lysis by the Natural killer cells and the T cells [46, 47].
Conclusion
Macrophages have possessed a key role in tumorigenic processes, ranging from tumor initiation to acceleration of tumor progression and metastasis. The abilities of these cells to move to specific sites, increase matrix remodeling and induce angiogenesis are essential during normal development and in normal physiological processes, such as wound healing and inflammation [2]. This continuous ‘on’ signal indicated that strategies that directed to dampen the recruitment and tumor-associated functions of macrophages could find an important place within the therapeutic arsenal against tumors. Macrophages do not harbor malignant mutations and therefore have a stable genome. They would be much less likely to develop drug resistance. This makes them a good target for cytostatic treatment of tumor progression to malignancy using small molecule inhibitors of selected macrophage functions. A complete knowledge on the signaling pathways which permitted the macrophages to promote tumor progression will provide new insights on the evolution of the tumor microenvironment which supports the invasion and metastasis, thereby offering targets for anticancer therapies. Thus, a wide knowledge on the wide array of biological effects within the tumor microenvironment will bring in novel methods of therapeutic intervention [1].
Acknowledgments
The authors thank the co-workers, Department of Oral Pathology, Saveetha Dental College, for their immense contribution and support to prepare this review.
Footnotes
Conflicts of Interest
The authors declare no conflict of interest.
Authors' Contribution
Dr. Suhashini Ramanathan has made a compilation of all the materials required for the review and Dr. Nithya Jagannathan prepared this manuscript (data gathering and writing).
REFERENCES
- 1.Pollard JW. Tumour-educated macrophages promote tumor progression and metastasis. Nat Rev Cancer. 2004;4(1):71–8. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 2.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–7. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7(3):211–17. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Bingle L, Brown NJ, Lewis CE. The role of tumor associated macrophages in tumor progression: implications for new anticancer therapies. J. Pathol. 2002;196(3):254–65. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 5.Swann JB, Vesely MD, Silva A, Sharkey J, Akira S, Schreiber RD, et al. Demonstration of inflammation-induced cancer and cancer immune editing during primary tumor genesis. Proc Natl Acad Sci USA. 2008;105(2):652–6. doi: 10.1073/pnas.0708594105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pollard JW. Macrophages define the invasive microenvironment in breast cancer. J Leukoc Biol. 2008;84(3):623–30. doi: 10.1189/jlb.1107762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pollard JW. Trophic macrophages in development and disease. Nature Reviews Immunology. 2009;9(4):259–270. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon S. Alternative activation of macrophages. Nature Reviews Immunology. 2003;3(1):23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 9.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25(12):677–86. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 10.Biswas SK, Mantovani A. Orchestration of metabolism by macrophages. Cell Metabolism. 2012;15(4):432–7. doi: 10.1016/j.cmet.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 11.Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Current opinion in immunology. 2010;22(2):231–7. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Lingen MW. Role of leukocytes and endothelial cells in the development of angiogenesis in inflammation and wound healing. Archives of pathology & laboratory medicine. 2001;125(1):67–71. doi: 10.5858/2001-125-0067-ROLAEC. [DOI] [PubMed] [Google Scholar]
- 13.Nathan C. Points of control in inflammation. Nature. 2002;420(6917):846–52. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- 14.Bottazzi B, Polentarutti N, Acero R, Balsari A, Boraschi D, Ghezzi P, et al. Regulation of the macrophage content of neoplasms by chemo attractants. Science. 1983;220(4593):210–12. doi: 10.1126/science.6828888. [DOI] [PubMed] [Google Scholar]
- 15.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4(7):540–50. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 16.Cortez-Retamozo V, Etzrodt M, Newton A, Ryan R, Pucci F, Sio SW, et al. Angiotensin II drives the production of tumor-promoting macrophages. Immunity. 2013;38(2):296–308. doi: 10.1016/j.immuni.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH, Wang XZ, et al. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. . PLoS One. 2012;7(12):e50946. doi: 10.1371/journal.pone.0050946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Connolly KM, Elgert KD. Regulation of T cell mixed lymphocyte reaction reactivity: demonstration of enhancing and inhibitory activity in tumor-bearing host macrophage supernatants. Cell Immunol. 1979;45(1):107–94. doi: 10.1016/0008-8749(79)90365-4. [DOI] [PubMed] [Google Scholar]
- 19.Herberman RB, Holden HT, Djeu JY, Jerrells TR, Varesio L, Tagliabue A, et al. Macrophages as regulators of immune responses against tumors. AdvExp Med Biol. 1979;121:361–79. doi: 10.1007/978-1-4684-8914-9_35. [DOI] [PubMed] [Google Scholar]
- 20.Evans R. Macrophage requirement for growth of a murine fibrosarcoma. British Journal of Cancer. 1978;37(6):1086–9. doi: 10.1038/bjc.1978.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aoe T, Okamoto Y, Saito T. Activated macrophages induce structural abnormalities of the T cells receptor-CD3 complex. . The Journal of experimental medicine. 1995;181(5):1881–6. doi: 10.1084/jem.181.5.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 23.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454(7203):436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 24.Lin EY, Gouon-Evans V, Nguyen AV, Pollard JW. The macrophage growth factor CSF-1 in mammary gland development and tumor progression. Journal of mammary gland biology and neoplasia. 2002;7(2):147–62. doi: 10.1023/a:1020399802795. [DOI] [PubMed] [Google Scholar]
- 25.Wyckoff J, Wang W, Lin E. Y, Wang Y, Pixley F, Stanley ER, et al. A paracrine loop between tumor cells and macrophages is required for tumor cell migration in mammary tumors. . Cancer Res. 2004;64(19):7022–9. doi: 10.1158/0008-5472.CAN-04-1449. [DOI] [PubMed] [Google Scholar]
- 26.Leek RD, Harris AL. tumor-associated macrophages in breast cancer. Journal of mammary gland biology and neoplasia. 2002;7(2):177–89. doi: 10.1023/a:1020304003704. [DOI] [PubMed] [Google Scholar]
- 27.Mytar B, Woloszyn M, Szatanek R, Baj-Krzyworzeka M, Siedlar M, Ruggiero I, et al. Tumor cell-induced deactivation of human monocytes. . J Leukoc Biol. 2003;74(6):1101–1094. doi: 10.1189/jlb.0403140. [DOI] [PubMed] [Google Scholar]
- 28.Giavazzi R, Garofalo A, Bani MR, Abbate M, Ghezzi P, Boraschi D, et al. Interleukin 1-induced augmentation of experimental metastases from a human melanoma in nude mice. . Cancer Res. 1990;50(15):4771–5. [PubMed] [Google Scholar]
- 29.Folkman J, Sning Y. Angiogenesis. . J Biol Chem. 1992;267(16):10931–34. [PubMed] [Google Scholar]
- 30.Friesel RE, Maciag T. Molecular mechanisms of angiogenesis: Fibroblast growth factor signal. FASEB J. 1995;9(10):919–25. doi: 10.1096/fasebj.9.10.7542215. [DOI] [PubMed] [Google Scholar]
- 31.Olofsson B, Pajusola K, Kaipainen A. Von Euler G, Joukov V, Saksela O, et al. Vascular endothelial growth factor B, a novel growth factor for endothelial cells. . Proceedings of the National Academy of Sciences. 1996;93(6):2576–81. doi: 10.1073/pnas.93.6.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelly CP, Keates S, Siegenberg D. IL-8 secretion and neutrophil activation by HT-29 colonic epithelial cells. Am J Physiol. 1994;267(6):G991–G7. doi: 10.1152/ajpgi.1994.267.6.G991. [DOI] [PubMed] [Google Scholar]
- 33.Connolly DT, Stoddard BL. HarakasNK Human fibroblast-derived growth factor is a mitogen and chemoattractant for endothelial cells. Biochim Biophys Acta. 1987;144(2):705–12. doi: 10.1016/s0006-291x(87)80022-0. [DOI] [PubMed] [Google Scholar]
- 34.Fukumura D, Xavier R, Sugiura T, Chen Y, Park EC, Lu N, et al. Tumour induction of VEGF promoter activity in stromal cells. Cell. 1998;94(6):715–25. doi: 10.1016/s0092-8674(00)81731-6. [DOI] [PubMed] [Google Scholar]
- 35.Lewis JS, Landers RJ, Underwood JC, Harris AL, Lewis CE. Expression of vascular endothelial growth factor by macrophages is up-regulated in poorly vascularized areas of breast carcinomas. J Pathol. 2000;192(2):150–8. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH687>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 36.Condeelis J, Singer RH, Segall JE. The great escape: when cancer cells hijack the genes for chemo taxis and motility. Annual Review of Cell and Developmental Biology. 2005;21:718–695. doi: 10.1146/annurev.cellbio.21.122303.120306. [DOI] [PubMed] [Google Scholar]
- 37.Wang W, Goswami S, Sahai E, Wyckoff JB, Segall JE, Condeelis JS. tumor cells caught in the act of invading: their strategy for enhanced cell motility. Trends Cell Biol. 2005;15(3):138–45. doi: 10.1016/j.tcb.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 38.Ueno T, Toi M, Saji H, Muta M, Bando H, Kuroi K, et al. Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. . Clinical Cancer Research. 2000;6(8):3282–9. [PubMed] [Google Scholar]
- 39.Smith HO, Anderson PS, Kuo DY, Goldberg GL, DeVictoria CL, Boocock CA, et al. The role of colony-stimulating factor 1 and its receptor in the pathogenesis etiology of endometrial adenocarcinoma. Clinical Cancer Research. 1995;1(3):313–25. [PubMed] [Google Scholar]
- 40.Russell JS, Brown JM. The irradiated tumor micro environment: role of tumor associated macrophages invascular recovery. Front Physiol. 2013;4:157. doi: 10.3389/fphys.2013.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chiang CS, Syljuäsen RG, Hong JH, Wallis A, Dougherty GJ, McBride WH. Effects of IL-3 gene expression on tumor response to irradiation in vitro and in vivo. . Cancer Res. 1997;57(18):3899–903. [PubMed] [Google Scholar]
- 42.Tsai CS, Chen FH, Wang CC, Huang HL, Jung SM, Wu CJ, et al. Macrophages from irradiated tumors express higher levels of iNOS, arginase-I and COX-2, and promote tumor growth. International Journal of Radiation Oncology* Biology* Physics. 2007;68(2):499–507. doi: 10.1016/j.ijrobp.2007.01.041. [DOI] [PubMed] [Google Scholar]
- 43.Jenkins J, Ruckerl D, Cook PC, Jones LH, Finkelman FD, Van Rooijen N, et al. Local macrophage proliferation, rather than recruitment from the blood, is signature of TH2 inflammation. Science. 2011;332(6035):1284–88. doi: 10.1126/science.1204351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang SC, Yu CF, Hong JH, Tsai CS, Chiang CS. Radiation Therapy-Induced Tumor Invasiveness Is Associated with SDF-1-Regulated Macrophage Mobilization and Vasculogenesis. . PLoS One. 2013;8(8):e69182. doi: 10.1371/journal.pone.0069182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lewis CE, Pollard JW. Distinct Role of Macrophages in Different Tumour microenvironments. Cancer res. 2006;66(2):605–12. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 46.Noman MZ, Messai Y, Carré T, Akalay I, Méron M, Janji B, et al. Microenvironmental hypoxia orchestrating the cell stoma cross talk, tumor progression and antitumor response. Crit Rev Immunol. 2011;31(5):357–77. doi: 10.1615/critrevimmunol.v31.i5.10. [DOI] [PubMed] [Google Scholar]
- 47.Fingleton B, Vargo-Gogola T, Crawford HC, Matrisian LM. Matrilysin [MMP-7] expression selects for cells with reduced sensitivity to apoptosis. Neoplasia (New York, NY). 2001;3(6):459–68. doi: 10.1038/sj.neo.7900190. [DOI] [PMC free article] [PubMed] [Google Scholar]