Significance
Drug release from implants and coatings provides a means for local administration while minimizing systemic toxicity. Controlled release can provide a slowly eluting drug reservoir to maintain elevated therapeutic levels. Devices based on degradable polymer matrices can control drug release for multiple weeks, but longer durations typically require bulky, nondegradable devices. Using a combination of a polymer–drug conjugate and its electrostatic thin film assembly, we discovered a predictable long-term sustained release of more than 14 mo, far exceeding the duration noted in most previous reports, especially those from biodegradable matrices. Because of the substantial drug loading, nanoscale films were able to maintain significant concentrations that remained highly potent. We report a versatile, long-term drug delivery platform with broad biomedical implications.
Keywords: NSAID, polyelectrolyte multilayers, polymer prodrug
Abstract
Long-term, localized delivery of small molecules from a biodegradable thin film is challenging owing to their low molecular weight and poor charge density. Accomplishing highly extended controlled release can facilitate high therapeutic levels in specific regions of the body while significantly reducing the toxicity to vital organs typically caused by systemic administration and decreasing the need for medical intervention because of its long-lasting release. Also important is the ability to achieve high drug loadings in thin film coatings to allow incorporation of significant drug amounts on implant surfaces. Here we report a sustained release formulation for small molecules based on a soluble charged polymer–drug conjugate that is immobilized into nanoscale, conformal, layer-by-layer assembled films applicable to a variety of substrate surfaces. We measured a highly predictable sustained drug release from a polymer thin film coating of 0.5–2.7 μm that continued for more than 14 mo with physiologically relevant drug concentrations, providing an important drug delivery advance. We demonstrated this effect with a potent small molecule nonsteroidal anti-inflammatory drug, diclofenac, because this drug can be used to address chronic pain, osteoarthritis, and a range of other critical medical issues.
There is a compelling need for long-term, controlled drug release for sustained treatment of chronic or recalcitrant medical conditions and diseases, especially when using small molecules with abundant targets throughout the body. For example, antibacterial therapy typically requires sustained local concentrations for periods of days to weeks, whereas some diseases, such as tuberculosis, necessitate daily doses of antibiotics for at least 6 mo (1). Other diseases also have demonstrated benefits from a long-term multimonth drug regimen, including cystic fibrosis (2), ankylosing spondylitis (3), and chronic uveitis (4).
Chronic pain may best illustrate the positive multifaceted impact of long-term drug treatment. Described as pain that extends beyond the expected period of healing, it is a widespread and debilitating ailment underpinning an unwanted dependency on narcotic medications. Often misdiagnosed and undertreated, chronic pain affects roughly 20% of US adults, and 25% of those age >45 y (5). Sometimes considered a silent epidemic, chronic pain calls for advanced pain treatments (6).
Nonsteroidal anti-inflammatory drugs (NSAIDs), one of a number of approaches to pain relief, strike a preferable balance between analgesic effectiveness and adverse reactions (7). Although the potentially adverse effects of NSAIDs are minimal compared with alternatives, prolonged and frequent use can lead to adverse drug reactions, especially in adults aged 65 and older (8). Given that NSAIDs are the most frequently prescribed medications for osteoarthritis (9), the ability to treat this persistent pain while avoiding the associated adverse drug reactions could have tremendously positive effects for millions of people.
Localized drug delivery is an attractive route for substantially reducing systemic dosages while maintaining localized saturation (10). Locally delivered diclofenac has shown similar analgesia to systemically delivered diclofenac but with significantly fewer adverse reactions (11), which led the way for Food and Drug Administration approval of topical formulations: Voltaren Gel (Novartis) and Pennsaid (Mallinckrodt). Although topical diclofenac has demonstrated the promise of localized delivery, it requires frequent administration (4 times daily), with bioelimination occurring within hours (12). Less frequent dosing has shown a positive influence on outcome (13), which would tremendously benefit several other applications including orthopedics and wound healing.
Long-term small molecule release is typically difficult, and formulations based on biodegradable materials, such as poly(lactic-co-glycolic acid) (PLGA), can extend release for days up to several weeks and, in some notable cases, multiple months (10). Factors including water permeation, decreased pH owing to acid buildup in the case of polymers like PLGA, structural instability (e.g., crack formation), erosion, and drug diffusivity, among others, form a complex picture with a ceiling on release duration (14). Their small size and hydrophobicity makes controlling the release of small molecules especially difficult, because they diffuse rapidly (15). Sustained release also can be achieved from other polymeric vehicles, ranging from gels to bulk degradable plastics (16, 17); these materials have been able to sustain drug release for up to a few months at best when mitigating the aforementioned obstacles. In addition, these inherent limitations make achieving extensive release periods difficult because of the need for excessive quantities of material, changes in material properties with time, and ultimate constraints on drug loading density owing to the thermodynamic constraints of traditional polymer molecular blends. Achieving sustained multiple-month long-term release usually requires a nondegradable physical release device, such as Illuvien (Alimera Sciences), a cylindrical intraocular insert with a semipermeable membrane that can sustain release for more than 1 y (18). In pain management, some nondegradable implants capable of sustained release for a few months have been investigated (19), but a biodegradable, long-term, sustained drug delivery formulation remains highly desired.
The layer-by-layer (LbL) assembly technique has uniquely demonstrated the construction of thin films under benign aqueous conditions for controlled release of numerous therapeutics, including proteins, growth factors, peptides, and small molecules (20). This technique relies on numerous intermolecular interactions (e.g., electrostatic) for assembly and stability; thus, the use of high molecular weight polyelectrolytes can result in films that are extremely robust and capable of coating a variety of materials and substrate geometries. A common challenge in any small molecule drug delivery system is the incorporation and subsequent controlled release of poorly charged or low molecular weight species. Our laboratory has investigated a number of LbL-based biodegradable strategies for controlled small molecule release, including direct incorporation of drug into the film (21, 22) and physical entrapment in other charged systems (23, 24), with a longest sustained release of 3 wk. Changes in the aqueous environment, such as pH or ionic strength, also can facilitate undesirable film deterioration or drug elution that dramatically affects release kinetics (25).
Here we report the development of a polymer–drug conjugate and its assembly into an LbL thin film for long-term sustained drug delivery. We used diclofenac as a model small molecule and measured its release for longer than 14 mo from biodegradable conformal coatings. Films as thin as ∼500 nm demonstrate the capability for substantial diclofenac loading, and once released, the drug maintains its anti-inflammatory activity and potency despite prolonged hydration at elevated temperatures. The possibility of such long-term controlled release of active small molecule drugs from a biodegradable, nanoscale thin film has implications for drug delivery in a broad spectrum of fields.
Results and Discussion
Polymer–Drug Conjugate.
LbL assembly is based on alternating adsorption of charged (or otherwise complementary) species. We used the biologically derived polyacid poly(l-glutamatic acid) (PGA), because it is composed of a natural l-amino acid and ultimately can be broken down and resorbed by the body. In addition, the pendant carboxylates allows for a high level of drug conjugation while maintaining sufficient free acid groups for electrostatic complexation. Similar PGA-based LbL approaches have demonstrated in vitro (26) and in vivo (27) bioactivity from functionalized films, as well as for cellular delivery of doxorubicin (28).
To generate our polymer–drug conjugate, we relied on a two-step strategy that first forms a diclofenac prodrug with hydroxyl functionality and is subsequently conjugated to the polymer backbone. As shown in Fig. 1, the carboxylic acid of diclofenac 1 was activated with 1,1′-carbonyldiimidazole and then esterified with excess triethylene glycol (TriEG). The resulting prodrug, a TriEG-diclofenac conjugate (TriEG-Diclof) 2, was purified and subsequently coupled to PGA via Steglich esterification using N,N′-dicyclohexylcarbodiimide and 4-dimethylaminopyridine. This resultant polymer–drug conjugate, PGA-TriEG-Diclof 3, had 9.8 mol% (19.5 wt%) of the PGA monomer repeat units functionalized with diclofenac. Diclofenac, similar to other small molecules, has a limited aqueous solubility of 2.4 μg/mL (29). Although 2 was found to be water-insoluble (30), 3 was well solubilized at 50 mg/mL, equivalent to 9.8 mg/mL diclofenac. By virtue of attachment to a large polyacid, diclofenac’s solubility in water increased 4,000-fold over its acid form and more than fivefold over its sodium salt [1.9 mg/mL (29)], indicating that significant stability is imparted by conjugation to a hydrophilic backbone, analogous to observations with other PGA–drug conjugates (31). Furthermore, the high drug density of the polymer conjugate provides the opportunity to achieve substantial film loading of the drug in LbL films.
Fig. 1.
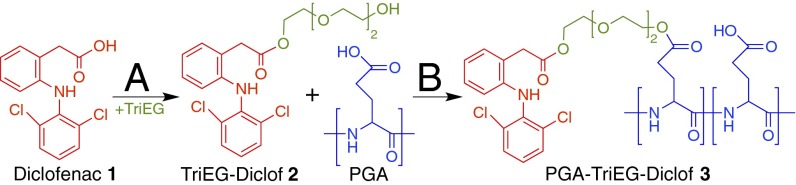
Synthetic scheme of the polymer–drug conjugate. (A) The carboxylic acid moiety of diclofenac 1 is activated with 1,1′-carbonyldiimidazole and subsequently esterified with TriEG to form the TriEG-Diclof prodrug 2. (B) After purification, 2 is then conjugated to poly(l-glutamic acid) via Steglich esterification with N,N′-dicyclohexylcarbodiimide and 4-dimethylaminopyridine to yield the PGA-TriEG-Diclof conjugate 3.
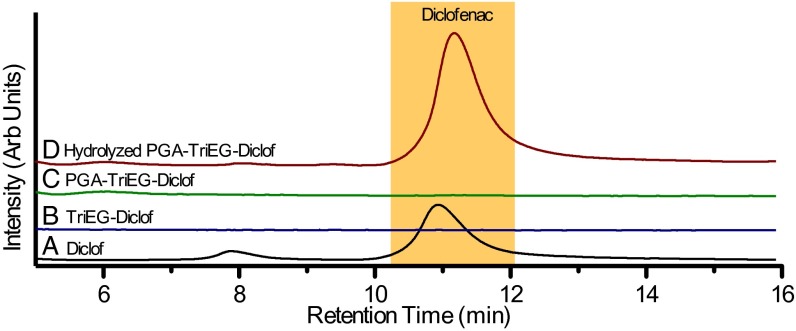
To characterize diclofenac tethering to the PGA backbone, we analyzed our various diclofenac-based compounds by HPLC. A typical chromatogram for diclofenac is shown in Fig. 2A with a small peak at 8 min that appears to be a low-level impurity. Neither TriEG-Diclof (Fig. 2B) nor PGA-TriEG-Diclof (Fig. 2C) solutions produced a significant HPLC signal, indicating that chemical modification substantially changes diclofenac’s characteristic affinity for the C18 HPLC column, and that the product contains no detectable free diclofenac, reaffirming complete covalent modification. Quantitative ester hydrolysis of PGA-TriEG-Diclof with sodium hydroxide (100 mM, pH ∼11) overnight at 37 °C showed that diclofenac is regenerated (Fig. 2D).
Fig. 2.
Chromatograms for the fluorescent intensity of stock diclofenac 1 (A), the TriEG-Diclof prodrug 2 (B), the polymer–drug conjugate 3 (C), and hydrolyzed polymer–drug conjugate (D) as analyzed by HPLC.
Before the incorporation of PGA conjugates into LbL films, we first investigated the rate of ester hydrolysis of the PGA conjugates under physiological conditions, which is an important determinant of whether therapeutically relevant concentrations of diclofenac can be generated on a biologically important time scale. Using pseudo first-order reaction kinetics, we calculated the initial rates of diclofenac release in PBS at pH 7.4 to determine their rate constants (kobs) at 19 °C, 37 °C, and 50 °C (Table 1). As expected, hydrolysis accelerated with increasing temperature, as characterized by the positive activation energy (Ea) of 67 ± 12 kJ/mol. In analogous polymer–drug systems, the half-life (t1/2) at pH 7.4 and 37 °C of small molecules conjugated to dextran can vary greatly with the drug type and polymer backbone. For example, 5-fluorouracil (t1/2 = 0.73 d) (32), benzoate (t1/2 = 7.5 d) (33), naproxen (t1/2 = 7.6 d) (34), and ketoprofen/ibuprofen/naproxen (t1/2 = 7.8 d/5.3 d/4.2 d) (35) have half-lives of roughly 1 wk or less, whereas other variations, such as starch-naproxen (t1/2 = 33 d) (34) and hyaluronic acid-cortisone (t1/2 = 3.6 d) (36), demonstrate a potentially broad range. We found that our polymer–drug compound has a substantially longer half-life compared with most reported conjugates (Table 1), making it an ideal candidate for long-term sustained release formulations.
Table 1.
Hydrolysis kinetics of PGA-TriEG-Diclof in PBS, pH 7.4
| T, °C | kobs, × 10−8 s−1 | t1/2, d |
| 19 | 0.311 ± 0.005 | 2,577 |
| 37 | 2.29 ± 0.03 | 350 |
| 50 | 4.14 ± 0.29 | 194 |
Numerous factors can affect the rate of ester hydrolysis. For example, nearest- neighbor groups are influential (37), with negative charges along the polymer backbone slowing (38) and positive charges accelerating bond cleavage (39). In addition, electron- donating or -withdrawing groups near the ester can modulate hydrolysis (40), as can steric bulk that limits accessibility (34). If a linker is present, as in our case, its hydrophobicity can slow ester cleavage (41). The combination of a high negative charge along the PGA backbone, which is increased as the ester hydrolyzes, and hydrophobicity of the pendant TriEG-Diclof is likely a significant factor contributing to the slow release kinetics that we observed.
In addition to the linker’s hydrolytic susceptibility, the hydrophobicity of the pendant side-chains along a charged, hydrophilic backbone has also shown the propensity to form colloidal aggregates that exhibit a hydrophilic outer shell and hydrophobic core (42). Some amphiphilic polymers, such as those with randomly functionalized pendant hydrophobic moieties, also can generate hydrophobic intramolecular nanodomains within a single molecule (43). Our studies of PGA-TriEG-Diclof in aqueous solution at 1 mg/mL by dynamic light scattering showed hydrodynamic diameters of 124.9 ± 1.6 nm in 100 mM sodium acetate, pH 6.3 (conditions used for assembly with chitosan), and 28.8 ± 4.7 nm in 10 mM sodium phosphate, pH 7.4 (conditions used for assembly with PLL). In addition, their critical micelle concentrations were 4.2 μg/mL and 9.9 μg/mL, respectively. These measurements reveal the presence of micellar aggregates due to the hydrophobicity of the pendant diclofenac and hydrophilicity of the PGA backbone. The difference in size depending on solution conditions is likely a consequence of the lower ionization and increased charge shielding occurring in 100 mM sodium acetate, pH 6.3 compared with 10 mM sodium phosphate, pH 7.4; the reduced electrostatic repulsion in the former allows for higher aggregation numbers and thus larger particle sizes (44). Because of the self-assembly into micellar aggregates, pendant ester linkages are sequestered into the hydrophobic core, which is likely a major factor in the slow diclofenac release kinetics that we observed.
Multilayer Films.
Solution-phase kinetics indicate that controlled diclofenac release can be facilitated by ester hydrolysis from a polymer backbone; however, the polymer–drug conjugate itself is soluble in water and cannot be used to create a stable thin film coating or similar construct for controlled localized release. Using an LbL assembly technique, we were able to immobilize nanoscale layers of PGA-TriEG-Diclof from aqueous solutions via polyelectrolyte complexation with a cationic polymer atop a substrate surface. Repeated deposition of (Polycation/PGA-TriEG-Diclof) layers allows for control over film thickness and drug payload. These bilayers were constructed with polycations of poly(l-lysine) (PLL) at pH 7.4 and chitosan at pH 5.0, with PGA-TriEG-Diclof at pH 7.4 and 6.3, respectively. Their film growth revealed a concomitant increase in thickness with the number of bilayers, a characteristic feature of LbL-based films (45) (Fig. 3 A and B).
Fig. 3.
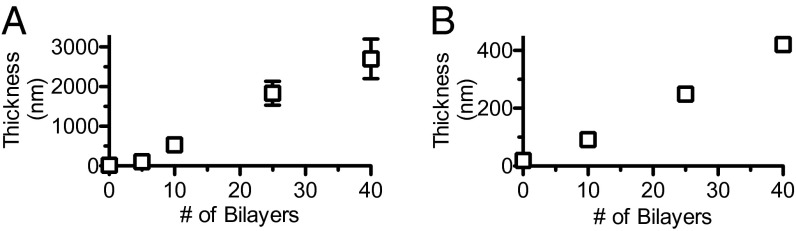
Growth curves of (PLL/PGA-TriEG-Diclof)n (A) and (chitosan/PGA-TriEG-Diclof)n (B) films.
The bilayer thickness of an LbL film can vary from subnanometer to tens of nanometers depending on various factors, including pH, ionic strength, and degree of ionization of the adsorbing polymer chains. These effects, among others, can mechanistically influence the polyelectrolyte’s ability to diffuse into, out of, and within hydrated multilayer films during film assembly (46). In addition, the size of the PGA-TriEG-Diclof micellar aggregate may restrict its diffusivity within the film. Other electrostatic-based LbL films with block copolymer micelles have shown linear growth behavior after the initial few layers, with bilayer thicknesses of ∼100 nm, suggesting the deposition of multiple micelle layers, a large fraction of the other film component, or a combination thereof. We observed a similar growth behavior in (PLL/PGA-TriEG-Diclof)n films in which relatively little material is deposited for the first few layers, but the assembly transitions to considerably thicker deposition beyond five bilayers (Fig. 3A).
PLL (pka∼9.9 (47)) is significantly charged with a random coil conformation (48) at pH 7.4 during deposition, and its intediffusivity can be a considerable factor, given that films of (PLL/PGA)n are well known to demonstrate exponential growth owing to interdiffusion (49), with the diffusivity of PLL independent of the diffusivity of the polyanion (50). Linear regression of this latter growth phase (Fig. 3A) revealed a deposit of 75.1 ± 5.1 nm (R2 = 0.991) per bilayer, suggesting considerable deposition. In contrast, (chitosan/PGA-TriEG-Diclof)n films were considerably thinner with no observed induction period, with 11.0 ± 0.3 nm (R2 = 0.9994) deposited per bilayer. The growth behavior of a (chitosan/PGA)n film can range from linear to exponential depending on the polyelectrolyte’s charge density during film deposition (51), which contributes significantly to the thickness of film per bilayer (52). When chitosan [pKa∼6.5 (53)] is well ionized and attains a flat and extended conformation, it demonstrates linear film growth (51), as we observed. The seemingly small contribution to thickness of the polymer–drug conjugate despite its micelle size (∼125 nm) is likely related to spreading of the aggregates and possible rearrangement of the structures on adsorption.
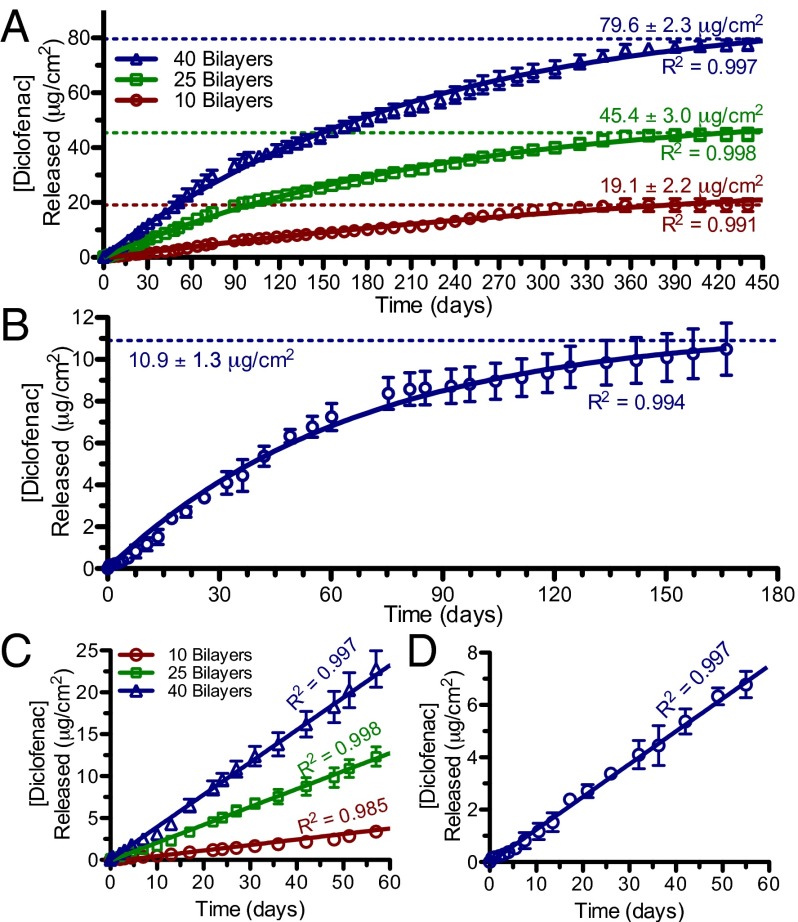
The elution of diclofenac from these films demonstrates a long-lasting multimonth release. We measured release for more than 14 mo in the PLL-based films and for more than 5 mo in the chitosan-based films (Fig. 4). In the latter case, diclofenac release from the thinner 10- and 25-bilayer films was too low for reliable detection by HPLC. Overall, the release kinetics of diclofenac from these LbL films can be fit to pseudo first-order kinetics, as shown by the curves in Fig. 4 A and B. This is not surprising, given that diclofenac release by ester hydrolysis in the hydrated LbL film occurs through the same mechanism as in solubilized PGA-TriEG-Diclof. Film assembly through electrostatic complexation of the polyanionic backbone accommodates its immobilization into the film while maintaining the polymer–drug conjugate’s aqueous solvation and accessibility, thereby allowing its use as a localized drug delivery strategy. The kinetic rate constants, kobs, can be similarly calculated from these release curves; we found values of 5.6 × 10−8 s−1 for the PLL-based film and 1.7 × 10−7 s−1 for the chitosan-based film. Both of these film-based rate constants are greater than that of solubilized PGA-TriEG-Diclof (Table 1) under identical conditions of PBS, pH 7.4 at 37 °C. This film-based rate acceleration is likely a consequence of a combination of the aforementioned micellar disruption on film assembly and the polyion’s electrostatic complexation, which neutralizes much of the backbone nearest-neighbor negative charges and thus may reduce the inhibition of ester side-chain hydrolysis (38).
Fig. 4.
Diclofenac release profiles from (PLL/PGA-TriEG-Diclof)n films (A and C) and (chitosan/PGA-TriEG-Diclof)40 films (B and D). Full-scale (A and B) and the first 60 d (C and D) are shown. The symbols represent measured data, and solid lines represent first-order fits (A and B) or linear regressions (C and D).
A highly desirable feature for controlled release systems is zeroth-order release kinetics, in which the rate of drug release can balance its bioelimination to sustain a therapeutic concentration (10). Linear regression of the drug released from each of our films in the first 2 mo demonstrated constant rates of diclofenac elution from both PLL-based (Fig. 4C) and chitosan-based (Fig. 4D) systems. An initial burst release of drug is commonly observed in controlled-release systems for small molecules owing to their low molecular weights (54); this is frequently undesirable because it can be uncontrollable, irreproducible, and toxic resulting from a rapid elevation in local drug concentration (55). With our strategy of using a polymer–drug conjugate that is electrostatically immobilized into a film, the drug release is mediated by ester hydrolysis, eliminating this potentially deleterious initial burst release.
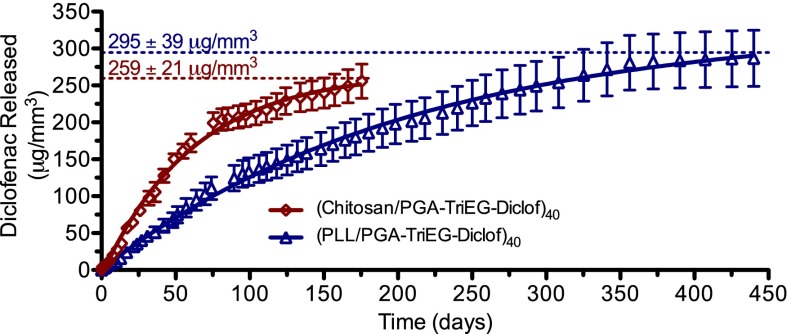
The differences in diclofenac release rates from PLL-based and chitosan-based films become more evident when their release profiles from 40-bilayer films are presented in terms of the mass of diclofenac released per film volume, as shown in Fig. 5. Our observation of faster release rates from chitosan-based films compared with PLL-based films likely reflects the compositional differences within the films, including less water accessibility for the latter, given that greater hydrophobicity has been found to slow hydrolysis, whereas increased hydrophilicity accelerates hydrolysis (56). We suspect that the polycation charge density (e.g., PLL and chitosan), ionic strength, and pH of assembly conditions also contribute to differences in the effective ionic cross-link density and degree of water swelling within the films, affecting hydrolysis rates and influencing the presence of hydrophobic regions [i.e., self-assembly of pendant diclofenac into hydrophobic pockets (57)]. We gauged the relative water content in these films by comparing their swelling on hydration in PBS, pH 7.4. The PLL-based films showed relatively moderate swelling (38 ± 12%), whereas chitosan-based films revealed significantly greater swelling (116 ± 14%), indicating its greater hydrophilicity. This also corresponds with the more rapid diclofenac elution seen from chitosan-based films. Furthermore, the change in pH between the assembly conditions and release conditions for chitosan films also may facilitate increased water accessibility of the ester linkages, given that pH-induced morphological changes in LbL films can cause film swelling and rearrangement and, consequently, increased water access to moieties previously hidden in hydrophobic pockets (58).
Fig. 5.
Mass of diclofenac per film volume released from (PLL/PGA-TriEG-Diclof)40 and (chitosan/PGA-TriEG-Diclof)40 films. Symbols represent measured data; solid lines, first-order fits.
A myriad of factors can contribute to the characteristics of controlled-release films, and, interestingly, our system showed significantly higher loading and extended release compared with another LbL-film based on a polymer–drug conjugate, (chitosan/hyaluronic acid-paclitaxel)n films (59). For our PLL-based and chitosan-based films of 40 bilayers (Fig. 5), diclofenac loading densities were 295 μg/mm3 (30 wt%) and 259 μg/mm3 (26 wt%), respectively. These PLL-based and chitosan-based films are also significantly greater in drug loading and release duration of diclofenac than in other nonconjugate LbL-based (24, 25), PLGA-based (60), and lipid nanoparticle-based systems (61).
Although ester hydrolysis facilitates diclofenac liberation and elution, the polypeptide-based film remains intact. We found that after 10.6 mo of incubation and drug release, the (PLL/PGA-TriEG-Diclof)40 dry film thickness was 2.9 ± 0.2 μm, comparable to its thickness before release (2.7 ± 0.5 μm), indicating that any small changes in charge distribution and net charge balance within the LbL films during diclofenac release are insufficient to cause destabilization of the films. We propose that during hydration, the film effectively swells and the pendant drug is believed to uniformly undergo hydrolysis-based release over time. This leaves the poly(l-glutamic acid) backbone intact in the LbL film, thereby making the film less sensitive to some of the complexities (e.g., autocatalysis, nonuniformity, pore formation, cracks, structural collapse) that plague certain other drug release systems (14). This behavior also differs from the previously developed erosion-based, hydrolytically degradable LbL films used for controlled delivery that rely on poly(β-amino ester)s with cleavable ester linkages along the polymer backbone. The hydrophobicity of these degradable polycations can be tuned, as can the release kinetics from the film (62). Differential rates of water uptake and, consequently, hydrolysis-mediated film erosion in such systems allow for diverse release kinetics, but are not as easily adapted to the controlled release of small molecules, which are bound to undergo some rapid diffusion with swelling, given that they rely on trapping of molecules within the ionic network rather than on covalent conjugation.
Whereas film degradation by polypeptide bond hydrolysis is relatively inconsequential compared with the rate of diclofenac release, its proteolysis can accelerate disassembly. This biodegradability is a coveted feature for implantable materials, and its rate is highly dependent on the biological fluid’s enzyme composition. Investigations into tuning of proteolytic susceptibility have shown that although some human proteases have poor activity against LbL films (63), film characteristics including covalent cross-linking (64), l- or d-polypeptides (65), and terminal layer charge (66), among others, can enhance or inhibit degradation. In addition, antifouling modifications like PEGylation (67) and mixed-charge nanodomains (68) can introduce a protein resistance that reduces enzyme contact with the film. This versatility in LbL-assembled films, coupled with the nonenzymatic hydrolytic release mechanism, can readily accommodate a variety of modifications that specifically tune film characteristics to the desired application.
Anti-Inflammatory Activity.
We examined the efficacy of diclofenac derived from our various formulations to identify potentially deleterious effects from the synthesis, film assembly, and release process. By investigating diclofenac’s inhibition of COX-1 activity in vitro, we can gain insight into its ability to reduce the downstream effects of inflammation and pain. As shown in Fig. 6, stock diclofenac yielded substantial COX inhibition, whereas polymer conjugation (i.e., PGA-TriEG-Diclof) eliminated it. This inactivation is likely related to the masking of diclofenac’s carboxylic acid by esterification and the introduction of steric bulkiness from the polymer-linker component, both of which can prevent binding with the COX enzyme (69). To regenerate activity, we hydrolyzed the ester linkages and found that diclofenac recovered from the polymer–drug conjugate also retained its functionality for COX inhibition (Fig. 6). In our controlled-release films, we incorporated PGA-TriEG-Diclof into electrostatically assembled LbL films incubated under physiological conditions of PBS, pH 7.4 at 37 °C for multiple months, possibly submitting diclofenac to the degradative effects of prolonged hydration and elevated temperature. Therefore, we tested the undiluted release of diclofenac at 0.5, 3.0, and 6.0 mo from (PLL/PGA-TriEG-Diclof)40 films and found that the drug, as released, retained substantial COX inhibition at a similar level as stock diclofenac solutions. We have previously found that therapeutics released from LbL films remain active, even after weeks of incubation, possibly stemming from the stabilization provided by the milieu of intermolecular interactions with the surrounding matrix. In addition, the degradation pathways for diclofenac involve mainly intramolecular cyclization with its carboxylic acid (70), which may be inhibited by its esterification.
Fig. 6.
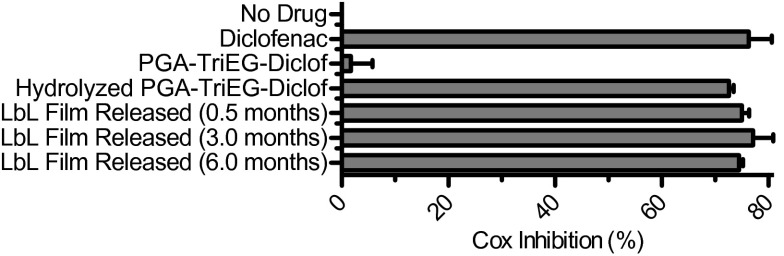
COX inhibition activity of diclofenac-based compounds. Diclofenac released from LbL films was examined for COX inhibition activity at the concentration released into PBS, pH 7.4.
We also examined the intrinsic potency of the LbL-incorporated diclofenac by measuring the IC50 (concentration required for 50% inhibition) of COX. As shown in Table 2, the diclofenac IC50 was comparable in an unfunctionalized diclofenac control, hydrolyzed drug from PGA-TriEG-Diclof film, and drug released from (PLL/PGA-TriEG-Diclof)40 film. This finding confirms that none of the steps—including synthesis of the polymer–drug conjugate, LbL assembly into a film, long-term hydration and incubation at elevated temperature, and ester hydrolysis to regenerate the drug—has a deleterious effect; drug efficacy was maintained throughout the long-term release.
Table 2.
Inhibiting concentrations of various materials necessary to elicit 50% inhibition of COX activity
| Source | IC50, ng/mL |
| Unfunctionalized diclofenac (71) | 24.2 ± 5.7 |
| Hydrolyzed PGA-TriEG-Diclof | 26.4 ± 12.4 |
| LbL film released Diclof | 25.8 ± 4.8 |
Conclusions
Long-term small molecule treatment from a biodegradable device can have a highly positive impact on a broad spectrum of chronic or recalcitrant medical issues. We have generated a polymer–drug conjugate capable of relatively slow release based on ester hydrolysis and incorporated it into LbL films, yielding a robust nanoscale film with substantial payload that elutes active drug for more than 14 mo. This versatile strategy is applicable to a broad range of therapeutics and represents a promising approach to treating a multitude of targets.
Materials and Methods
Multilayer films were assembled by incubation for 10 min in polycation solution; followed by 10-s, 20-s, and 30-s H2O rinses; then 10 min of polyanion solution; and finally more 10-s, 20-s, and 30-s H2O rinses. For (PLL/PGA-TriEG-Diclof)n films, both PLL and PGA-TriEG-Diclof were formulated at 1 mg/mL in 10 mM sodium phosphate, pH 7.4. For (chitosan/PGA-TriEG-Diclof)n films, 2 mg/mL chitosan in 100 mM sodium acetate, pH 5.0, and 1 mg/mL PGA-TriEG-Diclof in 100 mM sodium acetate, pH 6.3, were used. (The latter pH was chosen because of the limited solubility of PGA-TriEG-Diclof below pH 6.3.) Film-based diclofenac release was studied by immersion in 1 mL of PBS, pH 7.4 at 37 °C for predetermined times, after which films were transferred to fresh PBS solution. Films released into solution were analyzed by HPLC for COX-1 inhibition. COX-1 inhibition activity was quantified with a COX fluorescent inhibitor screening assay kit (Cayman Chemicals) according to the manufacturer’s directions. More details regarding the synthesis of PGA-TriEG-Diclof and its characterization in solution and in multilayer films are provided in SI Materials and Methods.
Supplementary Material
Acknowledgments
This research was supported by the US Army Research Office (Contract W911NF-13-D-0001) and by the US Air Force (Contract W911NF-07-D-0004).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. G.D. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1323829111/-/DCSupplemental.
References
- 1.Connolly LE, Edelstein PH, Ramakrishnan L. Why is long-term therapy required to cure tuberculosis? PLoS Med. 2007;4(3):e120. doi: 10.1371/journal.pmed.0040120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Konstan MW, Schluchter MD, Xue W, Davis PB. Clinical use of ibuprofen is associated with slower FEV1 decline in children with cystic fibrosis. Am J Respir Crit Care Med. 2007;176(11):1084–1089. doi: 10.1164/rccm.200702-181OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kroon F, Landewé R, Dougados M, van der Heijde D. Continuous NSAID use reverts the effects of inflammation on radiographic progression in patients with ankylosing spondylitis. Ann Rheum Dis. 2012;71(10):1623–1629. doi: 10.1136/annrheumdis-2012-201370. [DOI] [PubMed] [Google Scholar]
- 4.Hazirolan D, Pleyer U. Think global—act local: Intravitreal drug delivery systems in chronic noninfectious uveitis. Ophthalmic Res. 2013;49(2):59–65. doi: 10.1159/000345477. [DOI] [PubMed] [Google Scholar]
- 5.Blackwell D, Clarke T. Percentage of adults aged ≥18 years who often had pain in the past 3 months, by sex and age group—National Health Interview Survey, United States 2010–2011. MMWR Morb Mortal Wkly Rep. 2013;62(17):342. [Google Scholar]
- 6.Institute of Medicine . Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington, DC: National Academies Press; 2011. [PubMed] [Google Scholar]
- 7.Zhang W, et al. OARSI recommendations for the management of hip and knee osteoarthritis: part III: Changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage. 2010;18(4):476–499. doi: 10.1016/j.joca.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 8.Franceschi M, et al. Prevalence, clinical features and avoidability of adverse drug reactions as cause of admission to a geriatric unit: A prospective study of 1756 patients. Drug Saf. 2008;31(6):545–556. doi: 10.2165/00002018-200831060-00009. [DOI] [PubMed] [Google Scholar]
- 9.Richette P, et al. Comparison of general practitioners and rheumatologists’ prescription patterns for patients with knee osteoarthritis. BMC Musculoskelet Disord. 2011;12:72. doi: 10.1186/1471-2474-12-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Siepmann J, Siegel RA, Rathbone MJ, editors. Fundamentals and Applications of Controlled Release Drug Delivery. New York: Springer; 2012. [Google Scholar]
- 11.Tugwell PS, Wells GA, Shainhouse JZ. Equivalence study of a topical diclofenac solution (pennsaid) compared with oral diclofenac in symptomatic treatment of osteoarthritis of the knee: A randomized controlled trial. J Rheumatol. 2004;31(10):2002–2012. [PubMed] [Google Scholar]
- 12.Willis JV, Kendall MJ, Flinn RM, Thornhill DP, Welling PG. The pharmacokinetics of diclofenac sodium following intravenous and oral administration. Eur J Clin Pharmacol. 1979;16(6):405–410. doi: 10.1007/BF00568201. [DOI] [PubMed] [Google Scholar]
- 13.Richter A, Anton SE, Koch P, Dennett SL. The impact of reducing dose frequency on health outcomes. Clin Ther. 2003;25(8):2307–2335. doi: 10.1016/s0149-2918(03)80222-9. [DOI] [PubMed] [Google Scholar]
- 14.Fredenberg S, Wahlgren M, Reslow M, Axelsson A. The mechanisms of drug release in poly(lactic-co-glycolic acid)-based drug delivery systems—a review. Int J Pharm. 2011;415(1-2):34–52. doi: 10.1016/j.ijpharm.2011.05.049. [DOI] [PubMed] [Google Scholar]
- 15.Klose D, Siepmann F, Elkharraz K, Krenzlin S, Siepmann J. How porosity and size affect the drug release mechanisms from PLGA-based microparticles. Int J Pharm. 2006;314(2):198–206. doi: 10.1016/j.ijpharm.2005.07.031. [DOI] [PubMed] [Google Scholar]
- 16.Goldberg M, Langer R, Jia X. Nanostructured materials for applications in drug delivery and tissue engineering. J Biomater Sci Polym Ed. 2007;18(3):241–268. doi: 10.1163/156856207779996931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uhrich KE, Cannizzaro SM, Langer RS, Shakesheff KM. Polymeric systems for controlled drug release. Chem Rev. 1999;99(11):3181–3198. doi: 10.1021/cr940351u. [DOI] [PubMed] [Google Scholar]
- 18.Del Amo EM, Urtti A. Current and future ophthalmic drug delivery systems: A shift to the posterior segment. Drug Discov Today. 2008;13(3-4):135–143. doi: 10.1016/j.drudis.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 19.Sprintz M, et al. Nanomedicine: Ushering in a new era of pain management. Eur J Pain Suppl. 2011;5(S2):317–322. [Google Scholar]
- 20.Hyder MN, Shah NJ, Hammond PT. Design and translation of nanolayer assembly processes: electrochemical energy to programmable pharmacies. In: Decher G, Schlenoff JB, editors. Multilayer Thin Films. 2nd Ed. Weinheim, Gemany: Wiley-VCH; 2012. [Google Scholar]
- 21.Moskowitz JS, et al. The effectiveness of the controlled release of gentamicin from polyelectrolyte multilayers in the treatment of Staphylococcus aureus infection in a rabbit bone model. Biomaterials. 2010;31(23):6019–6030. doi: 10.1016/j.biomaterials.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shukla A, Avadhany SN, Fang JC, Hammond PT. Tunable vancomycin- releasing surfaces for biomedical applications. Small. 2010;6(21):2392–2404. doi: 10.1002/smll.201001150. [DOI] [PubMed] [Google Scholar]
- 23.Kim B-S, Park SW, Hammond PT. Hydrogen-bonding layer-by-layer-assembled biodegradable polymeric micelles as drug delivery vehicles from surfaces. ACS Nano. 2008;2(2):386–392. doi: 10.1021/nn700408z. [DOI] [PubMed] [Google Scholar]
- 24.Smith RC, Riollano M, Leung A, Hammond PT. Layer-by-layer platform technology for small-molecule delivery. Angew Chem Int Ed Engl. 2009;48(47):8974–8977. doi: 10.1002/anie.200902782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shukla A, Fuller RC, Hammond PT. Design of multi-drug release coatings targeting infection and inflammation. J Control Release. 2011;155(2):159–166. doi: 10.1016/j.jconrel.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 26.Chluba J, et al. Peptide hormone covalently bound to polyelectrolytes and embedded into multilayer architectures conserving full biological activity. Biomacromolecules. 2001;2(3):800–805. doi: 10.1021/bm015529i. [DOI] [PubMed] [Google Scholar]
- 27.Schultz P, et al. Polyelectrolyte multilayers functionalized by a synthetic analogue of an anti-inflammatory peptide, α-MSH, for coating a tracheal prosthesis. Biomaterials. 2005;26(15):2621–2630. doi: 10.1016/j.biomaterials.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 28.Ochs CJ, Such GK, Yan Y, van Koeverden MP, Caruso F. Biodegradable click capsules with engineered drug-loaded multilayers. ACS Nano. 2010;4(3):1653–1663. doi: 10.1021/nn9014278. [DOI] [PubMed] [Google Scholar]
- 29.Fini A, Fazio G, Feroci G. Solubility and solubilization properties of non-steroidal anti-inflammatory drugs. Int J Pharm. 1995;126(1-2):95–102. [Google Scholar]
- 30.Bonina FP, et al. In vitro and in vivo evaluation of polyoxyethylene esters as dermal prodrugs of ketoprofen, naproxen and diclofenac. Eur J Pharm Sci. 2001;14(2):123–134. doi: 10.1016/s0928-0987(01)00163-4. [DOI] [PubMed] [Google Scholar]
- 31.Li C, et al. Complete regression of well-established tumors using a novel water-soluble poly(L-glutamic acid)-paclitaxel conjugate. Cancer Res. 1998;58(11):2404–2409. [PubMed] [Google Scholar]
- 32.Hao AJ, et al. Degradation kinetics of fluorouracil-acetic-acid-dextran conjugate in aqueous solution. Drug Dev Ind Pharm. 2006;32(6):757–763. doi: 10.1080/03639040600683436. [DOI] [PubMed] [Google Scholar]
- 33.Larsen C, Johansen M. Macromolecular prodrugs, 1: Kinetics and mechanism of hydrolysis of O-benzoyl dextran conjugates in aqueous buffer and in human plasma. Int J Pharm. 1985;27(2-3):205–218. [Google Scholar]
- 34.Larsen C. Macromolecular prodrugs, 12: Kinetics of release of naproxen from various polysaccharide ester prodrugs in neutral and alkaline-solution. Int J Pharm. 1989;51(3):233–240. [Google Scholar]
- 35.Wu C, Xie J, Branford-White C, Quan J, Zhu L. In vitro controlled release of polymeric drug–saccharide conjugates with ketoprofen, ibuprofen, and naproxen pendants. J Appl Polym Sci. 2011;121(3):1654–1660. [Google Scholar]
- 36.Rajewski LG, Stinnett AA, Stella VJ, Topp EM. Enzymatic and nonenzymatic hydrolysis of a polymeric prodrug—hydrocortisone esters of hyaluronic acid. Int J Pharm. 1992;82(3):205–213. [Google Scholar]
- 37.McCoy CP, Morrow RJ, Edwards CR, Jones DS, Gorman SP. Neighboring group-controlled hydrolysis: Towards “designer” drug release biomaterials. Bioconjug Chem. 2007;18(1):209–215. doi: 10.1021/bc060238y. [DOI] [PubMed] [Google Scholar]
- 38.Kondo S, Murase K, Kuzuya M. Mechanochemical solid-state polymerization, 7: The nature of hydrolysis of novel polymeric prodrugs prepared by mechanochemical copolymerization. Chem Pharm Bull (Tokyo) 1994;42(12):2412–2417. [Google Scholar]
- 39.Jo YS, Gantz J, Hubbell JA, Lutolf MP. Tailoring hydrogel degradation and drug release via neighboring amino acid controlled ester hydrolysis. Soft Matter. 2009;5(2):440–446. [Google Scholar]
- 40.Heller J, Helwing RF, Baker RW, Tuttle ME. Controlled release of water-soluble macromolecules from bioerodible hydrogels. Biomaterials. 1983;4(4):262–266. doi: 10.1016/0142-9612(83)90025-x. [DOI] [PubMed] [Google Scholar]
- 41.Schoenmakers RG, van de Wetering P, Elbert DL, Hubbell JA. The effect of the linker on the hydrolysis rate of drug-linked ester bonds. J Control Release. 2004;95(2):291–300. doi: 10.1016/j.jconrel.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 42.Kataoka K, Harada A, Nagasaki Y. Block copolymer micelles for drug delivery: Design, characterization and biological significance. Adv Drug Deliv Rev. 2001;47(1):113–131. doi: 10.1016/s0169-409x(00)00124-1. [DOI] [PubMed] [Google Scholar]
- 43.Deo P, Deo N, Somasundaran P, Jockusch S, Turro NJ. Conformational changes of pyrene-labeled polyelectrolytes with pH: Effect of hydrophobic modifications. J Phys Chem B. 2005;109(44):20714–20718. doi: 10.1021/jp051743f. [DOI] [PubMed] [Google Scholar]
- 44.Lee AS, Gast AP, Bütün V, Armes SP. Characterizing the structure of pH-dependent polyelectrolyte block copolymer micelles. Macromolecules. 1999;32(13):4302–4310. [Google Scholar]
- 45.Decher G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science. 1997;277(5330):1232–1237. [Google Scholar]
- 46.Porcel C, et al. From exponential to linear growth in polyelectrolyte multilayers. Langmuir. 2006;22(9):4376–4383. doi: 10.1021/la053218d. [DOI] [PubMed] [Google Scholar]
- 47.Dos A, Schimming V, Tosoni S, Limbach H-H. Acid-base interactions and secondary structures of poly-L-lysine probed by 15N and 13C solid-state NMR and ab initio model calculations. J Phys Chem B. 2008;112(49):15604–15615. doi: 10.1021/jp806551u. [DOI] [PubMed] [Google Scholar]
- 48.Myer YP. The pH-induced helix-coil transition of poly-L-lysine and poly-L-glutamic acid and the 238-mμ dichroic band. Macromolecules. 1969;2(6):624–628. [Google Scholar]
- 49.Lavalle P, et al. Comparison of the structure of polyelectrolyte multilayer films exhibiting a linear and an exponential growth regime: An in situ atomic force microscopy study. Macromolecules. 2002;35(11):4458–4465. [Google Scholar]
- 50.Picart C, et al. Molecular basis for the explanation of the exponential growth of polyelectrolyte multilayers. Proc Natl Acad Sci USA. 2002;99(20):12531–12535. doi: 10.1073/pnas.202486099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song Z, et al. Layer-by-layer buildup of poly(L-glutamic acid)/chitosan film for biologically active coating. Macromol Biosci. 2009;9(3):268–278. doi: 10.1002/mabi.200800164. [DOI] [PubMed] [Google Scholar]
- 52.Shiratori SS, Rubner MF. pH-dependent thickness behavior of sequentially adsorbed layers of weak polyelectrolytes. Macromolecules. 2000;33(11):4213–4219. [Google Scholar]
- 53.Wang QZ, et al. Protonation constants of chitosan with different molecular weight and degree of deacetylation. Carbohydr Polym. 2006;65(2):194–201. [Google Scholar]
- 54.Huang X, Brazel CS. On the importance and mechanisms of burst release in matrix-controlled drug delivery systems. J Control Release. 2001;73(2-3):121–136. doi: 10.1016/s0168-3659(01)00248-6. [DOI] [PubMed] [Google Scholar]
- 55.Allison SD. Analysis of initial burst in PLGA microparticles. Expert Opin Drug Deliv. 2008;5(6):615–628. doi: 10.1517/17425247.5.6.615. [DOI] [PubMed] [Google Scholar]
- 56.Pitt CG, Cha Y, Shah SS, Zhu KJ. Blend of PVA and PGLA: Control of the permeability and degradability of hydrogels by blending. J Control Release. 1992;19(1-3):189–199. [Google Scholar]
- 57.Fini A, Fazio G, Rabasco AM, Hervas MJF. Self-association properties of diclofenac. Farmaco. 1994;49(2):141–146. [Google Scholar]
- 58.Li Y, Kwon GS. Methotrexate esters of poly(ethylene oxide)-block-poly(2-hydroxyethyl-L-aspartamide), part I: Effects of the level of methotrexate conjugation on the stability of micelles and on drug release. Pharm Res. 2000;17(5):607–611. doi: 10.1023/a:1007529218802. [DOI] [PubMed] [Google Scholar]
- 59.Thierry B, et al. Delivery platform for hydrophobic drugs: Prodrug approach combined with self-assembled multilayers. J Am Chem Soc. 2005;127(6):1626–1627. doi: 10.1021/ja045077s. [DOI] [PubMed] [Google Scholar]
- 60.Tunçay M, et al. Diclofenac sodium incorporated PLGA (50:50) microspheres: Formulation considerations and in vitro/in vivo evaluation. Int J Pharm. 2000;195(1-2):179–188. doi: 10.1016/s0378-5173(99)00394-4. [DOI] [PubMed] [Google Scholar]
- 61.Liu D, et al. Solid lipid nanoparticles for transdermal delivery of diclofenac sodium: Preparation, characterization and in vitro studies. J Microencapsul. 2010;27(8):726–734. doi: 10.3109/02652048.2010.513456. [DOI] [PubMed] [Google Scholar]
- 62.Zhang J, Fredin NJ, Janz JF, Sun B, Lynn DM. Structure/property relationships in erodible multilayered films: Influence of polycation structure on erosion profiles and the release of anionic polyelectrolytes. Langmuir. 2006;22(1):239–245. doi: 10.1021/la052360b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Craig M, Bordes R, Holmberg K. Polypeptide multilayer self-assembly and enzymatic degradation on tailored gold surfaces studied by QCM-D. Soft Matter. 2012;8(17):4788–4794. [Google Scholar]
- 64.Picart C, et al. Controlled degradability of polysaccharide multilayer films in vitro and in vivo. Adv Funct Mater. 2005;15(11):1771–1780. [Google Scholar]
- 65.Jessel N, et al. Bioactive coatings based on a polyelectrolyte multilayer architecture functionalized by embedded proteins. Adv Mater. 2003;15(9):692–695. doi: 10.1016/j.bioeng.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 66.Serizawa T, Yamaguchi M, Akashi M. Enzymatic hydrolysis of a layer-by-layer assembly prepared from chitosan and dextran sulfate. Macromolecules. 2002;35(23):8656–8658. [Google Scholar]
- 67.Heuberger R, Sukhorukov G, Vörös J, Textor M, Möhwald H. Biofunctional polyelectrolyte multilayers and microcapsules: Control of non-specific and bio-specific protein adsorption. Adv Funct Mater. 2005;15(3):357–366. [Google Scholar]
- 68.Wong SY, et al. Drastically lowered protein adsorption on microbicidal hydrophobic/hydrophilic polyelectrolyte multilayers. Biomacromolecules. 2012;13(3):719–726. doi: 10.1021/bm201637e. [DOI] [PubMed] [Google Scholar]
- 69.Sidhu RS, Lee JY, Yuan C, Smith WL. Comparison of cyclooxygenase-1 crystal structures: Cross-talk between monomers comprising cyclooxygenase-1 homodimers. Biochemistry. 2010;49(33):7069–7079. doi: 10.1021/bi1003298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Galmier MJ, et al. Identification of degradation products of diclofenac by electrospray ion trap mass spectrometry. J Pharm Biomed Anal. 2005;38(4):790–796. doi: 10.1016/j.jpba.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 71.Kato M, Nishida S, Kitasato H, Sakata N, Kawai S. Cyclooxygenase-1 and cyclooxygenase-2 selectivity of non-steroidal anti-inflammatory drugs: Investigation using human peripheral monocytes. J Pharm Pharmacol. 2001;53(12):1679–1685. doi: 10.1211/0022357011778070. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.