Significance
The taxon “Homo floresiensis” was termed “the most important find in human evolution for 100 years.” The name was invented for several fragmentary skeletons found on one Indonesian island, all less than 100,000 y old (some as recent as 12,000 y), all coeval with only Homo sapiens existing everywhere else in the world. Defining taxonomic features appear in just a single specimen, LB1, which has the only known skull and femora. Key features of 380 mL and 1.06 m are shown here to be underestimates, supportable as species-defining only by overlooking asymmetry and disproportion that are general signs of abnormal development. Logically, patent isolated individual abnormality obviates new species status even without diagnosis of a particular syndrome.
Keywords: atavism, biogeography, developmental morphology, Pleistocene, probability
Abstract
The original centrally defining features of “Homo floresiensis” are based on bones represented only in the single specimen LB1. Initial published values of 380-mL endocranial volume and 1.06-m stature are markedly lower than later attempts to confirm them, and facial asymmetry originally unreported, then denied, has been established by our group and later confirmed independently. Of nearly 200 syndromes in which microcephaly is one sign, more than half include asymmetry as another sign and more than one-fourth also explicitly include short stature. The original diagnosis of the putative new species noted and dismissed just three developmental abnormalities. Subsequent independent attempts at diagnosis (Laron Syndrome, Majewski osteodysplastic primordial dwarfism type II, cretinism) have been hampered a priori by selectively restricted access to specimens, and disparaged a posteriori using data previously unpublished, without acknowledging that all of the independent diagnoses corroborate the patent abnormal singularity of LB1. In this report we establish in detail that even in the absence of a particular syndromic diagnosis, the originally defining features of LB1 do not establish either the uniqueness or normality necessary to meet the formal criteria for a type specimen of a new species. In a companion paper we present a new syndromic diagnosis for LB1.
Excavations at Liang Bua Cave on the island of Flores in Indonesia have yielded what is termed “the most important find in human evolution for 100 years” [Wood B. 28 October 2004. Anthropologist says new skeleton discovery most significant in 100 years. Interview by Alison Caldwell. AM: Australia ABC Local Radio. (www.abc.net.au/am/content/2004/s1229506.htm)]. This skeletal sample is unusual, with only LB1 presenting an anomalous array of anatomical variants, most of which are unmatched in accompanying more fragmentary skeletons. No explanation of the LB1 individual can render it ordinary, but neither does discovery of an atypical individual necessitate the creation of a novel species (1); other hypotheses are possible (2).
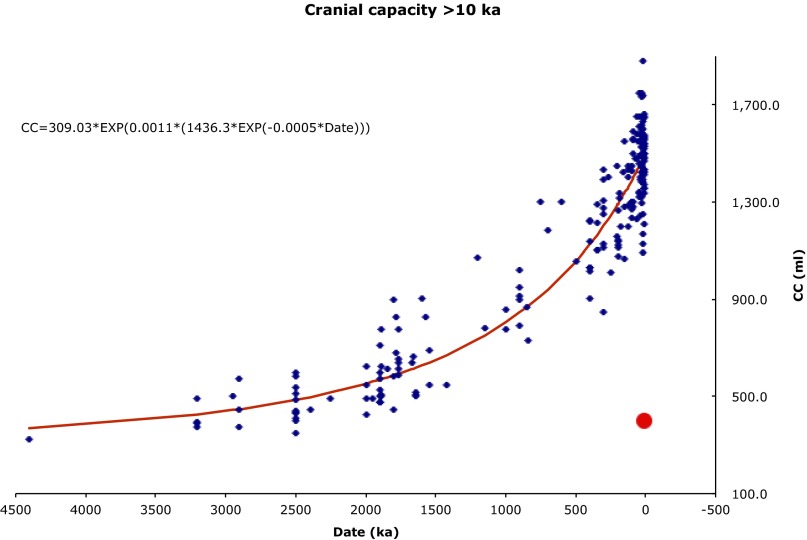
Rather than being the type specimen of a unique new species, LB1 represents the counterpart of what in mathematics is termed a singularity: a point at which an equation, surface, or other entity blows up or becomes degenerate. By analogy with mathematical use, “Homo floresiensis” is a purely hypothetical point in paleoanthropological space; one cannot get to its locus by plausible pathways of evolutionary biology (Fig. 1).
Fig. 1.
Endocranial volume of the LB1 (red dot) skull lies markedly below the long term trend in hominin endocranial volume.
About a quarter of a century ago, one of us (3) wrote about another episode in earth history: “Expressed in conventional wisdom, the inverse relation between frequency and magnitude is a modified form of the ‘Murphy’s Law’: anything that could happen, will happen in due time.” This insight applies to any low-frequency event in the geological record, including excavation of a skeleton with unexpected anomalies. Originally, however, the quote referred to the mass extinction over a short span of geological time at the end of the Cretaceous era (then referred to as the K/T boundary, K for German Kreide, chalk, the signature Cretaceous sediments, and T for Tertiary period; now K/Pg for Cretaceous/Paleogene), from the impact on the earth of a huge extraterrestrial bolide. Both the extinction event and its probable cause now approach universal acceptance, although not in 1980, when within a month of each other first Hsü (4) and then Alvarez et al. (5) hypothesized that an extremely large comet or asteroid had struck the earth, catalyzing the disappearance of a vast number of taxa from dinosaurs and all terrestrial animals larger than 25 kg through microscopic foraminiferan and nannoplankton. The impact theory was opposed vigorously, with many conventional paleontologists arguing over nearly a decade for terrestrial causes. Opinions divided chiefly along lines of gradualist terrestrial vs. catastrophic cosmic causation, the former camp including many paleontologists (6, 7), the latter mainly geologists (4) and physicists (5).
As often is the case in important controversies, many papers were published and debate ranged widely along disciplinary lines for years. When doubt was cast on some consequences of an extraterrestrial bolide strike, for a time the entire cosmic impact hypothesis was declared defunct. Extensive research on nannofossil records did render the narrow “impact winter” scenario untenable (8). Using later data (9), Hsü (3) calculated that the terminal Cretaceous event lasted about 30,000 y, with the pace of extinction attaining 15,000 times the background rate, easily qualifying objectively as catastrophic. Among the various alternative terrestrial mechanisms that could explain catastrophic extinction, only explosive volcanism (7, 10) could cover such essential elements as the high iridium concentration anomaly in boundary sediments. Finally, the sole distinguishing element that could be explained by extraterrestrial bolide impact but not terrestrial volcanism was the presence of shock-quartz grains in the K/T impact boundary sediments, but not in vulcan pyroclastics.
The protracted arguments over massive Cretaceous extinctions have their counterparts in the insistence that the Liang Bua skeletal remains must represent a new (formulaically conceived) hominin species because they manifest a combination of anatomical uniquenesses with some primitive features. In both cases (K/Pg extinction, Liang Bua Cave skeletons) many ancillary elements simply are distractions.
Here we show that asymmetry (particularly craniofacial) and disproportion (of braincase with face, femur with other long bones and foot, and so forth) are the shock-quartz grains of LB1; here they signal the impact of abnormal ontogenic development on the phenotype of that single individual, the diagnostic features of which cannot be “explained” by positing a new hominin species, unless its defining Bauplan is pervasive abnormality. All of the epiphenomenological arguments about the absolutely small LB1 brain being large and complex enough to make tools, whether its possessor was threatened by giant storks or Komodo dragons, even whether the entire population of which it was a member was wiped out by a volcanic eruption, and so forth, are diversions. Competing explanations for the defining oddities of the “new species”—basically properties of LB1 alone—juxtapose island dwarfing (11) versus colonization at an unknown time from an unidentified African source by an undiscovered lineage that left no traces over more than 8,000 km and a million years (12). Both origin hypotheses for the “new species” mytheme (13) are offered to account for anatomical peculiarities, from a skull unmatchable among normal hominins to femora so short that they are said to suggest limb proportions recalling Australopithecus afarensis. These constructs represent the Liang Bua Cave counterpart of “multiple working hypotheses gone mad” (14).
Murphy’s Law in Paleontology: Uncommon Events, Morphologies, and the Inevitability of the Improbable
The first description and diagnosis of H. floresiensis focused entirely on LB1 (aside from LB2, a single left P3, from which neither body nor brain size can be inferred), and was couched in canonical terms: many of the specimen’s anatomical characteristics are unusual (although not unique, as said then and reiterated until now), so the stereotypical paleoanthropological response designated it as a new species. A competing hypothesis came within days: the disharmonious craniofacial features of LB1 signaled developmental abnormality of these unfossilized remains (2). This alternative was opposed reflexively. Ignoring that dates for the Liang Bua Cave remains were encompassed entirely within those of anatomically modern Homo sapiens, paleoanthropologists assumed, statistically reasonably as a first approximation in studies of genuine very old rare fossils, that a specimen showing a previously unknown morphological pattern was typical of the population from which it was sampled. However, a working hypothesis never should be accepted as an axiom immune from further scrutiny. That LB1 was typical of its taxon is circular, because the initial diagnosis (3) designated LB1 as the type specimen of H. floresiensis, so LB1 was defined as representative—literally typical—a priori. That LB1 might be abnormal was considered improbable and the hypothesis dismissed perfunctorily (15). Thus, the initially possible (new species) became unquestionable, and a plausible alternative (developmental abnormality) was redefined ever more obdurately as unacceptable.
A probabilistic assessment of a developmentally abnormal individual occurring in populations present or past is possible. About a dozen individuals with endocranial volumes comparable to LB1 are known among archaeological remains of H. sapiens dating back as far as Magdalenian Period, 11 ka (2). Another extremely small human skull in yet another archaeological site several thousand years older is unsurprising.
The Probability of Developmental Abnormalities in the Paleontological Record
At birth, ∼3% of human infants have some major physical abnormality (16) affecting phenotypic structural or functional characteristics, with one-third of these disorders (1%) influencing the brain and >0.1% shaping limbs. Approximately half of all congenital abnormalities are sporadic (unknown causation). About 20–25% of anomalies are multifactorial, resulting from interaction of genetic and environmental elements; another 12–25% are attributable to exclusively genetic causes, chiefly chromosomal abnormalities (deletions or duplications of portions of chromosomes, trisomies, monosomies, and so forth). Full or partial genetic influence can play a role in up to half of all neonatal abnormalities, many affecting skeletal development.
Congenital anomalies now encountered neonatally occurred in previous human populations as well as their hominoid ancestors. Chromosomal and phenotypic counterparts of human trisomy 21 are known in chimpanzees (17) and orangutans (www.orangutanprotection.com/indexina.php?menu=show_weblog.php&id=170&lang=eng). Some early historic human skeletal remains manifest attributes of Down syndrome (18, 19). Frequencies may have varied earlier because some syndromes are influenced by maternal age. Survival rates may have been lower without medical care and support networks.
Morphological Evidence: Argument for Uniqueness
Features used to create and sustain the impression that the skeletons from Liang Bua Cave represent a new species have come in several successive waves. “When considered as a whole, the cranial and postcranial skeleton of LB1 combines a mosaic of primitive, unique and derived features not recorded for any hominin” (15). Conflation of data and interpretation continues, with some inferences presented as if they were observations. Thus, the first abstract (15) noted “Here we report the discovery, from the late Pleistocene of Flores, Indonesia, of an adult hominin with stature and endocranial volume approximating 1 m and 380 mL, respectively—equal to the smallest known australopithecines.” The 380-mL endocranial volume was a direct measurement (although incorrectly underestimated); the reported stature is not. It is an extrapolation compounding a datum (femur length of LB1), with formulae derived from an African pygmy population (rather than a more appropriate Asian one), assuming developmental normality of femur length, then diminished further in a commentary (20). However, in addition to Down syndrome and Turner syndrome, more than two dozen other dysplasiae and syndromes have short femora as one phenotypic feature (21).
The initial description of LB1 is confounded by comparison with earlier hominin populations, ranging from Homo erectus to Australopithecus and Paranthropus. However, no hominin other than H. sapiens is known worldwide during the lifetime of LB1. Many traits characterized as unique, and hence diagnostic, of a new species (e.g., enlarged, block-like P3 teeth, P3 and P4 teeth with Tomes roots, rotated P4 teeth, mandible lacking a chin) are known, along with unusually short stature (in global context) among Australomelanesian populations, particularly those still living on Flores. Of the very few features originally reported as unique, each trait or small trait cluster deviates from the expected regional pattern in its own incongruent way: for example, very low endocranial volume and craniofacial asymmetry are developmentally abnormal by objective clinical standards (22, 23). The humerus with a low degree of torsion can be matched in other human populations (24–26), and also explained in several alternative ways related to activity patterns, negating decisively assertions of its “uniqueness.” The clavicle described as S-shaped in dorsal profile can be matched at low frequencies in living humans, both normal and developmentally abnormal. None of the originally reported traits (15, 26) are so unusual as to have justified the invention of a new hominin species at the time (22). Features subsequently proposed as species diagnostic (e.g., anatomy of the foot) also misrepresent anomaly as taxonomic distinctiveness.
In this report we examine an array of morphological characteristics that originally were cited as unique or primitive defining features of H. floresiensis. They are neither unique nor archaic; all have less extreme alternative (sometimes multiple) explanations not requiring invention of a novel species. In the study of human evolution, we have been here before, having a new hominin taxon based on little primary evidence nonetheless being widely supported, often for considerable periods of time, before being shown to have been invalid (Supporting Information, SI1).
Morphological Evidence: Unusual Is Not Unique
Skull.
A decade past the original discovery there still is only one cranium known from Liang Bua Cave on Flores. Its major calvarial bones comprise the paired temporals and parietals, plus the frontal and occipital; sphenoid and ethmoid also contribute to the skull vault. The sphenoid and base of the occipital influence skull length and when compromised developmentally, as in Down syndrome and some other pathologies, produce brachycephaly (Supporting Information, SI2). The upper facial skeleton is reasonably well preserved save for nasal bones; this and the mandible are discussed separately in detail in Supporting Information, SI3. Preservation of the more fragile bones argues strongly against taphonomic deformation as the explanation for the unusual asymmetry (22, 27), countering attempts to minimize the extent of asymmetry (28, 29), with ref. 28 also exaggerating effects of taphonomic distortion.
Before the excavation of Liang Bua Cave, at least 12 other skeletons had been unearthed on Flores (30, 31). Where skulls were available, these specimens all had endocranial volumes in the low end of the range for recent H. sapiens, nearly three times the volume of LB1; for example, CF (31) has an endocranial volume of 1,258 mL. Normal skulls recovered on Palau are in the range of about 1,000 mL (32). Asymmetry aside, facial skeletal dimensions of LB1 differ less from those of developmentally normal modern humans than does its endocranial volume (see figure 1 in ref. 2), with the exception of orbits unusually high in relation to width.
Postcranial Skeleton.
Upper limb.
“…The upper limb presents a unique mosaic of derived (human-like) and primitive morphologies, the combination of which never is found in either healthy or pathological modern humans” (25). A statement of that form falls in the same philosophical category as that of Bertrand Russell’s hypothetical orbiting teapot. There is no logical burden on anyone to disprove such assertions because “never is found” implies an observation set that is nonexistent. Positive observations do carry weight. Aside from multiple monozygotic births, each human is unique genetically, and even genetically identical individuals have different life histories that produce detectable phenotypic distinctions. If more were meant than mundanely definitional individual distinctiveness, readers of the published reports might assume that some systematic research has been performed on variation, particularly into the hundreds of pathologies that are pertinent to assessment of LB1. There is no clear evidence that such investigations in fact have been carried out. Of the 23 references cited (25), none are primary studies of abnormal syndromes pertinent to understanding the anomalous morphology of LB1, particularly in a geographic region that widely lacks medical care and research that could document regional variation in even common developmental abnormalities, such as Down syndrome, let alone rarer disorders. Characteristics of the larger bones of the upper limb (scapula, clavicle, humerus, radius, ulna) are reviewed in detail in Supporting Information, SI4. In contrast to other individuals, such as LB6, which on the scant available evidence appear normal, the upper limb elements of LB1 show numerous developmental abnormalities.
The island isolation model (15) for the taxonomic novelty of H. floresiensis was based on anatomical features (endocranial volume, reconstructed stature and limb proportions, humeral and dental features, and so forth). Among some supporters of the new species the island isolation model was supplanted, and for yet others supplemented (despite contradictory implications) as analyses of wrist bone morphology were published (33–36). The effect of these papers has been to introduce a new—and different—set of claims for anatomical uniquenesses, but now centered on the wrist, shortly after the first set had been negated (22). The shift in emphasis on evidence from previous “uniquenesses” already disproved to new ones—focused on the wrist bones—created contradiction, because the substitute species-defining features are incompatible with the previous derivation via devolution from H. erectus. Required was a new origin tale: that the small brain and body size of H. floresiensis had existed before arrival of this hominin lineage on the island, thus somehow producing elsewhere the effects attributed previously to “island isolation” on Flores (note that the morphology has not changed), evidently without needing an island. The new hypothesis has its own set of problems; among others, the anomalous wrist bones ally H. floresiensis to no particular previously known hominin, showing only diffuse hominoid resemblances, as might be expected for an atavism.
Chiefly because the carpal evidence was not part of the original set of supposed unique traits defining H. floresiensis, the wrist offers not a solution to the species definition but a new conundrum. Because vague “plesiomorphy” of a sort might imply anything from heightened familial resemblance because of inbreeding, through atavism and other possibilities too numerous to explore here, and further because claims made for the phylogenetic significance of the carpals are so diffuse, discussion of them appears elsewhere (Supporting Information, SI5). Although from the outset the hypothesis of island isolation was implausible, it is all of the more puzzling that some advocates of H. floresiensis now try to derive that hypothetical taxon from an unknown Plio-Pleistocene antecedent that can neither be located nor reconstructed coherently.
Lower limb.
Much early diagnosis (15, 26) has been repeated (36), with additional comments that the limb proportions of LB1 were “similar to Australopithecus and distinct from humans and early Homo erectus” (37). Note that LB1 is the only individual for whom both key lower limb and upper limb elements are known, sans radius, the length of which has been estimated from ulnar length to be 190 mm, as noted separately (Supporting Information, SI4). Therefore, statements about the limb proportions of H. floresiensis are descriptive only of the LB1 individual. This is no small reservation, because the tibia of the LB8 adult is markedly shorter (216 mm) than that of LB1 (estimated at 235–240 mm), and the LB6 radius (157 mm) is dramatically shorter than the 190 mm estimated for LB1 but even more markedly shorter than that of the LB3 adult (210 mm), which in turn exceeds that of LB1 by more than 10%. Our studies of primary lower limb data have identified many separate enigmas in this small sample of bones. Consequently detailed analyses are given in Supporting Information, SI6. Overall, the wide individual variations among the few Liang Bua Cave limb skeletal elements do not support the H. floresiensis taxonomic speculations. These variations include its alleged uniformity in general and its limb proportions (again, inferred virtually exclusively from LB1) in particular. There are multiple objective indications that the LB1 individual is developmentally abnormal, with fuller analysis and diagnosis in a companion paper, making the more complete case for that inference (38).
Conclusions
For various reasons, some holes in the earth take on greater scientific import than others. Examples are the Chicxulub impact crater on the Yucatán Peninsula of Mexico and the Liang Bua Cave excavation on the island of Flores in Indonesia. The first location marks the passing of thousands of species; the second location marks the demise of a few individuals from just one species (on the balance of evidence, a regional variant of H. sapiens). When such scientific problems are perceived as unusually important their causes can remain long in dispute.
For the Cretaceous mass extinction, the iridium-isotope anomaly and carbon-isotope perturbation were important pieces of evidence; correspondingly, in the Liang Bua Cave assemblage, anatomical features of cranial and postcranial bones are important elements. Skeletal asymmetry and disproportion in LB1 are the shocked quartz grains of the Liang Bua Cave skeletal puzzle. Ignore them and several different explanations might fit the other data. Admit them and a “species” that is acknowledged even by its supporters to have evolved an unusual abundance of adaptive handicaps (literally from shrunken head to flat foot, although only in LB1) before becoming extinct might better be comprehended instead as an individual who overcame multiple developmentally related challenges to survive for several decades among a normal cohort.
In this paper we have not presented a specific diagnosis for the developmental anomaly that marks the LB1 individual, although we believe that a compelling argument can be made for such a diagnosis, the subject of a companion paper (38). This course of action is purposefully taken. The case that we make here is a necessary first step, establishing that the existing evidence has from the first been insufficient to support LB1 as a tenable type specimen for a separate species, H. floresiensis. To make this point it is not necessary to diagnose any particular developmental abnormality or medical syndrome, contra the statement “We understand the cognitive dissonance that the discovery of Homo floresiensis has created in some scientific circles, and we encourage efforts to frame testable, alternative hypotheses to account for these surprising hominins. We submit that ‘pathology,’ however, is not a scientific explanation unless a differential diagnosis is made specific, plausible, and testable” (29). Ignoring the nonce ad hominem psychologizing (see also www.liangbuacave.org), the answer to that assertion already has been provided: “While the statement by Falk and colleagues seems superficially plausible, the formulation simply is fundamentally illogical. It is philosophically comparable to investigators coming into a room and discovering on the floor a human body that exhibited the ambient temperature, was stiff, and showed no evidence of pulse or heartbeat, but with the investigators asserting that it would be impossible to render a verdict that the recumbent individual was deceased, until a specific cause of death could be known” (39).
For LB1 it is logically sufficient to show the presence of the several elements common to a great many disorders (asymmetry, disproportion, reduced brain size, short stature) in the type specimen, which then is ineligible according to the International Code of Zoological Nomenclature, section1.3.2. for teratological specimens as such (40); there is no other specimen from Liang Bua Cave that embodies the key originally defining features attributed to the type specimen of H. floresiensis.
Of critical importance but often overlooked is the principle that the implicit burden of proof is on those asserting an unusual claim: here, that the Liang Bua Cave skeletons represent a new species. When this principle is violated, a consequence is that the burden of proof is placed unfairly on critics of the claim (36). This shift introduces yet another logical fallacy: that a proposition is true if it has not yet been proven false. In the case of the Flores skeletons, Bertrand Russell’s version of Occam’s Razor is particularly apposite: “Whenever possible, substitute constructions out of known entities for inferences to unknown entities.” Such problems are compounded further if attempts at hypothesis testing are impeded by restricting critics’ access to the primary data whose interpretation is in question, as demonstrably is the case here.
Two of us (M.H. and R.B.E.) were able to study the specimens briefly in February 2005, limited by pressure on Teuku Jacob to return the Liang Bua Cave skeletons to the Indonesian National Archaeological Research Center, after which we (and most other qualified researchers) have been permitted no further access to the specimens or casts, with periodic requests for information also ignored or rejected. Physicists have experienced the ill effects arising when critics are blocked from access to critical data needed to test extreme claims (41).
The available data on LB1 raise sufficient cautions in their own right. The most attention-getting features, extremely small brain size and stature, have been misreported. Both were demonstrably underestimated, although in different ways and to different extents. None of the other original species-defining descriptive features are unique. Other investigators (42–47) who have not had the benefit of direct—even if limited—access to the Liang Bua Cave skeletal sample, nonetheless, have attempted to account for the abnormalities seen largely if not exclusively in the LB1 specimen. Criticism of these various hypotheses by the defenders of H. floresiensis has obscured their common observational core: LB1 empirically represents a singularity. Although derived from mathematics, the concept of singularity (a point at which an equation, surface, or other entity blows up or becomes degenerate) fits H. floresiensis because that hypothetical entity “blows up” as a species, with the singular specimen LB1 manifesting pervasive aspects of developmental disturbance common to atavisms.
The geologist William Whewell noted the complex relationship between observation and interpretation, commenting famously that “there is a mask of theory over the whole face of nature” (48). In our own time the blurred boundaries between the empirical and inferential elements of science might give even Whewell pause. In particular, for the last decade there has been widespread, seemingly determined, confusion of the observable (if only by some) LB1 skeletal features with a nearly inextricable epiphenomenological panoply of biogeography, material culture, inferred behavior, morphology, taxonomy, and phylogeny involved in the reification of Homo floresiensis.
Materials and Methods
Studies of the skeletal sample from Liang Bua Cave, Indonesia, were made by two of the authors (R.B.E. and M.H.) in February 2005, at the Laboratory of Bioanthropology and Paleoanthropology at Gadjah Mada University (Yogyakarta, Indonesia) using standard osteometric instruments for skeletal measurements. These observations were used to test published descriptions by others of LB1 and highly fragmentary skeletons of the associated individuals. A whole-body scanner (Toshiba) was used for CT scans of the Liang Bua skeletons. A subsequent pilot study was designed to collect comparative human data on subjects whose phenotypes combined unusually short stature with a variety of other skeletal displasiae. This study was approved by the Human Subjects Institutional Review Board of the Pennsylvania State University. Tests included full-body CT, MRI. Trial results are reported by way of illustration here for one subject who, after informed consent was obtained, was subjected to a full-body, thin bone CT scan on a Siemens Sensation 40, 120 kv, 51 mAs, 1.000-mm slice interval in the transverse plane. In addition, a thin bone CT scan was obtained of the wrist. Isolation and segmentation of the relevant bones (e.g., clavicle, reported here, Supporting Information, SI4) was accomplished using Mimics 14 (Materialise). For further analysis, the models generated were analyzed (by A.S.W.) using Geomagic Studio (Geomagic) to map deviations from symmetry. As deemed necessary, manual segmentation adjustment, smoothing, and triangle reduction adjustments were executed. Images were generated to correspond with perspectives previously published, to facilitate comparison. Two-dimensional projections were created, which were used as the basis for comparison with published images and measurements. Given the high resolution of the scans, measurements on them should approximate closely those taken on the physical material.
Supplementary Material
Acknowledgments
We thank John De Vos for cordially hosting R.B.E. at Naturalis Biodiversity Center while disagreeing with his conclusions; David A. Eckhardt (School of Computer Sciences at Carnegie Mellon University) for donating a decade of inimitable technical assistance and insight; Emily Unger for her avid and able cooperation with an important early hypothesis test; many Penn State students, but especially Amy Seybert, who elucidated the development and measurement of humeral retroversion and Sarah Tedesco, who organized extensive background research on carpal bones; the many supportive colleagues at Penn State, particularly Robert L. Sainburg in Kinesiology for helping to clarify the cranial and cerebral asymmetry of LB1 that was not reflected on the wall of the cave; and Bonita De Klerk for providing a timely copy of her doctoral thesis that does much to place our own work into an appropriate regional context. Hashim Djojohadikusumo provided financial support to one of our colleagues (since deceased) and, hence indirectly to all of us. R.B.E. thanks Dr. Patrick M. Byrne, Chairman and CEO of Overstock.com, for research funding and exemplary behavior.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1407385111/-/DCSupplemental.
References
- 1.Walker A, Zimmerman MR, Leakey REF. A possible case of hypervitaminosis A in Homo erectus. Nature. 1982;296(5854):248–250. doi: 10.1038/296248a0. [DOI] [PubMed] [Google Scholar]
- 2.Henneberg M, Thorne A. 2004. Flores human may be pathological Homo sapiens. Before Farming 2004/4, article 1.
- 3.Hsü KJ. Catastrophic extinctions and the inevitably of the improbable. J Geol Soc London. 1989;146(4):749–754. [Google Scholar]
- 4.Hsü KJ. Terrestrial catastrophe caused by cometary impact at the end of Cretaceous. Nature. 1980;285(5762):201–203. [Google Scholar]
- 5.Alvarez LW, Alvarez W, Asaro F, Michel HV. Extraterrestrial cause for the Cretaceous-Tertiary extinction. Science. 1980;208(4448):1095–1108. doi: 10.1126/science.208.4448.1095. [DOI] [PubMed] [Google Scholar]
- 6.Hallam A. End-cretaceous mass extinction event: Argument for terrestrial causation. Science. 1987;238(4831):1237–1242. doi: 10.1126/science.238.4831.1237. [DOI] [PubMed] [Google Scholar]
- 7.Officer CB, Hallam A, Drake CL, Devine JD. Late Cretaceous and paroxysmal Cretaceous/Tertiary extinctions. Nature. 1987;326(6100):143–149. [Google Scholar]
- 8.Perch-Nielsen K. Geologic events and the distribution of calcareous nannofossils—Some speculations. Bull Cent Rech Explor Prod Elf-Aquitaine. 1986;10:431–432. [Google Scholar]
- 9.Perch-Nielsen K, McKenzie J, He Q. Biostratigraphy and isotope stratigraphy and the “catastrophic” extinction of nannoplankton at the Cretaceous/Tertiary boundary. Spec Pap Geol Soc Am. 1982;190:353–372. [Google Scholar]
- 10.McLean DM. Deccan traps mantle degassing in the terminal Cretaceous marine extinctions. Cretaceous Res. 1985;6(3):235–259. [Google Scholar]
- 11.Dennell RW, Louys J, O’Regan HJ, Wilkinson DM. The origins and persistence of Homo floresiensis on Flores: Biogeographical and ecological perspectives. Quaternary Sci Rev. 2014;96:98–107. [Google Scholar]
- 12.Barras C. Our Asian origins. New Sci. 2013;218(2916):41–43. [Google Scholar]
- 13.Lévi-Strauss C. “La Structure des Mythes”. Anthropologie Structurale. Paris: Plon; 1958. pp. 227–255. [Google Scholar]
- 14.Vogt PR, Holden JC. 1979. The end-Cretaceous extinctions: A study of the multiple working hypotheses method gone mad. Cretaceous-Tertiary Boundary Events Symposium, eds Christensen WK, Birkelund T (Univ of Copenhagen, Copenhagen) Proceedings 2:49.
- 15.Brown P, et al. A new small-bodied hominin from the Late Pleistocene of Flores, Indonesia. Nature. 2004;431(7012):1055–1061. doi: 10.1038/nature02999. [DOI] [PubMed] [Google Scholar]
- 16.Kumar V, Abbas AK, Fausto N, Aster J. Robbins & Cotran Pathologic Basis of Disease. 8th Ed. Philadelphia: Elsevier Health Sciences; 2010. [Google Scholar]
- 17.McClure HM, Belden KH, Pieper WA, Jacobson CB. Autosomal trisomy in a chimpanzee: Resemblance to Down’s syndrome. Science. 1969;165(3897):1010–1012. doi: 10.1126/science.165.3897.1010. [DOI] [PubMed] [Google Scholar]
- 18.Czarnetski A, Blin N, Pusch CM. Down’s syndrome in ancient Europe. Lancet. 2003;362(9388):1000. doi: 10.1016/s0140-6736(03)14384-x. [DOI] [PubMed] [Google Scholar]
- 19.Starbuck J. On the antiquity of trisomy 21: Moving toward a quantitative diagnosis of Down syndrome in historic material culture. Journal of Contemporary Anthropology. 2011;II(1):18–44. [Google Scholar]
- 20.Diamond J. Anthropology. The astonishing micropygmies. Science. 2004;306(5704):2047–2048. doi: 10.1126/science.1107565. [DOI] [PubMed] [Google Scholar]
- 21.Ugurlucan FG, Kayserli H, Yuksel A. Prenatal evaluation of fetuses presenting with short femurs. In: Choy RKW, Leung TY, editors. Prenatal Diagnosis—Morphology, Scan and Invasive Methods. Rijeka, Croatia: Intech Europe; 2012. [Google Scholar]
- 22.Jacob T, et al. Pygmoid Australomelanesian Homo sapiens skeletal remains from Liang Bua, Flores: Population affinities and pathological abnormalities. Proc Natl Acad Sci USA. 2006;103(36):13421–13426. doi: 10.1073/pnas.0605563103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaifu Y, et al. Brief communication: “Pathological” deformation in the skull of LB1, the type specimen of Homo floresiensis. Am J Phys Anthropol. 2009;140(1):177–185. doi: 10.1002/ajpa.21066. [DOI] [PubMed] [Google Scholar]
- 24.Larson SG. Evolutionary transformation of the hominin shoulder. Evol Anthropol. 2007;16(5):555–570. [Google Scholar]
- 25.Larson SG, et al. Descriptions of the upper limb skeleton of Homo floresiensis. J Hum Evol. 2009;57(5):555–570. doi: 10.1016/j.jhevol.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 26.Morwood MJ, et al. Further evidence for small-bodied hominins from the Late Pleistocene of Flores, Indonesia. Nature. 2005;437(7061):1012–1017. doi: 10.1038/nature04022. [DOI] [PubMed] [Google Scholar]
- 27.Kaifu Y, et al. Craniofacial morphology of Homo floresiensis: description, taxonomic affinities, and evolutionary implication. J Hum Evol. 2011;61(6):644–682. doi: 10.1016/j.jhevol.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 28.Baab KL, McNulty KP. Size, shape, and asymmetry in fossil hominins: The status of the LB1 cranium based on 3D morphometric analyses. J Hum Evol. 2009;57(5):608–622. doi: 10.1016/j.jhevol.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 29.Falk D, et al. The type specimen (LB1) of Homo floresiensis did not have Laron syndrome. Am J Phys Anthropol. 2009;140(1):52–63. doi: 10.1002/ajpa.21035. [DOI] [PubMed] [Google Scholar]
- 30.Van der Plas M. A new model for the evolution of Homo sapiens from the Wallacean islands. PalArch’s Journal of Vertebrate Palaeontology. 2007;1(1):1–121. [Google Scholar]
- 31.Villa C, Persson L, Alexandersen V, Lynnerup N. A small skull from Flores dated to the 20th century. Homo. 2012;63(1):12–20. doi: 10.1016/j.jchb.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 32.De Klerk B. 2012. Size variation and body proportions in an isolated Holocene-aged population from Palau, Micronesia and its impact on our understanding of variation in extinct hominids. PhD dissertation. (Univ of Witwatersrand, Johannesburg, South Africa)
- 33.Tocheri MW, et al. The primitive wrist of Homo floresiensis and its implications for hominin evolution. Science. 2007;317(5845):1743–1745. doi: 10.1126/science.1147143. [DOI] [PubMed] [Google Scholar]
- 34.Orr CM, et al. New wrist bones from Homo floresiensis. Amer J Phys Anthropol Suppl S. 2011;52:230–231. [Google Scholar]
- 35.Orr CM, et al. New wrist bones of Homo floresiensis from Liang Bua (Flores, Indonesia) J Hum Evol. 2013;64(2):109–129. doi: 10.1016/j.jhevol.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 36.Jungers WL, et al. Descriptions of the lower limb skeleton of Homo floresiensis. J Hum Evol. 2009;57(5):538–554. doi: 10.1016/j.jhevol.2008.08.014. [DOI] [PubMed] [Google Scholar]
- 37.Argue D, Donlon D, Groves C, Wright R. Homo floresiensis: Microcephalic, pygmoid, Australopithecus, or Homo? J Hum Evol. 2006;51(4):360–374. doi: 10.1016/j.jhevol.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 38.Henneberg M, Eckhardt RB, Chavanaves S, Hsü KJ. Evolved developmental homeostasis disturbed in LB1 from Flores, Indonesia, denotes Down syndrome and not diagnostic traits of the invalid species Homo floresiensis. Proc Natl Acad Sci USA. 2014;111:11967–11972. doi: 10.1073/pnas.1407382111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eckhardt RB. 2010. Apportioning human variation: the regional dimension of biomedical research. Before Farming 2010/4, article 5:1–9.
- 40.Ride WDL. International Code of Zoological Nomenclature. Oxford: International Trust for Zoological Nomenclature; 1999. [Google Scholar]
- 41.Reich ES. Plastic Fantastic. New York: Palgreave Macmillan; 2009. [Google Scholar]
- 42.Martin RD, et al. Comment on “The Brain of LB1, Homo floresiensis”. Science. 2006;312(5776):999, author reply 999. doi: 10.1126/science.1121144. [DOI] [PubMed] [Google Scholar]
- 43.Martin RD, Maclarnon AM, Phillips JL, Dobyns WB. Flores hominid: New species or microcephalic dwarf? Anat Rec A Discov Mol Cell Evol Biol. 2006;288(11):1123–1145. doi: 10.1002/ar.a.20389. [DOI] [PubMed] [Google Scholar]
- 44.Hershkovitz I, Kornreich L, Laron Z. Comparative skeletal features between Homo floresiensis and patients with primary growth hormone insensitivity (Laron Syndrome) Am J Phys Anthropol. 2007;134(2):198–208. doi: 10.1002/ajpa.20655. [DOI] [PubMed] [Google Scholar]
- 45.Obendorf PJ, Oxnard CE, Kefford BJ. Are the small human-like fossils found on Flores human endemic cretins? Proc Biol Sci. 2008;275(1640):1287–1296. doi: 10.1098/rspb.2007.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oxnard C, Obendorf PJ, Kefford BJ. Post-cranial skeletons of hypothyroid cretins show a similar anatomical mosaic as Homo floresiensis. PLoS ONE. 2010;5(9):e13018. doi: 10.1371/journal.pone.0013018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oxnard C, Obendorf PJ, Kefford BJ, Dennison J. More on the Liang Bua finds and modern human cretins. Homo. 2012;63(6):407–412. doi: 10.1016/j.jchb.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 48.Whewell W. The Philosophy of the Inductive Sciences, Founded Upon their History. London: J & JJ Deighton; 1840. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.