Abstract
DNA is subject to many endogenous and exogenous insults that impair DNA replication and proper chromosome segregation. DNA double-strand breaks (DSBs) are one of the most toxic of these lesions and must be repaired to preserve chromosomal integrity. Eukaryotes are equipped with several different, but related, repair mechanisms involving homologous recombination, including single-strand annealing, gene conversion, and break-induced replication. In this review, we highlight the chief sources of DSBs and crucial requirements for each of these repair processes, as well as the methods to identify and study intermediate steps in DSB repair by homologous recombination.
Double-strand breaks are one of the most toxic DNA lesions. Eukaryotes possess several repair mechanisms involving homologous recombination, including single-strand annealing, gene conversion, and break-induced replication.
EXOGENOUS AND ENDOGENOUS SOURCES OF DNA DOUBLE-STRAND BREAKS
DNA damage can occur as a result of endogenous metabolic reactions and replication stress or from exogenous sources like radiation and chemotherapeutics. Damage comes in several different varieties: base lesions, intra- and interstrand cross-links, DNA-protein cross-links, and both single- and double-strand breaks (DSBs) (Lindahl 1993). Some types of damage, such as oxidative damage to DNA bases, arise, and are repaired, as often as 105 lesions per cell each day (Hoeijmakers 2009). Much less frequent are DNA DSBs, in which the phosphate backbones of the two complementary DNA strands are broken simultaneously, and these are one of the most cytotoxic forms of lesion.
Some well-known exogenous DNA damaging agents (clastogens) are anticancer chemotherapeutic drugs and ionizing radiation (IR). Chemotherapeutic drugs include DNA-alkylating agents such as methyl methanosulfonate and temozolomide, cross-linking agents such as mitomycin C and cisplatin, and radiomimetic compounds such as bleomycin or phleomycin (Chen and Stubbe 2005; Wyrobek et al. 2005). Another class are topoisomerase inhibitors such as camptothecin and etoposide, which induce the formation of single-strand breaks (SSBs) and DSBs, respectively, by trapping covalently linked topoisomerase-DNA cleavage complexes (Koster et al. 2007). Other drugs, such as hydroxyurea and aphidicolin, impair the progression of replication by depleting deoxyribonucleotide pools or inhibiting DNA polymerase.
Ionizing radiation leads to extensive base damage and, additionally, creates DNA SSBs by producing radiolysis radicals that attack the sugar-phosphate backbone (Ward 1994; Thompson 2012). Frequently, at high doses of irradiation, two such nicks are present in complementary DNA strands within one helical turn leading to DSBs (Milligan et al. 1995). There are about 10 SSBs for each DSB created by IR (Ma et al. 2012). IR breakage frequently leaves “dirty ends,” consisting of phosphoglycolates and terminal nucleotides, that cannot be ligated to “clean” ends consisting of a 5′ phosphate and 3′-OH group, such as those created by endonucleases (Weinfeld and Soderlind 1991).
Even in the absence of exogenously inflicted stress during an unperturbed cell cycle, DNA is vulnerable to suffer damage during replication, which, if unrepaired, can promote genomic instability. There are numerous natural impediments that lead to pausing or blocking of a replication fork, such as unusual DNA and chromatin structures or collisions with transcription machinery (Prado and Aguilera 2005; Aguilera and Gaillard 2014) or DNA-binding proteins (Mirkin and Mirkin 2007; Merrikh et al. 2012). The types of damage produced by normal cellular processes are very similar to those caused by some environmental agents (De Bont and van Larebeke 2004).
One way to estimate the frequency of spontaneous DSBs is to count them in cells in which DSB repair is prevented. In budding yeast, one can examine the fate of a single G1 cell lacking the RAD52 gene that is required for DSB repair by homologous recombination (HR). Approximately one cell in eight gives rise to a pair of daughter cells, one of which is inviable (J Haber, unpubl., cited in Coïc et al. 2008). This finding implies that there is a DSB that arises during DNA replication that would normally be repaired by sister chromatid recombination in a recombination-proficient cell. Given a genome size of ∼1.2 × 107 bp, this result, hence, suggests that there is about one spontaneous DSB per 108 bp. Another study estimates that, in normal human cells, ∼1% of single-strand lesions are converted to ∼50 DSBs per cell per cell cycle, that is, about one DSB per 108 bp (Vilenchik and Knudson 2003). In vertebrate cells such as chicken DT40, depleted for yet another key recombination protein, Rad51, the estimated rate of breakage is of the same magnitude (Sonoda et al. 2001).
An alternate way to count DSBs in a cell is to monitor the formation of damage-induced foci, either by indirect immunofluorescent staining or the use of fluorescent proteins fused to proteins that are recruited to the sites of DNA damage as part of the DNA damage response. In vertebrate cells, phosphorylation of the minor histone H2A variant, H2AX, to produce so-called γ-H2AX, is often used as an indicator of the incidence of DSBs; however, it is now becoming evident that γ-H2AX can be associated with DNA damage other than DSBs (Soutoglou and Misteli 2008; Löbrich et al. 2010; Valdiglesias et al. 2013) and thus may overestimate their incidence. Binding of other key DNA repair proteins, such as 53BP1, also serves as a surrogate for monitoring DSBs, as do the appearance of RPA (replication protein A) and Rad51 foci (Haaf et al. 1995; Raderschall et al. 1999; Noon and Goodarzi 2011). In budding yeast, the most frequently used live-cell marker of DSB damage is the recruitment of Rad52-YFP (or other colors) into damage-induced foci. The fact that even multiple DSBs result in a single Rad52 focus has been interpreted as evidence that DSBs aggregate into a repair center (Lisby et al. 2003); however, recent studies have suggested that Rad52-fluorescent protein fusion proteins have a remarkable ability to aggregate, so that they mark strongly only one of several independent DSBs (M Brown, I Fitzgerald, B Glick, and D Bishop, unpubl.; C-S Lee and J Haber, unpubl.).
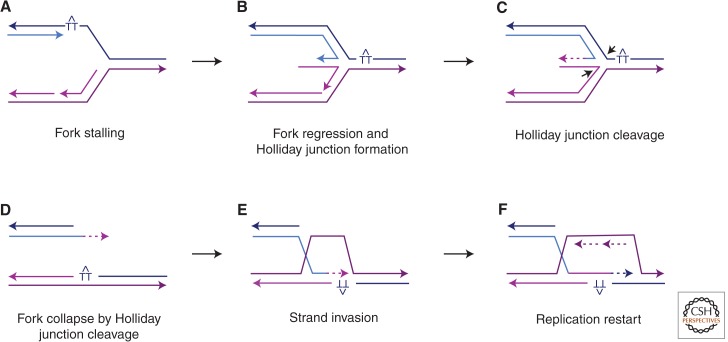
The fact that the majority of spontaneous DSBs appear in the context of DNA replication (Syeda et al. 2014) could suggest that DNA may be nicked before the passage of the replication fork, thus creating a broken chromatid, and, with the arrival of another replication fork in the opposite direction, an intact sister chromatid. However, such breaks can also arise by the processing of stalled replication forks. Stalled forks have been studied by impairing replication, for example, by adding hydroxyurea or introducing replication fork-blocking sequences. Stalled forks can regress by the unwinding and annealing of the newly synthesized strands to form a “chicken foot” structure, which is known as a Holliday junction (Fig. 1). These Holliday junctions (HJs) can be cleaved by structure-selective nucleases or HJ resolvases, such as Mus81-Mms4 or Yen1, to create a broken end and intact chromatid (Wyatt and West 2014). The broken end can then be processed by 5′ to 3′ resection (Symington 2014) and, after binding Rad51, engage in recombination-dependent replication restart, that is, break-induced replication (BIR) (Hanada et al. 2007; Petermann and Helleday 2010).
Figure 1.
Replication fork stalling and restart. DNA replication can be stalled at UV-induced thymidine dimers (TT), as well as DNA secondary structures. A stalled replication fork (A) can undergo regression and pairing of the newly synthesized strands to form a HJ “chicken foot” intermediate (B). The HJ can be cleaved by HJ resolvases (C) to lead to a collapsed fork, effectively a one-ended chromosome break (D). The free end can initiate HR by strand invasion (E) to bypass the lesion and resume replication (F). Newly synthesized DNA is depicted as dashed lines in the same color as the template; arrowheads indicate 3′ ends.
A growing body of evidence implicates transcription as one of the leading causes of DSBs and associated genome instability. Apart from hindering replication fork progression (Prado and Aguilera 2005), R-loops, three-strand nucleic acid structures formed by an RNA:DNA hybrid, plus a displaced single-stranded DNA (ssDNA) identical to the RNA molecule, have been linked to mutagenesis, recombination, and chromosome rearrangements (Huertas and Aguilera 2003; Kim and Jinks-Robertson 2009; Lin et al. 2010). R-loops are mainly formed by defects in RNA metabolism, but also seem to arise naturally in wild-type cells (Helmrich et al. 2011; Wahba et al. 2011). It was recently reported that formation of these RNA:DNA hybrids, in certain yeast transcription repression and RNA degradation mutants, requires the involvement of the HR machinery, including Rad51 and Rad52 (Wahba et al. 2013). Several high-throughput screens in budding yeast and mammalian cells have identified multiple RNA biogenesis factors whose depletion leads to R-loops-mediated DSB formation and the activation of the DNA damage response (Li and Manley 2005; Paulsen et al. 2009; Stirling et al. 2012; Gavalda et al. 2013). The evolutionarily conserved RNase H (in prokaryotes) or RNase H1 (in eukaryotes), which specifically degrades the RNA portion of hybrids, plays a major role in suppression and removal of such structures and, hence, helps maintain genome stability (Lin et al. 2010; Helmrich et al. 2011; Wahba et al. 2011). Thus, a complex network is emerging that links DNA damage and RNA metabolism.
PROGRAMMED DSBs
Although DSBs are among the most deleterious lesions, several physiologically and developmentally important processes require the generation of programmed site-specific DSBs and their subsequent repair by various pathways. In mammals, DSBs are produced by the RAG protein complex to initiate V(D)J recombination for assembling immunoglobulin antigen receptor genes, as well as T-cell receptor genes. DSBs also arise during immunoglobulin class switching (Soulas-Sprauel et al. 2007). A recent claim that there are DSBs in stimulated mouse neurons (Suberbielle et al. 2013), possibly dependent on Spo11 endonuclease, will require further investigation. DSB-induced recombination and subsequent crossover formation are crucial for faithful segregation of homologous chromosomes of different parental origins by the meiotic spindle during sexual reproduction (Lam and Keeney 2014). This is catalyzed by the generation of hundreds of programmed DSBs at nonrandom hotspots by the evolutionarily conserved meiosis-specific topoisomerase-II-like Spo11 endonuclease (Keeney 2008).
Ironically, missegregated chromosomes, although undergoing cytokinesis, can also promote the direct and indirect acquisition of DNA damage that may further lead to unbalanced translocations in the daughter cells (Janssen et al. 2011; Ganem and Pellman 2012). Segregation defects formed by merotelic kinetochore attachments or other causes of chromosome nondisjunction may foster the formation of ultrafine chromosome bridges during anaphase. Any delay in clearing the chromosomes from the central spindle and out of the path of the incoming actin-myosin cytokinetic ring can lead to cleavage of the chromatin and formation of DSBs (Hoffelder et al. 2004; Samoshkin et al. 2009; Janssen et al. 2011; Quevedo et al. 2012). Similar problems arise in the mitosis of dicentric chromosomes (Haber and Thorburn 1984; Kramer et al. 1994) or when intertwined sister chromatids are not disentangled by topoisomerase II (Spell and Holm 1994; Baxter and Diffley 2008). These broken fragments are recombinogenic and can generate chromosome translocations in the next cell cycle (Janssen et al. 2011; Quevedo et al. 2012). Suppression of cytokinetic furrowing can rescue the formation of DSBs at such chromosome bridges, confirming that they are formed as a result of cytokinesis (Baxter and Diffley 2008; Janssen et al. 2011). Often, anaphase-lagging chromosomes are left so severely behind that on telophase they form their own independent nuclear envelope, creating a micronucleus. It has recently been shown that, because of inefficient nuclear import, micronuclei fail to attain all of the required replication and repair components and, hence, show higher replication stress and DNA fragmentation (Crasta et al. 2012).
In many organisms, the excision of transposable elements creates DSBs that are usually repaired by gene conversion (GC) with a sister chromatid from which excision has not occurred. Recombinational repair can also occur with ectopic homologous sequences (Gloor et al. 1991). In the ciliate Paramecium, during the development of the somatic nucleus, programmed DSBs initiate the extensive genome rearrangements that take place at each sexual cycle. In particular, thousands of short noncoding germline sequences, called internal eliminated sequences, are spliced out (Betermier 2004). These developmentally programmed DNA DSBs depend on the domesticated transposase PiggyMac (Baudry et al. 2009) and are repaired using components of the nonhomologous end-joining (NHEJ) pathway (Kapusta et al. 2011).
In budding and fission yeasts, programmed switching of mating-type genes begins with a DSB within the mating-type locus that is repaired by ectopic recombination with donor sequences on the same chromosome, encoding opposite mating-type alleles. In Schizosaccharomyces pombe, mat1 switching depends on a programmed ssDNA nick that, during replication, is converted into a DSB (Arcangioli 2000; Klar 2007). MAT switching in Saccharomyces cerevisiae uses a site-specific homothallic (HO) endonuclease, which cleaves a degenerate 24-bp sequence to generate a DSB with 4-bp, 3′-OH overhanging ends (Haber 2012). Curiously, in another budding yeast, Kluyveromyces lactis, a very similar MAT switching event involving evolutionarily related mating-type sequences occurs without HO endonuclease; instead, a transposon-like sequence apparently excises from the MAT locus and leaves hairpin-closed ends that are opened to initiate DSB-mediated switching (Barsoum et al. 2010). Another well-characterized, specialized endonuclease is the mitochondrial enzyme I-Sce1, which is encoded by the optional intron of the 21S ribosomal RNA gene and responsible for intron mobility (Colleaux et al. 1988). Much of the detailed description of DSB repair in budding yeast has relied on the inducible expression of HO endonuclease or a codon-optimized I-SceI, which can cut cleavage sites introduced in different chromosomal contexts (Krogh and Symington 2004; Haber 2006; Wyman and Kanaar 2006; Kass and Jasin 2010).
Thus, DNA DSB formation and repair can be both deleterious and beneficial, and together have played a major role in the evolutionary development and survival of all living organisms.
REPAIR OF DSBs CAN OCCUR IN SEVERAL WAYS
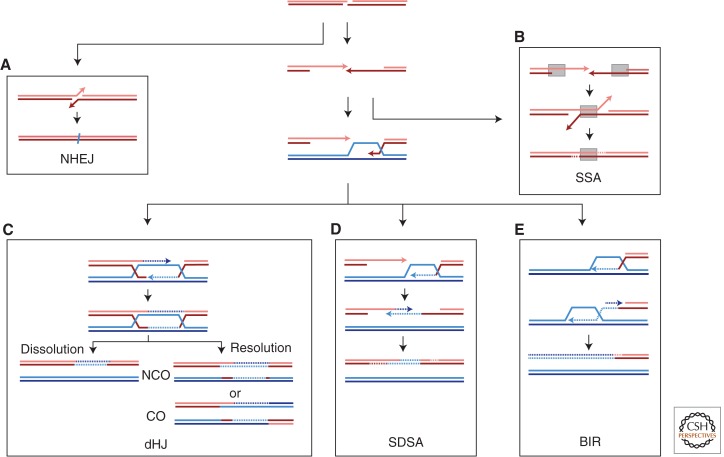
Two mechanistically distinct sets of pathways have evolved to repair DSBs: NHEJ and HR (Fig. 2). This review focuses on HR in somatic cells, but a brief summary of NHEJ is provided.
Figure 2.
Pathways of DNA DSB repair. DSBs are efficiently repaired by HR and NHEJ. DSBs are processed by 5′ to 3′ end resection producing 3′ single-stranded tails. (A) NHEJ involves ligation of broken ends, with little or no base pairing, to produce small deletions or insertions. (B) Single-strand annealing (SSA) takes place when resection reveals flanking homologous repeats that can anneal, leading to deletion of the intervening sequences. (C,D) Repair by two different mechanisms of GC results in a short patch of new DNA synthesis to repair the DSB. (C) In synthesis-dependent strand-annealing (SDSA), the newly synthesized strand dissociates from the D-loop and results in a noncrossover (NCO) outcome with no change to the template DNA. (D) The double Holliday Junction (dHJ) pathway involves second end capture to stabilize the D-loop. The dHJ structure can be resolved either by helicase and topoisomerase-mediated dissolution to give NCO or cleaved by HJ resolvases to produce both crossover (CO) or NCO outcomes. (E) BIR involves both leading and lagging strand synthesis and results in loss of heterozygosity or, if the template is located ectopically, a nonreciprocal translocation. Newly synthesized DNA is depicted as dashed lines in the same color as the template; arrowheads indicate 3′ ends.
NHEJ involves modification and ligation of the broken DNA ends with very little or no homology, often creating small deletions or insertions (Fig. 2A). Although NHEJ can occur throughout the cell cycle, it is especially important in the G1 stage, when a key initial step in HR, the 5′ to 3′ resection of DSB ends, is blocked (Aylon et al. 2004; Ira et al. 2004). In the special case of haploid cells, such as in the well-studied budding or fission yeasts, G1 cells lack a homologous chromosome and, hence, can only use NHEJ until chromosomal replication creates a sister chromatid that can be used as a template to repair a DSB (Moore and Haber 1996). NHEJ essentially consists of several related but distinct mechanisms that have different genetic requirements and lengths of microhomology at the junctions. Precise end joining of 3′-overhanging ends (e.g., religating the ends of a restriction endonuclease cleavage) requires the Ku70 and Ku80 proteins, as well as DNA ligase IV; whereas, more extensive deletions with longer microhomologies at the junction prove to be Ku independent and, in mammals, DNA ligase IV independent. This alternative NHEJ or microhomology-mediated end-joining (MMEJ) pathway is evident even in wild-type cells. The reader is encouraged to read several excellent reviews (Bennardo et al. 2008; McVey and Lee 2008; Mladenov and Iliakis 2011; Chiruvella et al. 2013; Decottignies 2013).
HR
DNA DSBs can be repaired by several different HR pathways (Fig. 2). Single-strand annealing (SSA) is the simplest mechanism, allowing the formation of a deletion between homologous sequences flanking a DSB. Other types of HR all depend on the recognition and pairing of broken DNA ends with intact homologous sequences present on a sister chromatid, an allelic locus, or at some ectopic location in the genome (Pâques and Haber 1999; Krogh and Symington 2004). We will focus on two major mechanisms of HR: GC and BIR. An additional HR mechanism, gene targeting, can account for the integration of foreign DNA into a homologous chromosomal locus (Rothstein 1983). We further distinguish two distinct pathways of GC: one involving the formation of a pair of HJs that often leads to crossovers (COs) accompanying repair and one in which COs are rare.
All HR repair mechanisms require proteins belonging to the evolutionarily conserved RAD52 epistasis group. Astonishingly, the Rad52 protein itself, which is the most recombination-essential gene in budding and fission yeasts, appears to have an inconsiderable role in HR in metazoans. Rad52 is absent in flies and worms and, although it is present in mammals, it plays a minor role in repair, apparently limited to strand annealing. Instead Rad52’s central role is replaced by orthologs of the mammalian BRCA2 protein (Jensen et al. 2010; Liu et al. 2010; Holloman 2011). It seems that budding and fission yeasts may have lost their BRCA2 orthologs because another yeast, Ustilago maydis, has retained it and is dependent on it for HR (Yang et al. 2005). GC and most BIR depend on Rad51 recombinase, the homolog of bacterial RecA. Rad51 is a DNA-dependent ATPase that forms a filament on ssDNA and promotes strand invasion with a homologous double-stranded partner.
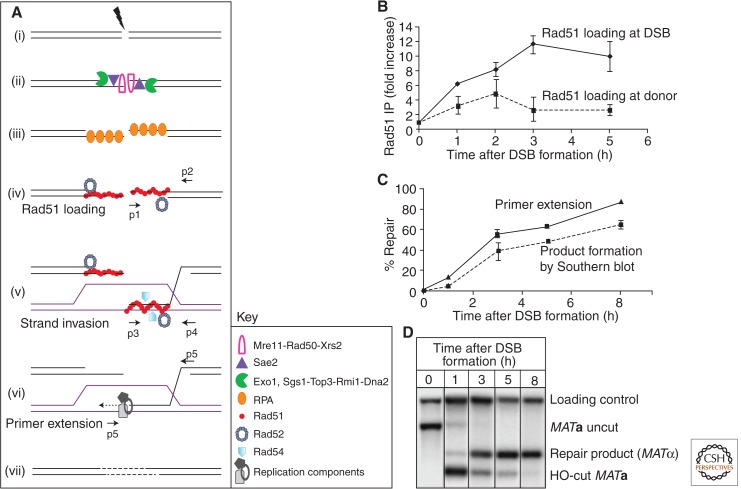
The series of biochemical steps in HR have been best studied in budding yeast, in which several early steps can be monitored in real time by a variety of techniques analyzing DNA intermediates and the proteins bound to these intermediates after inducing a DSB by the site-specific HO endonuclease (White and Haber 1990; Hicks et al. 2011) or other nucleases such as I-SceI (Fig. 3) (Plessis et al. 1992). Efficiency of DSB formation in a population can be gauged by a Southern blot (Fig. 3D) or monitoring the loss of a polymerase chain reaction (PCR) product with primers placed on opposite sides of the DSB site.
Figure 3.
Key intermediate steps of HR and methods to study them. (A) Key proteins are depicted in sequential early steps in GC in budding yeast. DSB formation (i) is immediately followed by 5′ to 3′ resection (ii). The 3′ tails are stabilized by RPA (iii), which is then replaced with Rad51 recombinase with assistance from accessory proteins like Rad52 (iv). Once the homologous donor is found, strand invasion occurs, resulting in formation of a D-loop (v) by displacement of the identical strand and base pairing with the complementary strand at the donor. (vi) Various components of the replication machinery assemble to start copying from the donor template. (vii) The break is sealed. Small arrows denote positions of PCR primer pairs used to analyze intermediate steps, shown on the right for MAT switching in S. cerevisiae. (B) Recruitment of Rad51 at DSB site (the MATa locus) by chromatin immunoprecipitation (ChIP) followed by quantitative PCR (qPCR) using primers p1 and p2 (solid line). Rad51 binding to the donor template (budding yeast HMLα locus) using primers p3 and p4 for qPCR (dotted line). Error bars indicate standard error of the mean. (C) The initiation of new DNA synthesis by primer extension is detected by using PCR primers p5 and p6, which amplifies a unique fragment once new DNA synthesis has been initiated (solid line). A dotted line shows quantitative densitometric analysis of Southern blot (D) that follows GC progression in budding yeast as MATa switches to MATα. Data from the investigators (A Mehta and J Haber, unpubl.).
All HR repair mechanisms are reliant on a common critical initial step: extensive 5′ to 3′ processing of the broken DNA ends (Fig. 3Aii). Whereas NHEJ and MMEJ involve limited processing of DNA ends for ligation, a prerequisite for HR is that broken ends are considerably resected to generate 3′-ended ssDNA tails that, once bound by Rad51 recombinase, act as the functionally active agents in searching for homologous template sequences to repair the DSB. 5′ to 3′ resection is a surprisingly complex process. An initial incision, removing about 100 nt, is performed by the Mre11-Rad50-Xrs2 complex along with the Sae2 protein. Subsequently, two other excision machines remove DNA at a rate of about 4 kb/hr. Exo1 chews off mononucleotides, whereas Sgs1-Top3-Rmi1-Dna2 acts as a helicase/endonuclease to clip off short oligonucleotides from the 5′ strand (Huertas et al. 2008; Mimitou and Symington 2008; Zhu et al. 2008; Cejka et al. 2010; Niu et al. 2010). Resection of a single HO endonuclease-induced DSB is highly limited in G1 phase of the cell cycle, when cyclin-dependent kinase Cdk1 (Cdc28) activity is low. The rate of resection increases once cells progress through the cell cycle and initiate DNA synthesis followed by mitosis in the G2/M phase, when Cdk1 activity is high (Aylon et al. 2004; Ira et al. 2004; Barlow et al. 2008). Additionally, Exo1 is impaired by binding of the Ku70-Ku80 proteins that facilitate NHEJ (Balestrini et al. 2013). Analogous machinery is found in mammalian cells, in which Sae2’s ortholog, CtIP, plays a central role and Sgs1’s ortholog, BLM helicase, appears to be coupled with both EXO1 and DNA2 (Sartori et al. 2007; Nimonkar et al. 2011; Sun et al. 2012).
Initially, the multimeric replication protein A (RPA) binds to ssDNA overhangs presumably to take out kinks and melt DNA secondary structures (internal base pairing) (Fig. 3Aiii). RPA is then replaced with the Rad51 recombinase, which forms a filament along the ssDNA (Fig. 3Aiv). As with the best-studied case of Escherichia coli RecA protein, the ssDNA is stretched within the Rad51 nucleoprotein filament to about 1.5 times the length of B-form DNA (Chen et al. 2008). However, this stretching is not uniform. The three bases bound by the RecA recombinase, and presumably by Rad51, are in almost a B-DNA configuration; the stretching happens predominantly between the 3-bp units. This arrangement may facilitate pairing through canonical Watson–Crick hydrogen bonds with complementary triplets in the donor duplex DNA; when RecA or Rad51 binds to dsDNA, it also becomes stretched to 150% of its normal length (Chen et al. 2008). The creation of the Rad51 filament is a complex process. After the binding of RPA, with the aid of the recombination mediator proteins (in yeast Rad52; in vertebrates BRCA2) plus several Rad51 paralog proteins (in yeast Rad55 and Rad57; in vertebrates RAD51B, RAD51C, RAD51D, XRCC2, and XRCC3) (Daley et al. 2014; Morrical 2014), RPA is displaced and the Rad51 nucleoprotein filament is formed. These steps can be seen in budding yeast in real time through the use of ChIP, using anti-RPA and anti-Rad51 antibodies (Fig. 3B) (Sugawara et al. 2003; Wang and Haber 2004). RPA binding is detected ∼10 min before Rad51 binding; no Rad51 binding occurs without Rad52.
ChIP can also be used to visualize the key next step in DSB repair: Rad51 nucleoprotein filament-mediated search for a distant homologous sequence and subsequent strand invasion (synapsis) between the resected end of the DSB and its duplex homologous donor sequence (Fig. 3Av). As shown in Figure 3B, these are remarkably slow steps. In the absence of special pairing sequences (i.e., the recombination enhancer that facilitates MAT switching between MATa and HMLα) (Li et al. 2012), Rad51 and its invading ssDNA strand becomes associated with the donor duplex DNA only after 30–45 min (Hicks et al. 2011; A Mehta and J Haber, unpubl.). In addition to Rad52, the Swi2/Snf2 homolog Rad54 also facilitates functional strand invasion (Sugawara et al. 2003; Kiianitsa et al. 2006; Hicks et al. 2011).
Following strand invasion, new DNA synthesis using either DNA polymerase (Pol)δ or Polε occurs using the 3′ invading end as a primer. Initiation of new DNA synthesis can be monitored by a PCR-based primer extension assay using two primers: one complementary to sequences distal to the DSB break-site and one within the donor (Fig. 3Avi,C) (White and Haber 1990). Several pathways can then occur (discussed below), ensuing in completion of repair. Southern blot analysis is used to accurately reveal the rate of repair product formation (Fig. 3C,D). DSB repair by HR can occur with less than 100 bp of homology with the DSB ends, but efficient repair is achieved with a few hundred bp (Sugawara and Haber 1992).
SSA
SSA is restricted to repair of DNA breaks that are flanked by direct repeats that can be as short as 30 nt (Sugawara et al. 2000; Villarreal et al. 2012). Resection exposes the complementary strands of homologous sequences, which recombine resulting in a deletion containing a single copy of the repeated sequence (Fig. 2B). SSA is therefore considered to be highly mutagenic. The nonhomologous single-stranded tails are removed by the Rad1-Rad10 endonuclease (XPF-ERCC1 in mammals) in a complex that includes both the Msh2-Msh3 mismatch repair proteins and “scaffold” proteins Slx4 and Saw1 (Sugawara et al. 1997; Li et al. 2008; Toh et al. 2010). After tail clipping, remaining gaps must be filled in by DNA synthesis and sealed by ligation. SSA requires the strand-annealing activity of Rad52 and is aided by the Rad52 homolog Rad59 (Sugawara et al. 2000); however, SSA does not involve DNA-strand invasion and thus is independent of the Rad51 recombinase (Ivanov et al. 1996).
GC
GC was initially defined as a nonreciprocal transfer of genetic information from one chromosome to its homolog during meiosis, but its meaning has been broadened to include DSB repair events in which a short patch of new DNA synthesis is copied from the homologous template. In budding yeast, about 10%–20% of interchromosomal allelic mitotic GCs are associated with crossing over, whereas COs are less frequent when the DSB uses an ectopic template that shares only a few kb with the regions around the break (Pâques and Haber 1999; Ira et al. 2003).
GC does not require many components of the normal DNA replication machinery, including DNA Polα-primase or Cdc45-GINS (complex consisting of four proteins: Sld5, Psf1, Psf2, and Psf3)-Mcm helicase complex (Wang et al. 2004; Lydeard et al. 2010). The efficiency of GC is also not significantly reduced by three mutations that severely impair the alternative HR repair mechanism of BIR, which can involve the synthesis of hundreds of kilobases of DNA: the nonessential DNA Polδ subunit pol32Δ (Lydeard et al. 2007; Jain et al. 2009), the 5′ to 3′ helicase pif1Δ (Chung et al. 2010; Wilson et al. 2013), and the proliferating cell nuclear antigen (PCNA) mutation pol30-FF248,249AA (Lydeard et al. 2010). The fact that GC does not require many of the normal DNA replication processivity factors might explain why it is highly susceptible to mutagenesis. Most mutations have the signatures of template switching during repair DNA synthesis (Strathern et al. 1995; Hicks et al. 2010).
Two different mechanisms, both of which are supported by substantial experimental data, can explain GCs and the different outcomes.
The Double Holliday Junction Mechanism
The double-strand break repair model, now known as the double Holliday junction or dHJ model, was first suggested by Resnick (1976) and later extended and elaborated by Szostak et al. (1983). Following resection and invasion into an intact homologous template, the 3′ Rad51-coated nucleoprotein filament base pairs with the complementary strand creating a displacement loop (D-loop) structure consisting of a region of heteroduplex DNA and displaced single strand of DNA. The D-loop can be extended by the initiation of new DNA synthesis from the 3′ end of the invading strand or the action of helicases, so that the ssDNA of the opposite side of the DSB can anneal, thus forming a dHJ intermediate (Fig. 2C). Alternatively, two independent strand invasions from both DSB ends, followed by simultaneous DNA synthesis and annealing could also result in a dHJ intermediate. No experimental evidence has shown whether recombination depends on one end or whether both undergo strand invasion. These HJs can be cleaved by one of several HJ resolvases, and, depending on which pair of strands is cut, can yield a noncrossover (NCO) or CO outcome. As an alternative to cleavage, dHJs can be “dissolved” to yield exclusively NCO outcomes (Wu and Hickson 2003).
The formation of dHJs has been extensively studied in budding yeast meiosis, but they have also been visualized in mitotic cells after a DSB is initiated by expression of a site-specific I-SceI endonuclease (Bzymek et al. 2010). Evidence for the presence of dHJ intermediates in budding yeast comes from ectopic recombination experiments in which the components of the dHJ “dissolvase” (Sgs1-Top3-Rmi1, or STR) are deleted. The proportion of GC events accompanied by a CO—in this case, a reciprocal translocation—increases from 4%–∼12% (Ira et al. 2003) when Sgs1 or other cofactors are absent. Assuming that sgs1Δ does not alter the proportion of events that form the dHJ intermediate, these results suggest 2/3 of the dHJs are normally dissolved. The proportion of COs increases even more when both Sgs1 and another 3′ to 5′ helicase, Mph1, are deleted. In this instance, it appears that deleting MPH1 shifts more of the repair events into the dHJ pathway (Prakash et al. 2009). Another estimate of the fraction of dHJs processed by the STR complex was obtained by Mitchel et al. (2013) by studying the position of heteroduplex DNA relative to the initiating gap in a plasmid-based gap-repair system. Their studies suggested that the fraction of dHJs processed by “resolution” is roughly equal to that processed by “dissolution.”
The Synthesis-Dependent Strand-Annealing Mechanism
Synthesis-dependent strand-annealing (SDSA) begins with the common step of strand invasion and formation of a D-loop (Fig. 2D). This is followed by new DNA synthesis (as monitored by primer extension assay) initiated by DNA Polδ or Polε from the invading 3′ end. There is mounting evidence that the principal repair polymerase is Polδ (Li et al. 2009a; Sebesta et al. 2011; Prindle and Loeb 2012), but repair is not eliminated either in a temperature-sensitive mutation of Polδ or Polε, suggesting both can be used (Lydeard et al. 2007). Polα primase is, however, not required (Wang et al. 2004). A carboxy-terminal truncation mutation of Polδ, pol3-ct, which does not affect replication, results in short DNA repair tracts in both mitosis and meiosis (Maloisel et al. 2004, 2008). If one examines mutations created during DSB repair (Hicks et al. 2010), defects of the 3′ to 5′ proofreading exonuclease activity of Polε resulted in the appearance of +1 frameshift mutations that were much less evident in wild-type repair. However, a much more dramatic effect was seen in the absence of proofreading activity of Polδ; several classes of mutations characterized as template switching defects (−1 frameshifts, quasi-palindrome mutations, and interchromosomal template switches) are surprisingly eliminated, leading to the suggestion that the wild-type Polδ enzyme is responsible for most of these events.
In Saccharomyces, there is little effect of ablating either of two translesion DNA polymerases, Polζ or Polη, although the error-prone Polζ polymerase has been shown to be the major contributor to mutations that arise in ssDNA regions, including those adjacent to the region in which both strands are newly copied during SDSA (Rattray et al. 2002; Yang et al. 2008). In vertebrates, evidence suggests that the translesion DNA polymerase, Polη, is a more important player in recombinational repair (Kawamoto et al. 2005). This conclusion is supported by demonstrations that Polη can perform repair synthesis in vitro (McIlwraith et al. 2005; Sneeden et al. 2013).
What makes SDSA synthesis unusual is that it is not semiconservative, in which the newly copied strand remains base paired to the donor template. Instead, the newly synthesized strand appears to be displaced from a migrating replication “bubble” and eventually pairs with the resected 3′ ssDNA on the other side of the DSB, resulting in an NCO outcome (Fig. 2D). Evidence in favor of SDSA includes the fact that the great majority of mitotic DSB repair events occur without an associated CO. Moreover, a number of studies in which GC is associated with the appearance of repair-associated mutations have found that all of the alterations are found in the recipient locus, whereas the donor remains unchanged—a result more consistent with SDSA than the dHJ mechanism (although dissolving dHJs would also result in this outcome) (Pâques and Haber 1999). Direct evidence for this mechanism was obtained by “heavy-light” density transfer experiments that showed that all of the newly copied DNA was located at the recipient locus, whereas the donor sequences were unaltered (Ira et al. 2006), indicating a conservative mode of strand inheritance. How the nascent DNA strand is unwound from the template is not known in detail, but both Mph1 and Srs2 3′ to 5′ helicases appear to be involved. As noted above, mph1Δ strains appear to direct a larger proportion of outcomes to the dHJ pathway, as would occur if Mph1 promoted the strand displacement. In contrast, srs2Δ strains show a 70% drop in viability, which results from a specific deficit in recovering NCO outcomes (Ira et al. 2003). This result suggests that Srs2 is required for completing SDSA, but not the dHJ pathway.
Although SDSA is presumed to yield exclusively NCO outcomes, one should not discount models that include the possibility of crossing over wherein the migrating D-loop associated with synthesizing the first strand can be captured by the second end, leading to the formation of a dHJ intermediate that can be resolved into a CO associated with GC (Ferguson and Holloman 1996; Pâques et al. 1998).
BIR
Under some circumstances, a broken chromosome may present only one end for repair, for instance, at collapsed forks during S-phase and in lengthening of telomeres (Doksani and de Lange 2014) in telomerase-deficient cells in pathways known as “alternative lengthening of telomeres” (McEachern and Haber 2006). When only one end of a chromosomal DSB shares homology with a template, cells rely on recombination-dependent DNA replication (i.e., BIR), a process in which strand invasion sets up a unidirectional replication fork capable of copying all of the sequences distal to the site of homology up to the telomere (Fig. 2E) (Malkova et al. 1996, 2005; Davis and Symington 2004). Repair by this mode results in a nonreciprocal translocation and, if the template is a homologous chromosome, extensive loss of heterozygosity. BIR has been observed with as little as 70 bp of shared homology between the DSB end and an ectopic template (Bosco and Haber 1998), but is much more efficient with longer regions of homology.
BIR has been analyzed in budding yeast both by transformation of a linear plasmid that has telomere-forming sequences at one end and homology with a chromosomal template on the other (Morrow et al. 1997; Davis and Symington 2004; Marrero and Symington 2010), and creating broken chromosomes using HO or I-SceI endonucleases (Bosco and Haber 1998; Malkova et al. 2005; Lydeard et al. 2007; Ruiz et al. 2009). The initial steps of BIR, up to homology search and strand invasion appear to be identical to those of GC and require all of the same proteins (Davis and Symington 2004; Jain et al. 2009). However, the subsequent repair DNA synthesis occurs very differently. BIR requires all of the replication factors and the three major DNA polymerases required for leading and lagging strand synthesis, except some proteins that are needed exclusively for the assembly of the prereplication complex at origins of replication (Lydeard et al. 2007, 2010).
Nevertheless, in several respects, BIR is quite different from normal leading- and lagging-strand S-phase replication. First, BIR is remarkably mutagenic compared to normal replication (Deem et al. 2011). Second, at least for the first several kb that are copied, BIR shows a very high frequency of template switching, from one homologous chromosomal template to the other (Smith et al. 2007). This instability may be a reflection of the finding that BIR can be initiated without DNA Polε, but requires this normally leading-strand polymerase for its completion. As noted above, unlike GC, BIR is significantly reduced by the deletion of POL32 (Lydeard et al. 2007) or PIF1 (Chung et al. 2010; Wilson et al. 2013), or the PCNA mutation pol30-FF248,249AA (Lydeard et al. 2010). This PCNA mutation was found as a suppressor of the cold sensitivity of pol32Δ, however, it is by itself as defective for BIR as pol32Δ and does not suppress pol32Δ’s BIR defect. The mutant has no effect on normal replication; moreover, the location of the mutated sites is unrelated to PCNA mutations involved in recruiting translesion DNA polymerases during replication fork stalling. Analogous mutations created in the Xenopus PCNA protein show a defect in an in vitro replication restart assay (Hashimoto et al. 2012). These differences point toward unique features of BIR, either in the establishment of the repair replication fork or its efficiency.
Recently, an explanation for the differences between BIR and normal replication was provided by the work from the Malkova and Lobachev laboratories (Saini et al. 2013), which analyzed intermediates of BIR by DNA combing and two-dimensional gel electrophoresis. These analyses produced several critical revelations. First, all of the newly copied DNA is found on the extension of the originally broken molecule, a conclusion also reached by Donnianni and Symington (2013). This “conservative” mode of DNA synthesis suggests either that semiconservatively replicated DNA is unwound by branch migration of a HJ formed behind the replication fork or the second new strand is copied from the first (Fig. 2E). Indeed, the latter possibility seems to be the case as the migration of replication intermediates is consistent with that expected for a migrating D-loop (as first proposed by Formosa and Alberts 1986) with a long ssDNA extension. In this respect, BIR would share certain common steps with SDSA but, as noted above, none of the BIR-specific factors (Pol32, Pif1, or the PCNA mutant) have much effect on repair by GC. One can rationalize the lack of effect of mutating these factors by the fact that most GC assays require copying of no more than ∼1 kb of new DNA. Indeed, when cells are required to repair a DSB in which the homologous ends of the DSB invade a template separated by ≥5 kb, the events become dependent on POL32 (Jain et al. 2009) and are impaired by the PCNA mutation, pol30-FF248,249AA (A Mehta, T Ryu, and J Haber, unpubl.).
Another interesting distinction between GC and BIR is that the latter shows a dramatic delay in the initiation of new DNA synthesis. This delay reflects a replication execution checkpoint (REC) that somehow monitors whether only one or both ends of a DSB can successfully pair with homologous template sequences on the same template and in the proper orientation and distance to complete GC (Jain et al. 2009). The kinetics of strand invasion, as monitored by the appearance of Rad51 at the donor locus by ChIP, are the same for GC and BIR, so the REC acts before the initiation of new DNA synthesis and is seen even when both ends of the DSB can pair with donor sequences, when they are separated by ≥5 kb. The strong kinetic barrier to promoting BIR also helps to explain the hierarchy of DSB repair events; in a situation in which both GC and BIR are possible, GC is by far the predominant outcome, although when homology is removed from one end, BIR proves to be quite efficient (Malkova et al. 2005; Jain et al. 2009).
PERSPECTIVES
In this review, we have summarized the various sources of DSBs, pathways to repair them, and ways to monitor the intermediate steps of repair. Faithful and efficient repair of all DNA lesions is essential to maintain genome integrity and cell viability. Deficiencies and mutations in repair pathways are associated with multiple human diseases and with aging. It is curious to note that DSBs can be detrimental as well as beneficial to organisms. The molecular details connecting DSB repair to other cellular processes are emerging. One interesting aspect that is yet to be sufficiently explored is the role of chromatin structure in HR.
Most of our understanding of HR comes from work in bacteria, yeast, and other model systems. Comparable studies in mammalian systems are now beginning to elucidate the intricacies of DNA repair. The exceedingly more complex mammalian system is likely to require additional hitherto unknown components. Indeed, several proteins, including ssDNA-binding protein (SSB) (Li et al. 2009b) and RAD51-associated protein 1 (RAD51AP1) (Modesti et al. 2007; Wiese et al. 2007) have been identified that have no homologs in lower eukaryotes.
Any deficiency in HR directs the cells along more error-prone pathways like NHEJ. Ironically, recent studies have shown that HR itself is highly error-prone and could be one of the leading causes of mutagenesis (Malkova and Haber 2012). Thus, HR is a double-edged sword whose precise regulation is critical. HR components are targets for emerging therapies, especially in cancer therapeutics. Hence, further elucidation of the mechanisms of HR repair, especially in mammals, is vital.
ACKNOWLEDGMENTS
Work from the Haber laboratory is supported by NIH Grants GM20056, GM61766, and GM76020 to JEH.
Footnotes
Editors: Stephen Kowalczykowski, Neil Hunter, and Wolf-Dietrich Heyer
Additional Perspectives on DNA Recombination available at www.cshperspectives.org
REFERENCES
*Reference is also in this collection.
- *.Aguilera A, Gaillard H 2014. Transcription and recombination: When RNA meets DNA. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a016543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcangioli B 2000. Fate of mat1 DNA strands during mating-type switching in fission yeast. EMBO Rep 1: 145–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aylon Y, Liefshitz B, Kupiec M 2004. The CDK regulates repair of double-strand breaks by homologous recombination during the cell cycle. EMBO J 23: 4868–4875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balestrini A, Ristic D, Dionne I, Liu XZ, Wyman C, Wellinger RJ, Petrini JH 2013. The Ku heterodimer and the metabolism of single-ended DNA double-strand breaks. Cell Rep 3: 2033–2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow JH, Lisby M, Rothstein R 2008. Differential regulation of the cellular response to DNA double-strand breaks in G1. Mol Cell 30: 73–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsoum E, Martinez P, Astrom SU 2010. α3, a transposable element that promotes host sexual reproduction. Genes Dev 24: 33–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudry C, Malinsky S, Restituito M, Kapusta A, Rosa S, Meyer E, Betermier M 2009. PiggyMac, a domesticated piggyBac transposase involved in programmed genome rearrangements in the ciliate Paramecium tetraurelia. Genes Dev 23: 2478–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter J, Diffley JF 2008. Topoisomerase II inactivation prevents the completion of DNA replication in budding yeast. Mol Cell 30: 790–802 [DOI] [PubMed] [Google Scholar]
- Bennardo N, Cheng A, Huang N, Stark JM 2008. Alternative-NHEJ is a mechanistically distinct pathway of mammalian chromosome break repair. PLoS Genet 4: e1000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betermier M 2004. Large-scale genome remodelling by the developmentally programmed elimination of germ line sequences in the ciliate Paramecium. Res Microbiol 155: 399–408 [DOI] [PubMed] [Google Scholar]
- Bosco G, Haber JE 1998. Chromosome break-induced DNA replication leads to nonreciprocal translocations and telomere capture. Genetics 150: 1037–1047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzymek M, Thayer NH, Oh SD, Kleckner N, Hunter N 2010. Double Holliday junctions are intermediates of DNA break repair. Nature 464: 937–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cejka P, Cannavo E, Polaczek P, Masuda-Sasa T, Pokharel S, Campbell JL, Kowalczykowski SC 2010. DNA end resection by Dna2-Sgs1-RPA and its stimulation by Top3-Rmi1 and Mre11-Rad50-Xrs2. Nature 467: 112–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Stubbe J 2005. Bleomycins: Towards better therapeutics. Nat Rev Cancer 5: 102–112 [DOI] [PubMed] [Google Scholar]
- Chen Z, Yang H, Pavletich NP 2008. Mechanism of homologous recombination from the RecA-ssDNA/dsDNA structures. Nature 453: 484–489 [DOI] [PubMed] [Google Scholar]
- Chiruvella KK, Liang Z, Wilson TE 2013. Repair of double-strand breaks by end joining. Cold Spring Harbor Perspect Biol 5: a012757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung WH, Zhu Z, Papusha A, Malkova A, Ira G 2010. Defective resection at DNA double-strand breaks leads to de novo telomere formation and enhances gene targeting. PLoS Genet 6: e1000948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coïc E, Feldman T, Landman AS, Haber JE 2008. Mechanisms of Rad52-independent spontaneous and UV-induced mitotic recombination in Saccharomyces cerevisiae. Genetics 179: 199–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colleaux L, D’Auriol L, Galibert F, Dujon B 1988. Recognition and cleavage site of the intron-encoded omega transposase. Proc Natl Acad Sci 85: 6022–6026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crasta K, Ganem NJ, Dagher R, Lantermann AB, Ivanova EV, Pan Y, Nezi L, Protopopov A, Chowdhury D, Pellman D 2012. DNA breaks and chromosome pulverization from errors in mitosis. Nature 482: 53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Daley JM, Gaines WA, Kwon YH, Sung P 2014. Regulation of DNA pairing in homologous recombination. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a017954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis AP, Symington LS 2004. RAD51-dependent break-induced replication in yeast. Mol Cell Biol 24: 2344–2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bont R, van Larebeke N 2004. Endogenous DNA damage in humans: A review of quantitative data. Mutagenesis 19: 169–185 [DOI] [PubMed] [Google Scholar]
- Decottignies A 2013. Alternative end-joining mechanisms: A historical perspective. Front Genet 4: 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deem A, Keszthelyi A, Blackgrove T, Vayl A, Coffey B, Mathur R, Chabes A, Malkova A 2011. Break-induced replication is highly inaccurate. PLoS Biol 9: e1000594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Doksani Y, de Lange T 2014. The role of DSB repair pathways at functional and dysfunctional telomeres. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a016576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnianni RA, Symington LS 2013. Break-induced replication occurs by conservative DNA synthesis. Proc Natl Acad Sci 110: 13475–13480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson DO, Holloman WK 1996. Recombinational repair of gaps in DNA is asymmetric in Ustilago maydis and can be explained by a migrating D-loop model. Proc Natl Acad Sci 93: 5419–5424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formosa T, Alberts BM 1986. DNA synthesis dependent on genetic recombination: Characterization of a reaction catalyzed by purified bacteriophage T4 proteins. Cell 47: 793–806 [DOI] [PubMed] [Google Scholar]
- Ganem NJ, Pellman D 2012. Linking abnormal mitosis to the acquisition of DNA damage. J Cell Biol 199: 871–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavalda S, Gallardo M, Luna R, Aguilera A 2013. R-loop mediated transcription-associated recombination in trf4Δ mutants reveals new links between RNA surveillance and genome integrity. PloS ONE 8: e65541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloor GB, Nassif NA, Johnson-Schlitz DM, Preston CR, Engels WR 1991. Targeted gene replacement in Drosophila via P element-induced gap repair. Science 253: 1110–1117 [DOI] [PubMed] [Google Scholar]
- Haaf T, Golub EI, Reddy G, Radding CM, Ward DC 1995. Nuclear foci of mammalian Rad51 recombination protein in somatic cells after DNA damage and its localization in synaptonemal complexes. Proc Natl Acad Sci 92: 2298–2302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber JE 2006. Transpositions and translocations induced by site-specific double-strand breaks in budding yeast. DNA Repair 5: 998–1009 [DOI] [PubMed] [Google Scholar]
- Haber JE 2012. Mating-type genes and MAT switching in Saccharomyces cerevisiae. Genetics 191: 33–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber JE, Thorburn PC 1984. Healing of broken linear dicentric chromosomes in yeast. Genetics 106: 207–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada K, Budzowska M, Davies SL, van Drunen E, Onizawa H, Beverloo HB, Maas A, Essers J, Hickson ID, Kanaar R 2007. The structure-specific endonuclease Mus81 contributes to replication restart by generating double-strand DNA breaks. Nat Struct Mol Biol 14: 1096–1104 [DOI] [PubMed] [Google Scholar]
- Hashimoto Y, Puddu F, Costanzo V 2012. RAD51- and MRE11-dependent reassembly of uncoupled CMG helicase complex at collapsed replication forks. Nat Struct Mol Biol 19: 17–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmrich A, Ballarino M, Tora L 2011. Collisions between replication and transcription complexes cause common fragile site instability at the longest human genes. Mol Cell 44: 966–977 [DOI] [PubMed] [Google Scholar]
- Hicks WM, Kim M, Haber JE 2010. Increased mutagenesis and unique mutation signature associated with mitotic gene conversion. Science 329: 82–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks WM, Yamaguchi M, Haber JE 2011. Real-time analysis of double-strand DNA break repair by homologous recombination. Proc Natl Acad Sci 108: 3108–3115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeijmakers JHJ 2009. DNA damage, aging, and cancer. N Engl J Med 361: 1475–1485 [DOI] [PubMed] [Google Scholar]
- Hoffelder DR, Luo L, Burke NA, Watkins SC, Gollin SM, Saunders WS 2004. Resolution of anaphase bridges in cancer cells. Chromosoma 112: 389–397 [DOI] [PubMed] [Google Scholar]
- Holloman WK 2011. Unraveling the mechanism of BRCA2 in homologous recombination. Nat Struct Mol Biol 18: 748–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huertas P, Aguilera A 2003. Cotranscriptionally formed DNA:RNA hybrids mediate transcription elongation impairment and transcription-associated recombination. Mol Cell 12: 711–721 [DOI] [PubMed] [Google Scholar]
- Huertas P, Cortes-Ledesma F, Sartori AA, Aguilera A, Jackson SP 2008. CDK targets Sae2 to control DNA-end resection and homologous recombination. Nature 455: 689–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ira G, Malkova A, Liberi G, Foiani M, Haber JE 2003. Srs2 and Sgs1-Top3 suppress crossovers during double-strand break repair in yeast. Cell 115: 401–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ira G, Pellicioli A, Balijja A, Wang X, Fiorani S, Carotenuto W, Liberi G, Bressan D, Wan L, Hollingsworth NM, et al. 2004. DNA end resection, homologous recombination and DNA damage checkpoint activation require CDK1. Nature 431: 1011–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ira G, Satory D, Haber JE 2006. Conservative inheritance of newly synthesized DNA in double-strand break-induced gene conversion. Mol Cell Biol 26: 9424–9429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov EL, Sugawara N, Fishman-Lobell J, Haber JE 1996. Genetic requirements for the single-strand annealing pathway of double-strand break repair in Saccharomyces cerevisiae. Genetics 142: 693–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S, Sugawara N, Lydeard J, Vaze M, Tanguy Le Gac N, Haber JE 2009. A recombination execution checkpoint regulates the choice of homologous recombination pathway during DNA double-strand break repair. Genes Dev 23: 291–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen A, van der Burg M, Szuhai K, Kops GJ, Medema RH 2011. Chromosome segregation errors as a cause of DNA damage and structural chromosome aberrations. Science 333: 1895–1898 [DOI] [PubMed] [Google Scholar]
- Jensen RB, Carreira A, Kowalczykowski SC 2010. Purified human BRCA2 stimulates RAD51-mediated recombination. Nature 467: 678–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapusta A, Matsuda A, Marmignon A, Ku M, Silve A, Meyer E, Forney JD, Malinsky S, Betermier M 2011. Highly precise and developmentally programmed genome assembly in Paramecium requires ligase IV-dependent end joining. PLoS Genet 7: e1002049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass EM, Jasin M 2010. Collaboration and competition between DNA double-strand break repair pathways. FEBS Lett 584: 3703–3708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto T, Araki K, Sonoda E, Yamashita YM, Harada K, Kikuchi K, Masutani C, Hanaoka F, Nozaki K, Hashimoto N, et al. 2005. Dual roles for DNA polymerase η in homologous DNA recombination and translesion DNA synthesis. Mol Cell 20: 793–799 [DOI] [PubMed] [Google Scholar]
- Keeney S 2008. Spo11 and the formation of DNA double-strand breaks in meiosis. Genome Dyn Stab 2: 81–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiianitsa K, Solinger JA, Heyer WD 2006. Terminal association of Rad54 protein with the Rad51-dsDNA filament. Proc Natl Acad Sci 103: 9767–9772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim N, Jinks-Robertson S 2009. dUTP incorporation into genomic DNA is linked to transcription in yeast. Nature 459: 1150–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klar AJ 2007. Lessons learned from studies of fission yeast mating-type switching and silencing. Annu Rev Genet 41: 213–236 [DOI] [PubMed] [Google Scholar]
- Koster DA, Palle K, Bot ES, Bjornsti MA, Dekker NH 2007. Antitumour drugs impede DNA uncoiling by topoisomerase I. Nature 448: 213–217 [DOI] [PubMed] [Google Scholar]
- Kramer KM, Brock JA, Bloom K, Moore JK, Haber JE 1994. Two different types of double-strand breaks in Saccharomyces cerevisiae are repaired by similar RAD52-independent, nonhomologous recombination events. Mol Cell Biol 14: 1293–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogh BO, Symington LS 2004. Recombination proteins in yeast. Annu Rev Genet 38: 233–271 [DOI] [PubMed] [Google Scholar]
- *.Lam I, Keeney S 2014. Mechanism and regulation of meiotic recombination initiation. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a016634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Manley JL 2005. Inactivation of the SR protein splicing factor ASF/SF2 results in genomic instability. Cell 122: 365–378 [DOI] [PubMed] [Google Scholar]
- Li F, Dong J, Pan X, Oum JH, Boeke JD, Lee SE 2008. Microarray-based genetic screen defines SAW1, a gene required for Rad1/Rad10-dependent processing of recombination intermediates. Mol Cell 30: 325–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Stith CM, Burgers PM, Heyer WD 2009a. PCNA is required for initiation of recombination-associated DNA synthesis by DNA polymerase δ. Mol Cell 36: 704–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Bolderson E, Kumar R, Muniandy PA, Xue Y, Richard DJ, Seidman M, Pandita TK, Khanna KK, Wang W 2009b. hSSB1 and hSSB2 form similar multiprotein complexes that participate in DNA damage response. J Biol Chem 284: 23525–23531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Coic E, Lee K, Lee CS, Kim JA, Wu Q, Haber JE 2012. Regulation of budding yeast mating-type switching donor preference by the FHA domain of Fkh1. PLoS Genet 8: e1002630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Dent SY, Wilson JH, Wells RD, Napierala M 2010. R loops stimulate genetic instability of CTG.CAG repeats. Proc Natl Acad Sci 107: 692–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T 1993. Instability and decay of the primary structure of DNA. Nature 362: 709–715 [DOI] [PubMed] [Google Scholar]
- Lisby M, Mortensen UH, Rothstein R 2003. Colocalization of multiple DNA double-strand breaks at a single Rad52 repair centre. Nat Cell Biol 5: 572–577 [DOI] [PubMed] [Google Scholar]
- Liu J, Doty T, Gibson B, Heyer WD 2010. Human BRCA2 protein promotes RAD51 filament formation on RPA-covered single-stranded DNA. Nat Struct Mol Biol 17: 1260–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löbrich M, Shibata A, Beucher A, Fisher A, Ensminger M, Goodarzi AA, Barton O, Jeggo PA 2010. γH2AX foci analysis for monitoring DNA double-strand break repair: Strengths, limitations and optimization. Cell Cycle 9: 662–669 [DOI] [PubMed] [Google Scholar]
- Lydeard JR, Jain S, Yamaguchi M, Haber JE 2007. Break-induced replication and telomerase-independent telomere maintenance require Pol32. Nature 448: 820–823 [DOI] [PubMed] [Google Scholar]
- Lydeard JR, Lipkin-Moore Z, Sheu YJ, Stillman B, Burgers PM, Haber JE 2010. Break-induced replication requires all essential DNA replication factors except those specific for pre-RC assembly. Genes Dev 24: 1133–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Halweg CJ, Menendez D, Resnick MA 2012. Differential effects of poly(ADP-ribose) polymerase inhibition on DNA break repair in human cells are revealed with Epstein–Barr virus. Proc Natl Acad Sci 109: 6590–6595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkova A, Haber JE 2012. Mutations arising during repair of chromosome breaks. Annu Rev Genet 46: 455–473 [DOI] [PubMed] [Google Scholar]
- Malkova A, Ivanov EL, Haber JE 1996. Double-strand break repair in the absence of RAD51 in yeast: A possible role for break-induced DNA replication. Proc Natl Acad Sci 93: 7131–7136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkova A, Naylor ML, Yamaguchi M, Ira G, Haber JE 2005. RAD51-dependent break-induced replication differs in kinetics and checkpoint responses from RAD51-mediated gene conversion. Mol Cell Biol 25: 933–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloisel L, Bhargava J, Roeder GS 2004. A role for DNA polymerase δ in gene conversion and crossing over during meiosis in Saccharomyces cerevisiae. Genetics 167: 1133–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloisel L, Fabre F, Gangloff S 2008. DNA polymerase δ is preferentially recruited during homologous recombination to promote heteroduplex DNA extension. Mol Cell Biol 28: 1373–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrero VA, Symington LS 2010. Extensive DNA end processing by exo1 and sgs1 inhibits break-induced replication. PLoS Genet 6: e1001007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEachern MJ, Haber JE 2006. Break-induced replication and recombinational telomere elongation in yeast. Annu Rev Biochem 75: 111–135 [DOI] [PubMed] [Google Scholar]
- McIlwraith MJ, Vaisman A, Liu Y, Fanning E, Woodgate R, West SC 2005. Human DNA polymerase η promotes DNA synthesis from strand invasion intermediates of homologous recombination. Mol Cell 20: 783–792 [DOI] [PubMed] [Google Scholar]
- McVey M, Lee SE 2008. MMEJ repair of double-strand breaks (director’s cut): Deleted sequences and alternative endings. Trend Genet 24: 529–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrikh H, Zhang Y, Grossman AD, Wang JD 2012. Replication-transcription conflicts in bacteria. Nat Rev Microbiol 10: 449–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan JR, Ng JY, Wu CC, Aguilera JA, Fahey RC, Ward JF 1995. DNA repair by thiols in air shows two radicals make a double-strand break. Radiat Res 143: 273–280 [PubMed] [Google Scholar]
- Mimitou EP, Symington LS 2008. Sae2, Exo1 and Sgs1 collaborate in DNA double-strand break processing. Nature 455: 770–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirkin EV, Mirkin SM 2007. Replication fork stalling at natural impediments. Microbiol Mol Biol Rev 71: 13–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchel K, Lehner K, Jinks-Robertson S 2013. Heteroduplex DNA position defines the roles of the Sgs1, Srs2, and Mph1 helicases in promoting distinct recombination outcomes. PLoS Genet 9: e1003340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mladenov E, Iliakis G 2011. Induction and repair of DNA double strand breaks: The increasing spectrum of non-homologous end joining pathways. Mutat Res 711: 61–72 [DOI] [PubMed] [Google Scholar]
- Modesti M, Budzowska M, Baldeyron C, Demmers JA, Ghirlando R, Kanaar R 2007. RAD51AP1 is a structure-specific DNA binding protein that stimulates joint molecule formation during RAD51-mediated homologous recombination. Mol Cell 28: 468–481 [DOI] [PubMed] [Google Scholar]
- Moore JK, Haber JE 1996. Cell cycle and genetic requirements of two pathways of nonhomologous end-joining repair of double-strand breaks in Saccharomyces cerevisiae. Mol Cell Biol 16: 2164–2173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Morrical SW 2014. DNA pairing and annealing processes in homologous recombination and homology-directed repair. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a016444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow DM, Connelly C, Hieter P 1997. “Break copy” duplication: A model for chromosome fragment formation in Saccharomyces cerevisiae. Genetics 147: 371–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimonkar AV, Genschel J, Kinoshita E, Polaczek P, Campbell JL, Wyman C, Modrich P, Kowalczykowski SC 2011. BLM-DNA2-RPA-MRN and EXO1-BLM-RPA-MRN constitute two DNA end resection machineries for human DNA break repair. Genes Dev 25: 350–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu H, Chung WH, Zhu Z, Kwon Y, Zhao W, Chi P, Prakash R, Seong C, Liu D, Lu L, et al. 2010. Mechanism of the ATP-dependent DNA end-resection machinery from Saccharomyces cerevisiae. Nature 467: 108–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noon AT, Goodarzi AA 2011. 53BP1-mediated DNA double strand break repair: Insert bad pun here. DNA Repair 10: 1071–1076 [DOI] [PubMed] [Google Scholar]
- Pâques F, Haber JE 1999. Multiple pathways of recombination induced by double-strand breaks in Saccharomyces cerevisiae. Microbiol Mol Biol Rev 63: 349–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pâques F, Leung WY, Haber JE 1998. Expansions and contractions in a tandem repeat induced by double-strand break repair. Mol Cell Biol 18: 2045–2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulsen RD, Soni DV, Wollman R, Hahn AT, Yee MC, Guan A, Hesley JA, Miller SC, Cromwell EF, Solow-Cordero DE, et al. 2009. A genome-wide siRNA screen reveals diverse cellular processes and pathways that mediate genome stability. Mol Cell 35: 228–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petermann E, Helleday T 2010. Pathways of mammalian replication fork restart. Nat Rev Mol Cell Biol 11: 683–687 [DOI] [PubMed] [Google Scholar]
- Plessis A, Perrin A, Haber JE, Dujon B 1992. Site-specific recombination determined by I-SceI, a mitochondrial group I intron-encoded endonuclease expressed in the yeast nucleus. Genetics 130: 451–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prado F, Aguilera A 2005. Impairment of replication fork progression mediates RNA polII transcription-associated recombination. EMBO J 24: 1267–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash R, Satory D, Dray E, Papusha A, Scheller J, Kramer W, Krejci L, Klein H, Haber JE, Sung P, et al. 2009. Yeast Mph1 helicase dissociates Rad51-made D-loops: Implications for crossover control in mitotic recombination. Genes Dev 23: 67–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prindle MJ, Loeb LA 2012. DNA polymerase δ in DNA replication and genome maintenance. Environ Mol Mutagen 53: 666–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevedo O, Garcia-Luis J, Matos-Perdomo E, Aragon L, Machin F 2012. Nondisjunction of a single chromosome leads to breakage and activation of DNA damage checkpoint in G2. PLoS Genet 8: e1002509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raderschall E, Golub EI, Haaf T 1999. Nuclear foci of mammalian recombination proteins are located at single-stranded DNA regions formed after DNA damage. Proc Natl Acad Sci 96: 1921–1926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattray AJ, Shafer BK, McGill CB, Strathern JN 2002. The roles of REV3 and RAD57 in double-strand-break-repair-induced mutagenesis of Saccharomyces cerevisiae. Genetics 162: 1063–1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick MA 1976. The repair of double-strand breaks in DNA; a model involving recombination. J Theor Biol 59: 97–106 [DOI] [PubMed] [Google Scholar]
- Rothstein RJ 1983. One-step gene disruption in yeast. Methods Enzymol 101: 202–211 [DOI] [PubMed] [Google Scholar]
- Ruiz JF, Gomez-Gonzalez B, Aguilera A 2009. Chromosomal translocations caused by either pol32-dependent or pol32-independent triparental break-induced replication. Mol Cell Biol 29: 5441–5454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini N, Ramakrishnan S, Elango R, Ayyar S, Zhang Y, Deem A, Ira G, Haber JE, Lobachev KS, Malkova A 2013. Migrating bubble during break-induced replication drives conservative DNA synthesis. Nature 502: 389–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samoshkin A, Arnaoutov A, Jansen LE, Ouspenski I, Dye L, Karpova T, McNally J, Dasso M, Cleveland DW, Strunnikov A 2009. Human condensin function is essential for centromeric chromatin assembly and proper sister kinetochore orientation. PloS ONE 4: e6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartori AA, Lukas C, Coates J, Mistrik M, Fu S, Bartek J, Baer R, Lukas J, Jackson SP 2007. Human CtIP promotes DNA end resection. Nature 450: 509–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebesta M, Burkovics P, Haracska L, Krejci L 2011. Reconstitution of DNA repair synthesis in vitro and the role of polymerase and helicase activities. DNA Repair 10: 567–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CE, Llorente B, Symington LS 2007. Template switching during break-induced replication. Nature 447: 102–105 [DOI] [PubMed] [Google Scholar]
- Sneeden JL, Grossi SM, Tappin I, Hurwitz J, Heyer WD 2013. Reconstitution of recombination-associated DNA synthesis with human proteins. Nucleic Acids Res 41: 4913–4925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonoda E, Morrison C, Yamashita YM, Takata M, Takeda S 2001. Reverse genetic studies of homologous DNA recombination using the chicken B-lymphocyte line, DT40. Philos Trans R Soc Lond B Biol Sci 356: 111–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soulas-Sprauel P, Rivera-Munoz P, Malivert L, Le Guyader G, Abramowski V, Revy P, de Villartay JP 2007. V(D)J and immunoglobulin class switch recombinations: A paradigm to study the regulation of DNA end-joining. Oncogene 26: 7780–7791 [DOI] [PubMed] [Google Scholar]
- Soutoglou E, Misteli T 2008. Activation of the cellular DNA damage response in the absence of DNA lesions. Science 320: 1507–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spell RM, Holm C 1994. Nature and distribution of chromosomal intertwinings in Saccharomyces cerevisiae. Mol Cell Biol 14: 1465–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling PC, Chan YA, Minaker SW, Aristizabal MJ, Barrett I, Sipahimalani P, Kobor MS, Hieter P 2012. R-loop-mediated genome instability in mRNA cleavage and polyadenylation mutants. Genes Dev 26: 163–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathern JN, Shafer BK, McGill CB 1995. DNA synthesis errors associated with double-strand-break repair. Genetics 140: 965–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suberbielle E, Sanchez PE, Kravitz AV, Wang X, Ho K, Eilertson K, Devidze N, Kreitzer AC, Mucke L 2013. Physiologic brain activity causes DNA double-strand breaks in neurons, with exacerbation by amyloid-β. Nat Neurosci 16: 613–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara N, Haber JE 1992. Characterization of double-strand break-induced recombination: Homology requirements and single-stranded DNA formation. Mol Cell Biol 12: 563–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara N, Paques F, Colaiacovo M, Haber JE 1997. Role of Saccharomyces cerevisiae Msh2 and Msh3 repair proteins in double-strand break-induced recombination. Proc Natl Acad Sci 94: 9214–9219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara N, Ira G, Haber JE 2000. DNA length dependence of the single-strand annealing pathway and the role of Saccharomyces cerevisiae RAD59 in double-strand break repair. Mol Cell Biol 20: 5300–5309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugawara N, Wang X, Haber JE 2003. In vivo roles of Rad52, Rad54, and Rad55 proteins in Rad51-mediated recombination. Mol Cell 12: 209–219 [DOI] [PubMed] [Google Scholar]
- Sun J, Lee KJ, Davis AJ, Chen DJ 2012. Human Ku70/80 protein blocks exonuclease 1-mediated DNA resection in the presence of human Mre11 or Mre11/Rad50 protein complex. J Biol Chem 287: 4936–4945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Syeda AH, Hawkins M, McGlynn P 2014. Recombination and replication. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a016550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Symington LS 2014. Processing of DNA breaks: Mechanism and regulation. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a016536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szostak JW, Orr-Weaver TL, Rothstein RJ, Stahl FW 1983. The double-strand-break repair model for recombination. Cell 33: 25–35 [DOI] [PubMed] [Google Scholar]
- Thompson LH 2012. Recognition, signaling, and repair of DNA double-strand breaks produced by ionizing radiation in mammalian cells: The molecular choreography. Mutat Res 751: 158–246 [DOI] [PubMed] [Google Scholar]
- Toh GW, Sugawara N, Dong J, Toth R, Lee SE, Haber JE, Rouse J 2010. Mec1/Tel1-dependent phosphorylation of Slx4 stimulates Rad1-Rad10-dependent cleavage of non-homologous DNA tails. DNA Repair 9: 718–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdiglesias V, Giunta S, Fenech M, Neri M, Bonassi S 2013. γH2AX as a marker of DNA double strand breaks and genomic instability in human population studies. Mutat Res 753: 24–40 [DOI] [PubMed] [Google Scholar]
- Vilenchik MM, Knudson AG 2003. Endogenous DNA double-strand breaks: Production, fidelity of repair, and induction of cancer. Proc Natl Acad Sci 100: 12871–12876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal DD, Lee K, Deem A, Shim EY, Malkova A, Lee SE 2012. Microhomology directs diverse DNA break repair pathways and chromosomal translocations. PLoS Genet 8: e1003026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahba L, Amon JD, Koshland D, Vuica-Ross M 2011. RNase H and multiple RNA biogenesis factors cooperate to prevent RNA:DNA hybrids from generating genome instability. Mol Cell 44: 978–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahba L, Gore SK, Koshland D 2013. The homologous recombination machinery modulates the formation of RNA-DNA hybrids and associated chromosome instability. eLife 2: e00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Haber JE 2004. Role of Saccharomyces single-stranded DNA-binding protein RPA in the strand invasion step of double-strand break repair. PLoS Biol 2: E21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Ira G, Tercero JA, Holmes AM, Diffley JF, Haber JE 2004. Role of DNA replication proteins in double-strand break-induced recombination in Saccharomyces cerevisiae. Mol Cell Biol 24: 6891–6899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward JF 1994. The complexity of DNA damage: Relevance to biological consequences. Int J Radiat Biol 66: 427–432 [DOI] [PubMed] [Google Scholar]
- Weinfeld M, Soderlind KJ 1991. 32P-postlabeling detection of radiation-induced DNA damage: Identification and estimation of thymine glycols and phosphoglycolate termini. Biochemistry 30: 1091–1097 [DOI] [PubMed] [Google Scholar]
- White CI, Haber JE 1990. Intermediates of recombination during mating type switching in Saccharomyces cerevisiae. EMBO J 9: 663–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese C, Dray E, Groesser T, San Filippo J, Shi I, Collins DW, Tsai MS, Williams GJ, Rydberg B, Sung P, et al. 2007. Promotion of homologous recombination and genomic stability by RAD51AP1 via RAD51 recombinase enhancement. Mol Cell 28: 482–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, Kwon Y, Xu Y, Chung WH, Chi P, Niu H, Mayle R, Chen X, Malkova A, Sung P, et al. 2013. Pif1 helicase and Polδ promote recombination-coupled DNA synthesis via bubble migration. Nature 502: 393–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Hickson ID 2003. The Bloom’s syndrome helicase suppresses crossing over during homologous recombination. Nature 426: 870–874 [DOI] [PubMed] [Google Scholar]
- *.Wyatt HDM, West SC 2014. Holliday junction resolvases. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a016485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyman C, Kanaar R 2006. DNA double-strand break repair: All’s well that ends well. Annu Rev Genet 40: 363–383 [DOI] [PubMed] [Google Scholar]
- Wyrobek AJ, Schmid TE, Marchetti F 2005. Relative susceptibilities of male germ cells to genetic defects induced by cancer chemotherapies. J Natl Cancer Inst Monogr 34: 31–35 [DOI] [PubMed] [Google Scholar]
- Yang H, Li Q, Fan J, Holloman WK, Pavletich NP 2005. The BRCA2 homologue Brh2 nucleates RAD51 filament formation at a dsDNA–ssDNA junction. Nature 433: 653–657 [DOI] [PubMed] [Google Scholar]
- Yang Y, Sterling J, Storici F, Resnick MA, Gordenin DA 2008. Hypermutability of damaged single-strand DNA formed at double-strand breaks and uncapped telomeres in yeast Saccharomyces cerevisiae. PLoS Genet 4: e1000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Chung WH, Shim EY, Lee SE, Ira G 2008. Sgs1 helicase and two nucleases Dna2 and Exo1 resect DNA double-strand break ends. Cell 134: 981–994 [DOI] [PMC free article] [PubMed] [Google Scholar]