Significance
Natural regulatory T cells (nTregs) play important roles in preventing autoimmune diseases, but they may be unstable in the presence of inflammation. Here we report that all-trans RA (atRA) but not rapamycin prevents human nTregs from converting to Th1/Th17 cells and sustains their suppressive function in inflammatory environments. Adoptive transfer of nTregs pretreated with atRA enhances their suppressive effects on xenograft-vs.-host diseases. Moreover, we show that atRA suppresses IL-1 receptor upregulation, accelerates IL-6 receptor downregulation, and affects the epigenetic modifications in Foxp3 locus in nTregs following inflammatory stimulation. We suggest that nTregs primed with atRA may represent a novel treatment strategy to control established chronic immune-mediated diseases.
Keywords: Treg cells, inflammation, epigenetic regulation, X-GVHD, epigenetics
Abstract
Recent studies have demonstrated that thymus-derived naturally occurring CD4+Foxp3+ regulatory T cells (Tregs) in human and mouse may be unstable and dysfunctional in the presence of proinflammatory cytokines. All-trans RA (atRA), the active derivative of vitamin A, has been shown to regulate Treg and T effector cell differentiation. We hypothesize atRA stabilizes human natural Tregs (nTregs) under inflammatory conditions. atRA prevents human nTregs from converting to Th1 and/or Th17 cells and sustains their Foxp3 expression and suppressive function in vitro or in vivo following encounters with IL-1 and IL-6. Interestingly, adoptive transfer of human nTregs pretreated with atRA significantly enhanced their suppressive effects on xenograft-vs.-host diseases (xGVHDs), and atRA- but not rapamycin-pretreated nTregs sustained the functional activity against xGVHD after stimulation with IL-1/IL-6. atRA suppresses IL-1 receptor (IL-1R) up-regulation, accelerates IL-6R down-regulation, and diminishes their signaling events as well as prevents the up-regulation of STIP1 homology and U-Box containing protein 1 on Foxp3+ cells following IL-1/IL-6 stimulation. atRA also increases histone acetylation on Foxp3 gene promoter and CpG demethylation in the region of Foxp3 locus (i.e., Treg-specific demethylated region). These results strongly implicate that nTregs primed with atRA may represent a novel treatment strategy to control established chronic immune-mediated autoimmune and inflammatory diseases.
Although natural Treg cells (nTregs) play important roles in preventing autoimmune diseases, most studies have demonstrated that these cells may be unstable and dysfunctional when adoptively transferred to established autoimmune and inflammatory diseases (1–4). Additionally, adoptive transfer of nTregs to Th17-mediated diseases is ineffective (5). nTreg plasticity under inflammatory conditions may explain their inability to suppress established diseases. Murine nTregs can convert into Th1, Th2, Th17, and T follicular helper cells and lose Foxp3 expression and functionality in vitro and in vivo when they encounter proinflammatory cytokines such as IL-6, IL-1, and IL-23 (1–4). Moreover, converted cells may become pathogenic and promote disease development (6). Likewise, human nTregs also may convert to Th17 cells under inflammatory conditions (7, 8), and as such, maintenance of nTreg stability becomes critical toward sustaining their functionality.
As a lineage fate determinant factor of nTregs, Foxp3 is essential and sufficient for its development and immunosuppressive activity (9–11), so Foxp3 induction and stability is critical to sustain high immunosuppressive activity of Tregs. Foxp3 expression level could be regulated by epigenetic modifications and posttranslational modifications. Several studies have shown that DNA demethylation of Foxp3 conserved noncoding sequence 2 (CNS2), also named Treg-specific demethylated region (TSDR), is critical for Foxp3 stable expression (12–15). It has been known that inflammatory cytokine IL-6 increases DNA methyltransferase 1 (DNMT1) binding and CpG methylation in the Foxp3 enhancer and results in repression of Foxp3 expression in nTreg (16). Acetylation and methylation status of histone H3 also correlates with Foxp3 expression level, especially gene-transcription permissive epigenetic modifications of H3K9/14Ac and H3K4me3, which are highly enriched on the Foxp3 promoter locus and which initiate Foxp3 gene transcription in nTregs (17).
We previously demonstrated that all-trans RA (atRA), a vitamin A metabolite, can maintain murine nTregs (18); however, corresponding studies on human nTregs remain elusive. Additionally, atRA promotes expansion of human nTregs in vitro in normal conditions (19), but whether it maintains this effect in an inflammatory environment is still unclear. Herein, we demonstrate that atRA not only markedly prevents human nTregs from becoming Th1, Th17, and double Th1/Th17 cells when stimulated with IL-1 and IL-6, but also sustains Foxp3 and other Treg-related markers and suppressive activities in vitro and in vivo. Moreover, these nTregs exhibited a markedly enhanced suppressive activity on xenogeneic graft-vs.-host-disease (xGVHD). We also demonstrated that atRA suppresses the expression of IL-1 and IL-6 receptors (IL-1R and IL-6R) by nTregs, reduces phosphorylated STAT1, IL-1R-associated kinase (IRAK), P-38, and STAT3 levels in nTregs following IL-1 or IL-6 stimulation. Additionally, atRA prevents the up-regulation of E3 ubiquitin ligase STIP1 homology and U-Box–containing protein 1 (Stub1) and enhances Foxp3 mRNA expression and suppresses DNMT1 mRNA expression by nTregs following IL-1 and IL-6 stimulation. Furthermore, atRA significantly promoted the accumulation of H3K9Ac and H3K4me3 in the promoter region and partially decreased methylation of CpG in the CNS regions of Foxp3 gene locus. By using a long-term culture and comparison experiment, we demonstrated that the ability of atRA to stabilize human nTregs can be maintained in 3-wk cultures and that atRA-, but not rapamycin-pretreated, nTregs sufficiently prolong the survival of xGHVD. These results strongly suggest that expansion of human nTregs with atRA has uniquely improved their quality and likely would lead to an innovative cell therapy for autoimmune and inflammatory diseases.
Results
Human nTregs Become Resistant to IL1/IL-6-Driven Th1 and Th17 Conversion Following atRA Treatment.
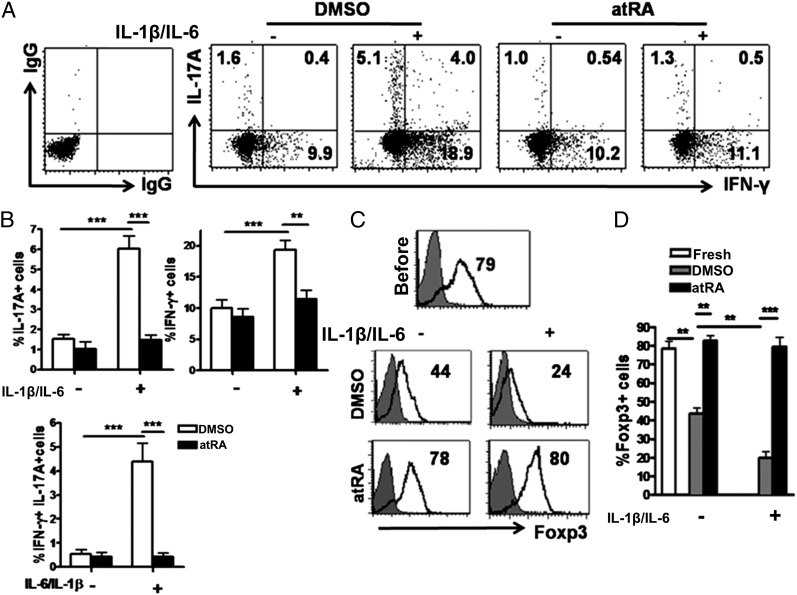
As confirmed from previous reports, ∼10% CD4+CD25+CD127− regulatory T cells sorted from human peripheral blood mononucleated cells (PBMCs) expressed IL-17A, >20% expressed IFN-γ, whereas approximately 4% expressed both IL-17A and IFN-γ when stimulated with IL-1 and IL-6 (7). Although these cells may consist of thymus-derived nTregs and induced Tregs (20), the majority of these cells express Helios and NrP-1, markers whose expression may distinguish nTregs from induced Tregs (21–23). Th2 and/or T follicular helper cell conversion was undetectable. The combination of IL-1 and IL-6 is important because either cytokine alone induced less Th1/Th17 conversion. Freshly isolated and expanded nTregs exhibited similar Th1/Th17 conversion. It is noted that only Tregs but not CD4+CD25−CD127+ non-Tregs converted to Th17 following IL-1/IL-6 stimulation, indicating that is a bona fide conversion of Tregs to T effector cells. However, human nTregs that had been primed with atRA displayed almost a complete resistance to Th1 and Th17 cell conversion (Fig. 1 A and B). The IL-17A and IFN-γ production in atRA-primed nTregs following IL-1 and IL-6 restimulation was comparable to nTregs that had been restimulated without these cytokines. ELISA-based measurements of IL-17A protein from these experiments confirmed our cytometric analysis (Fig. S1). We also further revealed that atRA suppresses the expression of IL-17A and IFN-γ in Helios+ Foxp3+ nTregs (Fig. S2). We found that atRA priming did not affect human nTreg activation and proliferation, suggesting that atRA may specifically inhibit the conversion of human nTregs to T effector cells in inflammatory conditions.
Fig. 1.
atRA stabilizes nTregs following stimulation with IL-1β and IL-6. Human nTregs were sorted from healthy PBMCs and expanded with anti-CD3/CD28–coated beads (one bead to three cells) and IL-2 (300 U/mL) with or without atRA solvent (DMSO) or atRA (0.1 µM) for 7 d. These cells were restimulated with anti-CD3/CD28 beads with or without IL-6 (10 ng/mL) and/or IL-1β (10 ng/mL). Three days later, these cells were collected for surface CD4, CD25, and intracellular IL-17 and IFN-γ staining. (A and B) Representative of Th1 and/or Th17 frequency from four separate experiments. Values indicate mean ± SEM of these experiments. (C and D) Representative of Foxp3 expression and values indicate mean ± SEM of four separate experiments (**P < 0.01 and ***P < 0.001).
Human nTregs Pretreated with atRA Sustain Foxp3 Levels Following in Vitro Expansion Even in the Presence of Inflammatory Cytokines.
Foxp3 level in Tregs usually reflects their functional status. It has been reported that IL-1/IL-6 combination can down-regulate Foxp3 expression in human nTregs although IL-6 alone is sufficient in mouse nTregs (1, 2). Here we revealed that, consistent with a previous report (24), Foxp3 expression decreased moderately 1 wk following nTreg expansion. Interestingly, the addition of atRA to the cultures completely sustained and even increased Foxp3 expression. As atRA suppresses Th1 and Th17 cells differentiation and expansion, it is likely that atRA might also suppress the expansion of non-Treg cell subsets mixed with a starting population of nTregs, thereby indirectly increasing the percentage of cells expressing Foxp3. To exclude this possibility, we have counted the total cell numbers following the expansion. We demonstrated that atRA priming did not decrease but conversely increased the total numbers of expanded nTregs, suggesting a Foxp3 maintenance role for atRA. This result implicates that the addition of atRA at appropriate doses does not affect the yield of expanded human nTregs for clinical use. We also calculated the total numbers of Foxp3+ and Foxp3− cells during the in vitro expansion and found that atRA predominately expanded the Foxp3+ cells, particularly in the presence of inflammatory cytokines (Fig. S3).
As anticipated, addition of IL-1 and IL-6 dramatically down-regulated Foxp3 expression in human nTregs (Fig. 1 C and D). This reduction was mostly associated with inflammatory cytokines rather than T-cell receptor (TCR) signaling (Fig. S4). In contrast, atRA-primed human nTregs completely prevented the decrease in Foxp3 expression mediated by these cytokines (Fig. 1 C and D). Although culture with IL-1 and IL-6 did not affect the expression of CD62L, CD103, CTLA-4, GITR, and PD1 on human nTregs, these cytokines did mediate a decrease in membrane-bound TGF-β levels on these cells. Of note, atRA treatment increased CD103, CTLA-4, GITR, and PD-1 expression on nTregs. Interestingly, atRA not only prevented the down-regulation of membrane-bound TGF-β, but actually increased its expression after stimulating with IL-1 and IL-6 (Fig. S5). It is likely that increased TGF-β is associated with improved suppressive activity (as detailed later). We also observed that atRA treatment diminished CD62L expression but increased CD44 expression on Tregs confirming that atRA promotes human T-cell differentiation (25).
Human nTregs Pretreated with atRA Maintain Their Suppressive Activities Following in Vitro Expansion Even in the Presence of Inflammatory Cytokines.
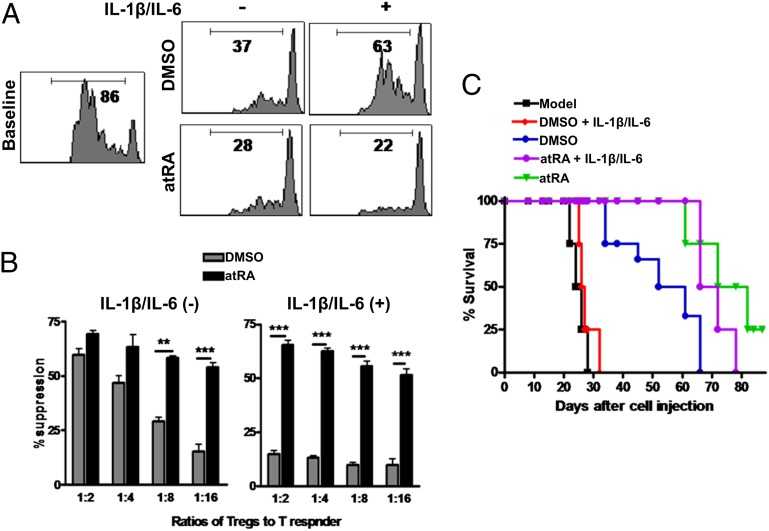
We have also investigated the functional activities of these Tregs in vitro. As shown in Fig. 2A, human nTregs primed with DMSO or atRA displayed a potent suppression of T-cell proliferation (1:2 ratio of Tregs to T responder cells). However, at low ratios of Tregs to responder cells, we observed that the suppressive activity was significantly lower in DMSO-primed nTregs than in atRA-primed nTregs (Fig. 2 A and B). Although some groups have demonstrated that human nTregs are stable following expansion in vitro, other studies have shown that nTregs lose suppressive activity when they are repeatedly stimulated (24). We found that the suppressive activity of untreated nTregs was almost completely lost when exogenous IL-1 and IL-6 were added to the cultures. Importantly, atRA-primed nTregs sustained their suppressive activity even in the presence of these proinflammatory cytokines.
Fig. 2.
atRA sustains and enhances the suppressive activities of nTregs in vitro and in vivo even following encounter with IL-1β and IL-6 in vitro. nTregs expanded as in Fig.1A were harvested and restimulated with or without IL-1β/IL-6 (10 ng/mL) for 3 d. These cells were added to CD25− T cells labeled with CFSE and stimulated with OKT3 (20 ng/mL) for 72 h in the presence of irradiated APCs (1:1). (A) The CFSE dilution was examined by flow cytometry and representative of four independent experiments. (B) Values indicate percentages of suppression with mean ± SEM of four independent experiments (**P < 0.01 and ***P < 0.001). (C) nTreg subsets (5 × 106) were cotransferred with CD25− PBMCs into NOG mice and survival was monitored. Each group includes eight mice, and data represent the integration of two independent experiments. nTregs/atRA vs. nTregs/DMSO (P = 0.0234); nTregs/atRA+IL-1+IL-6 vs. nTregs/DMSO+IL-1+IL-6 (P = 0.0003).
We further investigated whether this finding might have clinical relevance by using a humanized animal model. Previous studies have demonstrated that lightly irradiated nonobese diabetic SCID common γ-chain−/− (NOG) mice injected with 20 × 106 human CD25-depleted PBMCs will develop a human anti-mouse xGVHD whereby mice rapidly lose weight and die in 1 mo (26). The cotransfer of 5 × 106 human nTregs primed with DMSO or atRA along with the xGVHD-inducing PBMCs markedly prolonged survival. Interestingly, we found that the protective effect of atRA-primed nTregs is superior to that of DMSO-primed nTregs (Fig. 2C). Given that atRA also sustains Foxp3 expression by nTregs, these studies suggest that atRA-based expansion of human nTregs can enhance their function to levels superior to those observed by using current expansion approaches. More importantly, following stimulation with IL-1 and IL-6 nTregs lost their therapeutic effect on xGVHD whereas atRA-primed nTregs maintained their therapeutic efficacy. The atRA treatment of NOG mice injected with PBMCs did not significantly alter the disease courses, excluding the possibility that atRA directly suppresses xGVHD. These studies strongly suggest that human nTregs primed with atRA display increased suppressive activity and sustain the suppressive ability even under inflammatory conditions, providing a novel approach to the development of human nTregs for clinical therapy.
atRA Reduces IL-1R and IL-6R Expression and Activation by nTregs Following Cytokine Stimulation.
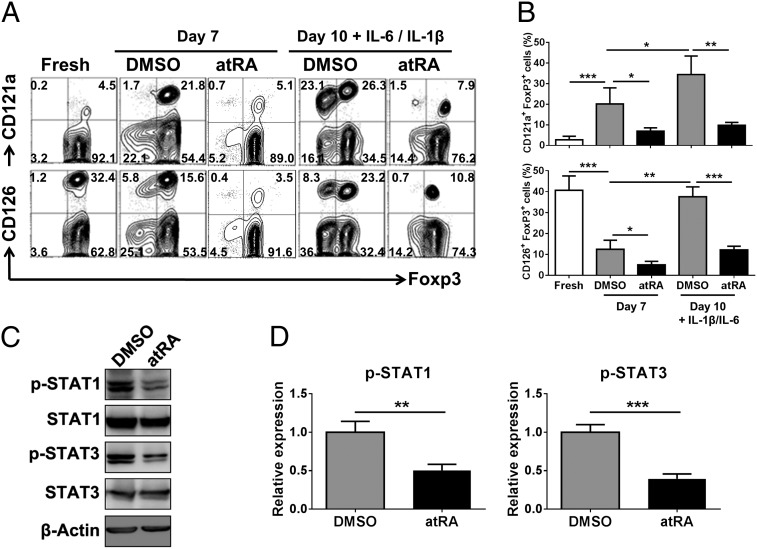
We sought to search the underlying mechanism whereby atRA sustains the stability of human nTregs in the presence of proinflammatory cytokines. It has been known that human T cells express IL-1R and IL-6R and therefore respond to these cytokines (7, 8). Our analyses revealed that freshly isolated nTregs expressed IL-6R, although IL-1R was only expressed at a low level. These cells are highly purified Treg cells because nearly 98% are Foxp3+. Following TCR stimulation, IL-1R expression significantly increased and IL-6R expression significantly decreased whereas the Foxp3 expression was reduced. IL-1R up-regulation was mostly located on the Foxp3+ cell population whereas IL-6R was expressed on Foxp3+ and Foxp3− cell populations. Interestingly, addition of atRA markedly suppressed up-regulation of IL-1R and increased degradation of IL-6R in Foxp3+ and Foxp3− cell populations (Fig. 3 A and B). Furthermore, IL-1R expression in nTregs markedly increased and IL-6R expression did not significantly decrease following IL-1 or IL-6 stimulation, indicating these cytokines regulate their role in nTregs with a feedback mechanism. Inflammatory cytokines also induced IL-1R expression on Foxp3− cell population, implicating the possibility that Foxp3− cell population comes from Treg cell conversion. Of note, the addition of atRA to the cultures has completely prevented the increase of IL-1R and IL-6R on nTregs following cultures with IL-1 and IL-6, if any; it actually decreased IL-6R expression on Treg cells. atRA also distinctly sustained Foxp3 expression on Treg cells, regardless of addition of IL-1 and IL-6 (Fig. 3 A and B). CD121a was used to identify the IL-1R because it recognizes and binds IL-1α and IL-1β, and IL-1/CD121a signal is involved in many cytokine and inflammation responses. Additionally, the CD121a is expressed on TCR-activated human nTregs (27). CD126 is IL-6 receptor that is expressed on nTregs and able to bind to IL-6 and mediate IL-6 signals into the cells through the interaction of gp130 (18, 28). These results are similar to the previous findings for mouse nTregs and imply that the down-regulation of IL-1R and IL-6R signal pathways may contribute to nTreg stability (18).
Fig. 3.
atRA decreases IL-1R and IL-6R expression and down-stream signal molecules on nTregs when stimulated with IL-1β and IL-6. nTregs were sorted and expanded as described earlier. The IL-1R (CD121a) and IL-6R (CD126) expression on freshly isolated or expanded nTregs pretreated with atRA or DMSO before (day 7) and after (day 10) IL-1β/IL-6 stimulation was examined. (A) Data are representative of three separate experiments (gated on Foxp3+ cells). (B) Values are mean ± SEM of three independent experiments. (C) The expanded nTregs primed with atRA or DMSO were stimulated with IL-1β and IL-6 for 30 min, and the levels of phosphorylated STAT1 and STAT3 were determined by Western blot. Data are representative of four separate experiments with the similar results. (D) The histograms show the relative phosphorylation level (mean ± SEM of four separate experiments) of STAT1 and STAT3 at Tyr705 or Tyr701 normalized to the expression of the total STAT1 and STAT3 protein. The DMSO vehicle has been arbitrarily assigned as 1 with the others represented to this value (**P < 0.01 and ***P < 0.001).
We then investigated the expression and activation of the downstream signal molecules for IL-1R and IL-6R. It has been well recognized that IL-1 and/or IL-6 exert their effects through the activation of STAT1 and STAT3, respectively (29–31). We hypothesized that the addition of atRA might reduce STAT1 and STAT3 activation following IL-1 and IL-6 stimulation. We observed that, although IL-1 and IL-6 stimulated STAT1 and STAT3, as expected, atRA markedly decreased STAT1 and STAT3 activation following IL-1 and IL-6 stimulation (Fig. 3 C and D). By using flow-cytometric analysis, we further showed that, following IL-1 and IL-6 stimulation, atRA decreased STAT1 and STAT3 activation in the Foxp3+ cell population (Fig. S6). Given that IRAK is needed for IL-1 to active Stat1 (32), we also investigated IRAK activation. As expected, IL-1 or IL-6 stimulation activated IRAK on nTregs. Because previous studies have shown that IL-1 receptor signaling usually activates p38 mitogen-activated protein kinases (p38 MAPK) and then activated p38 MAPK indirectly activates Stat1 (33), we also examined the levels of phosphorylated p38 MAPK (p-p38 MAPK) on nTregs, as shown in Fig. S7. We found that the expression of p-p38 MAPK on nTregs was significantly increased following IL-1 or IL-6 stimulation. The levels of phosphorylated IRAK and p38 MAPK paralleled that of activated Stat1 (Fig. 3 C and D and Fig. S7). Importantly, atRA treatment consistently suppressed the levels of phosphorylated Stat1, IRAK, and p38 MAPK (Fig. 3 C and D and Fig. S7). Taken together, these results suggest that atRA reduces IL-1R and IL-6R expression and their signaling capabilities in nTregs under proinflammatory conditions.
atRA Sustains Foxp3 Expression Through Epigenetic Modulation of Foxp3 Gene and Regulation of E3 Ligase Stub1 Expression in nTregs.
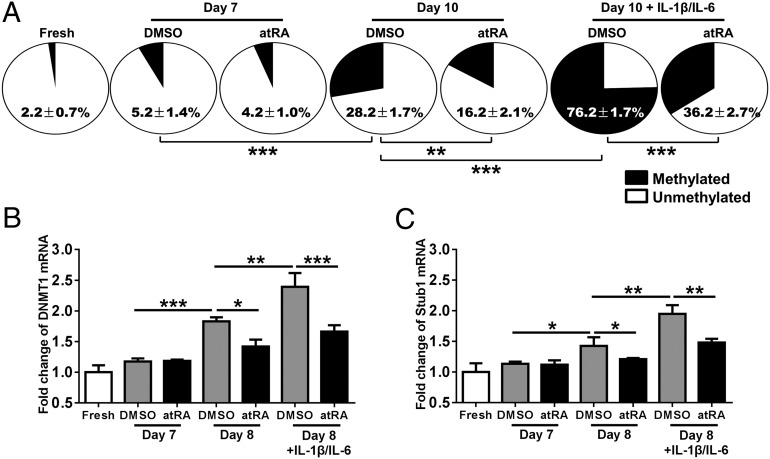
The epigenetic regulation in the Foxp3 locus leading to demethylation of CpG islands in the region of Foxp3 locus (i.e., TSDR) is considered to be an important hallmark for stability and functionality of Foxp3+ Tregs (12). As such, we used bisulfite sequence analysis to examine the methylation status of atRA-primed nTregs in the presence or absence of IL-1 and IL-6. As expected, freshly isolated nTregs exhibited few methylated CpG sites but underwent a slight increase following 7 d of expansion in vitro. Addition of atRA to the culture did not significantly change the CpG methylation on nTregs. When these cells were restimulated for an additional 3 d, they exhibited a significant increase in methylated CpG sites but atRA significantly reduced this methylation (Fig. 4A). Additionally, nTregs treated with IL-1 and IL-6 markedly increased the number of methylated CpG sites whereas atRA-primed nTregs had a significantly lower level of CpG methylation (76.2 ± 1.7% vs. 36.2 ± 2.7%; P < 0.001, Fig. 4A). Consistent with this observation, IL-1 and IL-6 stimulation also increased DNMT1 mRNA expression in nTregs whereas atRA treatment abrogated this response (Fig. 4B). DNMT1 increase and its recruitment to CpG islands in Foxp3 gene is associated with CpG methylation (16), further supporting that proinflammatory cytokines can induce Foxp3 gene methylation. We examined the DNMT1 mRNA expression on day 8 because its expression usually precedes the CpG methylation. We also found that the increase of DNMT1 mRNA mainly depends on IL-1 and IL-6, but TCR restimulation also plays a significant role in the early (24 h; Fig. 4B) but not late (72 h) stage (Fig. S8), alluding to the specific role of inflammatory cytokines in maintaining DNMT1 mRNA on nTreg cells. These results imply that atRA-mediated TSDR demethylation at least in part contributes to nTreg stability in the presence of proinflammatory cytokines. Others have previously reported that the stability of Tregs generated in vivo was associated with demethylated TSDR (34). We also found that the expression of Foxp3 mRNA was increased in atRA-primed nTregs with or without the addition of IL-1 and IL-6 (Fig. S9), demonstrating that atRA can regulate Foxp3 transcript in nTreg cells.
Fig. 4.
atRA sustains Foxp3 expression through epigenetic modulation of Foxp3 gene and regulation of E3 ligase Stub1 expression in nTregs. Fresh nTregs were sorted from human PBMCs and expanded in the presence of atRA or DMSO for 7 d, and then restimulated with IL-1β and IL-6 for another 1 d (day 8) or 3 d (day 10). Cells were harvested at each step for quantitative PCR or methylation status assay. (A) Methylation status of CpG motifs of TSDR of Foxp3 locus in aforementioned harvested nTregs were detected with bisulfite sequencing PCR. Numbers in filled sectors (methylated) and open sectors (unmethylated) of the pie charts indicate mean ± SEM in 11 CpG sites of TSDR from 12 replicates. (B and C) The expression level of DNMT1 and Stub1 were detected with aforementioned harvested nTregs as indicated by quantitative PCR. β-Actin was used as the internal reference. Fold changes were normalized with freshly isolated nTregs. Values indicate mean ± SEM of four separate experiments (n = 4 individual healthy donors). (*P < 0.05, **P < 0.01, and ***P < 0.001).
As atRA did not completely prevent the methylation of CpG sites in the TSDR, we also investigated the acetylation status of histones found in the Foxp3 gene locus. By using a ChIP assay, we found that in vitro expanded human nTregs displayed a somewhat enriched level of H3K9Ac and H3K4me3 in the Foxp3 promoter region when the cells were stimulated with IL-1, IL-6, or both together (Fig. S10A). Whereas atRA treatment indeed promoted the accumulation of transcriptional activation markers such as H3K9Ac and H3K4me3 in the promoter region of Foxp3, this was not observed in the CNS1-3 regions of the Foxp3 gene locus (Fig. S11).
Given that our recent report demonstrated that ubiquitin ligase Stub1 is responsible for Foxp3 degradation and instability of Treg cells (35), we also investigated the roles of inflammatory cytokines and atRA in Stub1 expression. We observed that inflammatory cytokines IL-1 and IL-6 indeed up-regulate Stub1 expression on human nTregs and atRA significantly prevents the up-regulation of Stub1 (Fig. 4C). It is likely that atRA also contributes to Treg stability through stabilizing Foxp3 protein in the inflammatory conditions.
Human nTregs Pretreated with atRA but Not with Rapamycin Sustain Foxp3 Levels and Functional Activity in the Presence of Inflammatory Cytokines.
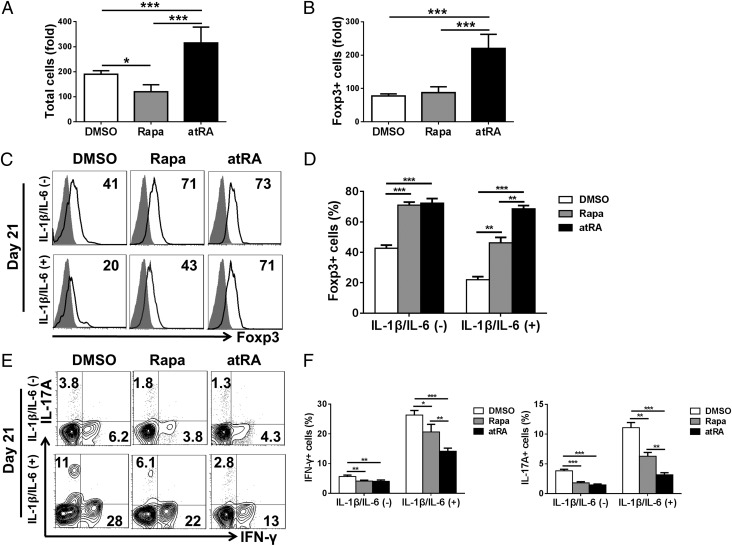
Given that human nTregs need to be repeatedly expanded ex vivo to gain the sufficient cell numbers for clinical use, we asked whether atRA can maintain its effect on nTreg stability for a more long-term culture process. In addition, because rapamycin has been shown to be effective in stabilizing human nTregs (19), we also tried to compare the effect of atRA and rapamycin on nTreg stability. A 21-d culture experiment was set up to evaluate the effect of atRA and rapamycin in nTreg stability during a long-term expansion. Following 21-d culture, total cells resulted in near 200-fold expansion. atRA significantly increased the total numbers whereas rapamycin did not increase but actually reduced the total numbers compared with DMSO-treated control cells (Fig. 5A). Similarly, atRA also significantly increased the total Foxp3+ cells whereas rapamycin did not expand the total Foxp3+ cells compared with the control (Fig. 5B).
Fig. 5.
atRA but not rapamycin stabilizes long-term expanded nTregs in the presence of inflammatory cytokines. nTregs were sorted and expanded for 21 d with DMSO (control), rapamycin (100 nM), or atRA (100 nM) and then restimulated with IL-1β and IL-6 for another 3 d. These cells were harvested for staining of Foxp3, IFN-γ, and IL-17A or for in vitro suppressive assay as described earlier. (A) The expansion folds of total cells on day 21. (B) The expansion folds of Foxp3+ cells on day 21. (C and D) Representative of Foxp3 expression and values indicate the mean ± SEM of four separate experiments. In each experiment, all Treg cells come from the same donor, and data are the summary of four independent experiments (n = 4). (E and F) Representative of Th1 and Th17 frequency from four separate experiments, and the values indicate mean ± SEM of these experiments (*P < 0.05, **P < 0.01, and ***P < 0.001).
We further investigated whether the inflammatory cytokines will affect the stability of nTregs following a long-term expansion. As shown in Fig. 5 C and D, human nTregs pretreated with rapamycin or with atRA mostly sustained Foxp3 expression compared with DMSO control in 21-d expansion. Interestingly, addition of IL-1 and IL-6 to the cultures, the Foxp3 expression on DMSO- or rapamycin-treated nTregs was significantly reduced, whereas atRA treatment still preserved the Foxp3 expression.
We also examined the conversion of human nTregs that had undergone a long-term expansion to T effector cells. Whereas ∼6.5% cells became Th1 cells and ∼4% cells became Th17 cells, atRA and rapamycin significantly suppressed Th1 and Th17 conversion. When restimulated with IL-1 and IL-6, the nTreg-to-Th1 and -Th17 conversion was markedly augmented. Although atRA and rapamycin suppressed this conversion, the effect of atRA on controlling Th1 and Th17 differentiation is evidently superior to the effect of rapamycin (Fig. 5 E and F).
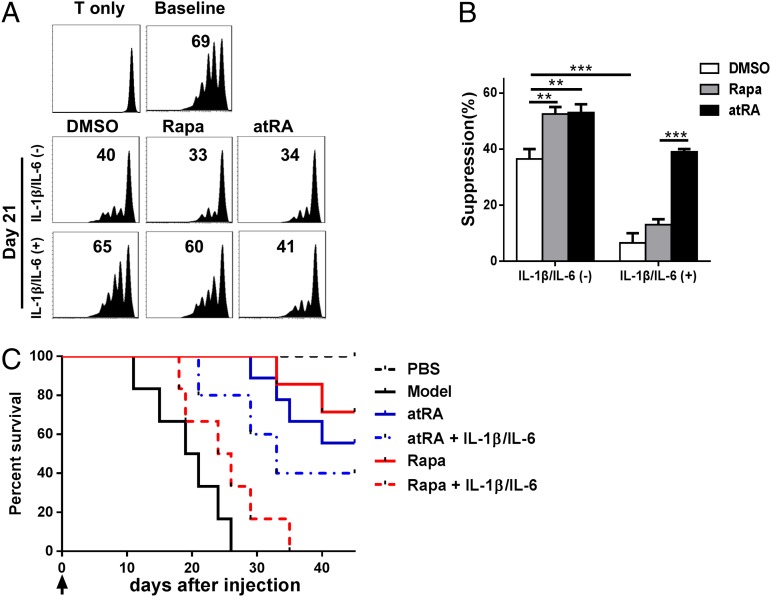
We then studied whether this stability regulation reflects their functional characteristics. By using a standard in vitro suppression assay described in Fig. 2, we observed that human nTregs following 21-d expansion displayed the suppressive activity and atRA- or rapamycin-treated human nTregs significantly enhanced their functional activities. However, the suppressive activity was almost completely lost when IL-1 and IL-6 were added to culture system, and rapamycin-treated nTregs did not display their effect in the inflammatory conditions. Interestingly, atRA-pretreated human nTregs mostly sustained their effect in the inflammatory conditions (Fig. 6 A and B). By using a humanized animal model, we observed that atRA- and rapamycin-treated human nTregs following 21-d expansion prolonged the survival of xGVHD mice, and, as expected, rapamycin-treated nTregs lost the protective role after they had been exposed to IL-1 and IL-6 whereas atRA-pretreated human nTregs preserved their therapeutic role in the similar conditions (Fig. 6C). These data strongly suggest that the method with atRA-pretreated and expanded human nTreg has markedly surpassed the current approaches established and has an important value of clinical translation in the treatment of immunologic diseases.
Fig. 6.
atRA but not rapamycin sustains the suppressive activities of long-term expanded nTregs in vitro and in vivo following encountering with inflammatory cytokines. nTregs were sorted and expanded in the presence of atRA or rapamycin for 21 d, and restimulated with IL-1β and IL-6 for another 3 d. These cells were harvested for in vitro or in vivo suppressive assay as described earlier. (A) Expanded nTreg subsets were added to CD25− T cells labeled with CFSE and stimulated with OKT3 (20 ng/mL) for 72 h in the presence of irradiated APCs (1:1). The CFSE dilution was examined by flow cytometry and is representative of four independent experiments. (B) Values indicate percentages of suppression with mean ± SEM of four independent experiments. (C) nTreg subsets (5 × 106) were cotransferred with CD25− PBMCs into NOG mice and survival was monitored. Each group includes eight mice, and data represent the integration of two independent experiments. nTregs/atRA+IL-1+IL-6 vs. nTregs/atRA (P = 0.4726); nTregs/atRA+IL-1+IL-6 vs. nTregs/RAPA+IL-1+IL-6 (P = 0.0423); nTregs/atRA+IL-1+IL-6 vs. model (P = 0.0036); nTregs/RAPA+IL-1+IL-6 vs. model (P = 0.1284).
Discussion
Treg cells and their stability play an important role in regulating homeostasis and preventing autoimmune diseases. Although Treg cells may be stable in normal conditions and in Th1-mediated inflammation in animal models (36), most studies have recently demonstrated that nTregs have plasticity in inflammatory conditions (1–4, 18). Moreover, these cells may become pathogenic cells when they lose Foxp3 expression and convert to Th1 and/or Th17 effector cells in mice (6).
Likewise, human nTregs can also convert to Th1 and/or Th17 cells in the presence of proinflammatory cytokines including IL-1 and IL-6 (7), atRA pretreatment prevents their conversion to pathogenic cells and sustains the Treg-specific phenotype and suppressive activities, both in vitro and in vivo. The effect of atRA on stabilizing human nTregs is consistent during the nTreg expansion and has a superior effect on stabilizing human nTregs in the inflammatory conditions compared with rapamycin-treated nTregs. These observations extend previous reports that atRA stabilizes mouse nTregs under inflammatory conditions (18). Because IL-1 and IL-6 levels are frequently elevated in patients with autoimmune diseases, our findings suggest that the proper manipulation of human nTregs can be used to stabilize their phenotype and function, thus controlling disease activity and progress. Previous studies have demonstrated that Treg numbers and function in patients with autoimmune diseases can indeed be impaired (37, 38).
Exactly how atRA stabilizes human nTregs remains incompletely understood. Engagement of IL-1 and IL-6 to their receptors appears to be important for STAT1, IRAK, p38 MAPK, and STAT3 activation, which in turn promotes the differentiation of Th cells (39). atRA treatment can decrease the expression of IL-1R and IL-6R, as well as STAT1, IRAK, p38 MAPK, and STAT3 activation, which might contribute to nTreg stability. This atRA-mediated nTreg stability seems to predominate over the differentiation of non-Treg toward the Treg phenotype. It is likely that atRA not only maintains Foxp3 but also inhibits the expansion of Foxp3− (non-Treg) cells. atRA has been known to suppress the differentiation of Th1 and Th17 effector cells (40, 41). Previous studies have demonstrated that atRA does not induce Foxp3 from Foxp3− cells (42); therefore, it is unlikely that Foxp3− cells are involved as precursors for Foxp3 induction. Although there is still debate concerning the mechanisms by which atRA affects Treg stability or non-Treg expansion, our results indicate that atRA predominately affects Foxp3+ nTregs rather than a non-Treg population. Importantly, the consequence is that atRA-treated nTreg cells have a high quality and purity that is beneficial to cell therapy in the clinical setting.
Epigenetic regulation in the Foxp3 locus leading to demethylation of CpG islands in the region of the Foxp3 locus (i.e., TSDR) is considered a hallmark of Treg stability and functionality (12), and our observations implicate atRA as being involved in this process. atRA is at least partially effective in the demethylation of the TSDR, but atRA also affects the acetylation status of the Foxp3 promoter region in Tregs. This observation is further validated by the expression of DNMT1, a DNA methyltransferase, for which increase and recruitment to CpG islands in Foxp3 gene is associated with CpG methylation (16). We have found that TCR stimulation and inflammatory cytokines induce DNMT1 expression whereas inflammatory cytokines also sustain DNMT1 expression on nTregs. The Foxp3 promoter region is mainly regulated through TCR/CD28 stimulation and IL-2 receptor signals, via NFAT and AP-1, resulting in Foxp3 stability (17). Our results suggest that atRA may stabilize nTregs through epigenetic regulation of the Foxp3 gene locus in a proinflammatory milieu. Although atRA may inhibit the expansion of non-Treg cells and then indirectly enhance demethylation levels of the TSDR in nTregs, it is evident that atRA also affects the histone acetylation status of the Foxp3 gene in human nTregs. Recent study has revealed that inflammatory stimuli such as IL-1 and LPS also up-regulate the expression of an E3 ligase Stub1 that is responsible for ubiquitinating Foxp3 and Foxp3 protein degradation (35). We have now confirmed that IL-1 and IL-6 can up-regulate Stub1 expression and observed that atRA prevents the Stub1 up-regulation on human nTreg following inflammatory stimuli. Thus, atRA may stabilize human nTregs through the modulation of Foxp3 gene and protein in the inflammatory conditions.
Current studies have demonstrated that rapamycin plays an important role in sustaining Foxp3 expression during human nTreg expansion (2, 3). Our study has confirmed this finding. However, we observed that rapamycin lost its effect on stabilizing human nTregs in the presence of inflammatory cytokines, whereas atRA sustains this function. Moreover, we also revealed that atRA has a constant role in stabilizing human nTregs with or without inflammatory cytokines. Although this observation is inconsistent with other reports (19, 43), it is possible that the methods and protocols have been used differently. For example, we have used IL-1 and IL-6 to mimic inflammation whereas Scottà et al. have used IL-21, IL-23, and TGF-β (43). As the combination of IL-1 and IL-6 is widely recognized to be able to convert human nTregs to become Th1/Th17 cells, we therefore favor the use of this mixture. In fact, this group also found that atRA-primed human nTregs are mostly stable when stimulated with IL-1, IL-6, and TGF-β (43).
In summary, our demonstration of an enhanced suppressive effect of atRA-pretreated human nTregs in the inflammatory condition by using in vitro and xGVHD in vivo model highlights the importance of Treg phenotype and stability in treating immune related diseases. Thus, human nTregs treated with atRA represent a novel therapeutic strategy for autoimmune and other immune diseases.
Materials and Methods
Treg Isolation and Expansion.
Human nTregs were sorted from PBMCs by gating on CD4+CD25brightCD127− cells (>98% purity), expanded with anti-CD3/CD28 beads (one bead to three cells; Invitrogen) and IL-2 (300 U/mL; BioLegend) with or without atRA (0.1 µM; Sigma) or DMSO for 7 d, and then restimulated with anti-CD3/28 beads (1:10) with or without IL-6 (10 ng/mL) and/or IL-1β (10 ng/mL) for 1–3 d. In some cultures, rapamycin (0.1 µM; Sigma) was added. Supernatants were subjected to human IL-17A and IFN-γ ELISA (BioLegend). Suppression assays were performed as previously described (44).
Flow Cytometry Analysis.
Cell suspensions were harvested and stained for flow cytometry analysis by using the following antibodies: anti-CD4 (A161A1), CD25 (M-A251), Foxp3 (150D), IL-17A (BL168), IFN-γ (4S.B3), CD126 (UV4), Helios (22F6), CD62L (DREG-56), CD103 (Ber-ACT8), CTLA4 (L3D10), GITR (621), and TGF-β (TW4-2F8) were purchased from BioLegend. Anti-CD121a (FAB269P) was purchased from R&D Systems. Anti-PD1 (EH12.1), pSTAT1 (pY701), and pSTAT3 (pY705) antibodies were purchased from BD Pharmingen. The staining procedures were performed as previously described (3). Results were obtained on a BD FACSCalibur flow cytometer and analyzed by using FlowJo (TreeStar).
In Vitro Suppression Assays.
In vitro suppression assays were performed as previously described (40). Briefly, expanded nTregs were harvested and restimulated with or without IL-1β/IL-6 (10 ng/mL) for 3 d. These cells were added to CD25- T cells labeled with carboxyfluorescein succinimidyl ester (CFSE) and stimulated with muromonab-CD3 (OKT3) (20 ng/mL) for 72 h in the presence of irradiated antigen-presenting cells (APCs; 1:1). The CFSE dilution was examined by flow cytometry. The suppression ratio was calculated as (baseline group − experiment group)/baseline group × 100%.
xGVHD Models.
NOG mice (Jackson Laboratory) received a 200-cGy γ-irradiation from a linear accelerator before injection of human PBMCs. On the same day, irradiated mice were injected with 20 × 106 human CD25-depleted PBMCs with or without 5 × 106 expanded human nTregs pretreated with atRA, rapamycin, or DMSO control. In some groups, the expanded human nTreg subsets had been exposed to IL-1 and IL-6 for 3 d and then were cotransferred with human PBMCs to NOG mice. Mouse survival was monitored twice per week.
Immunoblotting.
Cells (0.5 × 106) were lysed in RIPA buffer. The lysate was fractionated by 8% (wt/vol) SDS/PAGE and electroblotted. Polyclonal primary antibodies against STAT1, p-STAT1, STAT3, p-STAT3, IRAK and p-IRAK, P38 and p-P38 (Cell Signaling), and peroxidase-coupled secondary antibodies against mouse or rabbit epitopes (Jackson) were used.
Quantitative RT-PCR.
Total RNA was extracted from cells by using TRIzol reagent and used to determine the expression and relative level of the Foxp3, DNMT1, and Stub1 in nTreg cell subsets. The mRNA levels of targeted genes were measured by quantitative RT-PCR (7900HT; ABI) by using the iTaq Universal SYBR Green One-Step Kit (Bio-Rad). The relative expression of Foxp3, DNMT1, and Stub1 were determined by normalizing expression of each target to β-actin. (Foxp3, forward primer 5′-GTG GCC CGG ATG TGA GAA G-3; reverse primer 5′-GGA GCC CTT GTC GGA TGA TG-3; DNMT1, forward primer 5′-CCA TCA GGC ATT CTA CCA-3; reverse primer 5′-CGT TCT CCT TGT CTT CTC-3 T; Stub1, forward primer 5′-AGG CCA AGC ACG ACA AGT ACA T-3; reverse primer 5′-CTG ATC TTG CCA CAC AGG TAG T-3; β-actin, forward primer 5′-AGG CAC CAG GGC GTG AT-3; reverse primer 5′-GCC CAC ATA GGA ATC CTT CTG AC-3).
TSDR Methylation Status Assay.
Genomic DNA was isolated using the Mammalian genomic DNA extraction kit (Beyotime) and processed by using the EZ DNA Methylation-Direct kit (Zymo Research) according to the manufacturer’s protocol. Purified bisulfite-treated DNA was used in bisulfite sequencing PCR with follow a pair of TSDR amplification PCR primers: 5′-TTG GGT TAA GTT TGT TGT AGG ATA G-3′ and 5′-ATC TAA ACC CTA TTA TCA CAA CCC C-3′. The PCR products were purified and cloned into pMD-18T vector (Takara) and single clones were selected for sequencing. All sequencing results of bisulfite converted TSDR region were analyzed on BDPC DNA methylation analysis platform, and the average methylation status of 11 CpG sites in TSDR region were statistically analyzed.
ChIP Analysis.
ChIP analysis was performed as described previously with little modification (45). Briefly, 50,000 cells of each treated nTreg cell sample were harvested and cross-linked with 1% formaldehyde, and then lysed with 120 μL of lysis buffer [50 mM Tris⋅HCl, pH 8.0, 10 mM EDTA, 1% (wt/vol) SDS, protease inhibitor mix (1:100 dilution; Sigma), 1 mM PMSF, 20 mM Na-butyrate]. The chromatin in the lysate was sonicated to 500–800-bp fragments and then diluted with 800 μL of RIPA ChIP buffer [10 mM Tris⋅HCl, pH 7.5, 140 mM NaCl, 1 mM EDTA, 0.5 mM EGTA, 1% (vol/vol) Triton X-100, 0.1% (wt/vol) SDS, 0.1% (wt/vol) Na-deoxycholate, protease inhibitor mix (1:100 dilution; Sigma), 1 mM PMSF, and 20 mM Na-butyrate]. Dynabeads protein G (10 μL; Invitrogen) was incubated with 1 µg of H3K4me3 (Abcam) or H3K9ac (Cell Signaling) or normal rabbit IgG negative control ChIP-grade antibodies for 2 h separately. Then, 100 μL of the sheared chromatin was immunoprecipitated with pretreated antibody–bead complexes and another 100 μL of the sheared chromatin for total input DNA extraction separately. Immunoprecipitated DNA was quantified by real-time PCR with following primers: promoter, 5′-ACC GTA CAG CGT GGT TTT TC-3′ and 5′-CTA CCT CCC TGC CAT CTC CT-3′; CNS1, 5′- CCC AAG CCC TAT GTG TGA TT-3′ and 5′-GTG TGT CAG GCC TTG TGC TA-3′; CNS2, 5′-GTC CTC TCC ACA ACC CAA GA-3′ and 5′-GAC ACC ACG GAG GAA GAG AA -3′; and CNS3, 5′-AGG TGC CGA CCT TTA CTG TG-3′ and 5′- ACA ATA CGG CCT CCT CCT CT-3′.
Statistics.
Differences in Kaplan–Meier survival curves were analyzed by the log-rank test, and other statistical comparisons were performed by the Student t test by using Prism software (GraphPad). Differences were considered significant when P values were <0.05.
Supplementary Material
Acknowledgments
S.G.Z. was supported by the National Institutes of Health Grants AR059103 and AI084359, a Within Our Reach grant from the Rheumatology Research Foundation, Science and Technology Foundation of Shanghai Pudong Grant PKJ2009-Y41, the Arthritis Foundation, and the Wright Foundation. L.L. was supported by National Natural Science Foundation of China Grant 81100270.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1408780111/-/DCSupplemental.
References
- 1.Xu L, Kitani A, Fuss I, Strober W. Cutting edge: Regulatory T cells induce CD4+CD25-Foxp3- T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol. 2007;178(11):6725–6729. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- 2.Zheng SG, Wang J, Horwitz DA. Cutting edge: Foxp3+CD4+CD25+ regulatory T cells induced by IL-2 and TGF-beta are resistant to Th17 conversion by IL-6. J Immunol. 2008;180(11):7112–7116. doi: 10.4049/jimmunol.180.11.7112. [DOI] [PubMed] [Google Scholar]
- 3.Lu L, et al. Role of SMAD and non-SMAD signals in the development of Th17 and regulatory T cells. J Immunol. 2010;184(8):4295–4306. doi: 10.4049/jimmunol.0903418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445(7129):766–770. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 5.Huter EN, Stummvoll GH, DiPaolo RJ, Glass DD, Shevach EM. Cutting edge: Antigen-specific TGF beta-induced regulatory T cells suppress Th17-mediated autoimmune disease. J Immunol. 2008;181(12):8209–8213. doi: 10.4049/jimmunol.181.12.8209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou X, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat Immunol. 2009;10(9):1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koenen HJ, et al. Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood. 2008;112(6):2340–2352. doi: 10.1182/blood-2008-01-133967. [DOI] [PubMed] [Google Scholar]
- 8.Beriou G, et al. IL-17-producing human peripheral regulatory T cells retain suppressive function. Blood. 2009;113(18):4240–4249. doi: 10.1182/blood-2008-10-183251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4(4):330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 10.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 11.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4(4):337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 12.Floess S, et al. Epigenetic control of the foxp3 locus in regulatory T cells. PLoS Biol. 2007;5(2):e38. doi: 10.1371/journal.pbio.0050038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huehn J, Polansky JK, Hamann A. Epigenetic control of FOXP3 expression: The key to a stable regulatory T-cell lineage? Nature Rev. 2009;9(2):83–89. doi: 10.1038/nri2474. [DOI] [PubMed] [Google Scholar]
- 14.Polansky JK, et al. Methylation matters: Binding of Ets-1 to the demethylated Foxp3 gene contributes to the stabilization of Foxp3 expression in regulatory T cells. J Mol Med (Berl) 2010;88(10):1029–1040. doi: 10.1007/s00109-010-0642-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morikawa H, et al. FANTOM Consortium Differential roles of epigenetic changes and Foxp3 expression in regulatory T cell-specific transcriptional regulation. Proc Natl Acad Sci USA. 2014;111(14):5289–5294. doi: 10.1073/pnas.1312717110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lal G, et al. Epigenetic regulation of Foxp3 expression in regulatory T cells by DNA methylation. J Immunol. 2009;182(1):259–273. doi: 10.4049/jimmunol.182.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zheng Y, et al. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463(7282):808–812. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou X, et al. Cutting edge: All-trans retinoic acid sustains the stability and function of natural regulatory T cells in an inflammatory milieu. J Immunol. 2010;185(5):2675–2679. doi: 10.4049/jimmunol.1000598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Golovina TN, et al. Retinoic acid and rapamycin differentially affect and synergistically promote the ex vivo expansion of natural human T regulatory cells. PLoS ONE. 2011;6(1):e15868. doi: 10.1371/journal.pone.0015868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lan Q, et al. Induced Foxp3(+) regulatory T cells: A potential new weapon to treat autoimmune and inflammatory diseases? J Mol Cell Biol. 2012;4(1):22–28. doi: 10.1093/jmcb/mjr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thornton AM, et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184(7):3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yadav M, et al. Neuropilin-1 distinguishes natural and inducible regulatory T cells among regulatory T cell subsets in vivo. J Exp Med. 2012;209(10):1713–1722. doi: 10.1084/jem.20120822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weiss JM, et al. Neuropilin 1 is expressed on thymus-derived natural regulatory T cells, but not mucosa-generated induced Foxp3+ T reg cells. J Exp Med. 2012;209(10):1723–1742, S1721. doi: 10.1084/jem.20120914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffmann P, et al. Loss of FOXP3 expression in natural human CD4+CD25+ regulatory T cells upon repetitive in vitro stimulation. Eur J Immunol. 2009;39(4):1088–1097. doi: 10.1002/eji.200838904. [DOI] [PubMed] [Google Scholar]
- 25.Imaizumi M, Breitman TR. A combination of a T cell-derived lymphokine differentiation-inducing activity and a physiologic concentration of retinoic acid induces HL-60 to differentiate to cells with functional chemotactic peptide receptors. Blood. 1986;67(5):1273–1280. [PubMed] [Google Scholar]
- 26.Ito R, et al. Highly sensitive model for xenogenic GVHD using severe immunodeficient NOG mice. Transplantation. 2009;87(11):1654–1658. doi: 10.1097/TP.0b013e3181a5cb07. [DOI] [PubMed] [Google Scholar]
- 27.Tran DQ, et al. Selective expression of latency-associated peptide (LAP) and IL-1 receptor type I/II (CD121a/CD121b) on activated human FOXP3+ regulatory T cells allows for their purification from expansion cultures. Blood. 2009;113(21):5125–5133. doi: 10.1182/blood-2009-01-199950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Connor RA, Floess S, Huehn J, Jones SA, Anderton SM. Foxp3⁺ Treg cells in the inflamed CNS are insensitive to IL-6-driven IL-17 production. Eur J Immunol. 2012;42(5):1174–1179. doi: 10.1002/eji.201142216. [DOI] [PubMed] [Google Scholar]
- 29.Joshi VD, et al. A role for Stat1 in the regulation of lipopolysaccharide-induced interleukin-1beta expression. J Interferon Cytokine Res. 2006;26(10):739–747. doi: 10.1089/jir.2006.26.739. [DOI] [PubMed] [Google Scholar]
- 30.Sawa S, et al. Autoimmune arthritis associated with mutated interleukin (IL)-6 receptor gp130 is driven by STAT3/IL-7-dependent homeostatic proliferation of CD4+ T cells. J Exp Med. 2006;203(6):1459–1470. doi: 10.1084/jem.20052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nguyen H, et al. IRAK-dependent phosphorylation of Stat1 on serine 727 in response to interleukin-1 and effects on gene expression. J Interferon Cytokine Res. 2003;23(4):183–192. doi: 10.1089/107999003765027384. [DOI] [PubMed] [Google Scholar]
- 32.Koziczak-Holbro M, et al. IRAK-4 kinase activity is required for interleukin-1 (IL-1) receptor- and toll-like receptor 7-mediated signaling and gene expression. J Biol Chem. 2007;282(18):13552–13560. doi: 10.1074/jbc.M700548200. [DOI] [PubMed] [Google Scholar]
- 33.Goh KC, Haque SJ, Williams BR. p38 MAP kinase is required for STAT1 serine phosphorylation and transcriptional activation induced by interferons. EMBO J. 1999;18(20):5601–5608. doi: 10.1093/emboj/18.20.5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miyao T, et al. Plasticity of Foxp3(+) T cells reflects promiscuous Foxp3 expression in conventional T cells but not reprogramming of regulatory T cells. Immunity. 2012;36(2):262–275. doi: 10.1016/j.immuni.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 35.Chen Z, et al. The ubiquitin ligase Stub1 negatively modulates regulatory T cell suppressive activity by promoting degradation of the transcription factor Foxp3. Immunity. 2013;39(2):272–285. doi: 10.1016/j.immuni.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rubtsov YP, et al. Stability of the regulatory T cell lineage in vivo. Science. 2010;329(5999):1667–1671. doi: 10.1126/science.1191996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Valencia X, et al. TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood. 2006;108(1):253–261. doi: 10.1182/blood-2005-11-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyara M, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30(6):899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 39.Hibbert L, Pflanz S, De Waal Malefyt R, Kastelein RA. IL-27 and IFN-alpha signal via Stat1 and Stat3 and induce T-Bet and IL-12Rbeta2 in naive T cells. J Interferon Cytokine Res. 2003;23(9):513–522. doi: 10.1089/10799900360708632. [DOI] [PubMed] [Google Scholar]
- 40.Iwata M, Eshima Y, Kagechika H. Retinoic acids exert direct effects on T cells to suppress Th1 development and enhance Th2 development via retinoic acid receptors. Int Immunol. 2003;15(8):1017–1025. doi: 10.1093/intimm/dxg101. [DOI] [PubMed] [Google Scholar]
- 41.Mucida D, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317(5835):256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 42.Lu L, et al. All-trans retinoic acid promotes TGF-β-induced Tregs via histone modification but not DNA demethylation on Foxp3 gene locus. PLoS ONE. 2011;6(9):e24590. doi: 10.1371/journal.pone.0024590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scottà C, et al. Differential effects of rapamycin and retinoic acid on expansion, stability and suppressive qualities of human CD4(+)CD25(+)FOXP3(+) T regulatory cell subpopulations. Haematologica. 2013;98(8):1291–1299. doi: 10.3324/haematol.2012.074088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou X, et al. Isolation of purified and live Foxp3+ regulatory T cells using FACS sorting on scatter plot. J Mol Cell Biol. 2010;2(3):164–169. doi: 10.1093/jmcb/mjq007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dahl JA, Collas P. A rapid micro chromatin immunoprecipitation assay (microChIP) Nat Protoc. 2008;3(6):1032–1045. doi: 10.1038/nprot.2008.68. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.