Significance
For nearly a century, processes have been used to convert biomass-derived carbohydrates, such as glucose, into fuels and chemicals. However, plant cell walls also contain an aromatic polymer, lignin, that has not been cost-effectively converted into fuels or commodity chemicals. With the intensive development of lignocellulosic biorefineries around the world to produce fuels and chemicals from biomass-derived carbohydrates, the amount of waste lignin will dramatically increase, warranting new lignin upgrading strategies. In nature, some microorganisms have evolved pathways to catabolize lignin-derived aromatics. Our work demonstrates that the utilization of these natural aromatic catabolic pathways may enable new routes to overcome the lignin utilization barrier that, in turn, may enable a broader slate of molecules derived from lignocellulosic biomass.
Keywords: biofuels, lignocellulose, biorefinery, aromatic degradation
Abstract
Lignin is an energy-dense, heterogeneous polymer comprised of phenylpropanoid monomers used by plants for structure, water transport, and defense, and it is the second most abundant biopolymer on Earth after cellulose. In production of fuels and chemicals from biomass, lignin is typically underused as a feedstock and burned for process heat because its inherent heterogeneity and recalcitrance make it difficult to selectively valorize. In nature, however, some organisms have evolved metabolic pathways that enable the utilization of lignin-derived aromatic molecules as carbon sources. Aromatic catabolism typically occurs via upper pathways that act as a “biological funnel” to convert heterogeneous substrates to central intermediates, such as protocatechuate or catechol. These intermediates undergo ring cleavage and are further converted via the β-ketoadipate pathway to central carbon metabolism. Here, we use a natural aromatic-catabolizing organism, Pseudomonas putida KT2440, to demonstrate that these aromatic metabolic pathways can be used to convert both aromatic model compounds and heterogeneous, lignin-enriched streams derived from pilot-scale biomass pretreatment into medium chain-length polyhydroxyalkanoates (mcl-PHAs). mcl-PHAs were then isolated from the cells and demonstrated to be similar in physicochemical properties to conventional carbohydrate-derived mcl-PHAs, which have applications as bioplastics. In a further demonstration of their utility, mcl-PHAs were catalytically converted to both chemical precursors and fuel-range hydrocarbons. Overall, this work demonstrates that the use of aromatic catabolic pathways enables an approach to valorize lignin by overcoming its inherent heterogeneity to produce fuels, chemicals, and materials.
Lignocellulosic biomass represents a vast resource for the production of renewable fuels and chemicals to offset global fossil fuel use. For decades, research has been undertaken to develop processes for valorizing plant polysaccharides cellulose and hemicellulose (1–8). Lignin, an alkyl-aromatic polymer comprising 15–30% of biomass, is typically underused in selective conversion processes and is instead relegated for heat and power (2, 5, 6, 8). The need for lignin utilization is a problem of growing urgency because production of waste lignin will soar with the commercialization of lignocellulosic biofuels. The inability to valorize lignin, despite being the most energy dense polymer in plant cell walls because of its higher C-to-O ratio relative to carbohydrates, is due to its inherent heterogeneity and recalcitrance. Lignin is composed of three monomeric phenylpropanoid units connected by C-C and C-O bonds (9). Although lignin depolymerization has been studied across a broad range of catalytic, thermal, and biological routes (10, 11), the product slate obtained from depolymerization is almost invariably heterogeneous, making lignin valorization a daunting challenge.
In nature, some fungi and bacteria depolymerize lignin by using powerful oxidative enzymes (10, 12–14). This pool of aromatic compounds present during biomass decomposition likely triggered evolution of microbial pathways for using aromatic molecules as carbon sources. Many aromatic-catabolizing organisms use “upper pathways,” wherein a diverse battery of enzymes “funnels” aromatic molecules to central intermediates, such as catechol and protocatechuate (Fig. 1). From these intermediates, dioxygenase enzymes cleave carbon-carbon bonds in the aromatic rings to produce ring-opened species (15–18) that are metabolized via the β-ketoadipate pathway to central carbon metabolism (19, 20), thus enabling microorganisms to metabolize a broad range of aromatic species. These pathways have long been studied for their catalytic novelty in C-C bond cleavage in aromatic rings (15–17) and highlight the ability for microbes to evolve mechanisms for xenobiotic catabolism, often driven by man-made pollution (18). From a biomass conversion standpoint, these upper pathways offer a direct, versatile approach to funnel the heterogeneous portfolio of molecules produced from lignin depolymerization to targeted intermediates for upgrading to fuels, chemicals, and materials.
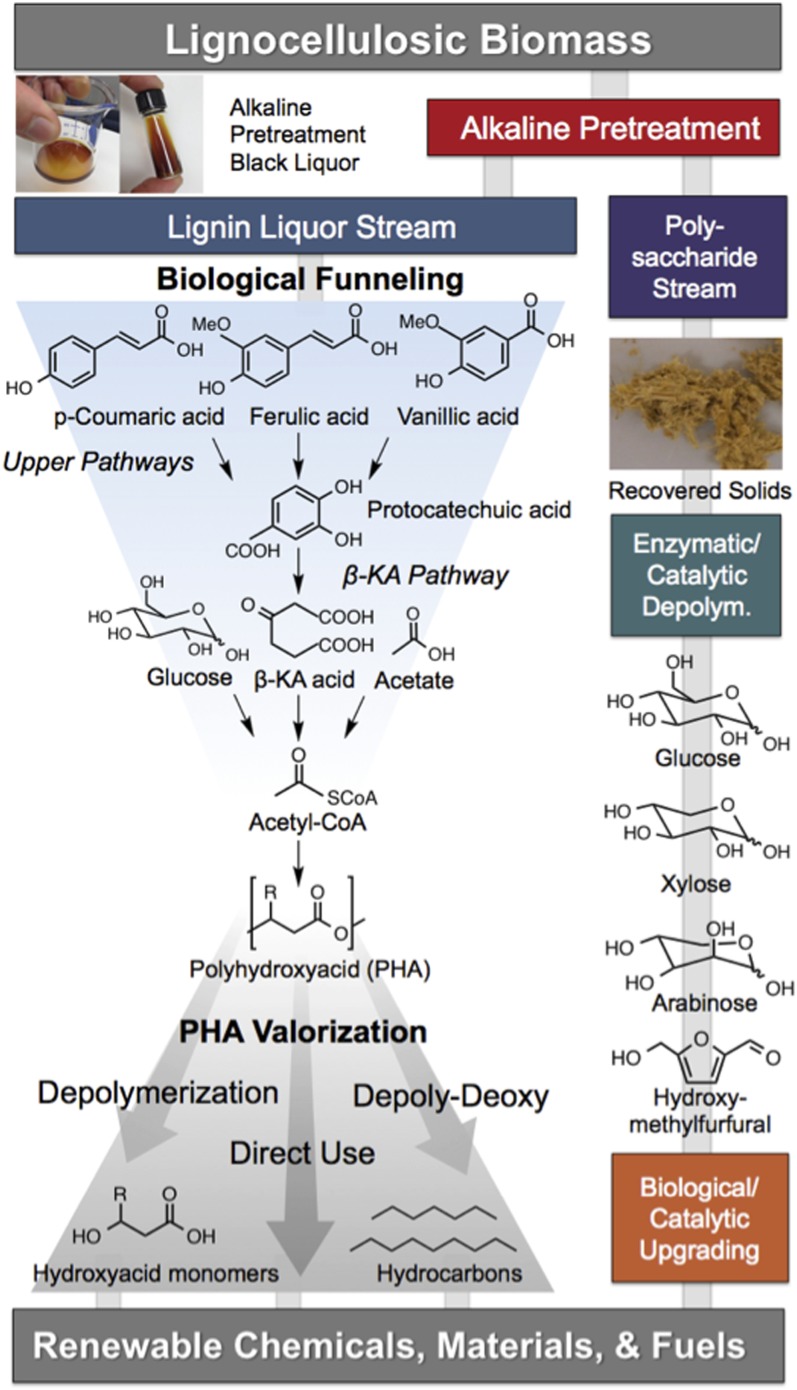
Fig. 1.
Integrated production of fuels, chemicals, and materials from biomass-derived lignin via natural aromatic catabolic pathways and chemical catalysis. Biomass fractionation can yield streams enriched in lignin and polysaccharides, which can be converted along parallel processes. The challenges associated with lignin’s heterogeneity are overcome in a “biological funneling” process through upper pathways that produce central intermediates (e.g., protocatechuic acid). Dioxygenases cleave the aromatic rings of these intermediates, which are metabolized through the β-ketoadipate (β-KA) pathway to acetyl-CoA. As shown, residual glucose and acetate present will also be metabolized to acetyl-CoA, the primary entry point to mcl-PHA production via fatty acid synthesis. We demonstrate mcl-PHA production, which are biodegradable polymers. mcl-PHAs are converted to alkenoic acids, and further depolymerized and deoxygenated (“depoly-deoxy”) into hydrocarbons, thus demonstrating the production of fuels, chemicals, and materials from lignin and other biomass-derived substrates.
As a demonstration of the potential of the aromatic catabolic pathways for lignin valorization, here we use an aromatic-catabolizing bacterium with a diverse metabolic repertoire (21), Pseudomonas putida KT2440 (hereafter P. putida), to produce medium chain-length (C6-C14) polyhydroxyalkanoates (mcl-PHAs) from lignin in an integrated process (Fig. 1). mcl-PHAs are high-value polymers that can serve as plastics or adhesives (22), or can be depolymerized and converted to chemical precursors (23) or methyl-ester–based fuels (e.g., biodiesel) (24). In P. putida, mcl-PHAs can be generated through fatty acid synthesis via the central metabolite, acetyl-CoA (25). To produce lignin-enriched streams for mcl-PHA production via biological funneling, pilot-scale alkaline pretreatment was used to depolymerize lignin from biomass to the aqueous phase (Fig. 1). The pretreated solids, which mainly consist of polysaccharides, can be valorized through known routes such as enzymatic hydrolysis and fermentation or via catalytic routes (2–4, 7). The resulting alkaline pretreated liquor (APL) was fed to P. putida, which under nitrogen depletion induces mcl-PHA production. After biological conversion of APL, the cells were harvested and the mcl-PHAs were extracted and characterized. We further demonstrate depolymerization of mcl-PHAs to alkenoic acids, which are precursors for diverse chemical applications. Subsequently, a bimetallic catalyst was used to convert alkenoic acids to alkanes. Overall, this study illustrates the concept of coupling upstream lignin depolymerization and downstream catalysis to a biological funneling by using bacterial aromatic catabolic pathways to overcome the intrinsic heterogeneity of lignin.
Results
To obtain a process stream enriched in lignin-derived compounds, alkaline pretreatment was first used to produce APL by using corn stover in a 1900-L pretreatment vessel with 70 mg of NaOH/g dry biomass. Anthraquinone was also cocharged to the reactor at a loading of 0.2% (wt/wt), which serves to maximize polysaccharide retention in the solids via minimization of polysaccharide “peeling” reactions (26). From pretreatment, 56% of the lignin is fractionated to the APL, whereas 95% of the glucan and 81% of the xylan are retained in the solids. Based on mass closure, the aqueous fraction of APL consists mostly of lignin, extractives, inorganic components, and acetate at 32%, 23%, 11%, and 8% wt/wt, respectively (SI Appendix, Fig. S1). The APL molecular mass distribution consists of major peaks at 200, 250, and 350 Da, suggesting that most APL components are monomers, dimers, and trimers (SI Appendix, Fig. S2). Primary components include p-coumaric acid, vanillic acid, ferulic acid, and acetate among others (SI Appendix, Table S1 and Fig. S3). Glucose, which is the only biomass-derived sugar in APL utilizable by P. putida KT2440, is present at only 0.13 g/L. The residual solids are enriched in polysaccharides and are readily digestible by an industrial cellulase mixture. In particular, ∼90% glucan conversion and greater than 50% xylan conversion are reached within 48 h by using the Novozymes CTec2 mixture, providing a stream for parallel carbohydrate upgrading through established routes (Fig. 1 and SI Appendix, Fig. S4) (1–7).
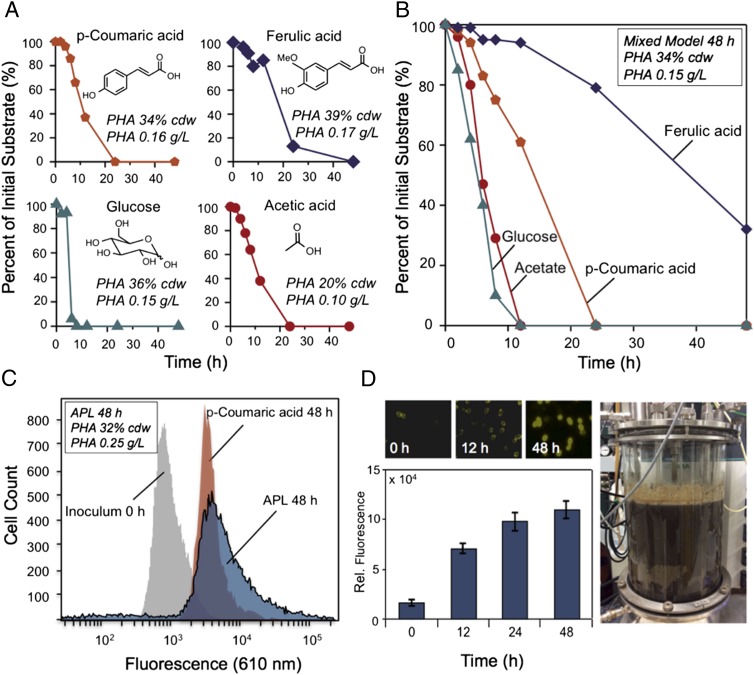
We subsequently evaluated the ability of P. putida to use several individual molecules derived from both lignin and polysaccharides present in APL for cell growth and mcl-PHA production. Based on identification of the most prevalent species in APL relative to previous genomics analyses of P. putida (21, 27), we predict that most of the aromatic molecules present in APL (SI Appendix, Table S1) will be catabolized via the β-ketoadipate pathway, as illustrated in SI Appendix, Fig. S5. To test this prediction, we then grew P. putida in 250-mL shake flasks with both single- and mixed-model carbon sources representative of APL components (Fig. 2). Flow cytometry was used in all cases to monitor the accumulation of mcl-PHAs (SI Appendix, Fig. S6). As shown in Fig. 2A, p-coumaric acid, ferulic acid, and glucose all led to mcl-PHA accumulation in P. putida at comparable levels, namely 34–39% cell dry weight (cdw). Acetate, which is quite prevalent in APL due to deacetylation of hemicellulose side chains in caustic conditions (28), is the exception, with only 20% cdw production of mcl-PHAs (Fig. 2A). The corresponding volumetric productivity ranges from 0.15 to 0.17 g/L mcl-PHAs, with acetate producing 0.10 g/L. P. putida, was also grown in a mixture of the same four-model compounds at equivalent starting concentrations, which reached 34% cdw and 0.15 g/L, indicating that lignin and carbohydrate-derived species are coconverted to mcl-PHAs (Fig. 2B). It is noted that p-coumaric acid, glucose, and acetate are all fully used by 24 h, whereas ferulic acid is only ∼70% used by 48 h. As shown in SI Appendix, Fig. S5, ferulic acid goes through many of the same enzymatic steps in P. putida as p-coumaric acid. Assuming that it is not regulated differently than p-coumaric acid, ferulic acid may simply be a poor substrate relative to p-coumaric acid for the overlapping enzymatic steps. Cultures grown with high acetate levels also exhibit coutilization with similar mcl-PHA productivity (33% cdw, 0.12 g/L), further suggesting that aromatic and carbohydrate-derived species will be used simultaneously in complex mixtures like APL (SI Appendix, Fig. S7).
Fig. 2.
Biological conversion of lignin-derived aromatic molecules and carbohydrate-derived products in APL to mcl-PHAs in P. putida. (A) Conversion and mcl-PHA production from representative model compounds present in APL, each at 2 g/L. (B) Conversion and mcl-PHA production of a mixture of four representative model compounds from APL, each loaded at 0.5 g/L. (C) Flow cytometry of Nile Red-stained cells for mcl-PHA accumulation. Cell counts are plotted as a function of fluorescence intensity in the initial inoculum (t = 0) and cultures at t = 48 h for a model substrate, p-coumaric acid, and APL grown in 250-mL shake flasks, with the corresponding total mcl-PHA production from APL shown in Inset. (D) Fluorescence imaging of cells at 0, 12, and 48 h stained with Nile Red demonstrates mcl-PHA production from APL (Upper). Fluorescence quantitation of P. putida cells from the APL conversion as a function of time adjusted to an equivalent cell density (Lower). Biological APL conversion by P. putida in a 14-L fermenter (Right).
Based on the ability of P. putida to convert individual compounds to mcl-PHAs, we examined mcl-PHA production in shake-flask conditions with APL as a sole carbon source (Fig. 2). Substrate conversion was not tracked, as conventional analytical methods are inadequate for quantitatively characterizing APL (29). Interestingly, P. putida grows quite well in APL without appreciable dilution, beyond adding a small amount of modified minimal M9 salts and without removal of potential inhibitors. Intracellular mcl-PHAs accumulate over 48 h with APL, with a fluorescence intensity distribution at 610 nm, similar to p-coumaric acid alone (Fig. 2C). The fluorescence data and yield of mcl-PHAs from APL (0.252 g/L at 32% cdw) are comparable to the model compound experiments, demonstrating that an aromatic-catabolizing organism can convert lignin-enriched streams derived from industrially relevant feedstocks to a value-added product (Fig. 2). To confirm that lignin monomers were incorporated into mcl-PHAs in APL, shake-flask experiments were performed by using APL supplemented with 13C-labeled p-coumaric acid. Analysis of the 13C/12C ratio for major derivatized hydroxyacids methyl esters (HAMEs) confirmed significant 13C-enrichment of the mcl-PHAs due to labeled p-coumaric acid incorporation (SI Appendix, Fig. S8), even with initial supplementation at trace levels (3.5 mg/L 13C from labeled p-coumaric acid of the 11,300 mg/L total APL carbon). Going forward, there are known means to improve mcl-PHA production via fermentation optimization, substrate feed concentration, and organism engineering (22), as outlined below.
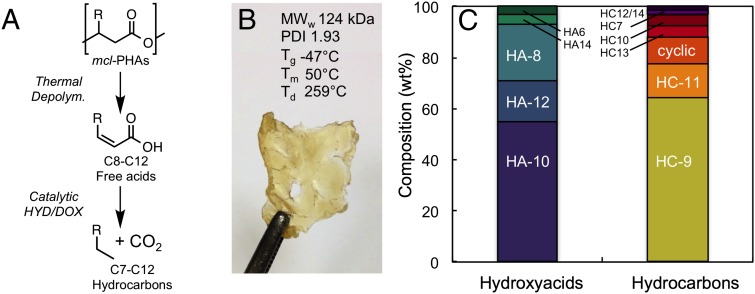
To produce sufficient quantities of mcl-PHAs for material characterization and processing, a 14-L batch cultivation of P. putida was grown in APL as a sole carbon source in a laboratory fermenter (Fig. 2D). Nile Red staining via fluorescence microscopy and quantitation per cell confirmed that the primary increase in fluorescence, reflective of mcl-PHA production, occurs within 24 h of cultivation. Additionally, flow cytometry data in the 14-L batch cultivation are comparable to shake-flask results (SI Appendix, Fig. S9). Characterization of APL-derived mcl-PHAs indicated that their physicochemical properties are comparable to those derived from carbohydrates (Fig. 3B and SI Appendix, Figs. S10 and S11) (22, 30–32). Analysis of the hydroxyacid monomer distribution showed that the mcl-PHA polymer primarily comprises 3-hydroxydecanoic acid (HA-10; 55%), 3-hydroxyoctanoic acid (HA-8; 22%), 3-hydroxydodecanoic acid (HA-12; 16%), 3-hydroxytetradecancoic acid (HA-14; 4%), and 3-hydroxyhexanoic acid (HA-6; 3%) (Fig. 3C).
Fig. 3.
APL-derived mcl-PHA physicochemical properties and catalytic upgrading to chemical precursors and fuels. (A) Example of thermal-catalytic upgrading pathway for mcl-PHAs to chemical precursors and hydrocarbon fuels. (B) APL-derived mcl-PHAs and physicochemical properties including weight-average molecular weight (MWw), polydispersity index (PDI), glass transition temperature (Tg), melting point (Tm), and 5% decomposition temperature (Td). (C) Initial mcl-PHA hydroxyacid composition (Left) and alkane distribution (Right) after thermal depolymerization and catalytic deoxygenation.
Monomers derived from mcl-PHAs can also be used for products beyond bioplastics, such as for chemical precursors or fuels. To that end, APL-derived mcl-PHAs were thermally depolymerized at 250 °C under inert atmosphere to produce alkenoic acids (33), which can serve as a platform intermediate for an array of chemicals, similar to other biologically derived acids (34–36). This thermal process results in dehydration and monomer products reflective of the parent polymer (SI Appendix, Fig. S12 and Table S2). Additionally, tandem thermal depolymerization of APL-derived mcl-PHAs and catalytic deoxygenation of the resulting alkenoic acids was used to produce fuel-range hydrocarbons (Fig. 3C). Catalytic deoxygenation was performed with a Pt-Re/C catalyst via hydrogenation and decarboxylation-decarbonylation (denoted as “HYD/DOX” in Fig. 3) at 300 °C in water under mild hydrogen pressure (2.5 MPa initial H2 loading at 25 °C) (Fig. 3 A and C) (37). The use of water facilitates reforming of renewable H2 donors in situ (38), whereas CO produced from decarbonylation can react with water to produce H2 via the water-gas-shift reaction, minimizing external H2 requirements. The product distribution is reflective of the mcl-PHA polymer and demonstrates conversion of mcl-PHAs to fuel-range hydrocarbons (SI Appendix, Fig. S13 and Table S3).
Discussion
The conversion of carbohydrates via fermentation has provided humanity with renewable transportation fuels and chemicals for well more than a century. Conversely, the only market for lignin to date on a scale concomitant with fuels and chemicals derived from carbohydrates is heat and power. On a much smaller scale, the primary market to date for lignin beyond niche materials is in the production of vanillin and lignosulfonates, markets that would be swamped by orders of magnitude given the potential for lignin production in the growing, worldwide biofuels economy. Thus, lignin valorization strategies that incorporate new approaches are desperately needed (6). From a biological conversion standpoint, previous studies have either focused on the modification of upper pathways to produce value-added aromatics, such as vanillin (39), or have shown that single aromatic model compounds can be converted to intermediates such as lipids (40, 41). Here, we show that the use of microbial, aromatic catabolic pathways on a mixture of biomass-derived lignin substrates can overcome the primary, inherent challenge in lignin valorization, namely the heterogeneity of lignin. It is important to note that significant improvements to mcl-PHA production with productivities of 1–3 g⋅L−1⋅hr−1 and volumetric concentrations near 100 g/L are required to reach economic viability (42). It has been shown that improvements of this magnitude are possible in mcl-PHA production in wild-type P. putida from different substrates (e.g., glucose or fatty acids) via fed-batch fermentation and concentrating the substrate (22). To this end, we note that a 5× concentration of APL produces an equivalent, per-cell PHA fluorescence intensity and culture density, thus demonstrating equivalent titers can be achieved in a concentrated APL stream (SI Appendix, Table S4). These previous results and techno-economic analyses suggest that further development and optimization of this approach via modifications to fermentation conditions could eventually enable the scalable production of mcl-PHAs from lignin-enriched substrates such as APL or other lignin-derived process streams (22, 42).
Additionally, mcl-PHAs have a broad range of potential markets, both for small- and large-volume applications. There are ongoing research efforts to tailor mcl-PHAs as neat or blended bioplastics for films, coatings, biocompatible drug-delivery and biomedical materials, and organic/inorganic composites (43). The extended carbon side chain of mcl-PHAs imparts unique properties by disrupting the regularity of the polymer backbone compared with short-chain PHAs, allowing for greatly reduced melting and glass transition temperature, as well as crystallinity (44). PHAs can be depolymerized by several routes, including thermal degradation (33), aqueous hydrolysis (45), and extracellular enzyme depolymerases (23). Alternatively, hydroxyacid monomers can be directly produced during fermentation by genetic knockout of PHA synthases (46). The resulting monomeric acids can potentially serve as a platform for producing an array of value-added chemicals including diols, ketones, amides, and nitriles, similar to other biologically derived carboxylic acids (e.g., lactic acid, levulinic acid, succinic acid) (34–36). Additionally, these monomers can be converted to fuel substitutes (4), as previously demonstrated with methyl esters (i.e., biodiesel) derived from mcl-PHAs (24), and fully deoxygenated hydrocarbon fuels, as described in this study. These carbon chain lengths fall primarily within the range of jet (C8-C16) and diesel grade (C8-C21) fuels, which is particularly promising because heavy-duty vehicle and airline fuel consumption is anticipated to grow substantially in the near future (47). These forms of transportation require power sources that are not readily substituted with other renewables (e.g., short-chain alcohols, electric, fuel cell), thus a market for mcl-PHAs derived from lignin and carbohydrates may also be eventually possible at a scale of renewable fuels.
Lastly, the approach developed here is merely an example of an integrated process for lignin valorization wherein a “biological funneling” step can be used with a lignin depolymerization step and biological or catalytic upgrading. There is significant versatility and modularity in each step, enabling adaptation to different biomass feedstocks and desired fuel and chemical portfolios, thus holding promise toward industrial application. Namely, lignin depolymerization is possible through many established routes and, thus, many depolymerization processes can likely be used. For example, biological lignin depolymerization is possible with oxidative fungal and bacterial enzymes (10, 12–14), transition metal catalysts (11, 48–51), homogeneous and heterogeneous alkaline catalysts (52–56), oxidation catalysts (11), and thermal routes. The selectivity of each of these depolymerization strategies will dictate the aromatic-derived substrates for biological conversion. This substrate range, in turn, will guide the selection of genes present needed in the upper pathways, not all of which may exist in a single organism, thus requiring pathway engineering. From a biological conversion standpoint, aromatic catabolic pathways can be engineered to funnel the products to different biological intermediates (i.e., not just mcl-PHAs), thus opening up a new field of metabolic engineering for lignin utilization. Additionally, process streams from industrial-scale biomass depolymerization may likely contain additional nonlignin-derived molecules, such as acetate or hemicellulose-derived sugars, which potentially can be simultaneously funneled to desired products, thus maximizing carbon utilization from biomass. Moreover, the production of biofuel intermediates similar to those produced from sugars, such as isoprenoids, fatty acids, or higher-chain alcohols, is plausible given the coupling of aromatic upper pathways with the proper downstream genetic modifications (7). Generally, the approach we demonstrate here can be combined with many lignin isolation and biocatalytic upgrading strategies to facilitate the development of an immense range of molecules derived from lignin.
Conclusions
The conversion of biomass-derived polysaccharides has provided mankind with renewable fuels and chemicals for well more than a century. Conversely, the only use for lignin to date on a scale concomitant with polysaccharide-derived fuels and chemicals is heat and power. Here, we demonstrate a flexible, integrated process that overcomes the inherent challenges in lignin valorization, namely lignin heterogeneity, thus enabling comprehensive utilization of biomass polymers to produce renewable fuels, chemicals, and materials for a sustainable energy economy.
Methods
Corn Stover Alkaline Pretreatment.
To obtain a lignin-rich stream for upgrading, corn stover from Idaho National Laboratory (100 kg, 1/4-inch hammer milled, Lot 4) was pretreated with 70 mg of NaOH/g dry stover and anthraquinone (0.2% charge wt/wt dry stover) at 7 wt% solids in a 1,900-L, jacketed paddle mixer (American Process Systems). The slurry was indirectly heated to 100 °C with 30–40 psi gauge of saturated steam on the vessel jackets, with a heat ramp of ∼2 h. After 30 min at temperature, the slurry was cooled to 60 °C with jacketed cooling water. APL at a pH of ∼12 was gravity drained. A continuous screw press (Vincent Corp. Model CP10) dewatered the residual pretreated stover to ∼20 wt% solids, and screw press-recovered APL was added to the gravity-drained APL. Biomass and pretreated solids were characterized by compositional analysis (SI Appendix, SI Methods). Pretreated solids were subjected to enzymatic hydrolysis (SI Appendix, SI Methods). APL was characterized by compositional analysis, gel permeation chromatography (GPC), liquid chromatography, and glucose concentrations were measured with a YSI life sciences 7100 MBS instrument (SI Appendix, SI Methods).
P. putida Cultivation.
mcl-PHA production with P. putida was initially examined in shake flask cultures. To support growth, APL was adjusted to pH 7.0 by using 5 M H2SO4 and supplemented with 10× modified M9 salts (per liter of 10×-M9: 6.78 g of Na2PO4, 3 g of KH2PO4, 0.5 g of NaCl, and 10 M NaOH to pH 7.0) at 10% volume. Subsequently, 2 mL of 1 M MgSO4, and 100 µL of 1 M CaCl2 were added to make 0.9× APL; 1× M9 medium, referred to as “M9-APL.” A 500-mL seed culture of P. putida KT2440 was grown overnight in LB at 30 °C, then diluted fivefold in LB with continued growth for 2 h to achieve a logarithmic growth phase. The culture was pelleted via centrifugation, washed once in PBS, and used to inoculate 1-L flasks containing different media to a total volume of 250 mL at OD at 600 nm. Seven conditions were set up in duplicate. Each contained 1xM9 salts with varied carbon sources: (i) 2 g/L glucose, (ii) 2 g/L acetate (sodium acetate), (iii), 2 g/L p-coumaric acid, (iv) 2 g/L transferulic acid, (v) mixed carbon medium at equal concentrations (0.5 g/L glucose, 0.5 g/L p-coumaric acid, 0.5 g/L transferulic acid, and 0.5 g/L acetate), (vi) mixed carbon medium at high acetate concentration (1.0 g/L acetate, 0.3 g/L glucose, 0.3 g/L p-coumaric acid, and 0.3 g/L transferulic acid), (vii) and APL (90% volume, 3 µm filtered). Cultures were grown at 30 °C for 48 h at 225 rpm and periodically sampled for analysis. Carbon utilization was monitored by HPLC for defined media (57). mcl-PHA production was monitored by flow cytometry via Nile Red staining (SI Appendix, SI Methods). To determine cell dry weight at 48 h, 200 mL of culture was centrifuged, washed in 10% PBS, recentrifuged, and lyophilized. mcl-PHAs were recovered by accelerated solvent extraction and ethanol precipitation (SI Appendix, SI Methods). 13C-enrichment of mcl-PHAs derived from APL supplemented with 13C-labeled monomers was performed in shake flask cultures, with mcl-PHAs derivatized and analyzed by gas chromatography isotope ratio mass spectroscopy (SI Appendix, SI Methods).
mcl-PHAs were produced in larger quantities for upgrading by using P. putida grown in a 14-L BioFlo 3000 batch reactor (New Brunswick Scientific) with M9-APL. Seed cultures were grown overnight in LB medium to an optical density at 600 nm (OD600) of 3.5–4.0, centrifuged, washed once in 1× M9 medium, and used to inoculate cultures to a starting OD600 of 0.05 in M9-APL. Supplemental nitrogen (NH4)2SO4 was either withheld or added at 1 mM. Excess nitrogen [10 mM (NH4)2SO4] was used as a negative control for flow cytometry analysis and to demonstrate the dependence of mcl-PHA production on nitrogen content in APL. The temperature was maintained at 30 °C, and mixing was achieved by using a bottom marine impeller and midheight Rushton impeller at 200 rpm. Aeration was set at 0.35 vessel volumes per min by using 100% air, and pH at 7.0 was controlled by using KOH/HCl. mcl-PHA accumulation was monitored by fluorescence microscopy and flow cytometry (SI Appendix, SI Methods). Cultivations ran for 72 h, followed by centrifugation and lyophilization to harvest cells. mcl-PHAs were recovered by accelerated solvent extraction and characterized by GPC and thermal analysis (SI Appendix, SI Methods). mcl-PHA monomer distribution was determined by methylation and gas chromatography (SI Appendix, SI Methods).
mcl-PHA Depolymerization and Upgrading.
mcl-PHAs were thermally depolymerized to produce free alkenoic acids for catalytic upgrading. Thermal depolymerization was performed by using a Parr 5000 Multireactor (Parr Instruments), outfitted with 75-mL reactor vessels. The reactor vessel was loaded with 445 mg of recovered mcl-PHAs and purged with Ar for three cycles. The gas purge line was then closed, and the reactor was heated to 250 °C for 30 min at temperature (58). The depolymerization products were recovered in dichloromethane, filtered [0.2-µm polytetrafluoroethylene (PTFE)], and identified by gas chromatography, as described in SI Appendix, SI Methods.
Depolymerized alkenoic acids derived from mcl-PHAs were catalytically converted to hydrocarbons over a platinum-rhenium (Pt-Re) catalyst supported on activated carbon by using water as a solvent. Pt-Re/C (5 wt% Pt, 4 wt% Re) was prepared by aqueous adsorption of Re, using ammonium perrhenate (Sigma Aldrich) as a precursor, onto commercial Pt/C (Sigma Aldrich), followed by in situ reduction at 200 °C using 1.4 MPa of H2 loaded into the reactor at ambient temperature. Catalyst material properties were characterized (37). Catalytic deoxygenation and reduction of thermally depolymerized mcl-PHAs was conducted by using the Parr 5000 Multireactor described above. The 75-mL reactor vessel was loaded with 270 mg of depolymerized mcl-PHA, 50 mg of Pt-Re/C, and 9.8 mL of deionized water. Before conversion, the vessel was purged with Ar for three cycles and pressurized to 2.75 MPa with H2 at room temperature. The reactors were heated to 300 °C under rapid stirring for 180 min at temperature. Catalysis products were recovered in CH2Cl2, filtered (0.2-µm PTFE), and the product distribution was determined by gas chromatography, as described in SI Appendix, SI Methods.
Supplementary Material
Acknowledgments
We thank E. Kuhn and R. Elander for APL; T. VanderWall for conducting the fermentations; M. Resch for running enzymatic hydrolysis; H. Pilath, A. Starace, D. Johnson, and W. Michener for analytical assistance; D. Reuss for analytical assistance and discussions; M. Biddy, T. Foust, and L. Moens for helpful discussions; and B. Knott for comments on the manuscript. We thank the US Department of Energy BioEnergy Technologies Office through the National Advanced Biofuels Consortium for funding. Support for D.R.V. and T.J.S. is also provided by National Science Foundation (NSF) Grant NSF-CBET-0746453 and NSF Graduate Research Fellowship NSF-DGE-1144245 (to D.R.V.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1410657111/-/DCSupplemental.
References
- 1.Ragauskas AJ, et al. The path forward for biofuels and biomaterials. Science. 2006;311(5760):484–489. doi: 10.1126/science.1114736. [DOI] [PubMed] [Google Scholar]
- 2.Himmel ME, et al. Biomass recalcitrance: Engineering plants and enzymes for biofuels production. Science. 2007;315(5813):804–807. doi: 10.1126/science.1137016. [DOI] [PubMed] [Google Scholar]
- 3.Stephanopoulos G. Challenges in engineering microbes for biofuels production. Science. 2007;315(5813):801–804. doi: 10.1126/science.1139612. [DOI] [PubMed] [Google Scholar]
- 4.Alonso DM, Bond JQ, Dumesic JA. Catalytic conversion of biomass to biofuels. Green Chem. 2010;12(9):1493–1513. [Google Scholar]
- 5.Chundawat SPS, Beckham GT, Himmel ME, Dale BE. Deconstruction of lignocellulosic biomass to fuels and chemicals. Annu Rev Chem Biomol Eng. 2011;2:121–145. doi: 10.1146/annurev-chembioeng-061010-114205. [DOI] [PubMed] [Google Scholar]
- 6.Tuck CO, Pérez E, Horváth IT, Sheldon RA, Poliakoff M. Valorization of biomass: Deriving more value from waste. Science. 2012;337(6095):695–699. doi: 10.1126/science.1218930. [DOI] [PubMed] [Google Scholar]
- 7.Peralta-Yahya PP, Zhang F, del Cardayre SB, Keasling JD. Microbial engineering for the production of advanced biofuels. Nature. 2012;488(7411):320–328. doi: 10.1038/nature11478. [DOI] [PubMed] [Google Scholar]
- 8.Ragauskas AJ, et al. Lignin valorization: Improving lignin processing in the biorefinery. Science. 2014;344(6185):1246843. doi: 10.1126/science.1246843. [DOI] [PubMed] [Google Scholar]
- 9.Boerjan W, Ralph J, Baucher M. Lignin biosynthesis. Annu Rev Plant Biol. 2003;54:519–546. doi: 10.1146/annurev.arplant.54.031902.134938. [DOI] [PubMed] [Google Scholar]
- 10.Kirk TK, Farrell RL. Enzymatic “combustion”: The microbial degradation of lignin. Annu Rev Microbiol. 1987;41:465–505. doi: 10.1146/annurev.mi.41.100187.002341. [DOI] [PubMed] [Google Scholar]
- 11.Zakzeski J, Bruijnincx PCA, Jongerius AL, Weckhuysen BM. The catalytic valorization of lignin for the production of renewable chemicals. Chem Rev. 2010;110(6):3552–3599. doi: 10.1021/cr900354u. [DOI] [PubMed] [Google Scholar]
- 12.Floudas D, et al. The Paleozoic origin of enzymatic lignin decomposition reconstructed from 31 fungal genomes. Science. 2012;336(6089):1715–1719. doi: 10.1126/science.1221748. [DOI] [PubMed] [Google Scholar]
- 13.Bugg TDH, Ahmad M, Hardiman EM, Singh R. The emerging role for bacteria in lignin degradation and bio-product formation. Curr Opin Biotechnol. 2011;22(3):394–400. doi: 10.1016/j.copbio.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 14.Bugg TDH, Ahmad M, Hardiman EM, Rahmanpour R. Pathways for degradation of lignin in bacteria and fungi. Nat Prod Rep. 2011;28(12):1883–1896. doi: 10.1039/c1np00042j. [DOI] [PubMed] [Google Scholar]
- 15.Harayama S, Kok M, Neidle EL. Functional and evolutionary relationships among diverse oxygenases. Annu Rev Microbiol. 1992;46(1):565–601. doi: 10.1146/annurev.mi.46.100192.003025. [DOI] [PubMed] [Google Scholar]
- 16.Bugg TD. Dioxygenase enzymes: Catalytic mechanisms and chemical models. Tetrahedron. 2003;59(36):7075–7101. [Google Scholar]
- 17.Vaillancourt FH, Bolin JT, Eltis LD. The ins and outs of ring-cleaving dioxygenases. Crit Rev Biochem Mol Biol. 2006;41(4):241–267. doi: 10.1080/10409230600817422. [DOI] [PubMed] [Google Scholar]
- 18.Fuchs G, Boll M, Heider J. Microbial degradation of aromatic compounds - from one strategy to four. Nat Rev Microbiol. 2011;9(11):803–816. doi: 10.1038/nrmicro2652. [DOI] [PubMed] [Google Scholar]
- 19.Harwood CS, Parales RE. The β-ketoadipate pathway and the biology of self-identity. Annu Rev Microbiol. 1996;50(1):553–590. doi: 10.1146/annurev.micro.50.1.553. [DOI] [PubMed] [Google Scholar]
- 20.Ornston LN, Stanier RY. The conversion of catechol and protocatechuate to β-ketoadipate by Pseudomonas putida. J Biol Chem. 1966;241(16):3776–3786. [PubMed] [Google Scholar]
- 21.Jiménez JI, Miñambres B, García JL, Díaz E. Genomic analysis of the aromatic catabolic pathways from Pseudomonas putida KT2440. Environ Microbiol. 2002;4(12):824–841. doi: 10.1046/j.1462-2920.2002.00370.x. [DOI] [PubMed] [Google Scholar]
- 22.Chen GQ. A microbial polyhydroxyalkanoates (PHA) based bio- and materials industry. Chem Soc Rev. 2009;38(8):2434–2446. doi: 10.1039/b812677c. [DOI] [PubMed] [Google Scholar]
- 23.Gangoiti J, Santos M, Llama MJ, Serra JL. Production of chiral (R)-3-hydroxyoctanoic acid monomers, catalyzed by Pseudomonas fluorescens GK13 poly(3-hydroxyoctanoic acid) depolymerase. Appl Environ Microbiol. 2010;76(11):3554–3560. doi: 10.1128/AEM.00337-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X, Luo R, Wang Z, Deng Y, Chen GQ. Application of (R)-3-hydroxyalkanoate methyl esters derived from microbial polyhydroxyalkanoates as novel biofuels. Biomacromolecules. 2009;10(4):707–711. doi: 10.1021/bm801424e. [DOI] [PubMed] [Google Scholar]
- 25.Rehm BHA, Mitsky TA, Steinbüchel A. Role of fatty acid de novo biosynthesis in polyhydroxyalkanoic acid (PHA) and rhamnolipid synthesis by pseudomonads: Establishment of the transacylase (PhaG)-mediated pathway for PHA biosynthesis in Escherichia coli. Appl Environ Microbiol. 2001;67(7):3102–3109. doi: 10.1128/AEM.67.7.3102-3109.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donald D, Göran G. 2010. Chemistry of Alkaline Pulping (CRC, Boca Raton, FL) pp 349–391.
- 27.Nelson KE, et al. Complete genome sequence and comparative analysis of the metabolically versatile Pseudomonas putida KT2440. Environ Microbiol. 2002;4(12):799–808. doi: 10.1046/j.1462-2920.2002.00366.x. [DOI] [PubMed] [Google Scholar]
- 28.Chen X, et al. Improved ethanol yield and reduced Minimum Ethanol Selling Price (MESP) by modifying low severity dilute acid pretreatment with deacetylation and mechanical refining: 1) Experimental. Biotechnol Biofuels. 2012;5(1):60. doi: 10.1186/1754-6834-5-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sjöström E, Alén R. 1999. Analytical Methods in Wood Chemistry, Pulping, and Papermaking (Springer, New York), pp 193–231.
- 30.Poirier Y, Nawrath C, Somerville C. Production of polyhydroxyalkanoates, a family of biodegradable plastics and elastomers, in bacteria and plants. Biotechnology (N Y) 1995;13(2):142–150. doi: 10.1038/nbt0295-142. [DOI] [PubMed] [Google Scholar]
- 31.El-Hadi A, Schnabel R, Straube E, Muller G, Henning S. Correlation between degree of crystallinity, morphology, glass temperature, mechanical properties and biodegradation of poly (3-hydroxyalkanoate) PHAs and their blends. Polym Test. 2002;21(6):665–674. [Google Scholar]
- 32.Abe H, Ishii N, Sato S, Tsuge T. Thermal properties and crystallization behaviors of medium-chain-length poly(3-hydroxyalkanoate)s. Polymer (Guildf) 2012;53(14):3026–3034. [Google Scholar]
- 33.Sato S, Ishii N, Hamada Y, Abe H, Tsuge T. Utilization of 2-alkenoic acids for biosynthesis of medium-chain-length polyhydroxyalkanoates in metabolically engineered Escherichia coli to construct a novel chemical recycling system. Polym Degrad Stabil. 2012;97(3):329–336. [Google Scholar]
- 34.Dusselier M, Van Wouwe P, Dewaele A, Makshina E, Sels BF. Lactic acid as a platform chemical in the biobased economy: The role of chemocatalysis. Energy Env. Sci. 2013;6(5):1415–1442. [Google Scholar]
- 35.Rackemann DW, Doherty WO. The conversion of lignocellulosics to levulinic acid. Biofuels Bioprod Bioref. 2011;5(2):198–214. [Google Scholar]
- 36.Cukalovic A, Stevens CV. Feasibility of production methods for succinic acid derivatives: A marriage of renewable resources and chemical technology. Biofuels Bioprod Bioref. 2008;2(6):505–529. [Google Scholar]
- 37.Vardon DR, et al. Hydrothermal catalytic processing of saturated and unsaturated fatty acids to hydrocarbons with glycerol for in situ hydrogen production. Green Chem. 2014;16:1507–1520. [Google Scholar]
- 38.Cortright RD, Davda RR, Dumesic JA. Hydrogen from catalytic reforming of biomass-derived hydrocarbons in liquid water. Nature. 2002;418(6901):964–967. doi: 10.1038/nature01009. [DOI] [PubMed] [Google Scholar]
- 39.Sainsbury PD, et al. Breaking down lignin to high-value chemicals: The conversion of lignocellulose to vanillin in a gene deletion mutant of Rhodococcus jostii RHA1. ACS Chem Biol. 2013;8(10):2151–2156. doi: 10.1021/cb400505a. [DOI] [PubMed] [Google Scholar]
- 40.Kosa M, Ragauskas AJ. Bioconversion of lignin model compounds with oleaginous Rhodococci. Appl Microbiol Biotechnol. 2012;93(2):891–900. doi: 10.1007/s00253-011-3743-z. [DOI] [PubMed] [Google Scholar]
- 41.Kosa M, Ragauskas AJ. Lignin to lipid bioconversion by oleaginous Rhodococci. Green Chem. 2013;15(8):2070–2074. [Google Scholar]
- 42.Hermann BG, Patel M. Today’s and tomorrow’s bio-based bulk chemicals from white biotechnology: A techno-economic analysis. Appl Biochem Biotechnol. 2007;136(3):361–388. doi: 10.1007/s12010-007-9031-9. [DOI] [PubMed] [Google Scholar]
- 43.Chen G-Q. 2007. Plastics from Bacteria: Natural Functions and Applications (Springer, New York) pp 17–37.
- 44.Rai R, Keshavarz T, Roether J, Boccaccini AR, Roy I. Medium chain length polyhydroxyalkanoates, promising new biomedical materials for the future. Mater Sci Eng Rep. 2011;72(3):29–47. [Google Scholar]
- 45.Saeki T, Tsukegi T, Tsuji H, Daimon H, Fujie K. Hydrolytic degradation of poly-R-3-hydroxybutyric acid in the melt. Polymer. 2005;46(7):2157–2162. [Google Scholar]
- 46.Chung A, Liu Q, Ouyang S-P, Wu Q, Chen G-Q. Microbial production of 3-hydroxydodecanoic acid by pha operon and fadBA knockout mutant of Pseudomonas putida KT2442 harboring tesB gene. Appl Microbiol Biotechnol. 2009;83(3):513–519. doi: 10.1007/s00253-009-1919-6. [DOI] [PubMed] [Google Scholar]
- 47.Milbrandt A, Kinchin C, McCormick R. The Feasibility of Producing and Using Biomass-Based Diesel and Jet Fuel in the United States. Golden, CO: Natl Renew Energy Lab; 2013. [Google Scholar]
- 48.Pepper JM, Hibbert H. Studies on lignin and related compounds; high pressure hydrogenation of maple wood. J Am Chem Soc. 1948;70(1):67–71. doi: 10.1021/ja01181a021. [DOI] [PubMed] [Google Scholar]
- 49.Pepper JM, Lee YW. Lignin and related compounds. I. A comparative study of catalysts for lignin hydrogenolysis. Can J Chem. 1969;47(5):723–727. [Google Scholar]
- 50.Rinaldi R, Schüth F. Design of solid catalysts for the conversion of biomass. Energy Env. Sci. 2009;2(6):610–626. [Google Scholar]
- 51.Parsell TH, et al. Cleavage and hydrodeoxygenation (HDO) of C–O bonds relevant to lignin conversion using Pd/Zn synergistic catalysis. Chem. Sci. 2013;4(2):806–813. [Google Scholar]
- 52.Miller JE, Evans L, Littlewolf A, Trudell DE. Batch microreactor studies of lignin and lignin model compound depolymerization by bases in alcohol solvents. Fuel. 1999;78(11):1363–1366. [Google Scholar]
- 53.Deng HB, et al. Perovskite-type oxide LaMnO3: An efficient and recyclable heterogeneous catalyst for the wet aerobic oxidation of lignin to aromatic aldehydes. Catal Lett. 2008;126(1-2):106–111. [Google Scholar]
- 54.Roberts VM, et al. Towards quantitative catalytic lignin depolymerization. Chemistry. 2011;17(21):5939–5948. doi: 10.1002/chem.201002438. [DOI] [PubMed] [Google Scholar]
- 55.Sturgeon MR, et al. Lignin depolymerisation by nickel supported layered-double hydroxide catalysts. Green Chem. 2014;16(2):824–835. [Google Scholar]
- 56.Karp EM, et al. Alkaline pretreatment of corn stover: Bench-scale fractionation and stream characterization. ACS Sust. Chem. Eng. 2014;2(6):1481–1491. [Google Scholar]
- 57.Franden MA, Pienkos PT, Zhang M. Development of a high-throughput method to evaluate the impact of inhibitory compounds from lignocellulosic hydrolysates on the growth of Zymomonas mobilis. J Biotechnol. 2009;144(4):259–267. doi: 10.1016/j.jbiotec.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 58.Sin MC, Gan SN, Annuar MSM, Tan IKP. Thermodegradation of medium-chain-length poly(3-hydroxyalkanoates) produced by Pseudomonas putida from oleic acid. Polym Degrad Stabil. 2010;95(12):2334–2342. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.