Significance
All lymphoid cells are considered to be products of hematopoietic stem cells (HSCs); however, it has been suggested, but not proven, that innate immune B-1 progenitor cells develop independently of HSCs in the fetal liver. B-1 cells, especially B-1a cells, are not replaced by adult bone marrow transplantation. Thus, it is critical to understand the origin and mechanisms required to sustain these cells in vivo because B-1 cells play important roles in the first line of defense against microbial infection and in preventing organ damage in autoimmune patients and infections in some patients after bone-marrow transplantation. We demonstrate that B-1 progenitor cells can develop independently of HSCs in the fetal liver and that their development relies critically on the expression of core-binding factor beta.
Keywords: TEK, hemogenic endothelial cell, immune layered model
Abstract
The fetal liver is a major hematopoietic site containing progenitor cells that give rise to nearly all blood cells, including B-1 cells. Because the fetal liver is not a de novo site of hematopoietic stem cell (HSC) or progenitor-cell emergence, it must be seeded by yolk sac (YS)-derived erythromyeloid progenitors at embryonic day (E) 8.5–E10 and aorta-gonado-mesonephros (AGM)-derived HSCs at E10.5–E11.5. Although the B-1 progenitor cell pool in the fetal liver is considered to be of HSC origin, we have previously proposed that YS-derived B-1 progenitors may also contribute to this pool. Until now, it has been impossible to determine whether HSC-independent B-1 progenitor cells exist in the fetal liver. Here, we demonstrate the presence of transplantable fetal-liver B-1 and marginal zone B progenitor cells in genetically engineered HSC-deficient embryos. HSC-deficient YS and AGM tissues produce B-1 progenitors in vitro and thus may serve as sites of origin for the B-1 progenitors that seed the fetal liver. Furthermore, we have found that core-binding factor beta (Cbfβ) expression is required for fetal-liver B-1 progenitor cell maturation and expansion. Our data provide, to our knowledge, the first evidence for the presence of B-1 progenitor cells in the fetal liver that arise independently of HSCs and implicate Cbfβ as a critical molecule in the development of this lineage.
The immune layered model proposed by Herzenberg and Herzenberg in 1989 (1) predicted B-1 and B-2 lineage production through three waves of B lymphopoiesis arising from different precursors during development (2): According to this model, the first wave gives rise exclusively to innate immune B cells in early embryonic life and may be derived from progenitor cells independent of hematopoietic stem cells (HSCs), challenging the stem-cell theory that all blood cells are products of HSCs. The second and third waves are comprised of HSCs and HSC-derived progenitors in the fetal liver, neonatal bone marrow (BM) (the second wave), and adult BM (the third wave), respectively. Importantly, AA4.1+CD19+B220lo-neg B-1 specific progenitors have been identified in the second wave (3). The second wave produces more B-1 cells than B-2 cells whereas the third wave displays an opposite skewing of B-cell differentiation (4–7). In fact, the B-1 cell-producing abilities of HSCs and common lymphoid progenitor cells decline with advancing age (6), and, in particular, CD5+B-1a cells are not produced by adult HSCs when examined by single HSC transplantation assay (7). Although the second and third waves have been examined in detail, it is unclear whether the first wave exists and contributes to innate immunity in postnatal life and whether the B-1 progenitor cells in wave 2 in the fetal liver are all HSC-derived or contain derivatives of the wave 1 HSC-independent embryonic progenitor cells.
Murine B-1 cells are innate immune cells (distinguished from conventional B-2 cells by specific surface markers such as IgMhiIgDloCD11b+), residing in the peritoneal and pleural cavities. These cells produce stereotypic natural antibodies in a T cell-independent manner and execute important roles in the first line of defense against microbial infection (8, 9). B-1 cells are segregated into CD5+B-1a and CD5−B-1b cells. Marginal zone (MZ) B cells, named after the restricted localization of these cells in the splenic marginal zone, are usually categorized as BM HSC-derived B-2 cells but share similar functions with B-1 cells, such as rapid production of IgM antibodies against bacterial pathogens in a T cell-independent manner. There is evidence that a portion of MZ B cells is also of embryonic or fetal origin (10–12).
We have recently reported that yolk sac (YS) and para-aortic-splanchnopleura (P-Sp) hemogenic endothelial cells (HECs) harvested before the first emergence of HSC give rise to transplantable, functional B-1a, B-1b, and MZ B cells in vitro and thus have provided supportive evidence for the first wave of B cells (13). However, because we isolated and cultured YS/P-Sp cells in vitro to allow them to differentiate into B-1 progenitor cells, whether YS/P-Sp–derived B progenitor cells seed the fetal liver in vivo and contribute to the B-1 progenitor cell pool or mature B-1 or MZ B cells in postnatal life has never been established. In other words, to address the question whether the first wave of B lymphopoiesis is present in vivo or not, we have to confirm the existence of HSC-independent B-1 progenitor cells in the fetal liver.
The fetal liver is an organ dependent upon hematopoietic stem/progenitor cell seeding from different hematopoietic tissues. It is an established concept that erythro-myeloid progenitors (EMPs) derived from embryonic day (E) 8.5–E10 YSs seed the fetal liver to support homeostatic hematopoiesis in the embryo whereas HSCs that emerge in the aorta-gonado-mesonephros (AGM) region seed the fetal liver at E11 and provide hematopoietic support later in development (14, 15). However, it is unknown whether the YS/P-Sp HEC-derived B-1 lymphoid progenitors seed the fetal liver. Because the B-1 progenitor cell pool in the fetal liver is considered to be an HSC derivative and because HSCs exist in the fetal liver concomitant with B-1 progenitor cells, it has been impossible to prove the existence of HSC-independent B lymphopoiesis in the fetal liver. To specifically address this question, we have used a unique mouse model devoid of HSC but known to possess some uncharacterized fetal-liver B cells (16, 17).
Core-binding factor beta (CBFβ) is the common non–DNA-binding subunit of the Cbf family of heterodimeric transcription factors. By associating with CBFα subunits, CBFβ increases the affinity of CBFα DNA-binding. Runt related transcription factor 1 (Runx1) also called core-binding factor alpha 2 (Cbfα2)−/− embryos die at E12 and display severe deficiencies in definitive EMP, lymphoid cells, and HSCs (18). CBFβ is required for Runx1 function, and loss of CBFβ in embryos leads to embryonic lethality at E11–E13 similar to Runx1−/− embryos with a severe defect in definitive hematopoiesis and HSCs (17, 19). However, when Cbfβ expression was restored in Cbfβ−/− embryos under transgenic control of the endothelial promoter endothelial-specific receptor tyrosine kinase (Tek) (Cbfβ−/−:Tek-GFP/Cbfβ), EMP formation and rare B and T cells were observed (16). In these rescued embryos, lymphoid progenitors (Lin−c-Kit+Flt3+) were detectable in the E14 fetal liver and produced B or T cells in OP9 or OP9-DL1 in vitro cocultures, respectively. However, E14 Cbfβ−/−:Tek-GFP/Cbfβ fetal-liver cells failed to reconstitute recipient peripheral blood (PB) and BM upon transplantation, indicating that Cbfβ−/−:Tek-GFP/Cbfβ embryos contain lymphoid progenitors without transplantable HSCs. It has been previously reported that lymphoid progenitor cells are present in the YS and P-Sp before HSC emergence at an early stage (E8.25–E9.5) of embryo development (13, 20–23). Furthermore, the presence of multipotent progenitor cells with myeloid, B-cell, and T-cell lineage potentials without long-term HSC activity also has been reported (24–26). Whether the B and T progenitor cells emerge from a common HEC or are independently generated has not been fully resolved. Nonetheless, the fact that lymphoid progenitor cells are present prior to HSC emergence suggests that the initial phases of developmental hematopoiesis may not follow the same paradigm as adult BM HSC development where all of the lymphoid progenitor cells are HSC-derived (15, 27). Indeed, transplantable HSCs were rescued when Cbfβ was expressed under transgenic control of the Ly6a promoter in the Cbfβ−/− embryos (Cbfβ−/−:Ly6a-GFP/Cbfβ), suggesting that Cbfβ is required in the Ly6a+ HECs for producing transplantable HSCs that repopulate myeloid and lymphoid lineages (16). Based on these findings and our previous reports that B-1 (but not B-2) and T lymphoid progenitor cells are present in the embryo before HSC emergence (13, 20), we hypothesized that the unspecified B cells detected in the Cbfβ−/−:Tek-GFP/Cbfβ embryos that do not harbor transplantable HSCs are HSC-independent B-1 progenitor cells that constitute the first wave of B lymphopoiesis.
In the present study, we confirm the existence of fetal-liver AA4.1+CD19+B220− B-1 progenitor cells in the absence of transplantable HSCs in vivo. YS and AGM cells from Cbfβ−/−:Tek-GFP/Cbfβ embryos produce B-1 progenitor cells in vitro. Our results clearly demonstrate the presence of B-1 and MZ B progenitor cells in the fetal liver independent of HSCs and suggest that these progenitor cells are likely to be derived in part from YS/P-Sp sites. However, these B-1 progenitor cells do not display persistent expression of GFP/Cbfβ because TEK is not expressed once HECs become hematopoietic cells. Therefore, this Cbfβ deficiency in B-1 progenitor population results in impaired maturation and expansion of these cells. Consistently, we show that overexpression of Cbfβ in Cbfβ−/−:Tek-GFP/Cbfβ fetal-liver cells via a retrovirus promotes engraftment of B-1 and MZ B cells in recipient immunodeficient mice. Thus, our findings demonstrate that persistent Cbfβ expression is required for the maturation and expansion of B-1 progenitor cells during in vivo development.
Results
B-1 Progenitor Cells Exist in the Fetal Liver in the Absence of HSCs.
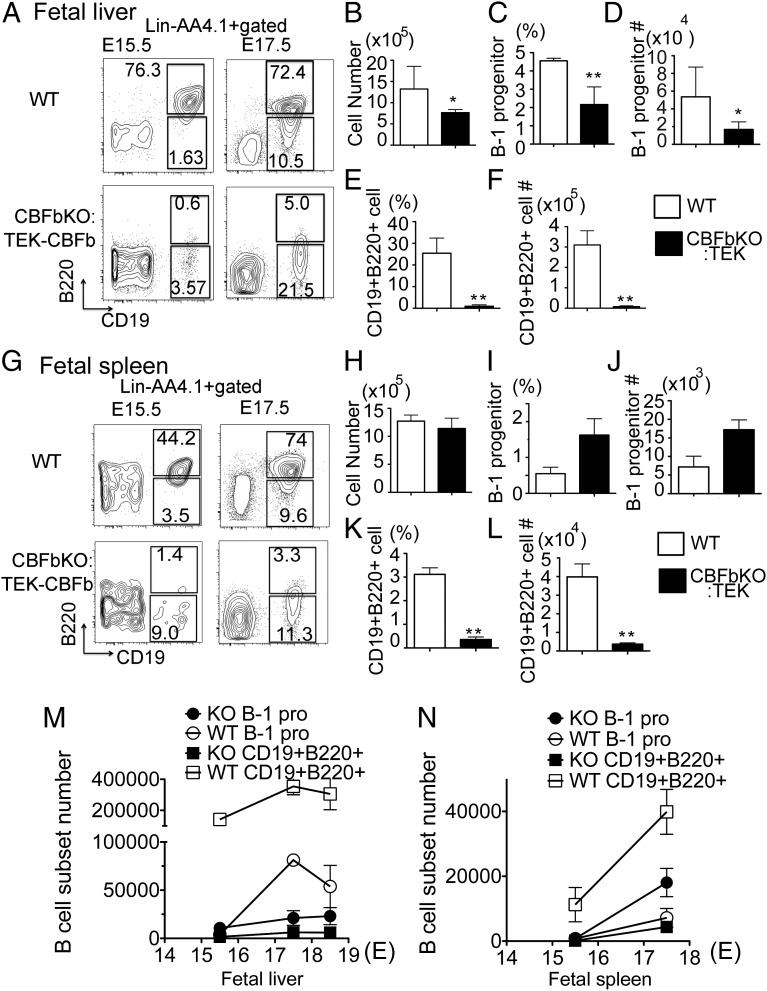
First, we examined whether AA4.1+CD19+B220lo-neg B-1 progenitor cells exist in the fetal liver of Cbfβ−/−:Tek-GFP/Cbfβ embryos. As previously reported (16), Cbfβ−/−:Tek-GFP/Cbfβ embryos can survive until birth in the absence of HSCs. The fetal livers of Cbfβ−/−:Tek-GFP/Cbfβ embryos at E15.5 and E17.5 were of similar size to the fetal liver of wild-type (WT) littermate embryos. Total fetal-liver mononuclear cell (MNC) counts from E17.5 Cbfβ−/−:Tek-GFP/Cbfβ embryos were significantly lower than WT embryos (Fig. 1B). As shown in Fig. 1A, lin−AA4.1+CD19+B220lo-neg B-1 progenitor cells were detected in the fetal livers of Cbfβ−/−:Tek-GFP/Cbfβ embryos from E15.5 until birth although the frequency and total number were significantly lower than WT values (Fig. 1 A–D and M). Furthermore, lin−AA4.1+CD19+B220lo-neg B-1 progenitor cells were also detected in the fetal spleen from E15.5 until birth (Fig. 1 G–J and N). Few AA4.1+CD19+B220+ B cells were detected in the fetal liver and spleen of Cbfβ−/−:Tek-GFP/Cbfβ embryos whereas WT fetal liver contained more AA4.1+CD19+B220+ B cells than B-1 progenitor cells (Fig. 1 A, E–G, K, and L). AA4.1+CD19+B220+ cells are overlapping with AA4.1+B220+CD43+ proB cells (5). Whereas adult BM AA4.1+CD19+B220+ cells give rise predominantly to B-2 cells, fetal-liver AA4.1+CD19+B220+ or AA4.1+B220+CD43+ cells are known to give rise mainly to B-1 cells upon transplantation (3, 5). Thus, the fetal-liver AA4.1+CD19+B220+ population contains more B-1 lineage than B-2 lineage cells and is considered to be differentiated from both HSCs (through AA4.1+CD19−B220+ phenotype) and B-1 progenitor cells as we have previously reported (13). Therefore, severe reduction of AA4.1+CD19+B220+ cells in the fetal liver (Fig. 1 E and F) and spleen (Fig. 1 K and L) is due to HSC deficiency and an impaired maturation of HSC-independent B-1 progenitor cells in the Cbfβ−/−:Tek-GFP/Cbfβ embryos. Nevertheless, it is of note that Cbfβ−/−:Tek-GFP/Cbfβ fetal spleen contains B progenitor cells (2.0 × 104 ± 1.7 × 104 cells) at 44% of the number measured in WT fetal spleen (4.7 × 104 ± 1.3 × 104 cells) (by calculating total B progenitor cells in E17.5 spleen) (Fig. 1N). This result suggests that at least 40% of B-1 progenitor cells in the fetal-neonatal spleen are HSC-independent, a point that contrasts with the common perception that all lymphoid cells are HSC-derived.
Fig. 1.
B-1 progenitor cells exist in the Cbfβ−/−:Tek-GFP/Cbfβ fetal liver (FL) and spleen. AA4.1+CD19+B220lo-neg B-1 progenitor phenotype was examined in the WT and Cbfβ−/−:Tek-GFP/Cbfβ fetal-liver and spleen cells at each day from E15.5 until birth (E18.5). The representative data for E15.5 and E17.5 are depicted. Lin−AA4.1+ gated FACS dot plots at E15.5 and E17.5 fetal liver (A) and fetal spleen (G) are depicted. Total MNCs of E17.5 FL (B) and spleen (H), percentage of B-1 progenitor cells in FL (C) and spleen (I), total cell number of B-1 progenitor cells in FL (D) and spleen (J), percentage of AA4.1+CD19+B220+ cells in FL (E) and spleen (K), and total cell number of AA4.1+CD19+B220+ cells in FL (F) and spleen (L) are shown. (B–F and H–L) Open bar, WT; filled bar, Cbfβ−/−:Tek-GFP/Cbfβ. The cell numbers of B-1 progenitors and CD19+B220+ cells from WT and Cbfβ−/−:Tek-GFP/Cbfβ FL (M) and spleen (N) at each embryonic day are shown (n = 4 for each group; *<0.05, **<0.01).
B-1 Progenitors in Cbfβ−/−:Tek-GFP/Cbfβ Fetal Liver Fail to Mature and Expand in Vitro.
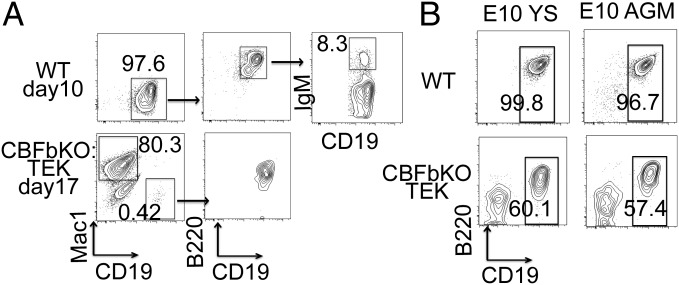
Although we have confirmed for the first time, to our knowledge, that B-1 progenitors are present in the HSC-deficient fetal liver, we have also observed a possible maturation defect of B-1 progenitor cells in Cbfβ−/−:Tek-GFP/Cbfβ embryos (Fig. 1). To examine the maturation capacity of B-1 progenitor cells in Cbfβ−/−:Tek-GFP/Cbfβ fetal liver, AA4.1+CD19+B220lo-neg B-1 progenitor cells from WT and Cbfβ−/−:Tek-GFP/Cbfβ fetal liver were plated on OP9 stromal cells with added IL-7 and Flt3-ligand. WT AA4.1+CD19+B220lo-neg cells proliferated, forming cobblestones beneath the stromal cells, and produced more than 106 nonadherent cells with CD19+B220+ (including IgM+) B cells in 7–10 d (Fig. 2A, Upper). In contrast, Cbfβ−/−:Tek-GFP/Cbfβ AA4.1+CD19+B220lo-neg cells did not proliferate as robustly although they produced cobble stones by 17 d with 106 nonadherent cells, including a few CD19+B220+ cells and many Mac1+ macrophages (Fig. 2A, Lower). Considering the fact that AA4.1+CD19+B220lo-neg cells were originally reported as B cell-macrophage bipotent progenitors (28), these results indicated that loss of Cbfβ may have contributed to the reduction of B lymphoid potential in this population. This is compatible with a previous report that the B lymphoid potential of lymphoid progenitor cells in Cbfβ−/−:Tek-GFP/Cbfβ embryos was 30-fold less than in WT embryos (16). Thus, proliferation and B-1 cell differentiation capacity were significantly impaired in Cbfβ−/−:Tek-GFP/Cbfβ B-1 progenitor cells at least under the coculture conditions we used in vitro.
Fig. 2.
(A) B-1 progenitor cells from Cbfβ−/−:Tek-GFP/Cbfβ fetal liver fail to expand in vitro. AA4.1+CD19+B220lo-neg B-1 progenitor cells were sorted from E17.5 WT and Cbfβ−/−:Tek-GFP/Cbfβ FL and plated on OP9 stromal cells with IL-7 and Flt3-ligand. FACS dot plots of WT culture on day 10 (Upper) and Cbfβ−/−:Tek-GFP/Cbfβ culture on day 17 (Lower) are depicted. Representative FACS dot plots from three separate experiments are depicted. (B) YS and AGM cells from Cbfβ−/−:Tek-GFP/Cbfβ embryos can produce B progenitor cells in vitro. E10 YS and AGM from WT (Upper) and Cbfβ−/−:Tek-GFP/Cbfβ (Lower) embryos were plated on OP9 stromal cells with IL-7 and Flt3-ligand. Cells after 10-d coculture were analyzed for their surface markers by flow cytometry. Representative FACS dot plots from two separate experiments (each experiment was done in triplicate) are depicted.
YS and AGM Can Produce B-1 Progenitor Cells in the Embryo Devoid of HSCs.
The fetal liver is not a de novo site of hematopoietic cell emergence and must be seeded by various hematopoietic progenitor/stem cells derived from other hematopoietic tissues (15). Because HSCs are not produced in Cbfβ−/−:Tek-GFP/Cbfβ embryos, we examined the possible source of B-1 progenitor cells that seed the fetal liver. We cocultured YS and P-Sp/AGM cells with OP9 stromal cells as previously reported (13). As expected, Cbfβ−/−:Tek-GFP/Cbfβ YS and AGM cells produced AA4.1+CD19+B220+ B cells but at a lower frequency compared with WT YS/AGM (Fig. 2B). These cultured YS/P-Sp/AGM-derived AA4.1+CD19+B220+ B progenitor cells were confirmed to become mature B-1 cells in vivo upon transplantation as previously reported (13). Although there is a study suggesting that placenta has the potential of producing B cells at a pre-HSC stage (29), we found maternal B cells in the placenta at this time point whereas YS and P-Sp derived B-1 cells were confirmed to be embryonic in origin (13). Thus, although we cannot exclude the possibility of the placenta as a production site for the first wave of B-1 progenitor cells in Cbfβ−/−:Tek-GFP/Cbfβ embryos, our data indicate that YS and AGM produce B-1 progenitor cells that may seed the fetal liver in the Cbfβ−/−:Tek-GFP/Cbfβ embryos devoid of HSCs.
B-1 Progenitor Cells Can Mature into B-1 and MZ B Cells in Vivo but at Low Efficiency.
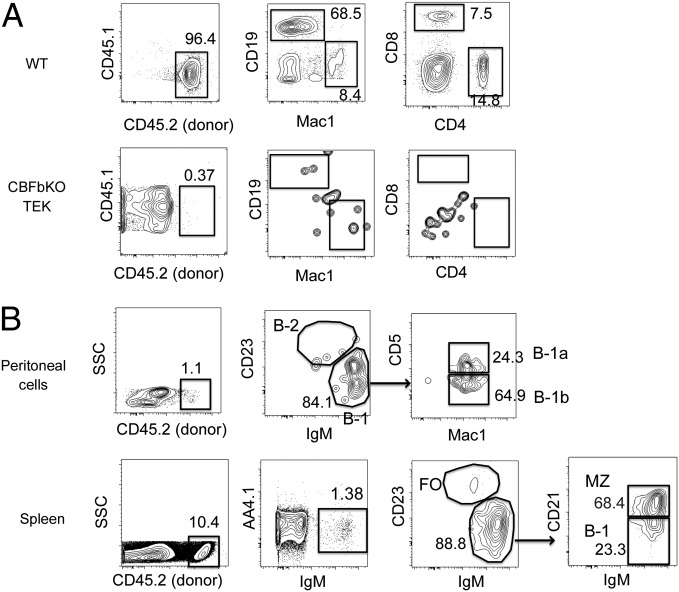
Lin−AA4.1+CD19+B220lo-neg B-1 progenitor cells were maintained until birth in Cbfβ−/−:Tek-GFP/Cbfβ mice (B progenitor cells were present at 40% of the number of WT mice in the spleen) (Fig. 1N), and mice died 1 d after birth as previously reported (16). Because mature B-1 cells are not detectable in the peritoneal cavity until 5 d after birth (30), we were unable to find mature peritoneal B-1 cells in the Cbfβ−/−:Tek-GFP/Cbfβ live-born neonates. To examine whether the B-1 progenitor cells found in the fetal liver of Cbfβ−/−:Tek-GFP/Cbfβ embryos can mature into B-1 cells in vivo, we transplanted fetal-liver MNCs from WT and Cbfβ−/−:Tek-GFP/Cbfβ embryos into irradiated NOD/SCID/IL2γc−/− (NSG) neonates. Although WT fetal-liver MNCs displayed multilineage repopulation in the PB of the recipient mice (Fig. 3A, Upper), no apparent engraftment was found in the PB of the recipient mice transplanted with Cbfβ−/−:Tek-GFP/Cbfβ fetal-liver MNCs as previously reported (16) (Fig. 3A, Lower). However, in the host peritoneal cavity and spleen, B-1 and MZ B cells were reconstituted from donor Cbfβ−/−:Tek-GFP/Cbfβ fetal-liver MNCs (Fig. 3B). No donor-derived B-2 cells or T cells were detected (Figs. 3B and 4E). These data indicated that HSC-deficient fetal-liver MNCs contain progenitor cells that can mature into only B-1 and MZ B cells in vivo upon transplantation.
Fig. 3.
Cbfβ−/−:Tek-GFP/CBFβ fetal-liver cells contain a few B-1 and MZ progenitor cells, but not HSCs. E15.5 FL MNCS from WT and Cbfβ−/−:Tek-GFP/Cbfβ embryos were transplanted into sublethally irradiated NSG neonates. PB analysis 8 wk after injection is depicted (A). PB of recipient mice transplanted with WT (Upper) and Cbfβ−/−:Tek-GFP/Cbfβ (Lower) fetal-liver cells are depicted. (B) The peritoneal (Upper) and spleen (Lower) cells in the recipient mice transplanted with Cbfβ−/−:Tek-GFP/Cbfβ fetal-liver cells are shown. FO, follicular B cell.
Fig. 4.
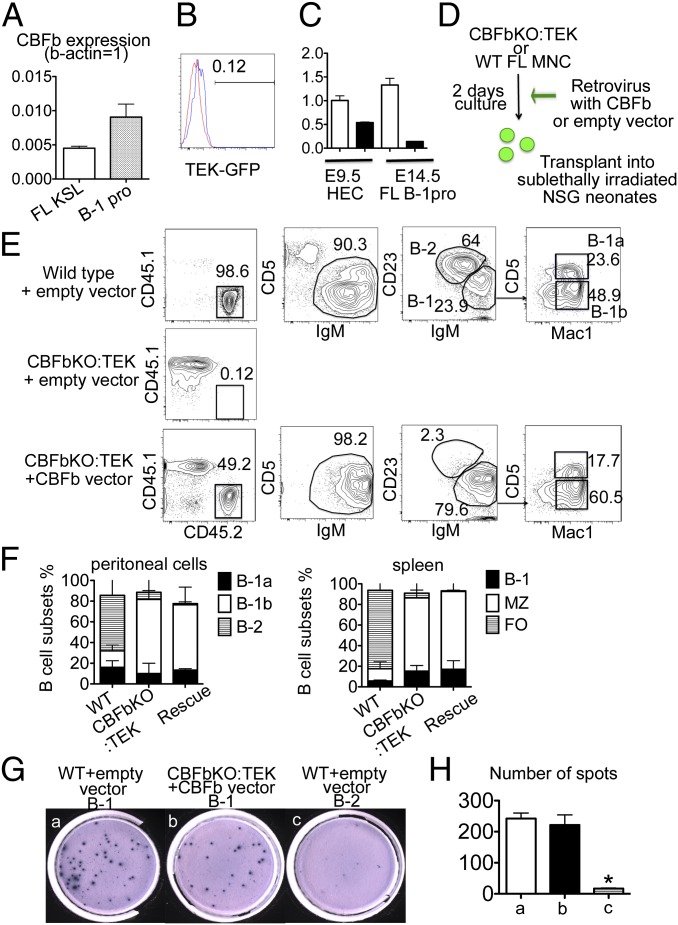
Overexpression of Cbfβ in Cbfβ−/−:Tek-GFP/Cbfβ fetal-liver cells restored B-1 and MZ cell engraftment. (A) Cbfβ expression in WT fetal-liver HSCs and B-1 progenitors by quantitative PCR is shown. Internal control: β-actin = 1.0. (B) Tek-GFP/Cbfβ expression within E15.5 fetal-liver AA4.1+CD19+B220dim B-1 progenitor cells in Cbfβ−/−:Tek-GFP/Cbfβ embryos. Red line, WT; blue line, Cbfβ−/−:Tek-GFP/Cbfβ fetal-liver B-1 progenitor cells. (C) Relative Cbfβ expression in E9.5 WT CD31+Tie2+ HECs, E9.5 Cbfβ−/−:Tek-GFP/Cbfβ HECs, E14.5 WT FL B-1progenitors, and E14.5 Cbfβ−/−:Tek-GFP/Cbfβ FL B-1 progenitors, compared with E9.5 WT HECs (=1). Open bar, WT; filled bar, Cbfβ−/−:Tek-GFP/Cbfβ. (D) Experimental design for rescuing Cbfβ expression in Cbfβ−/−:Tek-GFP/Cbfβ fetal-liver cells. (E) Peritoneal cells of recipient mice at 12 wk after transplantation with fetal-liver cells from WT (Top) or Cbfβ−/−:Tek-GFP/Cbfβ fetal-liver cells expressing empty vector (Middle) or Cbfβ (Bottom), respectively. (F) Donor-derived B-cell subsets in the recipient peritoneal cavity (Left) and spleen (Right) are depicted. Donor cells were WT fetal-liver MNC cells (left bars), Cbfβ−/−:Tek-GFP/Cbfβ fetal-liver cells (center bars), and Cbfβ−/−:Tek-GFP/Cbfβ fetal-liver cells infected with Cbfβ expressing retrovirus (rescue, right bars), in each panel. (G) IgM secretions from B-1 or B-2 cells stimulated with phosphorylcholine were detected by Elispot assay: (a) 1,000 B-1 cells from the recipient mice transplanted with WT fetal-liver cells with empty vector, (b) 650 B-1 cells derived from Cbfβ−/−:Tek-GFP/Cbfβ fetal-liver cells with Cbfβ expression vector, and (c) 1,000 B-2 cells derived from WT fetal-liver cells with empty vector were initially plated. (H) The numbers of spots per 1,000 B-1 or B-2 cells derived from recipient mice in Elispot assay (G, a, b, and c) are depicted. There was no significant difference between B-1 cells from WT fetal liver with empty vector and from Cbfβ−/−:Tek-GFP/Cbfβ fetal liver with Cbfβ expression vector. The number of IgM-secreting B-2 cells was significantly less than that of B-1 cells from WT or Cbfβ−/−:Tek-GFP/Cbfβ with Cbfβ expression vector (<0.05).
CBFβ Is Required for Maturation and Expansion of B-1 and MZ B Cells.
Although transplantable B-1 and MZ B-cell progenitors are present in HSC-deficient fetal liver, the level of chimerism was significantly lower than donor contributions from WT embryos (WT 97.8 ± 1.3% vs. Cbfβ−/−:Tek-GFP/Cbfβ 1.98 ± 0.3% in the peritoneal cavity and WT 92.8 ± 3.3% vs. Cbfβ−/−:Tek-GFP/Cbfβ 3.4 ± 2.4% in the spleen). It has been reported that Cbfβ is required in all blood-lineage cells for their development, but TEK-GFP/Cbfβ expression was not sustained once Tek-GFP/Cbfβ+ HECs become hematopoietic cells (16, 17). We confirmed that WT fetal-liver B-1 progenitor cells express Cbfβ at a similar level to HSCs (Fig. 4A). However, B-1 progenitor cells from Cbfβ−/−:Tek-GFP/Cbfβ do not express TEK-GFP/Cbfβ (Fig. 4B), thus having lost Cbfβ expression during differentiation from TEK+ HEC to B-1 progenitor cells (Fig. 4C). We have also recently determined that loss of Runx1 or Cbfβ in the B-cell lineage caused B-2 cell maturation defects (31). In this mouse model of Cbfβ deficient in B-cell lineage, we also observed a threefold reduction in B-1 cell number compared with WT mice, indicating that Cbfβ plays a critical role in B-1 cell development (Fig. S1) (31). Thus, we hypothesized that Cbfβ−/−:Tek-GFP/Cbfβ fetal-liver B-1 progenitor cells would require ongoing Cbfβ expression to mature into functional B-1 cells. To test the hypothesis, we overexpressed Cbfβ in Cbfβ−/−:Tek-GFP/Cbfβ fetal-liver MNCs using a retrovirus vector that constitutively produced CBFβ (Fig. 4D and Fig. S2A). Once retrovirally infected fetal-liver Cbfβ−/−:Tek-GFP/Cbfβ MNCs were transplanted into irradiated NSG neonates, Cbfβ−/−:Tek-GFP/Cbfβ-derived cells displayed dramatically improved engraftment capacity in up to 49% of donor cells in the peritoneal cavity (Fig. 4E) and improved maturation into IgM+ cells in the spleen (Fig. S3), and displayed only B-1 cell reconstitution in the peritoneal cavity and MZ and B-1 cells in the spleen 12 wk after transplantation (Fig. 4 E and F and Fig. S3). Nearly all (98%) of these Cbfβ−/−:Tek-GFP/Cbfβ-derived cells expressed retroviral Cbfβ-GFP (Fig. S2B) and displayed 15–25 times more Cbfβ mRNA expression compared with WT peritoneal B-1 cells (Fig. S2C). In contrast, Cbfβ−/−:Tek-GFP/Cbfβ fetal-liver cells infected with the empty vector control virus poorly engrafted (Fig. 4E, Middle). These results indicate that Cbfβ overexpression rescues maturation and expansion of impaired B-1 progenitor cells from Cbfβ−/−:Tek-GFP/Cbfβ embryos. Furthermore, Cbfβ expressing engrafted B-1 cells in the recipient mice were functional, displaying T cell-independent antiphosphorylcholine IgM secretion detected by enzyme-linked immunospot (Elispot) assay (Fig. 4 G and H). Taken together, Cbfβ reexpression restored engraftment and expansion/maintenance capacity of HSC-independent B-1 and MZ B cells from Cbfβ−/−:Tek-GFP/Cbfβ fetal liver in vivo, suggesting that Cbfβ plays important roles in B-1 cell development and proliferation, similar to B-2 cell development (31). Of note, HSCs were not restored by retrovirus Cbfβ overexpression in Cbfβ−/−:Tek-GFP/Cbfβ fetal-liver cells: no engraftment of any hematopoietic lineages other than B-1 or MZ B cells (Fig. 4E, Bottom, Fig. 4F, and Fig. S3), suggesting that the timing and dose of Cbfβ expression may be differentially required for the development of each hematopoietic lineage.
Discussion
In the current study, using unique HSC-deficient embryos, we have for the first time, to our knowledge, proven that progenitor cells that give rise to only B-1 and MZ cells exist in the fetal liver in the absence of HSCs. Although we were not able to quantitatively estimate the percentage contribution of the first wave of B cells in postnatal life (because the Cbfβ−/−:Tek-GFP/Cbfβ neonates die just after birth), it is probable that at least 40% of neonatal spleen B cells are derived from HSC-independent progenitors. Since fetal-liver cells do not produce any blood cells de novo but rely upon seeding from other sites, these B-1 progenitor cells must have been seeded from another site(s) of embryonic hematopoiesis, such as the yolk sac or embryo proper.
We also report that progenitor cells for MZ B cells developed in the absence of HSCs in the Cbfβ−/−:Tek-GFP/Cbfβ embryos. We previously identified YS/P-Sp–derived B-1 progenitor cells as giving rise to MZ B cells upon transplantation in vivo (13). The Cbfβ−/−:Tek-GFP/Cbfβ mouse model was instrumental in again validating the HSC-independent origin of MZ B cells. Because MZ B cells are also produced by adult BM HSCs and splenic lin−CD19+B220lo-negCD43− precursors (7, 32), we speculate that MZ B cells may comprise precursors from heterogenous sites. Ito et al. have reported less frequent generation of MZ B than B-1 cells from YS lymphoid progenitors (33) but still showed some MZ B-cell potential of the YS precursors. Differences in their results from our present work may be the result of differences in coculture lines and choice of cytokines or cytokine concentrations. Ectopic expression of Lin28b has been reported to reprogram adult hematopoietic stem/progenitor cells into cells with the ability to give rise to fetal lymphopoiesis (34). In this report, Lin28-expressing cells differentiated into predominantly B-1a and MZ B cells in the recipient mice, in agreement with our results. Thus, accumulating evidence supports a paradigm that a part of MZ B cells are derived from embryonic precursors, similar to B-1 cells.
Another important finding from our current work is that Cbfβ expression is required for B-1 cell development as well as B-2 cell development as previously reported (31). However, B-1 cell maturation is permissive in Cbfβ−/−:Tek-GFP/Cbfβ fetal liver-derived B-1 progenitor cells, and the reduction of mature B-1a cells in Cbfßflox/flox:MB1-cre mice is milder than the B-2 lineage. Cbfβ may play different roles in the B-1 and the B-2 cell lineages, and further study would be required to fully explore the roles played in each lineage.
In sum, the presence of transplantable B-1 and MZ progenitor cells in the fetal liver devoid of HSCs confirms the first wave of B lymphopoiesis in the immune layered model predicted more than 20 y ago (1). Many questions remain to be addressed. How do B-1 and MZ B-cell progenitors migrate into the fetal liver, spleen, and BM? How and where do the B-1 progenitor cells mature? How much do they really contribute to innate immunity compared with fetal-liver HSC-derived B-1 and MZ B cells? Lineage-tracing models would be required to resolve these questions, and answering those questions would help to understand the roles of B-1 cells in autoimmunity (35, 36), prevention of atherosclerosis (37), and prevention of infections in blood stem cell transplanted patients (38).
Materials and Methods
Mice.
Cbfβ+/−: Tek-GFP/Cbfβ mice were generated by crossing Cbfβ+/− mice and Tek-GFP/Cbfβ mice (kindly provided by Dr. Nancy Speck, University of Pennsylvania, Philadelphia). To obtain Cbfβ−/−: Tek-GFP/Cbfβ embryos, timed mating was performed by crossing Cbfβ+/−: Tek-GFP/Cbfβ males with Cbfβ+/−: Tek-GFP/Cbfβ or Cbfβ+/− females. Embryonic tail was used for genotyping.
Transplantation.
NSG neonates were used as recipients. One- to 5-d-old neonates were sublethally irradiated (150 rad), and fetal-liver cells from WT and Cbfβ−/−: Tek-GFP/Cbfβ embryos were injected into the peritoneal cavity of NSG neonates. Six to 12 wk after injection, PB, peritoneal, and spleen cells were analyzed donor cell types in the recipient mice.
OP9 Culture.
An OP9 stromal cell line was maintained with α-MEM and 20% (vol/vol) FBS. YS, P-Sp/AGM, or fetal-liver cells were plated on six-well plates confluent with OP9 stromal cells in induction medium [α-MEM, 10% (vol/vol) FBS and 5 × 10−5 M β-mercaptoethanol] supplemented with 10 ng/mL IL-7 and 10 ng/mL Flit3-ligand (PEPROTECH) as previously reported (13). Floating cells in the culture medium were collected at optimal time points, and we analyzed their surface markers by flow cytometry.
Flow Cytometry.
For analysis and sorting of B-1 progenitors, B-1, MZ B Cells, and each lineage of blood cells, the following antibodies were used at different fluorescent color combinations: anti-mouse AA4.1 (AA4.1), CD19 (1D3), B220 (RA3-6B2), IgM (II/41), CD21 (8D9), CD23 (B3B4), CD11b (M1/70), CD5 (53-7.3), CD3e (145-2C11), Ter119 (TER-119), c-kit (2B8), Sca-1 (D7), and IL-7Ra (A7R34) (all purchased from eBioscience). A lineage mixture containing anti-Ter119, CD11b, and CD3 was used for lineage negative gating. Cells were analyzed on LSRII or sorted on FACS Aria (Becton Dickinson).
Quantitative PCR.
Fetal-liver Lin−Sca-1+c-kit+ cells or AA4.1+CD19+B220lo-neg B-1 progenitors were sorted, and RNA was extracted using RNasy (QIAGEN) following the manufacturer’s instructions. cDNA was made using Super Scrip III fast strand Synthesis System (Invitrogen). Real-time PCR was performed using FastStart Universal SYBR Green Master (Rox) (Roche) by 7500 Real-Time PCR System (Life Technologies). The following sets of primers were used: Cbfβ, forward, GCAAGTTCGAGAACGAGGAG; reverse, CCCGTGTACTTAATCTCGCA; β-actin, forward, TCCTGTGGCATCCATGAAACT; reverse, GAAGCACTTGCGGTGCACGAT.
Expression Vectors and Retrovirus Production.
Full-length cDNA of mouse Cbfβ was purchased from TrueClone (catalog no. MC204426) and amplified by PCR. cDNA of mouse Cbfβ was inserted into an MSCV-IRES-GFP (MIG) plasmid. Retroviruses carrying empty vector (MIG-empty) or MIG-Cbfβ were produced using the Phoenix cell line. Virus supernatants were concentrated by centrifuging at 19,000 × g, 4 °C for 100 min. WT and Cbfβ−/−: Tek-GFP/Cbfβ fetal-liver MNCs were plated on retronectin-coated 24-well plates in induction medium with 10 ng/mL stem cell factor, 10 ng/mL IL-7, and 10 ng/mL Flit3-ligand. Concentrated virus supernatant was added into each well, and spin infection was performed by centrifuging at 1,000 × g, 32 °C for 100 min. The next morning, the medium was half changed, and second-spin infection was done. Three hours after second infection, cells were harvested and used for the transplantation assay. Infectious efficiency was confirmed by detecting GFP+ cells by flow cytometry.
Elispot.
Elispot was done as previously described (39). Donor-derived B-1 or B-2 cells in the peritoneal cavity of recipient mice were sorted on a FACS Aria (Becton Dickinson), suspended in Elispot medium (RPMI 1640 medium with 10% FBS and 5 × 10−5 M β-mercaptoethanol), supplemented with 10 ng/mL IL-5, and cultured for 36–48 h in 96-well plates precoated with phosphorylcholine-conjugated keyhole limpet hemocyanin. Five hundred to 1,000 cells were distributed into an Elispot MultiScreenHTS Filter Plate (Millipore) precoated with anti-mouse IgM and cultured in Elispot medium overnight. After washing, alkaline phosphatase-conjugated goat anti-mouse IgM (Southern Biotech) was applied to each well and incubated for 45 min. After washing, spots were visualized using BCIP/NBTplus substrate as described (Mabtech).
Statistics.
All experiments were completed in triplicate. The Student t test was used for statistical analysis.
Supplementary Material
Acknowledgments
We thank Drs. Mervin C. Yoder and Michael J. Ferkowicz for editorial comments. This work was partially supported by a Biomedical Research grant from Indiana University School of Medicine and the Riley Children’s Foundation.
Footnotes
*This Direct Submission article had a prearranged editor.
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1407370111/-/DCSupplemental.
References
- 1.Herzenberg LA, Herzenberg LA. Toward a layered immune system. Cell. 1989;59(6):953–954. doi: 10.1016/0092-8674(89)90748-4. [DOI] [PubMed] [Google Scholar]
- 2.Montecino-Rodriguez E, Dorshkind K. B-1 B cell development in the fetus and adult. Immunity. 2012;36(1):13–21. doi: 10.1016/j.immuni.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montecino-Rodriguez E, Leathers H, Dorshkind K. Identification of a B-1 B cell-specified progenitor. Nat Immunol. 2006;7(3):293–301. doi: 10.1038/ni1301. [DOI] [PubMed] [Google Scholar]
- 4.Hayakawa K, Hardy RR, Herzenberg LA, Herzenberg LA. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J Exp Med. 1985;161(6):1554–1568. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hardy RR, Hayakawa K. A developmental switch in B lymphopoiesis. Proc Natl Acad Sci USA. 1991;88(24):11550–11554. doi: 10.1073/pnas.88.24.11550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barber CL, Montecino-Rodriguez E, Dorshkind K. Reduced production of B-1-specified common lymphoid progenitors results in diminished potential of adult marrow to generate B-1 cells. Proc Natl Acad Sci USA. 2011;108(33):13700–13704. doi: 10.1073/pnas.1107172108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghosn EE, et al. Distinct B-cell lineage commitment distinguishes adult bone marrow hematopoietic stem cells. Proc Natl Acad Sci USA. 2012;109(14):5394–5398. doi: 10.1073/pnas.1121632109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herzenberg LA, et al. The Ly-1 B cell lineage. Immunol Rev. 1986;93:81–102. doi: 10.1111/j.1600-065x.1986.tb01503.x. [DOI] [PubMed] [Google Scholar]
- 9.Hardy RR, Hayakawa K. B cell development pathways. Annu Rev Immunol. 2001;19:595–621. doi: 10.1146/annurev.immunol.19.1.595. [DOI] [PubMed] [Google Scholar]
- 10.Carvalho TL, Mota-Santos T, Cumano A, Demengeot J, Vieira P. Arrested B lymphopoiesis and persistence of activated B cells in adult interleukin 7(-/)- mice. J Exp Med. 2001;194(8):1141–1150. doi: 10.1084/jem.194.8.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hao Z, Rajewsky K. Homeostasis of peripheral B cells in the absence of B cell influx from the bone marrow. J Exp Med. 2001;194(8):1151–1164. doi: 10.1084/jem.194.8.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carey JB, Moffatt-Blue CS, Watson LC, Gavin AL, Feeney AJ. Repertoire-based selection into the marginal zone compartment during B cell development. J Exp Med. 2008;205(9):2043–2052. doi: 10.1084/jem.20080559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshimoto M, et al. Embryonic day 9 yolk sac and intra-embryonic hemogenic endothelium independently generate a B-1 and marginal zone progenitor lacking B-2 potential. Proc Natl Acad Sci USA. 2011;108(4):1468–1473. doi: 10.1073/pnas.1015841108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lux CT, et al. All primitive and definitive hematopoietic progenitor cells emerging before E10 in the mouse embryo are products of the yolk sac. Blood. 2008;111(7):3435–3438. doi: 10.1182/blood-2007-08-107086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin Y, Yoder MC, Yoshimoto M. Lymphoid progenitor emergence in the murine embryo and yolk sac precedes stem cell detection. Stem Cells Dev. 2014;23(11):1168–1177. doi: 10.1089/scd.2013.0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen MJ, et al. Erythroid/myeloid progenitors and hematopoietic stem cells originate from distinct populations of endothelial cells. Cell Stem Cell. 2011;9(6):541–552. doi: 10.1016/j.stem.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller J, et al. The core-binding factor beta subunit is required for bone formation and hematopoietic maturation. Nat Genet. 2002;32(4):645–649. doi: 10.1038/ng1049. [DOI] [PubMed] [Google Scholar]
- 18.Okuda T, van Deursen J, Hiebert SW, Grosveld G, Downing JR. AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell. 1996;84(2):321–330. doi: 10.1016/s0092-8674(00)80986-1. [DOI] [PubMed] [Google Scholar]
- 19.Wang Q, et al. The CBFbeta subunit is essential for CBFalpha2 (AML1) function in vivo. Cell. 1996;87(4):697–708. doi: 10.1016/s0092-8674(00)81389-6. [DOI] [PubMed] [Google Scholar]
- 20.Yoshimoto M, et al. Autonomous murine T-cell progenitor production in the extra-embryonic yolk sac before HSC emergence. Blood. 2012;119(24):5706–5714. doi: 10.1182/blood-2011-12-397489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishikawa SI, et al. In vitro generation of lymphohematopoietic cells from endothelial cells purified from murine embryos. Immunity. 1998;8(6):761–769. doi: 10.1016/s1074-7613(00)80581-6. [DOI] [PubMed] [Google Scholar]
- 22.Böiers C, et al. Lymphomyeloid contribution of an immune-restricted progenitor emerging prior to definitive hematopoietic stem cells. Cell Stem Cell. 2013;13(5):535–548. doi: 10.1016/j.stem.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 23.Yamane T, Hosen N, Yamazaki H, Weissman IL. Expression of AA4.1 marks lymphohematopoietic progenitors in early mouse development. Proc Natl Acad Sci USA. 2009;106(22):8953–8958. doi: 10.1073/pnas.0904090106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohmura K, et al. Emergence of T, B, and myeloid lineage-committed as well as multipotent hemopoietic progenitors in the aorta-gonad-mesonephros region of day 10 fetuses of the mouse. J Immunol. 1999;163(9):4788–4795. [PubMed] [Google Scholar]
- 25.Ohmura K, et al. Immature multipotent hemopoietic progenitors lacking long-term bone marrow-reconstituting activity in the aorta-gonad-mesonephros region of murine day 10 fetuses. J Immunol. 2001;166(5):3290–3296. doi: 10.4049/jimmunol.166.5.3290. [DOI] [PubMed] [Google Scholar]
- 26.Inlay MA, et al. Identification of multipotent progenitors that emerge prior to hematopoietic stem cells in embryonic development. Stem Cell Rev. 2014;2(4):457–472. doi: 10.1016/j.stemcr.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishikawa SI, et al. All B cells are progeny of endothelial cells: A new perspective. Immunol Rev. 2000;175:112–119. [PubMed] [Google Scholar]
- 28.Montecino-Rodriguez E, Leathers H, Dorshkind K. Bipotential B-macrophage progenitors are present in adult bone marrow. Nat Immunol. 2001;2(1):83–88. doi: 10.1038/83210. [DOI] [PubMed] [Google Scholar]
- 29.Rhodes KE, et al. The emergence of hematopoietic stem cells is initiated in the placental vasculature in the absence of circulation. Cell Stem Cell. 2008;2(3):252–263. doi: 10.1016/j.stem.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montecino-Rodriguez E, Dorshkind K. Formation of B-1 B cells from neonatal B-1 transitional cells exhibits NF-κB redundancy. J Immunol. 2011;187(11):5712–5719. doi: 10.4049/jimmunol.1102416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seo W, Ikawa T, Kawamoto H, Taniuchi I. Runx1-Cbfβ facilitates early B lymphocyte development by regulating expression of Ebf1. J Exp Med. 2012;209(7):1255–1262. doi: 10.1084/jem.20112745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghosn EE, Sadate-Ngatchou P, Yang Y, Herzenberg LA, Herzenberg LA. Distinct progenitors for B-1 and B-2 cells are present in adult mouse spleen. Proc Natl Acad Sci USA. 2011;108(7):2879–2884. doi: 10.1073/pnas.1019764108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ito C, Yamazaki H, Yamane T. Earliest hematopoietic progenitors at embryonic day 9 preferentially generate B-1 B cells rather than follicular B or marginal zone B cells. Biochem Biophys Res Commun. 2013;437(2):307–313. doi: 10.1016/j.bbrc.2013.06.073. [DOI] [PubMed] [Google Scholar]
- 34.Yuan J, Nguyen CK, Liu X, Kanellopoulou C, Muljo SA. Lin28b reprograms adult bone marrow hematopoietic progenitors to mediate fetal-like lymphopoiesis. Science. 2012;335(6073):1195–1200. doi: 10.1126/science.1216557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chou MY, et al. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J Clin Invest. 2009;119(5):1335–1349. doi: 10.1172/JCI36800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grönwall C, Vas J, Silverman GJ. Protective Roles of Natural IgM Antibodies. Front Immunol. 2012;3:66. doi: 10.3389/fimmu.2012.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaw PX, et al. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J Clin Invest. 2000;105(12):1731–1740. doi: 10.1172/JCI8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moins-Teisserenc H, et al. CD19(+)CD5(+) B cells and B1-like cells following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19(6):988–991. doi: 10.1016/j.bbmt.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 39.Tumang JR, Francés R, Yeo SG, Rothstein TL. Spontaneously Ig-secreting B-1 cells violate the accepted paradigm for expression of differentiation-associated transcription factors. J Immunol. 2005;174(6):3173–3177. doi: 10.4049/jimmunol.174.6.3173. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.