Significance
The merging of lipid bilayer membranes, or membrane fusion, is a ubiquitous process in cellular trafficking in eukaryotic cells. The responsible proteins have long been known to be the soluble N-ethylmaleimide–sensitive factor attachment protein receptors (SNAREs); however, key mechanistic details regarding how they work remain elusive. Among them is the issue of cooperativity, which asks how many SNAREs are needed for fusion to take place. Hitherto, reports have addressed this question in terms of fixed numbers, providing a rather static picture of how SNAREs operate. Using an elaborate kinetic analysis, we provide strong biochemical evidence showing that cooperativity is highly variable and will depend on the energy barrier of the membrane fusion reaction in question, implying SNAREs are much more modular and dynamic than previously thought.
Keywords: exocytosis, fusion intermediate, liposome docking
Abstract
The soluble N-ethylmaleimide–sensitive factor attachment protein receptor (SNARE) complex drives the majority of intracellular and exocytic membrane fusion events. Whether and how SNAREs cooperate to mediate fusion has been a subject of intense study, with estimates ranging from a single SNARE complex to 15. Here we show that there is no universally conserved number of SNARE complexes involved as revealed by our observation that this varies greatly depending on membrane curvature. When docking rates of small (∼40 nm) and large (∼100 nm) liposomes reconstituted with different synaptobrevin (the SNARE present in synaptic vesicles) densities are taken into account, the lipid mixing efficiency was maximal with small liposomes with only one synaptobrevin, whereas 23–30 synaptobrevins were necessary for efficient lipid mixing in large liposomes. Our results can be rationalized in terms of strong and weak cooperative coupling of SNARE complex assembly where each mode implicates different intermediate states of fusion that have been recently identified by electron microscopy. We predict that even higher variability in cooperativity is present in different physiological scenarios of fusion, and we further hypothesize that plasticity of SNAREs to engage in different coupling modes is an important feature of the biologically ubiquitous SNARE-mediated fusion reactions.
Membrane fusion is an essential reaction common to intracellular trafficking and exocytosis in eukaryotic cells. Although the process involves an intricate interplay of several proteins, the fusion of membranes is dependent on the conserved family of proteins known as soluble N-ethylmaleimide–sensitive factor attachment protein receptors, or SNAREs (1, 2). In the important case of the fusion of synaptic vesicles (SVs), the SNAREs responsible are vesicular synaptobrevin 2 (syb) and plasma membrane proteins SNAP-25A (SN25) and syntaxin-1A (syx). A critical intermediate seems to be an acceptor complex consisting of a three-helix bundle formed by a 1:1 syx:SN25 complex, which serves as a binding site for syb (3, 4). According to the zipper hypothesis, the N termini of syb and the 1:1 syx:SN25 complex nucleate to form a parallel four-helix bundle called the SNARE complex. The directional assembly then proceeds toward the C termini, resulting in a pulling force between the membranes that leads to their fusion (4, 5). There is some consensus that the highly exergonic nature of the assembly of the SNARE complex provides the energy for overcoming the barrier for fusion (6, 7), although identification of putative fusion intermediates at molecular resolution as well as force measurement experiments suggest multiple energy barriers are present (7–10).
The question of whether and how SNAREs cooperate to mediate fusion has received substantial attention. Although some studies have left open the possibility that the number of SNARE complexes that cooperate during fusion is variable (11, 12), much attention has been given to the notion of a preferred number of SNARE complexes, with estimates varying from a single SNARE complex (13) to 15 (14), although more recent estimates vary between two and eight (12, 15–18). Unfortunately, this large disparity in results has not been appropriately explained, and it remains unclear whether the differences are a result of inherent properties of the particular set of SNAREs involved or rather originate from the biophysical characteristics of the fusing vesicles.
A commonly used approach to investigate how SNAREs work is by reconstituting complementary SNAREs into liposomes (19). We have previously demonstrated that SNAREs can mediate both lipid and contents mixing with similar kinetics (13), an important functional criterion for establishing membrane fusion (20). Using the SNAREs responsible for exocytosis of SVs as a model, we investigate here how the density of SNAREs affects fusion of liposomes to obtain mechanistic information on cooperativity. A rigorous way to address this is to vary the SNARE density in one or both membranes. Whereas it is experimentally straightforward for syb, this approach is problematic for SN25 and syx because they tend to associate into “off-pathway” complexes, compromising kinetic analysis (6, 21). To circumvent this, Pobbati et al. (22) introduced a stabilized acceptor complex that contains a C-terminal peptide of synaptobrevin (syb 49–96) (22). This 1:1 syx:SN25 complex (herein referred to as the ∆N complex) is stable and contains a free binding site for syb, allowing one to precisely define its concentration. Therefore, we used the ∆N complex in our experiments to rule out the effect of any side reactions related to the assembly/disassembly of the acceptor complex and thus simplify the kinetic analysis on fusion.
Results
Theoretical Considerations for Investigating SNARE Cooperativity in Bulk Lipid Mixing Experiments.
We decided to investigate cooperativity in fusion with reconstituted SNARE liposomes by measuring lipid mixing via Förster resonance energy transfer (FRET) between two fluorophore-labeled lipid analogs over a wide SNARE density range (16, 19, 23–25). Although we additionally attempted content mixing assays, we found the assay to yield results that were too variable for the purpose of our strategy described below. Regardless of the chosen fusion indicator, an often neglected aspect of these bulk assays is that they are rate-limited by docking (26). Consequently, observed changes in the kinetics of lipid mixing may have been misleadingly attributed to fusion when they actually were the result of preceding docking steps.
One way around this is to accelerate the rate of docking and thus enable the kinetics of lipid mixing to directly report the effect that SNARE density has on fusion. In principle this could be done by exploiting the bimolecular dependence of liposome concentration on the rate of docking and increase its concentration. However, many studies already use high amounts of liposomes with lipid concentrations in the 0.1–3 mM range (e.g., refs. 19, 24, and 27–29 and studies cited therein), suggesting that slow docking is mainly the result of the inefficiency of the assembly of the first trans SNARE complex. To overcome this, SNARE complex formation can be enhanced by using the ∆N complex, which accelerates this process by at least 30-fold (26). In a dilute liposome regime (0.02–0.1 mM total lipid) we have previously found that with the ∆N complex the rate of docking is accelerated to an extent similar to the rate of fusion, a kinetic condition known as partial-rate limiting (8, 30).
Based on the above considerations, we designed experiments to measure the cooperativity of the actual fusion step in liposome-based lipid mixing assays. Our approach is centered on the principle of leveled docking rates across a series of reactions with different SNARE densities under partial rate-limiting conditions, thus allowing both docking and fusion to be kinetically resolved. To introduce this approach, we consider the rate equation for the generation of docked liposomes [D]:
| [1] |
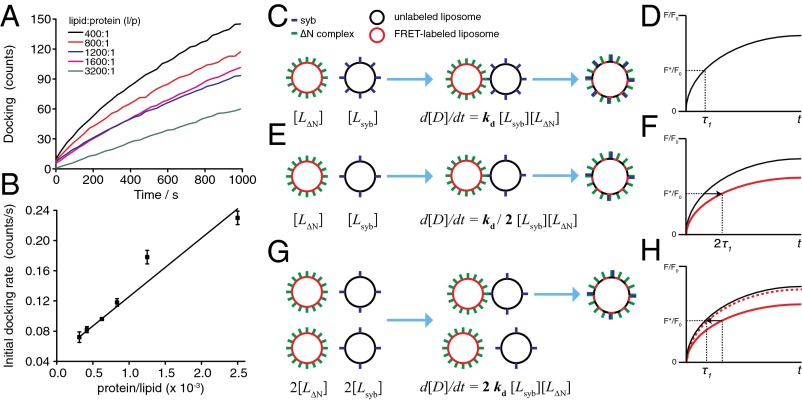
where [Lsyb] and [LΔN] are the concentrations of syb and ΔN complex liposomes, respectively, and kd(syb,ΔN) is the bimolecular docking constant, which is a function of both syb and ΔN complex density. To exclude that docking itself is not cooperative with regard to SNARE density, we used total internal reflection fluorescence (TIRF) microscopy, which can be used to distinguish docking and fusion of single liposomes (Fig. S1), to measure docking of liposomes containing varying concentrations of syb to a ΔN complex-containing supported lipid bilayer (Fig. 1A). This experiment revealed a linear (i.e., a noncooperative) relation between the initial docking rate and SNARE density (Fig. 1B). Having established noncooperative docking, Eq. 1 states that a decreased rate of docking resulting from a reduced SNARE density [a lower kd(syb,ΔN)] can be compensated by proportionally increasing the concentration of liposomes (a higher product of [Lsyb][LΔN]), thus leveling the original docking rate. Although it may initially seem counterintuitive, an analysis of Eq. 1 as presented in SI Materials and Methods shows that the amount by which concentrations of individual SNARE-liposomes should be increased to level docking rates is proportional to the change in kd(syb,ΔN), so that, for instance, both liposome concentrations need to be doubled if the density of only one of the SNAREs is reduced by one-half.
Fig. 1.
Schematic explanation of the principle of leveling docking for investigating SNARE-mediated cooperativity in fusion. (A) Small (∼40 nm) liposomes labeled with rhodamine-PE and containing syb at the indicated densities were added to a planar supported bilayer reconstituted with ΔN complex (l/p = 1,000:1). Docking was measured by the increase in total fluorescence intensity (counts) using TIRF microscopy (see ref. 18 and SI Materials and Methods for more details). Lines of best fit are presented. (B) Initial docking rates obtained by TIRF microscopy in A as a function of syb density, showing that docking is noncooperative within the range l/p = 400:1–3,200:1. Solid line: line of best fit (adjusted R2 = 0.94). Data are rates from fits of docking traces in A with error bars originating from fitting function. (C) Liposomes dock with a rate that depends on the concentrations of ΔN complex and syb liposomes ([LΔN] and [Lsyb], respectively), as well as a kinetic constant kd that itself depends on SNARE density. (D) If the total reaction rate is limited by docking, the recorded fluorescence signal F*/F0 for lipid mixing increases with the docking time constant τ1 (schematic trace shown). (E) Reducing the syb density by one-half will also reduce the docking rate by one-half of its original value. (F) Given that the docking reaction is still rate-limiting, the fluorescence signal F*/F0 now rises with a time constant of 2τ1, slowing down lipid mixing as schematically depicted in red. (G) This slowing down can be reversed by doubling both liposome concentrations (assuming halving the density does not affect fusion). The docking rate is now twice as fast as in C; however, because there are twice as many liposomes, the relative rate is now leveled to the original docking rate in C. (H) Thus, the relative lipid mixing normalized to the initial fluorescence F0 is unaffected, reestablishing the signal F*/F0 to the reference trace (schematically shown as a dashed red trace). The pattern of reducing syb density while increasing both liposome concentrations is continued iteratively until lipid mixing is no longer recovered from leveling docking rates, which would indicate reduced cooperativity with respect to fusion.
Using this principle of leveled docking rates, we prepared a set of syb liposomes over a wide range of syb densities and let them fuse with liposomes containing a fixed and excess amount of ΔN complex as schematically portrayed and explained in the legend for Fig. 1 C–H. Because ΔN complex is in excess, we will assume for now that all sybs within the vicinity of the first SNARE complex formed will readily find a binding partner and will initiate trans SNARE complex assembly. In the next two sections we describe experiments in which we applied this experimental approach to analyze and compare lipid mixing of small (∼40-nm diameter) and large (>80 nm) SNARE liposomes.
SNARE Density Has Negligible Effect on Membrane Fusion of Small Liposomes.
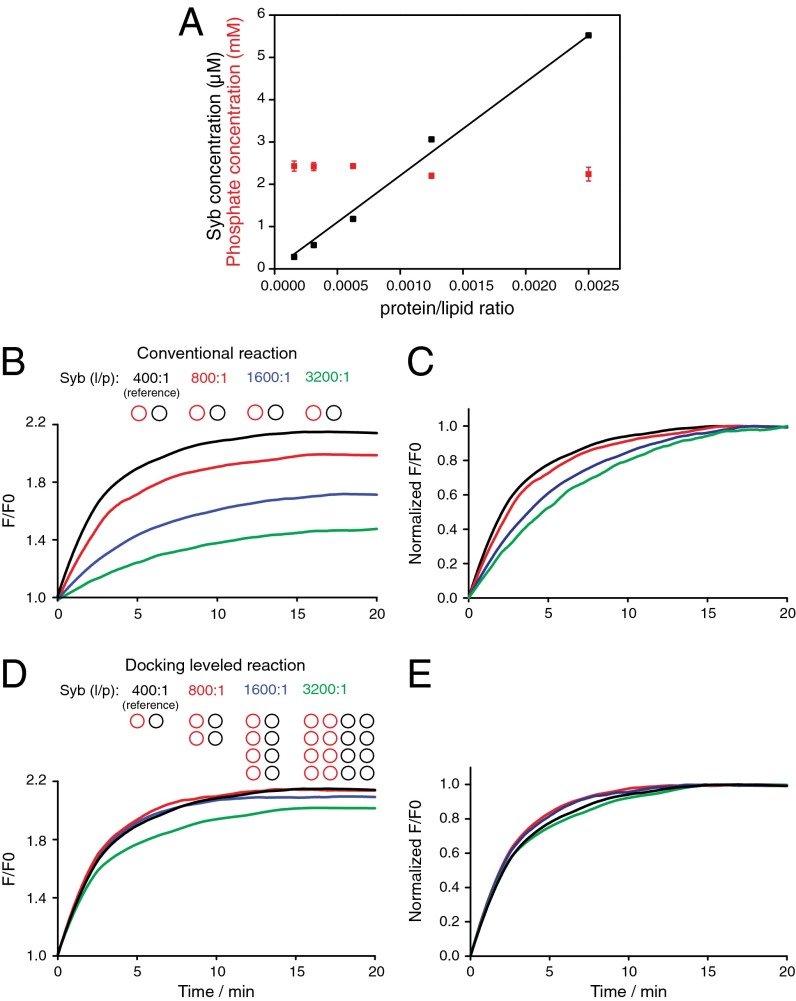
We prepared small liposomes with four different syb densities ranging from a lipid/protein (l/p) ratio of 400:1–3,200:1 (from now on denoted syb 400:1 and syb 3,200:1) and ΔN complex in relative excess (l/p = 300:1). We note that these densities are nominal and that the orientation of reconstituted syb is random whereas ΔN complex is predominantly right-side-out (13). Regardless of the absolute effective concentrations, it is essential that the syb concentrations are linearly scaled whereas the liposome concentrations of the different preparations are constant to quantitatively level docking rates. Quantification of syb by Western blot and of membrane lipid by assaying for liposome phosphate content confirm these requirements (Fig. 2A). We then measured lipid mixing in bulk for all densities as done conventionally by maintaining liposome concentrations unchanged. As expected, the lipid mixing levels decreased as the syb density was reduced (Fig. 2B), and the rate of lipid mixing was slowed as revealed by normalizing the traces to the final value of the reference reaction (Fig. 2C).
Fig. 2.
Leveled docking on small (∼40 nm) SNARE liposomes reveals negligible effect of SNARE density on lipid mixing. (A) A series of small syb liposomes were prepared at different syb densities and their syb protein (black) and phospholipid (red) contents were quantified by Western blot and organic phosphate determination (39). (B) Four small liposomes (unlabeled, black circles) with the depicted syb densities were mixed to ΔN complex liposomes (FRET-labeled, red circles, l/p = 300:1) at constant liposome concentration (conventional assay), showing a decline of lipid mixing as the syb density was decreased. (C) Same as B but normalized to the final F/F0 of the reference (black) trace after 20 min, showing that the lipid mixing rate is also reduced. (D) When liposome concentrations are increased in the relative amounts depicted, the leveling of docking causes almost all lipid mixing traces to converge to the reference level, indicating no effect of density on fusion. Only at nominal l/p = 3,200:1 is lipid mixing not fully recovered, suggesting a minor subpopulation of liposomes not having enough SNAREs for efficient fusion begins to emerge. (E) Normalization of traces in D to the final F/F0 of the reference trace (black) after 20 min reveals that the lipid mixing speeds are essentially identical.
When the same liposomes were mixed at increased leveled concentrations while SNARE densities were reduced, we observed the lipid mixing trace of the syb 800:1 liposomes converge to the reference reaction of the syb 400:1 liposomes (red trace in Fig. 2D). Thus, halving the syb density had no effect on lipid mixing, a behavior we confirmed with fusion measurements of single syb liposomes by TIRF microscopy (Fig. S2). Similarly, a fourfold reduction of syb density also had no effect on the efficiency of lipid mixing, further suggesting the reduction in lipid mixing in the conventional, not docking-leveled, reaction (Fig. 2B) is due to an effect on docking. However, when the syb density was reduced eightfold to l/p = 3,200:1, we observed a slight decline in efficiency as inferred from a partial and incomplete recovery of lipid mixing to the reference level (Fig. 2D). Interestingly, the lipid mixing speeds were the same for all reactions once docking rates were leveled, suggesting that efficiency but not speed begins to decrease at l/p 3,200:1 (Fig. 2E). Although we confirmed the validity of this approach by also varying the density of ΔN complex in small liposomes (Fig. S3), we will concentrate for the remainder of this study on the effect of syb density on liposome fusion.
Large Liposomes Require More SNAREs for Maximal Lipid Mixing Efficiency.
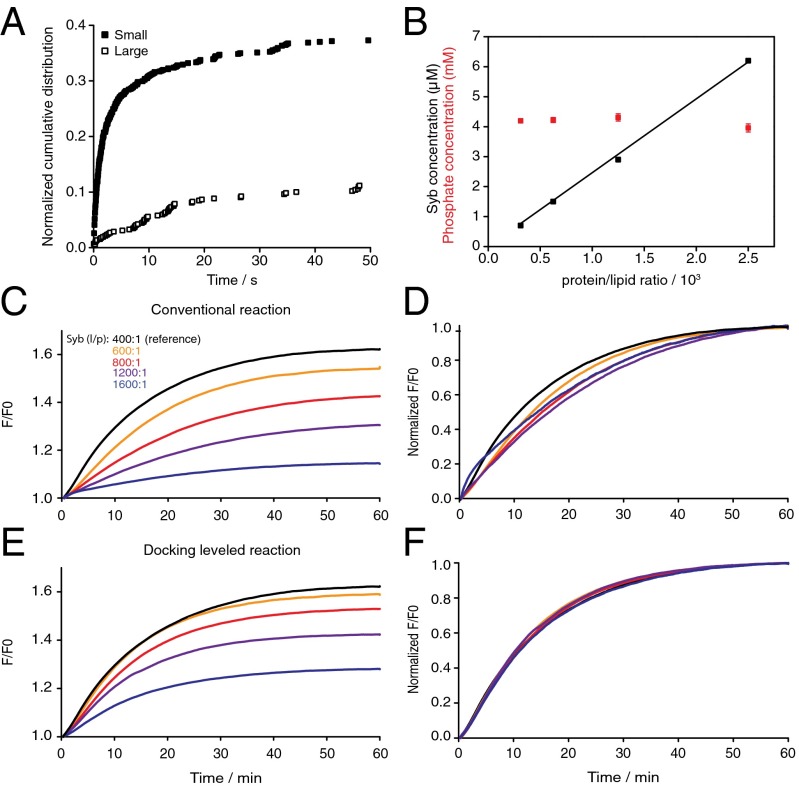
We next asked whether membrane curvature stress has an impact on the effect of SNARE density on lipid mixing. We prepared large (>80-nm diameter) liposomes that have approximately twofold less curvature than the small liposomes used previously (8). To rigorously compare the effect of curvature on fusion, we first recorded fusion single events by TIRF microscopy with small and large syb liposomes as they fuse with a planar supported bilayer with reconstituted ΔN complexes. This assay has the benefit that docking and fusion can be distinguished, allowing direct evaluation of the effect of curvature on fusion. Fig. 3A shows that large syb liposomes had a lower efficiency (two- to threefold) and speed (approximately sixfold) than small syb liposomes with similar l/p. Encouraged by this result, we conducted an extensive analysis of bulk liposome fusion measurements with large liposomes with different SNARE densities, similar to those described with small SNARE liposomes.
Fig. 3.
Lipid mixing on large (∼90 nm) SNARE liposomes is more sensitive to changes in SNARE density, indicating a higher requirement for SNARE cooperativity. (A) Cumulative distribution function obtained by TIRF microscopy of single fusion events showing small liposomes fuse more efficiently and rapidly than large syb liposomes (both at nominal l/p = 300:1) with a supported planar bilayer containing ΔN complex at l/p = 1,000:1. Lipid compositions of liposomes used for TIRF microscopy were quantitatively different from those used for bulk experiments (see SI Materials and Methods for details). (B) Syb protein concentration measured by Western blot (black) and phospholipid concentration (red) of four large liposome samples between the nominal ranges l/p = 400:1 and 3,200:1. (C) Lipid mixing of large labeled ΔN complex liposomes (l/p = 300:1) reacted with large syb liposomes at five different SNARE densities at constant liposome concentration (conventional assay). (D) Normalization of lipid mixing traces from C to the final F/F0 after 60 min of the reference reaction (black). (E) Syb and ΔN complex liposomes were reacted at same protein densities as in C, but liposome concentrations were increased proportionally to level docking rates revealing a partial recovery of lipid mixing. (F) The same traces as in E normalized to the final F/F0 after 60 min of the reference trace (black), indicating that the lipid mixing speeds are identical after docking is leveled.
As with small liposomes, the syb concentrations scaled linearly with density and the lipid phosphate concentrations remained constant over a wide range of SNARE densities (Fig. 3B). Liposome lipid mixing reactions performed in the conventional way (Fig. 3 C and D) and with leveled docking rates (Fig. 3 E and F) show that the lipid mixing levels were enhanced by increased syb density, but in almost all cases the enhancement did not restore lipid mixing to the reference level of syb 400:1. This shows that the relation between lipid mixing and SNARE density is distinct, with these larger liposomes and that SNARE densities become more limiting at much higher densities.
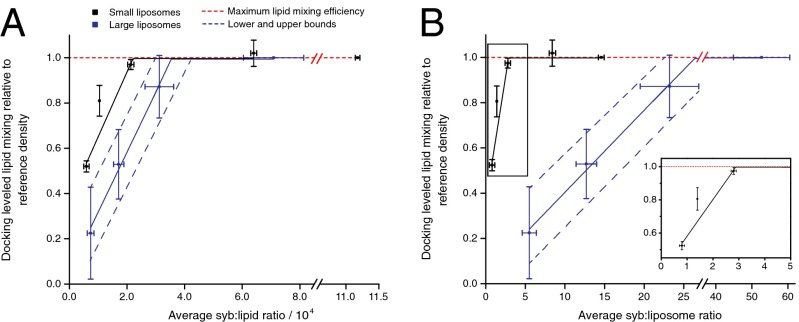
We repeated lipid mixing measurements for both small and large SNARE liposomes and plotted the final lipid mixing levels normalized to the lipid mixing of the reference density on a syb/lipid and syb/liposome basis (Fig. 4 A and B, respectively). These plots reveal the critical SNARE densities in both types of liposomes, below which the reactions can no longer be docking-rate leveled (i.e., the thresholds where SNARE densities become limiting for the fusion step in the overall reaction). The values in these plots are all corrected for total lipid and protein concentration as well as for the random orientation of syb on the liposomes. Therefore, syb/lipid or liposome values no longer match exactly between small and large liposomes. For both liposome sizes we noted two clearly distinguishable regions: a constant region at the higher SNARE densities where data points align closely to the dashed red line representing full lipid mixing efficiency and where lipid mixing is unaffected by changes in SNARE density and a sharply increasing linear region where lipid mixing decreases with increasing SNARE density up to the full efficiency level. The intersection between the two lines corresponds to the SNARE density below which lipid mixing efficiency is compromised. We refer to it as the lipid mixing efficiency threshold.
Fig. 4.
Quantitative comparison of lipid mixing of small and large liposomes provides insights on SNARE cooperativity. (A) The final lipid mixing value of all reactions normalized to the maximum lipid mixing efficiency (red dashed line) is plotted versus the corrected syb:lipid ratio (densities were converted from nominal l/p and from independently measured syb concentrations and phospholipid contents). Lines of best fit (solid lines) are shown for linearly increasing lipid mixing regions, with the intersection between the linear fit and the maximum efficiency (dashed horizontal line) denoting the lipid mixing threshold, below which fusion efficiency begins to be suboptimal. The threshold for large liposomes was treated as a range given the relatively high variability, with the dashed blue lines marking approximate upper and lower bounds. (B) The data of A replotted on a per-liposome basis to evaluate how lipid mixing varies when the total numbers of sybs per liposome are taken into account. For calculation we assumed a bilayer thickness of 4 nm and diameter of 40 nm for small liposomes and 90 nm for large liposomes obtained by light scattering (8) (see SI Materials and Methods for details). (Inset) A close-up view of the marked rectangular area showing the lipid mixing efficiency threshold on small liposomes. Ordinate error bars represent SDs from three to four independent experiments (including those shown in Figs. 2 B and C and 3 C and E), and abscissa error bars represent SDs from two to three organic phosphate determinations. Syb protein concentrations were measured by quantitative Western blot from one full series of SNARE liposomes and divided by 2 to account for the random orientation of sybs across the membrane (8, 13).
We observed that large liposomes require 1.5–3 times more sybs per lipid, that is, about 1 syb per 2,000 nm2 or 500 sybs per square micrometer, to reach the lipid mixing level of small liposomes. This is evident from the differences in both the slope of the linear regions and the lipid mixing efficiency threshold (see Fig. 4A for details). When the same comparison is made on a syb-per-liposome basis, the differences are even greater: Approximately three sybs are required to reach maximal lipid mixing efficiency of small liposomes, but 23–30 sybs are required to reach this threshold with large liposomes (Fig. 4B). These results strongly suggest that a greater number of SNAREs is needed to overcome the higher energy barrier for efficient fusion of the larger liposomes.
SNARE Depletion on Small Liposomes Is Responsible for Loss in Lipid Mixing Efficiency, Whereas High Threshold on Large Liposomes May Be Explained by Weak or Tight Cooperative Coupling.
Our results so far uncover a substantial difference in the ability of SNAREs to mediate lipid mixing on small and large liposomes, a behavior we attribute to the rapid and nonlinear reduction in curvature stress as liposome size is decreased (8). Still, we did not anticipate the curvature dependency of SNARE density threshold for efficient lipid mixing to be so large. How many SNAREs are then required to fuse small liposomes? Although at first sight the threshold would seem to suggest three (Fig. 4A), it is important to note that this is an average. SNAREs are distributed on liposomes with a random Poisson distribution, a feature that becomes more important to consider at very low SNARE densities (13). It has been shown that on small liposomes with an l/p of 8,000:1 and 16,000:1 (when taking into account externally orientated sybs) 25% and 45% of liposomes are without any sybs, respectively. This correlates well with our observation that at a corrected l/p of 9,500:1 and 17,000:1 (or an equivalent syb/lipid of 1.0 and 0.6 × 10−4 in Fig. 4A) the lipid mixing amount is 80% and 50% of the maximum, respectively. Thus, it is likely that the decrease in lipid mixing on small liposomes is not due to a diminishing cooperative effect as the SNARE density is reduced, but simply to the appearance of liposomes depleted of SNAREs. This strongly supports the conclusion that, at least for small liposomes, one SNARE complex is necessary and sufficient for fusion and is therefore noncooperative (13).
To evaluate whether SNARE depletion also affects the threshold on large liposomes, we simulated a random distribution of sybs and found that the population of large liposomes without any sybs is negligible at the lipid mixing efficiency threshold. Therefore, it cannot explain the drop in lipid mixing efficiency (Fig. S4). Because the lipid mixing threshold occurs at around 23–30 sybs per liposome (assuming an average diameter of 90 nm), the question arises as to whether these sybs are already locally present at initial membrane contact [either instantaneously or recruited by rapid diffusion before fusion is first observed (31)] or whether they are dispersed over a greater contact area. To gain more insight as to what may be occurring upon membrane contact, we constructed a simple geometric model to estimate the number of neighboring sybs that are present and within reach at the time when the first trans SNARE complex is formed (Fig. S5). Using several physical constraints (32) we find that two to four sybs (of the 23–30 sybs present) would be instantly within reach to form SNARE complexes (Table S1 and SI Materials and Methods). However, we cannot discard that more SNARE complexes may be recruited with or without expansion of the contact zone before fusion is experimentally observed.
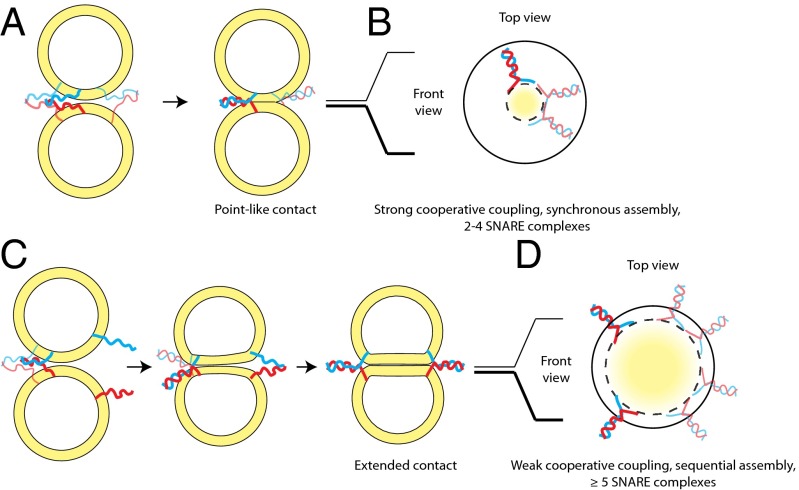
Based on these considerations, we suggest two models: (i) Fusion initiates at a single contact site and requires a localized and a synchronous cooperative assembly of SNAREs requiring only a subset of the 23–30 sybs (Fig. 5 A and B) or (ii) fusion involves an expanded contact site that forms over time as a larger number of cooperating SNAREs are recruited over larger distances (Fig. 5 C and D).
Fig. 5.
Cooperativity in SNARE-mediated fusion of large liposomes may be rationalized with two distinct coupling modes and may be further related to the morphology of the prefusion intermediate. (A) Strong cooperative coupling. If a sufficient number of SNAREs are locally present at the moment of the assembly of the first trans SNARE complex, additional SNARE complexes assemble in a near-synchronous manner. We refer to this scenario as strong coupling between SNARE complexes owing to the exerted force being focused on a point-like contact zone, which initiates membrane fusion. SNARE complexes drawn with bolder/fainter lines denote those oriented toward/away from the observer to give a 3D impression of the spatial arrangement. (B) Projection through membrane contact zone of A depicting the localized organization of the SNARE complexes in a strongly coupled mode. Based on the lipid mixing efficiency threshold on large liposomes and a simple geometric model (Fig. S5), we estimate two to four SNARE complexes could assemble in this mode. This scenario results in the formation of local deformations or point-like protrusions of the membranes, as supported by very recent ultrastructural evidence (35). (C) Weak cooperative coupling. When SNAREs are sparsely distributed in the membrane, the assembly of the first trans SNARE complex brings two extended bilayers together but does not immediately fuse them because it does not provide sufficient energy for surpassing the energy barrier. Additional SNAREs may be recruited from further away to assemble into complexes and exert force between the membranes over an extended contact area. We refer to this scenario as the weakly coupled cooperative mode. This weak coupling produces an extended contact zone between opposing membranes, a state that has been observed by cryo-electron microscopy of large liposomes (8, 9). (D) Projection through the membrane contact zone of C depicting the spatial organization of the SNARE complexes in the weakly coupled cooperativity. The dashed circle denotes the projected borders of the contact area between the membranes, and the solid circle is the outer diameter of the liposomes.
Discussion
The systematic comparison of the fusion of small and large SNARE liposomes presented here leads us to propose mechanistic features of SNARE-mediated fusion that deserve further attention. We have uncovered a high variability in cooperativity in a controlled in vitro system where the curvature of the membranes is decreased only two- to threefold, leading us to presuppose that in vivo even greater variability is to be expected. Our results suggest that when the energy barrier is low, as in highly curved small liposomes, one SNARE complex can suffice. However, fusion between large liposomes or in fusion of small liposomes with a planar membrane more SNAREs are needed to work in a cooperative manner to overcome the higher barrier. Thus, there seems to be no mechanistic requirement for a specific supramolecular rearrangement of SNAREs at the site of fusion. Because we observe this high variability with neuronal SNAREs at constant density but different curvature, a corollary of this conclusion is that we would expect that cooperativity of SNAREs involved in other trafficking pathways will also be highly variable, not because of their inherent biochemical properties, but rather owing to the particular biophysical characteristics of the trafficking vesicle. We therefore suggest that the reported estimates of numbers of involved SNARE complexes likely reflect the particular type of fusion studied and that there is no universally conserved number, as has been often assumed (13, 15–18, 33).
Our results highlight the importance of correcting for docking rates to uncover cooperativity in bulk liposome fusion experiments. In a recent study, Shi et al. (16) used a lipid mixing and a content release assay to investigate cooperativity in SNARE-mediated liposome-to-nanodisk fusion. As the authors pointed out, the rate-limiting step in these systems is docking due to slow initiation of SNARE complex assembly. Shi et al. (16) found their lipid mixing unchanged (in both efficiency and speed) even after a ninefold change in syb density, which is surprising because such behavior is incompatible with a docking rate-limited step. Content release, however, roughly decreased as syb density was reduced, a finding that was attributed to a requirement for several sybs to keep the fusion pore open. Although the observation that the lipid mixing dependence on SNARE density was unaltered remains to be explained, a simpler explanation for the SNARE density-dependent content release is that this behavior was due to slower docking rates at the lower SNARE densities.
Our observation that the efficiency threshold of 23–30 sybs for the fusion of large liposomes leaves open questions about how cooperativity operates. What is, then, the mechanistic interpretation of this high number? The efficiency threshold definition we have used here refers to the total number of sybs on a liposome at the point at which we observe a decrease in fusion (after taking docking into account). Our assumption has been that sybs adjacent to the first trans SNARE complex would readily form additional SNARE complexes (because ΔN complex is present in excess). However, at 23–30 sybs this assumption is not straightforward to apply owing to steric factors. Furthermore, estimates of the number of SNAREs involved in the homotypic fusion of early endosomes and the exocytosis of chromaffin granules are much lower than the total number of SNAREs available, suggesting that only local subsets of SNAREs take part in fusion (12, 34).
Based on these considerations, we have proposed two alternative mechanistic interpretations to explain our observations. In the first model, we suggest that although the threshold occurs at 23–30 sybs on the entire surface of the liposome this is the total amount required to locally enrich SNARE complexes, a process that implies stronger coupling and time synchronization in the cooperative assembly of the SNARE complexes. We have modeled a possible scenario and found that two to four SNARE complexes assembled in synchrony would fit in between two large liposomes as contact between membranes is initiated, in agreement with in vivo studies (12, 15, 33). However, we caution that this is a lower bound estimate because we cannot rule out lateral diffusion of neighboring SNAREs. It is interesting to note that this synchronous, strong cooperative coupling between SNARE complexes implies that membrane fusion is confined to small contact areas leading to protrusions (Fig. 5 A and B), a feature that has been recently identified by electron microscopy (35).
The second model we have considered involves SNARE complexes assembling at not one but multiple sites, which entails a weaker mode of cooperativity that operates over longer distances (Fig. 5 C and D). Recent electron microscopy studies have depicted contact zones as large as 90 nm in diameter, which could accommodate the assembly of greater numbers of SNARE complexes (8, 9). Interestingly, the morphology of this putative intermediate resembles very closely the vertex ring observed on yeast vacuoles, begging the question as to whether a feature of weak cooperative coupling is to generate extended docking intermediates such as those found in slower constitutive forms of fusion (36). In this regard, cooperativity may also critically depend on the required speed of a particular fusion reaction, an idea that we have not explored in the current work but that has been conceived in the context of dense core vesicle fusion in chromaffin cells (12). It is plausible that, for example, synaptic vesicle fusion, which requires fast speed, might proceed via a mechanism requiring much stronger coupling and thus higher cooperativity between SNAREs than, for instance, slow vacuolar fusion.
We recognize possible limitations arising from the use of the ΔN complex, which we used here to quantitatively fix the 1:1 syx:SN25 acceptor complex. In particular, the displacement of the syb 49–96 peptide requires overcoming a high activation energy barrier that reduces the force transmitted to the membranes (8). Although this does not alter our conclusion that cooperativity is variable, it does imply that the cooperativity threshold between large liposomes might be overestimated. Another factor to consider is that the displacement of the fragment may be rate-limiting and thereby hide cooperativity with regards to the speed of lipid mixing (see, for example, Figs. 2E and 3F). Thus, the cooperativity we have uncovered specifically refers to the efficiency of lipid mixing and it is understood that we cannot rule out another level of cooperativity with respect to speed.
Nevertheless, despite these limitations, we also note that the use of the ΔN complex in our study may have unraveled an otherwise occluded cooperativity behavior. For instance, when using full-length SNAREs, only a very low percentage of large SNARE liposomes undergo fusion and instead remain either stably docked or hemifused even at very high l/p ratios of 50 and 200:1 (37, 38), a finding we have also observed in our bulk assays. It is not clear why this is the case, but the observation that synaptotagmin 1 and Ca2+ substantially enhances fusion by presumably lowering the activation energy of certain intermediates might suggest that the energy barriers in these systems are higher than in ours. However, an alternative explanation may be that synchronized cooperative assembly of SNAREs is jeopardized by the dynamic generation of off-pathway 2:1 syx:SN25 acceptor complexes. This would reduce the effective concentration of 1:1 acceptor complexes at the site of fusion and desynchronize the strong cooperative coupling between complexes required for the execution of fusion.
Materials and Methods
Protein and liposome preparations, as well as TIRF measurements, have been previously described (8, 18). Bulk lipid mixing was measured using the standard NBD/rhodamine fluorescence dequenching assay (22). Experimental details, including all theoretical aspects developed in this work, are presented in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank F. A. Pérez-Lara, P. Halder, S. Jamshidi, E. Neher, S. Pantano, A. Stein, and G. Rivas for useful discussions and critical reading of the manuscript. This work was supported by Grant P01 GM72694 from the National Institutes of Health (to L.K.T. and R.J.), and by a grant from the Deutsche Forschungsgemeinschaft (SFB 803; to R.J.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1407435111/-/DCSupplemental.
References
- 1.Martens S, McMahon HT. Mechanisms of membrane fusion: Disparate players and common principles. Nat Rev Mol Cell Biol. 2008;9(7):543–556. doi: 10.1038/nrm2417. [DOI] [PubMed] [Google Scholar]
- 2.Südhof TC, Rothman JE. Membrane fusion: Grappling with SNARE and SM proteins. Science. 2009;323(5913):474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fasshauer D, Margittai M. A transient N-terminal interaction of SNAP-25 and syntaxin nucleates SNARE assembly. J Biol Chem. 2004;279(9):7613–7621. doi: 10.1074/jbc.M312064200. [DOI] [PubMed] [Google Scholar]
- 4.Jahn R, Scheller RH. SNAREs—engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7(9):631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 5.Jahn R, Fasshauer D. Molecular machines governing exocytosis of synaptic vesicles. Nature. 2012;490(7419):201–207. doi: 10.1038/nature11320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fasshauer D, Antonin W, Subramaniam V, Jahn R. SNARE assembly and disassembly exhibit a pronounced hysteresis. Nat Struct Biol. 2002;9(2):144–151. doi: 10.1038/nsb750. [DOI] [PubMed] [Google Scholar]
- 7.Gao Y, et al. Single reconstituted neuronal SNARE complexes zipper in three distinct stages. Science. 2012;337(6100):1340–1343. doi: 10.1126/science.1224492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hernandez JM, et al. Membrane fusion intermediates via directional and full assembly of the SNARE complex. Science. 2012;336(6088):1581–1584. doi: 10.1126/science.1221976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diao J, et al. Synaptic proteins promote calcium-triggered fast transition from point contact to full fusion. eLife. 2012;1:e00109. doi: 10.7554/eLife.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Min D, et al. Mechanical unzipping and rezipping of a single SNARE complex reveals hysteresis as a force-generating mechanism. Nat Commun. 2013;4:1705. doi: 10.1038/ncomms2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Domanska MK, Kiessling V, Tamm LK. Docking and fast fusion of synaptobrevin vesicles depends on the lipid compositions of the vesicle and the acceptor SNARE complex-containing target membrane. Biophys J. 2010;99(9):2936–2946. doi: 10.1016/j.bpj.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohrmann R, de Wit H, Verhage M, Neher E, Sørensen JB. Fast vesicle fusion in living cells requires at least three SNARE complexes. Science. 2010;330(6003):502–505. doi: 10.1126/science.1193134. [DOI] [PubMed] [Google Scholar]
- 13.van den Bogaart G, et al. One SNARE complex is sufficient for membrane fusion. Nat Struct Mol Biol. 2010;17(3):358–364. doi: 10.1038/nsmb.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montecucco C, Schiavo G, Pantano S. SNARE complexes and neuroexocytosis: How many, how close? Trends Biochem Sci. 2005;30(7):367–372. doi: 10.1016/j.tibs.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Sinha R, Ahmed S, Jahn R, Klingauf J. Two synaptobrevin molecules are sufficient for vesicle fusion in central nervous system synapses. Proc Natl Acad Sci USA. 2011;108(34):14318–14323. doi: 10.1073/pnas.1101818108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi L, et al. SNARE proteins: One to fuse and three to keep the nascent fusion pore open. Science. 2012;335(6074):1355–1359. doi: 10.1126/science.1214984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karatekin E, et al. A fast, single-vesicle fusion assay mimics physiological SNARE requirements. Proc Natl Acad Sci USA. 2010;107(8):3517–3521. doi: 10.1073/pnas.0914723107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Domanska MK, Kiessling V, Stein A, Fasshauer D, Tamm LK. Single vesicle millisecond fusion kinetics reveals number of SNARE complexes optimal for fast SNARE-mediated membrane fusion. J Biol Chem. 2009;284(46):32158–32166. doi: 10.1074/jbc.M109.047381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weber T, et al. SNAREpins: Minimal machinery for membrane fusion. Cell. 1998;92(6):759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- 20.Rizo J, Chen X, Araç D. Unraveling the mechanisms of synaptotagmin and SNARE function in neurotransmitter release. Trends Cell Biol. 2006;16(7):339–350. doi: 10.1016/j.tcb.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 21.Xiao W, Poirier MA, Bennett MK, Shin YK. The neuronal t-SNARE complex is a parallel four-helix bundle. Nat Struct Biol. 2001;8(4):308–311. doi: 10.1038/86174. [DOI] [PubMed] [Google Scholar]
- 22.Pobbati AV, Stein A, Fasshauer D. N- to C-terminal SNARE complex assembly promotes rapid membrane fusion. Science. 2006;313(5787):673–676. doi: 10.1126/science.1129486. [DOI] [PubMed] [Google Scholar]
- 23.Chen X, et al. SNARE-mediated lipid mixing depends on the physical state of the vesicles. Biophys J. 2006;90(6):2062–2074. doi: 10.1529/biophysj.105.071415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ji H, et al. Protein determinants of SNARE-mediated lipid mixing. Biophys J. 2010;99(2):553–560. doi: 10.1016/j.bpj.2010.04.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xue M, Ma C, Craig TK, Rosenmund C, Rizo J. The Janus-faced nature of the C(2)B domain is fundamental for synaptotagmin-1 function. Nat Struct Mol Biol. 2008;15(11):1160–1168. doi: 10.1038/nsmb.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith EA, Weisshaar JC. Docking, not fusion, as the rate-limiting step in a SNARE-driven vesicle fusion assay. Biophys J. 2011;100(9):2141–2150. doi: 10.1016/j.bpj.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen J, Tareste DC, Paumet F, Rothman JE, Melia TJ. Selective activation of cognate SNAREpins by Sec1/Munc18 proteins. Cell. 2007;128(1):183–195. doi: 10.1016/j.cell.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 28.Parlati F, et al. Rapid and efficient fusion of phospholipid vesicles by the alpha-helical core of a SNARE complex in the absence of an N-terminal regulatory domain. Proc Natl Acad Sci USA. 1999;96(22):12565–12570. doi: 10.1073/pnas.96.22.12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma C, Su L, Seven AB, Xu Y, Rizo J. Reconstitution of the vital functions of Munc18 and Munc13 in neurotransmitter release. Science. 2013;339(6118):421–425. doi: 10.1126/science.1230473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cypionka A, et al. Discrimination between docking and fusion of liposomes reconstituted with neuronal SNARE-proteins using FCS. Proc Natl Acad Sci USA. 2009;106(44):18575–18580. doi: 10.1073/pnas.0906677106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bacia K, Schuette CG, Kahya N, Jahn R, Schwille P. SNAREs prefer liquid-disordered over “raft” (liquid-ordered) domains when reconstituted into giant unilamellar vesicles. J Biol Chem. 2004;279(36):37951–37955. doi: 10.1074/jbc.M407020200. [DOI] [PubMed] [Google Scholar]
- 32.Li F, et al. Energetics and dynamics of SNAREpin folding across lipid bilayers. Nat Struct Mol Biol. 2007;14(10):890–896. doi: 10.1038/nsmb1310. [DOI] [PubMed] [Google Scholar]
- 33.Hua Y, Scheller RH. Three SNARE complexes cooperate to mediate membrane fusion. Proc Natl Acad Sci USA. 2001;98(14):8065–8070. doi: 10.1073/pnas.131214798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bethani I, et al. Endosomal fusion upon SNARE knockdown is maintained by residual SNARE activity and enhanced docking. Traffic. 2009;10(10):1543–1559. doi: 10.1111/j.1600-0854.2009.00959.x. [DOI] [PubMed] [Google Scholar]
- 35.Bharat TA, et al. SNARE and regulatory proteins induce local membrane protrusions to prime docked vesicles for fast calcium-triggered fusion. EMBO Rep. 2014;15(3):308–314. doi: 10.1002/embr.201337807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang L, Seeley ES, Wickner W, Merz AJ. Vacuole fusion at a ring of vertex docking sites leaves membrane fragments within the organelle. Cell. 2002;108(3):357–369. doi: 10.1016/s0092-8674(02)00632-3. [DOI] [PubMed] [Google Scholar]
- 37.Kyoung M, et al. In vitro system capable of differentiating fast Ca2+-triggered content mixing from lipid exchange for mechanistic studies of neurotransmitter release. Proc Natl Acad Sci USA. 2011;108(29):E304–E313. doi: 10.1073/pnas.1107900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lai Y, et al. Fusion pore formation and expansion induced by Ca2+ and synaptotagmin 1. Proc Natl Acad Sci USA. 2013;110(4):1333–1338. doi: 10.1073/pnas.1218818110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rouser G, Fkeischer S, Yamamoto A. Two dimensional then layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 1970;5(5):494–496. doi: 10.1007/BF02531316. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.