Significance
Fatty acids comprise a large class of compounds that serve broad roles in cells and society. These hydrophobic compounds provide integrity for biological membranes, make them impermeable to solutes and toxins, and modulate the cellular response to signals or stresses. Fatty acids, or the products derived from them, are also important as dietary supplements, lubricants, specialty chemicals, and fuels. Their potential utility in biology or industry could be increased by producing novel classes of fatty acids. This paper reports on the occurrence and synthesis of a newly discovered class of furan-containing fatty acid. It also provides evidence that furan-containing fatty acids scavenge toxic reactive oxygen species, suggesting a previously unnoticed role for this class of compounds in bacteria and other cells.
Keywords: radical scavenger, oxygenated fatty acid, fatty acyl methylase
Abstract
Fatty acids play important functional and protective roles in living systems. This paper reports on the synthesis of a previously unidentified 19 carbon furan-containing fatty acid, 10,13-epoxy-11-methyl-octadecadienoate (9-(3-methyl-5-pentylfuran-2-yl)nonanoic acid) (19Fu-FA), in phospholipids from Rhodobacter sphaeroides. We show that 19Fu-FA accumulation is increased in cells containing mutations that increase the transcriptional response of this bacterium to singlet oxygen (1O2), a reactive oxygen species generated by energy transfer from one or more light-excited donors to molecular oxygen. We identify a previously undescribed class of S-adenosylmethionine-dependent methylases that convert a phospholipid 18 carbon cis unsaturated fatty acyl chain to a 19 carbon methylated trans unsaturated fatty acyl chain (19M-UFA). We also identify genes required for the O2-dependent conversion of this 19M-UFA to 19Fu-FA. Finally, we show that the presence of 1O2 leads to turnover of 19Fu-Fa in vivo. We propose that furan-containing fatty acids like 19Fu-FA can act as a membrane-bound scavenger of 1O2, which is naturally produced by integral membrane enzymes of the R. sphaeroides photosynthetic apparatus.
Fatty acids have crucial, yet diverse, roles in biology. In cells and organelles, fatty acids maintain bilayer stability, provide a permeability barrier, act as secondary messengers in signaling pathways, and aid the function of integral membrane proteins (1–3). Fatty acids also help maintain viability in response to temperature and environmental changes and can be targets for modification by reactive oxygen species or membrane-active agents (2–8). Fatty acids, or the products derived from them, are valuable as food additives, specialty chemicals, and petroleum substitutes (9–12). Thus, there is considerable interest in understanding the suite of fatty acids that can be made by native or engineered pathways. We are studying the synthesis and role of fatty acids during stress responses.
Here, we demonstrate a previously unreported ability of the photosynthetic bacterium Rhodobacter sphaeroides to produce furan-containing fatty acids (Fu-FAs), an important, yet poorly understood, class of compounds. The presence of Fu-FAs has been reported previously in plants, fish, and some bacteria (13). Based on their chemical properties, it is proposed that Fu-FAs could provide bilayer protection against radicals or organic peroxides that reduce membrane function (13–15). The oxygen atom within Fu-FAs also provides a functional group for modifications that could increase their industrial value (13).
We discovered the 19-carbon furan-containing fatty acid 10,13-epoxy-11-methyl-octadecadienoate (9-(3-methyl-5-pentylfuran-2-yl)nonanoic acid) (19Fu-FA) in phospholipids isolated from an R. sphaeroides mutant lacking an antisigma factor, ChrR, that has increased transcription of genes that are normally activated in the presence of the reactive oxygen species (ROS) singlet oxygen (1O2). In this and other phototrophs, 1O2 is a byproduct of light energy capture in integral membrane complexes of the photosynthetic apparatus (5, 16, 17). Consequently, fatty acids or other membrane components are likely targets for damage by 1O2 (16, 17).
Despite the proposed roles of Fu-FAs, little is known about how they are synthesized (13). We report on proteins needed for the conversion of cis unsaturated fatty acids to 19Fu-FA. We show that a 1O2-inducible protein (RSP2144) is an S-adenosyl methionine (SAM)-dependent methylase that synthesizes a 19-carbon methylated trans unsaturated fatty acid (19M-UFA) from cis vaccenic acid both in vivo and in vitro. We also identify gene products needed for the O2-dependent conversion of 19M-UFA to 19Fu-FA. Further, we demonstrate that the presence of 1O2 leads to the disappearance of 19Fu-FA in vivo. Based on our findings, we propose a pathway for Fu-FA synthesis and propose a protective role for compounds in the presence of a ROS like 1O2.
Results
Increased σE Activity Alters Cellular Fatty-Acid Composition.
Fatty acids are targets for direct or indirect damage by ROS (1, 5–8, 16), particularly when ROS are produced by integral membrane enzymes in the respiratory chain or the photosynthetic apparatus (1, 7, 8, 16, 18). The R. sphaeroides σE protein activates a transcriptional stress response to 1O2, a ROS that is generated by integral membrane proteins of the photosynthetic apparatus (16, 17, 19). At least one ORF, which is a known member of the σE regulon, RSP2144, encodes a protein with amino acid similarity to an enzyme predicted to modify fatty acids (16, 17, 19–21). To test for σE-dependent alterations in fatty acid composition, we prepared fatty acid methyl esters (FAMEs) to compare the fatty acid content of wild-type cells and mutant cells (ΔChrR; see Table 1 for strain designations), which have high σE activity when grown aerobically in the absence of light because the antisigma factor ChrR that normally inhibits σE function has been inactivated (19, 20, 22, 23).
Table 1.
Strains and plasmids
| Strains/plasmids | Relevant genotype | Source |
| Strains | ||
| E. coli | ||
| DH5α | supE44 lacu169(Ф80 lacZ M15) hsdR178 recA1 endA1 gyrA96 thi-1 relA-1 | (52) |
| S17-1 | C600::RP-4 2-(Tc::Mu) (Kn::Tn7) thi pro hsdR Hsd M+recA | (53) |
| BL21(DE3) | F− ompT hsdSB (rB- mB-) gal dcm (DE3) | Novagen |
| JW1653 | cfa::kan of BW25113 Keio Collection | (48) |
| RLcfaK49-6 | cfa markerless deletion mutant of JW1653 | This study |
| R. sphaeroides | ||
| 2.4.1 | Wild type | (36) |
| TF18 | rpoE::drf | (23) |
| ΔChrR | chrR::drf | (54) |
| ΔRSP2144 | RSP2144::Ω SmrSpr | (20) |
| RSL1 | ΔchrR RSP2144::Ω SmrSpr | This study |
| 1091:spR/ΔChrR | ΩSpR insertion in RSP1091 coding sequence in ΔChrR | This study |
| Delta ΔRSP1091/ ΔChrR | In-frame deletion of both RSP1091 and ChrR | This study |
| Plasmids | ||
| pBlueScriptII KS- | Apr | Agilent Technologies |
| pRS2144 | RSP2144 in pBSII | (20) |
| pET-28a+ | His6 expression vector, Knr | Novagen |
| pRLhisRSP2144 | 1.2-kb RSP2144 fragment from pRS44 cloned into NdeI/EcoRI-cut pET-28a | This study |
| pIND5 | pIND4 NcoI site replaced with NdeI site, Knr | (20) |
| pRL101 | 1.3-kb fragment amplified from pRLhisRSP2144 cloned into NdeI/HindIII pIND5 | This study |
| pAYW19 | E. coli cfa gene on pGEM5, Apr | (49) |
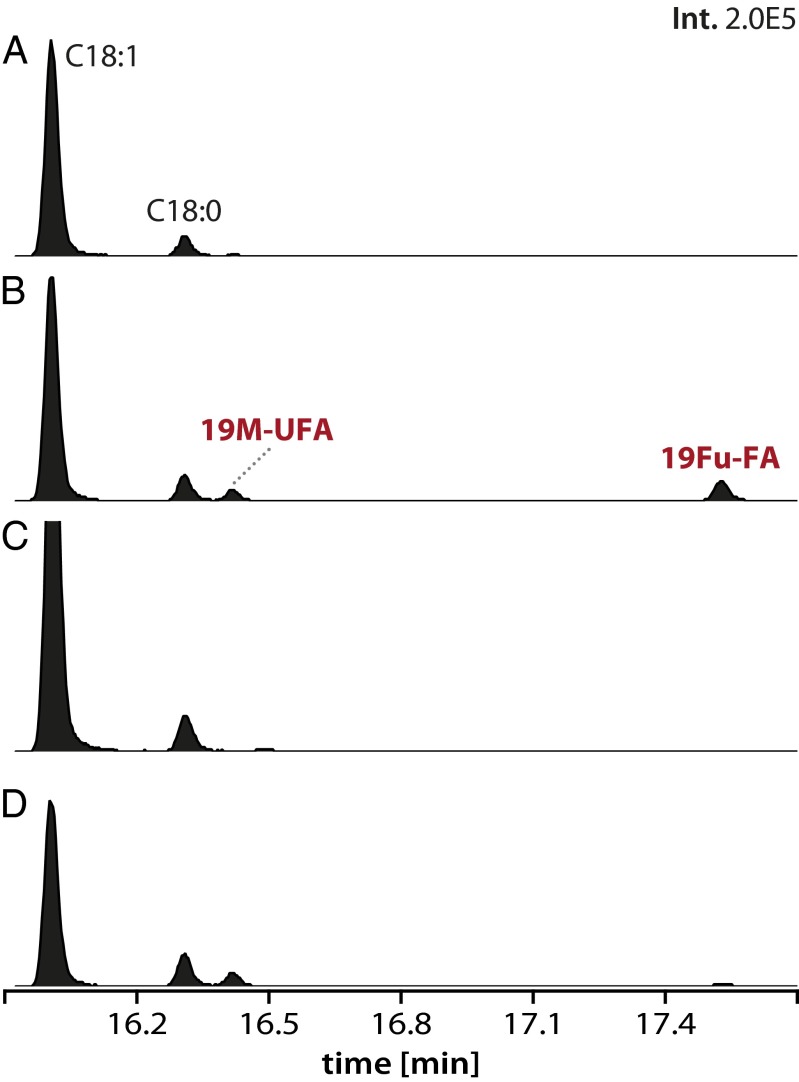
In wild-type cells, we found the expected major FAME products (C18:1, C18:0, C16:1, C16:0) (Table 2), based on published fatty-acid analysis of R. sphaeroides (24–27). In ΔChrR cells, we observed the accumulation of two additional FAME products (retention times of ∼16.4 and 17.5 min in Fig. 1) and lower levels of the vaccenic acid (C18:1) FAME compared with wild-type cells (Table 2). Thus, we conclude that increased σE activity alters the cellular fatty acid composition. However, neither of the two additional FAME products in cells containing increased σE activity elutes with compounds in bacterial fatty acid standard mixtures so we sought to determine their identity.
Table 2.
Relative cellular fatty acid content
| Strain/condition | C16:1 | C16:0 | C18:1 | C18:0 | 19M-UFA* | 19Fu-FA† | N |
| WT Aero | 5.3 (0.6) | 21.1 (3.1) | 45.9 (6.7) | 25.7 (2.9) | 1.3 (0.2) | 0.6 (0.2) | 3 |
| WT Photo | 5.1 (0.1) | 18.6 (0.1) | 48.3 (1.3) | 26.3 (0.7) | 1.7 (0.1) | ND | 2 |
| ΔChrR Aero | 5.7 (0.3) | 23.3 (1.2) | 40.0 (2.3) | 26.4 (1.1) | 2.5 (0.1) | 2.3 (0.2) | 3 |
| ΔChrR Photo | 5.4 (0.3) | 21.4 (2.0) | 42.8 (4.6) | 25.9 (3.4) | 4.6 (0.7) | ND | 3 |
| ΔUfaM Aero | 5.0 (0.2) | 21.4 (1.3) | 47.4 (2.4) | 26.0 (1.2) | ND | ND | 3 |
| ΔUfaM Photo | 4.2 (0.4) | 18.1 (5.1) | 51.8 (11.9) | 25.5 (6.4) | ND | ND | 2 |
| ΔChrR/ΔUfaM Aero | 5.4 (0.8) | 24.5 (3.0) | 45.3 (6.3) | 24.5 (2.5) | ND | ND | 3 |
| ΔChrR/ΔUfaM Photo | 4.5 (0.1) | 19.1 (0.5) | 49.7 (0.6) | 26.4 (1.4) | ND | ND | 3 |
| 1091:spR/ΔChrR2 Aero‡ | 5.0 (0.3) | 22.6 (0.3) | 40.1 (0.7) | 23.1 (0.5) | 9.2 (0.6) | ND | 2 |
| 1091:spR/ΔChrR2 Photo | 5.2 (0.4) | 19.9 (0.6) | 43.5 (2.6) | 24.6 (1.6) | 6.8 (0.1) | ND | 3 |
| Δ1091/ΔChrR2 Aero§ | 3.9 (1.2) | 21.5 (1.4) | 43.4 (3.2) | 22.5 (0.4) | 8.7 (1.7) | ND | 3 |
| Δ1091/ΔChrR2 Photo | 5.1 (0.2) | 21.6 (1.4) | 39.5 (2.4) | 26.8 (0.4) | 7.1 (0.5) | ND | 3 |
Percentage of the total fatty acid, with standard deviation in parentheses. Not determined (ND), <0.5% of the total FAME; N, number of biological replicates.
19M-UFA is 11-methyl-octadecenoate (n-6).
19Fu-FA is 10,13-epoxy-11-methyl-octadecadienoate (9-(3-methyl-5-pentylfuran-2-yl)nonanoic acid).
1091:spR cells contain a polar insertion of a spectinomycin-resistance gene in RSP1091.
Δ1091 cells contain an in-frame deletion in RSP1091.
Fig. 1.
Alterations in the fatty-acid profile of cells with increased σE activity (ΔChrR cells). Gas chromatograms of FAMEs are shown, and those FAMEs known to be present in wild-type R. sphaeroides (C18:0 and C18:1) are indicated, as well as two additional FAMEs (19M-UFA and 19Fu-FA) that are accumulated in ΔChrR cells. (A–D) The gas chromatograms of FAMEs from wild-type (A), ΔChrR (B), ΔRSP2144 (C), and ΔRSP2144 cells in which the RSP2144 gene is ectopically expressed from an isopropyl β-d-1-thiogalactopyranoside (IPTG)-inducible plasmid (D), respectively. The y and x axes show the relative abundance and retention time for each species, respectively.
Identification of Additional FAMEs in Cells with Increased σE Activity.
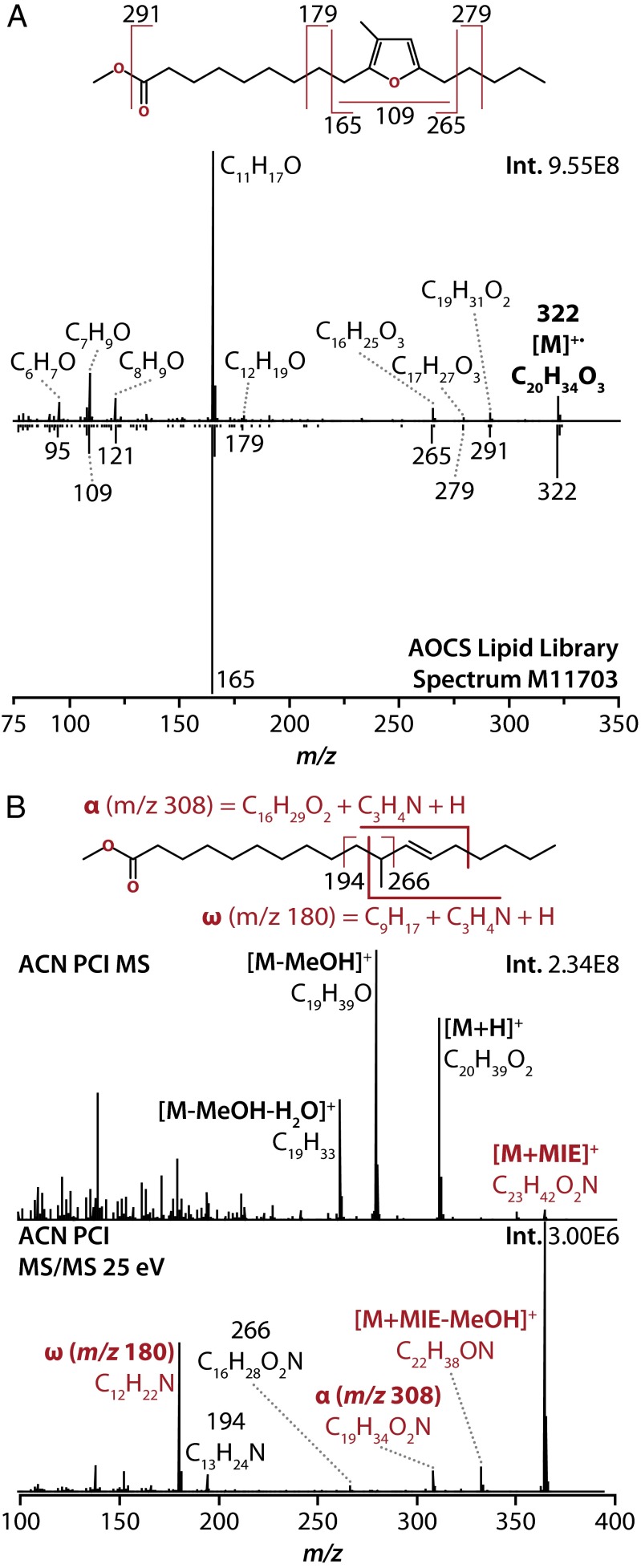
The electron ionization (EI) (70 eV) mass spectrum of one unknown FAME (retention time ∼17.5 min in Fig. 1), which accumulates in cells with increased σE activity, shows that it has an intact molecular ion mass of 322.2502 Da, corresponding to a molecular formula of C20H34O3 (Fig. 2A). The fragmentation pattern has good correlation with a methyl ester of a 19-carbon furan-containing fatty acid, 10,13-epoxy-11-methyl-octadecadienoate (9-(3-methyl-5-pentylfuran-2-yl)nonanoic acid), as seen by the comparison with the reference spectrum in Fig. 2A [spectrum M11703; American Oil Chemists’ Society (AOCS) Lipid Library]. This compound is hereafter referred to as 19Fu-FA.
Fig. 2.
(A) Identification of one of the unknown FAMEs (retention time ∼17.5 min in Fig. 1). Electron ionization (EI) spectrum and interpretation of major fragment ions (Upper), and comparison with reference library spectrum for methyl 10,13-epoxy-11-methyl-octadecadienoate (Lower). Library spectrum adapted from the AOCS Lipid Library, spectrum number M11703. (B) Identification of the other unknown FAME (retention time ∼16.4 min in Fig. 1) using acetonitrile (ACN) positive chemical ionization (PCI). Full-scan MS spectrum of this molecule (Upper) indicating key ACN PCI adducts of the intact species. MS/MS spectrum of the [M+MIE]+ ion of this molecule at 25 eV (Lower), showing key fragments that localize the double bond to position 12, as indicated in the diagram.
The other unidentified FAME (retention time ∼16.4 min in Fig. 1), which also accumulates in cells with increased σE activity, has an intact molecular ion mass of 310.2866 Da, corresponding to an elemental composition of C20H38O2 (Fig. 2B and Fig. S1). The EI mass spectrum of this FAME did not allow a definitive assignment of its identity so additional experiments were necessary. First, hydrogenation of the FAME led to a shift in retention time and an increase in the intact molecular ion mass by 2 Da (312.3023 Da, C20H40O2). The increase in the mass of this FAME after hydrogenation indicates that the untreated molecule is unsaturated (Fig. S1A). The EI mass spectrum of the 312-Da hydrogenated unknown contained diagnostic a and b fragment ions that localized a methyl branch at position 11 on the hydrogenated molecule, and by extension on the nonhydrogenated unknown. This spectrum correlates well with the reference spectrum of methyl 11-methyl-octadecanoate [spectrum 112141; National Institute of Standards and Technology (NIST) Library] (Fig. S1B). To then localize the position of the double bond in the acyl chain of the 310-Da unsaturated, faster-migrating unknown, we used a soft ionization technique (28), acetonitrile (ACN) positive chemical ionization (PCI), with subsequent isolation and MS/MS of a chemical ionization-derived molecular ion adduct {[M + 1-methyleneimino-1-ethenylium (MIE)]+} of the (nonhydrogenated) unknown FAME. The ACN PCI MS/MS fragmentation pattern of this compound contains diagnostic fragment ions, α and ω, that localize the double bond in the acyl chain to position 12 and thus identify this unknown FAME as methyl 11-methyl-C18:1 (n-6) (Fig. 2B), hereafter referred to as 19M-UFA. Finally, we showed that 19M-UFA has a trans configuration around the double bond (Fig. S2). 19M-UFA is derived from cis-vaccenic acid so unsaturated fatty acyl methylase (UfaM) activity alters the isomeric state of the fatty acyl molecule as is reported for SAM-dependent methylases involved in mycolic acid biosynthesis (29, 30).
To validate the assigned identity of these two FAMEs, we compared the behavior of synthetic standards of 19M-UFA and 19Fu-FA to those present in ΔChrR cells. We found that the fragmentation patterns of the synthetic 19M-UFA and 19Fu-FA were indistinguishable from the native 19M-UFA and 19Fu-FA FAMEs present in ΔChrR cells (Fig. S3). In addition, by using the synthetic FAMEs as quantitative standards, we estimated the relative cellular abundance of the 19M-UFA and 19Fu-FA. In aerobically grown wild-type cells, we found little of either the 19M-UFA or the 19Fu-FA (Fig. 1 and Table 2), presumably because these cells have low σE activity (19). In contrast, in aerobically grown ΔChrR cells (which contain high σE activity), ∼2.5% and ∼2.3% of the total FAME products are 19M-UFA and 19Fu-FA, respectively. In these cells, there is decreased abundance of vaccenic acid (C18:1) (Fig. 1 and Table 2), suggesting that both of these previously undescribed fatty acids were derived from vaccenic acid.
RSP2144 Is a SAM-Dependent Fatty Acyl Methylase.
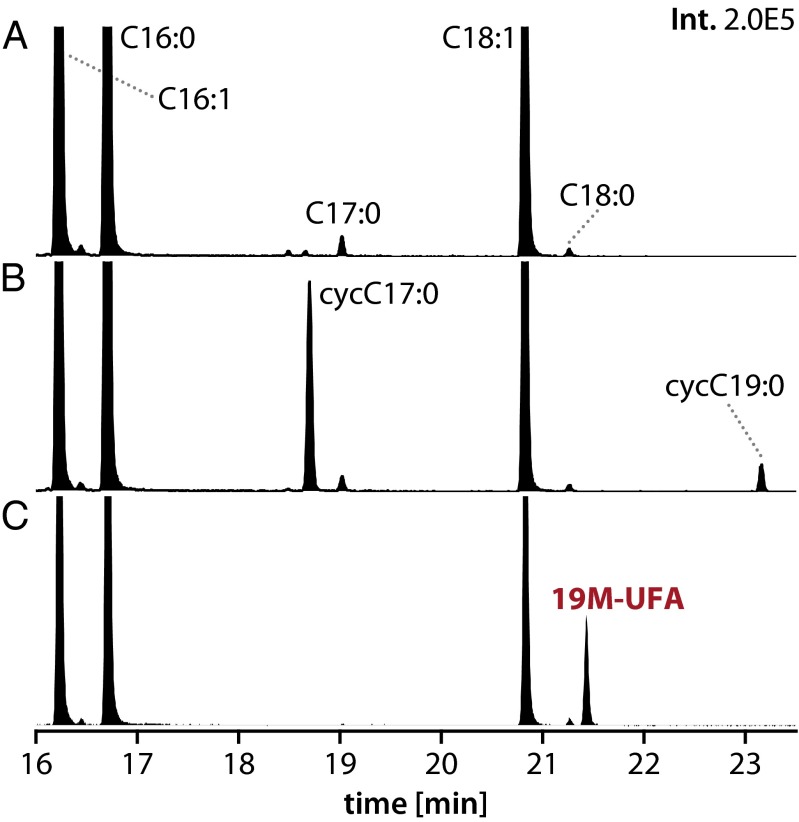
The accumulation of 19M-UFA and 19Fu-FA and the reduction in vaccenic acid in ΔChrR cells could reflect the use of a monounsaturated fatty acyl chain as a substrate for synthesis of one or both of these products. RSP2144 is annotated as a SAM-dependent fatty acyl-modifying enzyme with significant amino acid similarity to bacterial cyclopropane fatty acid synthase (16, 19, 21). However, RSP2144 does not appear to catalyze this reaction because ΔChrR cells, which have increased RSP2144 expression (19, 21), do not contain detectable levels of a C19 cyclopropane FAME (Fig. 1 and Table 2).
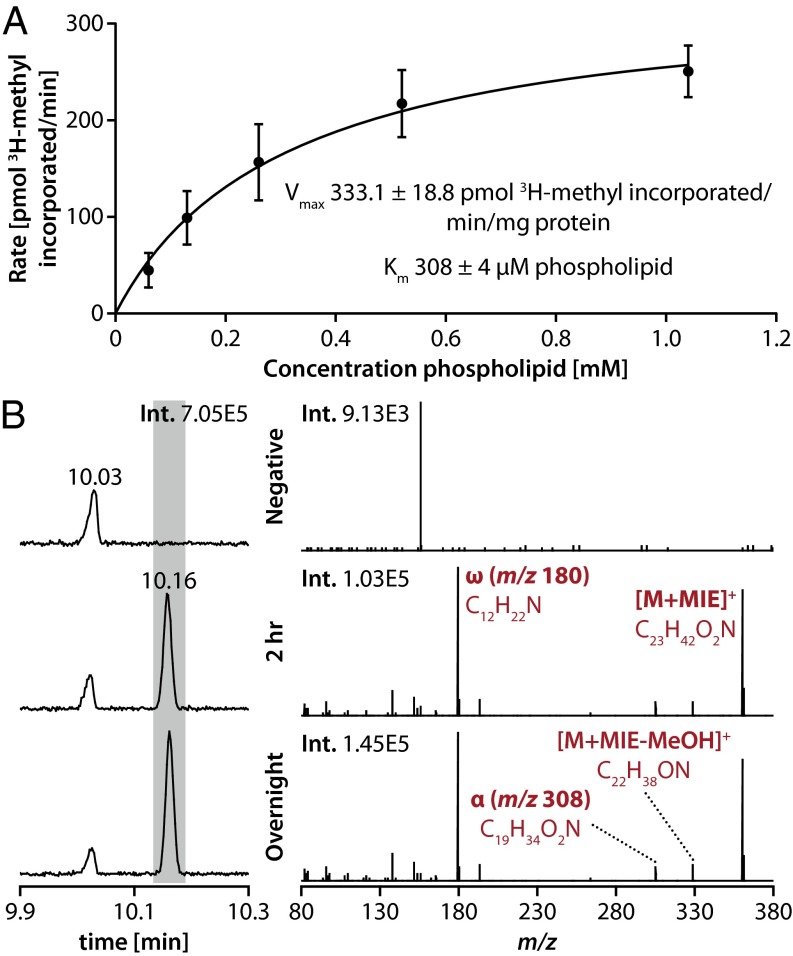
Thus, we considered the possibility that RSP2144 is a previously uncharacterized SAM-dependent unsaturated fatty acyl methylase (UfaM). To test this hypothesis, we asked whether purified recombinant His6-tagged RSP2144 was able to methylate fatty acids. We found that purified His6-RSP2144 catalyzed transfer of a 3H-methyl group from methyl-labeled SAM into tricholoracetic acid (TCA)-precipitated material when incubated with a phospholipid substrate mixture isolated from an R. sphaeroides ΔRSP2144 mutant. The activity of the recombinant RSP2144 enzyme (Vmax ∼ 331 pmol⋅min−1⋅mg−1) and its apparent affinity for phospholipid substrate (Km ∼ 308 µM) (Fig. 3A) were comparable with other SAM-dependent fatty acyl-modifying enzymes (31, 32). When the FAME products of this in vitro reaction were analyzed by GC-MS, we observed the accumulation of a product with a retention time and fragmentation pattern identical to the 19M-UFA [methyl 11-methyl-C18:1 (n-6)], which accumulates in ΔChrR cells (Fig. 3B).
Fig. 3.
Activity of RSP2144 in vitro. (A) Properties of a recombinant RSP2144 protein when assayed for incorporation of 3H-methyl–labeled SAM into tricholoroacetic acid insoluble material using micelles containing native R. sphaeroides phospholipids as a substrate. (B) FAME products obtained using R. sphaeroides lipids in the absence (negative) or presence of His6-RSP2144 protein (UfaM) and SAM (2 h and overnight time points). The chromatographic response of lipids before and after 2 h or overnight incubation with UfaM in vitro shows an increase in 19M-UFA concentration when incubated with UfaM (shaded in gray). The spectra (Right) show the ACN PCI [M+MIE]+ MS/MS (25 eV) spectra collected at the apex of the 19M-UFA peak in all three samples, with key fragment ions labeled. No 19M-UFA was detected in the reactions lacking UfaM (negative).
We also found that ectopic expression of His6-RSP2144 in either R. sphaeroides or an Escherichia coli cfa mutant leads to accumulation of 19M-UFA (Fig. 4C). Unlike R. sphaeroides, E. coli contains significant amounts of C16:1 (n-7) fatty acyl chains in its phospholipids (2, 3) so preferential accumulation of 19M-UFA and the absence of a detectable methyl C17 FAME in this host could indicate that RSP2144 has some selectivity for methylation of vaccenic acid. However, in E. coli, there is a bias for having a C16:1 chain at position 2 of phospholipids (33) so the lack of accumulation of a 17-carbon M-UFA in this bacterium could also reflect a preference for UfaM to methylate acyl chains at the 1 position. As a control, we found that ectopic expression of E. coli cfa in its native host led to accumulation of C17 and C19 cyclopropane FAMEs (Fig. 4B), as expected given the reported function of this enzyme (31, 32). Thus, we conclude that His6-RSP2144 is a previously uncharacterized SAM-dependent UFA methylase, which we hereafter call UfaM.
Fig. 4.
RSP2144 produces 19M-UFA in vivo. (A–C) Chromatograms of FAMEs accumulated in an E. coli ΔCfa mutant (JW1653) (A), an E. coli ΔCfa mutant containing E. coli cfa on a plasmid (B), and an E. coli ΔCfa mutant containing RSP2144 on a plasmid (C). The y and x axes show the relative abundance and retention time for each species, respectively.
RSP1091 Is Needed for Accumulation of 19Fu-FA.
Ectopic expression of His6-RSP2144 in ΔRSP2144 cells resulted in accumulation of 19M-UFA (Fig. 1D). However, both 19M-UFA and 19Fu-FA were accumulated in ΔChrR cells (Fig. 1B and Table 2), which have increased expression of RSP2144 and other proteins in the σE regulon (21). One interpretation of these data is that another σE-dependent gene is needed to synthesize 19Fu-FA. Other σE target genes in the putative RSP1091-1087 operon have amino acid sequence similarity to fatty acid-modifying enzymes (16, 19, 21). Thus, we asked whether any of these proteins had a previously unrecognized role in fatty-acid modification.
To test this hypothesis, we analyzed the FAME content of aerobic cells that lacked ChrR and RSP1091 (20). For this analysis, we used cells containing either an in-frame deletion in the RSP1091 coding sequence or ones that contained an insertion in RSP1091 that might have a polar (i.e., negative) effect on expression of the downstream genes RSP1090–1087 (20, 21). Fatty-acid analysis of either of the ChrR/RSP1091 double mutants showed that they lacked detectable levels of 19Fu-FA present in the ΔChrR mutant (Table 2). However, both the ChrR/RSP1091 double mutants tested contained 19M-UFA that is present in a ΔChrR mutant. Thus, we conclude that RSP1091 is needed for synthesis of 19Fu-FA although we cannot determine from this experiment whether other genes in the putative RSP1091-1087 operon are also involved in the conversion of 19M-UFA to 19Fu-FA (Discussion).
Based on only these data, it was deemed possible that the RSP1091 protein either directly converts vaccenic acid to 19Fu-FA or, alternatively, that 19M-UFA produced by the RSP2144 protein could be an intermediate in a RSP1091-dependent pathway for Fu-FA synthesis. To distinguish between these possibilities, we compared the FAME content of cells lacking both ChrR and RSP2144 with cells lacking only ChrR. Analysis of FAMEs from the ChrR/RSP2144 double mutant showed that it lacked both the 19M-UFA and 19Fu-FA that are accumulated in ΔChrR cells (Table 2). Thus, we concluded that 19M-UFA, as a product of RSP2144 activity, is needed to produce 19Fu-FA. In addition, we concluded that the RSP1091 protein, either alone or in conjunction with another σE target gene, is needed to convert 19M-UFA into 19Fu-FA.
O2 Is Needed for Accumulation of 19Fu-FA.
O2 is one potential source of the oxygen moiety in Fu-FAs (13), but experimental evidence in support of this notion is lacking. To test whether O2 was needed for accumulation of this bacterial 19Fu-FA, we compared the FAME profile of cells containing increased σE activity (ΔChrR cells) that were grown aerobically (30% O2 in the dark) or anaerobically (in the light by photosynthesis). Analysis of the FAME profile showed that 19Fu-FA is detected only when cells were grown in the presence of O2. In addition, we found that 19M-UFA is accumulated when this strain is grown either in the presence or absence of O2, suggesting that RSP2144 activity does not require O2 (Table 2). We conclude that O2 acts as a source of oxygen in this bacterial 19Fu-FA.
1O2 Causes Turnover of 19Fu-FA.
The above experiments showed accumulation of 19M-UFA and 19Fu-FA in ΔChrR cells that have increased σE activity. We wanted to determine whether changes in fatty acid content were observed when wild-type cells were exposed to 1O2, a signal that induces σE activity (16, 19, 20). When we exposed wild-type cells to 1O2 as a way to increase σE activity (16, 19, 20), there was no detectable accumulation of 19Fu-FA. This result was somewhat surprising because the conditions used to produce 1O2 are known to be sufficient to increase σE activity (19, 20) so we expected to see accumulation of 19Fu-FA.
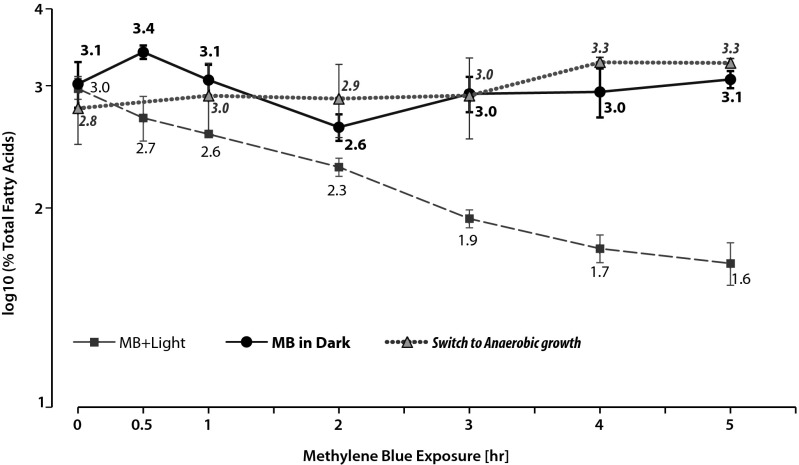
1O2 can directly oxidize furan moieties and produce fatty acyl radicals from unsaturated fatty acids so it has been proposed that Fu-FAs can act as a scavenger for this and other ROS (14, 15, 34, 35). Thus, the failure to observe alterations of the fatty-acid content when wild-type cells were exposed to 1O2 could reflect the ability of 19Fu-FA to scavenge this ROS or products of its action on bilayer constituents. To test this hypothesis, we monitored the effect of 1O2 on the fatty-acid content of ΔChrR cells that accumulate the furan fatty acid because they have increased σE activity (Table 2). We saw a time-dependent decrease in the abundance of 19Fu-FA after exposing ΔChrR cells to 1O2 generated by adding methylene blue to aerobically grown cultures in the presence of light (Fig. 5). This decrease in abundance of 19Fu-FA was not observed in a control aerobically grown ΔChrR culture that was exposed to methylene blue in the dark (Fig. 5) or when aerobically grown cells were transferred to dark anaerobic conditions at time 0 (Fig. 5). Thus, we conclude that this observed decrease in 19Fu-FA abundance required conditions that result in 1O2 formation. One explanation for this observation is that 19Fu-FA acts as a scavenger of fatty acyl radicals or other compounds that are produced in the presence of 1O2 (Discussion).
Fig. 5.
Impact of 1O2 exposure on 19Fu-FA abundance. Time-dependent changes in the cellular abundance of 19Fu-FA in ΔChrR cells that are (MB + Light, ■) and are not (MB in dark, ●) exposed to 1O2 with a control switched to anaerobic growth at time 0 (▲).
Discussion
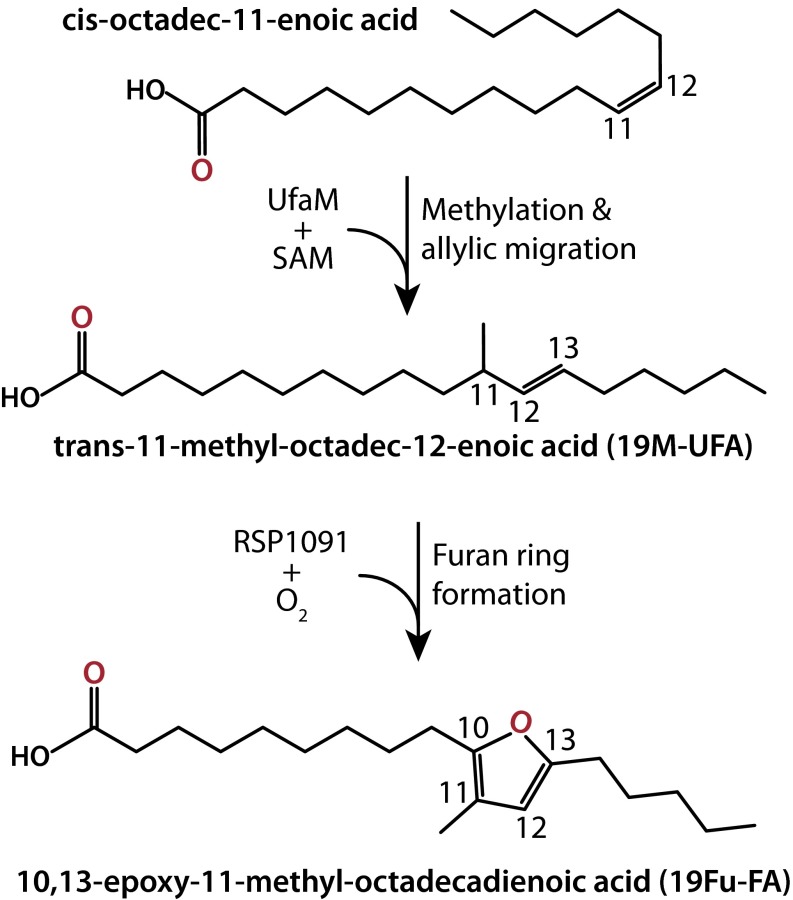
This report demonstrates the accumulation of methylated and furan-containing fatty acids in R. sphaeroides and shows that a previously unidentified class of a SAM-dependent methylase (RSP2144, UfaM) and hypothetical protein(s) (RSP1091), respectively, are needed for their production. Our data indicate that both 19M-UFA and 19Fu-FA are synthesized from unsaturated fatty acids in cellular phospholipids using a previously uncharacterized set of enzymes (Fig. 6). In addition, we show that formation of the ROS 1O2 leads to loss of 19Fu-FA, and we propose that this fatty acyl chain acts to scavenge reactive and potentially damaging products present in the bilayer upon 1O2 formation. Below, we place these observations in context, provide an explanation for our findings, and propose future experiments to answer questions posed by these results.
Fig. 6.
Proposed pathway for 19Fu-FA synthesis. In this model, UfaM (RSP2144) is a SAM-dependent methlyase that produces 19M-UFA from vaccenic acid. In an O2-dependent reaction, RSP1091 alone or in combination with other gene products converts 19M-UFA to 19Fu-FA (see Discussion).
Identification of Gene Products Needed to Produce 19M-UFA and 19Fu-FA.
We identified 19M-UFA and 19Fu-FA as unknown FAMEs present in a mutant strain of the photosynthetic bacterium R. sphaeroides. This mutant strain constitutively expresses stress response genes, such as RSP2144 and RSP1091, shown previously to be required for survival in the presence of 1O2 (16, 19, 20). We show that RSP2144 is a SAM-dependent methylase that synthesizes M-UFA in R. sphaeroides, both in vitro when a recombinant protein is incubated with purified native phospholipids, and in vivo when heterologously expressed in E. coli.
RSP2144 was previously annotated as a cyclopropane fatty acyl synthase; however, it does not produce detectable levels of cyclopropane fatty acids (CFAs) in vivo or in vitro under any conditions tested. Instead, our data indicate that RSP2144 is a previously undescribed enzyme that produces a 19-carbon methylated UFA product: thus, the name UfaM. In addition, UfaM could have a preference for methylating vaccenic acid (C18:1) because we observed only a C19 methyl product when this protein was expressed in E. coli (which contains more C16:1 than C18:1 fatty acyl chains). Furthermore, the production of the trans isomer of 19M-UFA from cis-vaccenic acid predicts that SAM-dependent fatty acyl methylation by UfaM uses a reaction mechanism similar to methylases involved in mycolic acid biosynthesis (29, 30).
This report also demonstrates that another σE target gene, RSP1091 (16, 19, 21), is needed for conversion of 19M-UFA to 19Fu-FA (Fig. 6). We show that this conversion requires growth of cells under aerobic conditions, suggesting that O2 is the source of the oxygen moiety in the furan ring. RSP1091 is annotated as a protein of unknown function (16, 19, 21), but it is predicted to contain an N-terminal Rossman fold (putative pyridine nucleotide binding domain), a flavin-binding domain, and to be a fatty acyl-modifying enzyme (36, 37). RSP1091 has yet to be characterized, but the presence of flavin and pyridine nucleotide cofactors could permit conversion of 19M-UFA into cognate 19Fu-FA in an O2-dependent manner. RSP1090 is also uncharacterized (19, 21, 36) so it is possible that RSP1091, along with RSP1090 and other proteins in the putative RSP1091-1087 operon, are needed for conversion of 19M-UFA into 19Fu-FA. Our data show that synthesis of 19Fu-FA requires the ability of cells to make 19M-UFA because the loss of UfaM prevents synthesis of 19Fu-FA. In this regard, it appears that methylation of the UFA creates a tertiary carbon in the acyl chain that is needed for subsequent conversion of 19M-UFA to 19Fu-FA.
Protective Role of 19Fu-FA in Scavenging ROS-Mediated Damage.
We show that the conditions that lead to formation of 1O2 also result in turnover of 19Fu-FA in vivo. Under the conditions we used, ∼50% of 19Fu-FA is removed in one cell doubling (∼3 h for R. sphaeroides). This decrease is probably an underestimate of the turnover of this fatty acid in the presence of 1O2 because these cells are also capable of synthesizing new 19Fu-FA under these conditions. In addition, it is unclear precisely how much 1O2 is formed inside or outside the cells under the conditions used. Thus, it is possible that the reactivity of 19Fu-FA is underestimated because fatty acyl chains in the inner or outer membrane of this Gram-negative bacterium are in the immediate vicinity of 1O2.
From the chemical properties of Fu-FAs, it is proposed that they can scavenge lipid peroxides, fatty acyl radicals, or even 1O2 (13–15). The loss of 19Fu-FA when cells generate 1O2 is, to our knowledge, the first report of their potential role as scavengers of ROS in bacteria. Wild-type R. sphaeroides retains growth after formation of 1O2 (19), and carotenoids have typically been considered the major route for quenching this ROS in photosynthetic bacteria and other microbes (38–40). Previous studies have shown that 1O2 kills cells lacking either UfaM (RSP2144) or RSP1091 proteins (20, 41). We now know that both of these strains are unable to make 19Fu-FA. Combined, these observations indicate that synthesis of 19Fu-FA is required for viability in the presence of 1O2, possibly because they can also scavenge and minimize cellular damage by this ROS.
Potential Role of 19Fu-FA as a Bacterial Second Messenger.
It is not surprising that previous analysis of the fatty-acid content of wild-type cells did not detect the presence of 19Fu-FA (24–27). Transcription of the genes needed to synthesize 19Fu-FA requires high activity of the alternative sigma factor σE, but, in the absence of 1O2, σE activity is inhibited because it is bound to an antisigma factor, ChrR (16, 19, 22). We have shown that 1O2 formation leads to 19Fu-FA turnover in dChrR cells, explaining why one does not observe time-dependent changes in levels of 19Fu-FA after exposing wild-type cells to 1O2.
In contrast to the situation in wild-type cells, mutants lacking either UfaM or RSP1091 have defects in increasing σE transcriptional activity (20, 41). Based on this observation and the results of the experiments in this study, we propose that a product of either gene is needed to promote dissociation of a σE–ChrR complex (20). For example, the ability of 19Fu-FA to scavenge 1O2 could lead to accumulation of lipid peroxides that act as a second messenger to promote dissociation of the σE–ChrR complex. In this model, the subsequent ChrR proteolysis in the presence of 1O2 (20, 41, 42) could be promoted by direct modification of this antisigma factor or by the activation of one or more proteases by a lipid peroxide.
ufaM (RSP2144) and the genes in the RSP1091-1087 operon are present across a wide group of α- and γ-proteobacteria (16, 21). In addition, in these other organisms, homologs of these genes are often predicted to be transcribed by a homolog of R. sphaeroides σE, suggesting that they are members of a core regulon that is conserved across the bacterial phylogeny (21). Thus, it would seem that 19Fu-FA synthesis in the presence of 1O2 and the potential use of the products of UfaM and RSP1091 activity as second messengers are conserved across bacteria.
In sum, we identified conditions and enzymes needed for bacterial synthesis of 19Fu-FA. Compounds predicted to be 19Fu-FA and 19M-UFA have been provisionally identified in bacteria before (43, 44), but information on their cellular abundance, the enzymes needed for their synthesis, and their cellular role have not yet been reported. We also identified conditions to increase production of 19Fu-FA in both native and foreign hosts, such as E. coli. These studies pave the way to identify and characterize enzymes needed to synthesize large quantities of 19Fu-FA in bacterial systems. With large amounts of 19Fu-FA available, one can probe the interaction of 1O2 with this Fu-FA, identify potential secondary messengers, and test the utility of Fu-FAs as food, chemical, or fuel additives.
Materials and Methods
Bacterial Strains and Growth.
E. coli and R. sphaeroides strains were grown as described (19). Mutant strains Δ1091/ΔChrR and 1091:spR/ΔChrR were made using methods described previously (20).
Purification of His6-RSP2144 Protein.
pRLhisRSP2144 was generated by cloning the RSP2144 coding region into the NdeI and EcoRI sites of pET-28a(+) to produce an N-terminally hexahistidine-tagged protein (His6-RSP2144). A 500-mL culture of log phase BL21DE3 E. coli cells, containing pRLhisRSP2144, was exposed to 1 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) for 4 h at 28 °C to induce expression of His6-RSP2144. The cells were harvested by centrifugation, and the resulting pellet was resuspended in lysis buffer [25 mM Hepes (pH 7.5), 150 mM KCl, 20 mM imidazole, 10% glycerol (vol/vol), 1 mg/mL lysozyme, and 1× Halt protease inhibitor (Pierce)], sonicated on ice, pulsing every 20 s, for 7 min, and centrifuged for 1 h at 50,000 × g. The resulting supernatant was passed over a 4-mL Ni-NTA agarose column (Novagen) and washed with 50 mL of wash buffer [25 mM Hepes (pH 7.5), 150 mM KCl, 50 mM imidazole, and 10% glycerol], and protein was removed with a 16-mL elution buffer [25 mM Hepes (pH 7.5), 150 mM KCl, 250 mM imidazole, and 10% glycerol]. Fractions containing the most protein were combined and concentrated using a YM10 centrifugal filter (Millipore) and dialyzed into 50 mM Hepes, 10 mM sodium bicarbonate, and 50% glycerol. Small portions were aliquotted and stored at −80 °C. Protein concentration was estimated using the Bradford Assay (Bio-Rad).
In Vitro Assay of His6-RSP2144 Activity.
The phospholipid substrate was purified from a ΔRSP2144 strain, and a phospholipid micelle solution (in water) was created (45) and quantitated by a lipid phosphorous assay (46). Each enzyme reaction contained 0.06–1.04 mM phospholipid, 4.4 µM His6-RSP2144 protein, 20 mM potassium phosphate buffer (pH 7.4), 0.5 mg/mL BSA, and 750 µM SAM (Sigma) with a specific activity of 25 µCi/µmol (Perkin-Elmer). The reactions were incubated at 30 °C, and individual time points were taken by placing 100-µL aliquots into 1 mL of 10% trichloroacetic acid (vol/vol). The solutions were filtered over Whatman GF/c glass filter fibers on a 1225 sampling manifold (Millipore), followed by three washes with 1 mL of 10% trichloroacetic acid and three washes with 1 mL of water. The filters were put into 5 mL of Optiphase scintillation fluid (Perkin-Elmer) and incubated at room temperature overnight before determining radioactivity on a scintillation counter. The results of duplicate assays were averaged, and the reaction rate was calculated by plotting radioactivity versus time for each concentration of phospholipid. The rates were averaged between two independent experiments.
Exposure to 1O2.
R. sphaeroides strains were exposed to 1O2 as described (19). Then, 1 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) was added to cells 1 h before 1O2 exposure to induce protein expression in cells containing a plasmid-encoded His6-RSP2144 protein. Cells were grown anaerobically by sparging cultures with a 95% N2/5% CO2 gas mixture.
Fatty Acid Methyl Ester Content.
All samples from methylene blue-treated cells were kept in the dark until FAMEs were generated and extracted into hexane before analysis by GC-MS. Equal cell numbers, of up to 4 mL of cell culture, were added to 8 mL of 1:1 vol/vol methanol:chloroform (9) containing 50 µg or 100 µg of pentadecanoic acid as a recovery standard (pentadecanoic acid is not detectable in R. sphaeroides fatty acids). The suspension was vigorously agitated and centrifuged at low speed to separate the phases. The organic phase was removed, dried under N2, and lyophilized for 1 h. FAMEs were prepared by resuspending the dried materials in 600 µL of anhydrous methanol (Sigma), adding 100 µL of sodium methoxide (Sigma), and incubating at room temperature for 3 h (47). The reaction was stopped by adding 600 µL of 2 M HCl, and FAMEs were extracted with 600 µL of hexane. Then, 1 µL of each sample was analyzed on an Agilent 7890A/5975C GC-MS with differing split ratios with an HP-5ms capillary column and He carrier gas (20 cm/s at 150 °C) using one of two oven programs: (i) 150 °C isothermal for 4 min, 4 °C/min ramp to 250 °C, and isothermal at 250 °C for 5 min; or (ii) 150 °C isothermal for 4 min, 6 °C/min ramp to 245 °C, isothermal at 245 °C for 2 min, 80 °C/min ramp to 325 °C, and isothermal at 325 °C for 2 min. Chromatograms and mass spectra were analyzed using Agilent GC-MS ChemStation (version E.02.00.493) and MassHunter software (version B.06.00; Agilent Technologies) and compared with the NIST MS Search 2011b library. For quantification, a set of appropriate FAME standard curves were created from a mix of Supelco C8-C24 standards (for C16:0, C16:1, C18:0), C15:0, C18:1 (Sigma), methyl 11-methyl-octadecenoate (n-6) (19M-UFA), and 10,13-epoxy-11-methyl-octadecadienoate (19Fu-FA) (Larodan). The MassHunter integration peak filter was set to >5% of the largest peaks; peak area was integrated for ions diagnostic for each FAME (m/z 74 for C15:0, C16:0, C18:1, and C18:0; m/z 55 for C16:1; m/z 69 for 19M-UFA; and m/z 165.1 for 19Fu-FA). The integrated areas were normalized to the recovery standard (C15:0), and each FAME was converted to a percentage of the total fatty acids, followed by averaging data from technical duplicates. Biological duplicates were averaged, and the SD was calculated.
Ectopic Expression of RSP2144 in R. sphaeroides and E. coli.
pRL101 was created by cloning the His6-RSP2144 gene from pRLhisRSP2144 into the NdeI and HindIII sites of pIND5. This plasmid and pAYW19 (containing E. coli cfa) were then transformed into the E. coli strain JW1653, which lacks cfa (48, 49). JW1653 was obtained from the Keio collection, and the Knr gene was removed before use (48). Triplicate biological cultures were separately treated with 1 mM isopropyl β-d-1-thiogalactopyranoside (IPTG) before preparing FAMEs (see Fatty Acid Methyl Ester Content section in Materials and Methods).
Hydrogenation of FAME Samples.
FAMEs were dried under N2, dissolved in 10 mL of (1:2 vol/vol) chloroform:methanol with 15 mg of 5% platinum on activated charcoal (50). The reaction tubes were fitted with stoppers and sparged with a 95% N2/5% H2 gas mixture for 1 h. The tubes were centrifuged twice to remove the charcoal, filtered through glass wool in a Pasteur pipet, and analyzed by GC-MS.
Identification of Unknown FAMEs.
Gas chromatography was performed on a Trace GC Ultra (Thermo Electron) equipped with a CTC Analytics GC PAL autosampler (Zwingen) using a 30 m × 0.25 mm (i.d.) × 0.25 μm (df) Crossbond 5% diphenyl/95% dimethyl polysiloxane column (Restek Rxi-5Sil MS) and He as carrier gas. Mass spectrometry was performed on a breadboard GC/quadrupole-Orbitrap MS.
A FAME mix of 26 compounds in methyl caproate was used for chromatographic and MS source optimization (Sigma). Samples in hexane (1 µL) were injected via the hot-needle technique at various split ratios depending on sample concentration, with an injector temperature of 250 °C, He flow rate of 1 mL/min, and the following oven program: 1 min isothermal at 150 °C, 15 °C/min to 250 °C, 1 min isothermal at 250 °C, 80 °C/min to 320 °C, and 2 min isothermal at 320 °C. The transfer line and source temperatures were 280 °C and 250 °C, respectively. Samples were ionized via EI or positive CI (PCI) using acetonitrile (ACN) as the reagent gas (70 eV). Full-scan analyses used a scan range of 75–400 Th, resolution of 17,500, automated gain control (AGC) target of 1E6, and maximum injection time of 100 ms. Targeted MS/MS analyses used a 3 Th isolation width, normalized collision energy of 25 eV, resolution of 17,500, AGC target of 1E6, and maximum injection time of 250 ms.
To enable ACN PCI, a 250-µm (i.d.) fused silica capillary connected an ACN reservoir (6 mL) directly to the MS source through the heated transfer line. A two-holed ferrule was used to permit entry of both the GC column and ACN capillary into the transfer line. Although the column extended into the source, the ACN capillary was set back ∼5 cm from the source to prevent interference with the GC eluent. A medium-flow metering valve (Swagelok) was placed between the reservoir and transfer line to regulate the flow of ACN into the source. A source pressure of 7.1E−5 Torr, ∼0.2 ms reagent injection time (at a 1E6 AGC target) and m/z 42 (protonated ACN)-to-m/z 54 (1-methyleneimino-1-ethenylium, or MIE) ratio of 5:1 were found to be optimal for generation of molecular ion MIE adducts of unsaturated FAMEs.
Identification of Fatty Acyl Isomers.
Identification of isomer configuration of 11-methyl-octadecanoate was determined by gas chromatography equipped with a flame ionization detector (6890N; Agilent technologies). Commercial FAME standards (18:0, 18:1Δ9cis, 18:1Δ11cis, 18:1Δ9trans, and M-UFAtrans) and biological samples were separated on a DB-23 capillary column, 30 m × 0.25 mm (i.d.) and 0.25-µm film thickness. The He flow rate was 1.5 mL/min, and the following oven program was run: 3 min isothermal at 140 °C, 5 °C /min to 230 °C, and isothermal at 230 °C for 3 min. Injector and detector were maintained at 250 °C throughout the analysis. Isomers in biological samples were identified by retention time comparison with FAME standards (51).
Supplementary Material
Acknowledgments
We thank Dr. John Ohlrogge (Michigan State University) for helpful discussions, Dr. John Cronan (University of Illinois) for providing the pAYW19 plasmid, and Becky Ciske for technical assistance early in the project. This work was supported in part by National Institute of General Medical Sciences Grants GM075273 (to T.J.D.) and GM107199 (to J.J.C.) and by Department of Energy, Office of Science Great Lakes Bioenergy Research Center Grant DE-FC02-07ER64494 (to T.J.D.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1405520111/-/DCSupplemental.
References
- 1.Mueller S, et al. General detoxification and stress responses are mediated by oxidized lipids through TGA transcription factors in Arabidopsis. Plant Cell. 2008;20(3):768–785. doi: 10.1105/tpc.107.054809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cronan JE. Phospholipid modifications in bacteria. Curr Opin Microbiol. 2002;5:202–205. doi: 10.1016/s1369-5274(02)00297-7. [DOI] [PubMed] [Google Scholar]
- 3.Cronan JE. Bacterial membrane lipids: Where do we stand? Annu Rev Microbiol. 2003;57(1):203–224. doi: 10.1146/annurev.micro.57.030502.090851. [DOI] [PubMed] [Google Scholar]
- 4.Chang YY, Cronan JE., Jr Membrane cyclopropane fatty acid content is a major factor in acid resistance of Escherichia coli. Mol Microbiol. 1999;33(2):249–259. doi: 10.1046/j.1365-2958.1999.01456.x. [DOI] [PubMed] [Google Scholar]
- 5.Girotti AW, Kriska T. Role of lipid peroxides in photo-oxidative stress signalling. Antioxid Redox Signal. 2004;6:301–310. doi: 10.1089/152308604322899369. [DOI] [PubMed] [Google Scholar]
- 6.Imlay JA. Pathways of oxidative damage. Annu Rev Microbiol. 2003;57:395–418. doi: 10.1146/annurev.micro.57.030502.090938. [DOI] [PubMed] [Google Scholar]
- 7.Sayre LM, De L, Quan Y, Xiaochun Z, Xiaoxia T. Protein adducts generated from products of lipid oxidation: Focus on HNE and ONE*. Drug Metab Rev. 2006;38(4):651–675. doi: 10.1080/03602530600959508. [DOI] [PubMed] [Google Scholar]
- 8.Watabe N, Ishida Y, Ochiai A, Tokuoka Y, Kawashima N. Oxidation decomposition of unsaturated fatty acids by singlet oxygen in phospholipid bilayer membranes. J Oleo Sci. 2007;56(2):73–80. doi: 10.5650/jos.56.73. [DOI] [PubMed] [Google Scholar]
- 9.Lennen RM, Braden DJ, West RA, Dumesic JA, Pfleger BF. A process for microbial hydrocarbon synthesis: Overproduction of fatty acids in Escherichia coli and catalytic conversion to alkanes. Biotechnol Bioeng. 2010;106(2):193–202. doi: 10.1002/bit.22660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peralta-Yahya PP, Zhang F, del Cardayre SB, Keasling JD. Microbial engineering for the production of advanced biofuels. Nature. 2012;488(7411):320–328. doi: 10.1038/nature11478. [DOI] [PubMed] [Google Scholar]
- 11.Connor MR, Liao JC. Microbial production of advanced transportation fuels in non-natural hosts. Curr Opin Biotechnol. 2009;20(3):307–315. doi: 10.1016/j.copbio.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 12.Lands B. Consequences of essential fatty acids. Nutrients. 2012;4(9):1338–1357. doi: 10.3390/nu4091338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spiteller G. Furan fatty acids: Occurrence, synthesis, and reactions. Are furan fatty acids responsible for the cardioprotective effects of a fish diet? Lipids. 2005;40(8):755–771. doi: 10.1007/s11745-005-1438-5. [DOI] [PubMed] [Google Scholar]
- 14.Okada Y, Kaneko M, Okajima H. Hydroxyl radical scavenging activity of naturally occurring furan fatty acids. Biol Pharm Bull. 1996;19(12):1607–1610. doi: 10.1248/bpb.19.1607. [DOI] [PubMed] [Google Scholar]
- 15.Okada Y, Okajima H, Konishi H. Antioxidant effect of naturally occurring furan fatty acids on oxidation of linoleic acid in aqueous dispersion. J Am Oil Chem Soc. 1990;67:858–862. [Google Scholar]
- 16.Ziegelhoffer EC, Donohue TJ. Bacterial responses to photo-oxidative stress. Nat Rev Microbiol. 2009;7(12):856–863. doi: 10.1038/nrmicro2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glaeser J, Nuss AM, Berghoff BA, Klug G. Singlet oxygen stress in microorganisms. In: Poole RK, editor. Advances in Microbial Physiology. Vol 58. New York: Academic; 2011. pp. 141–173. [DOI] [PubMed] [Google Scholar]
- 18.Koopman WJ, et al. Mammalian mitochondrial complex I: Biogenesis, regulation, and reactive oxygen species generation. Antioxid Redox Signal. 2010;12(12):1431–1470. doi: 10.1089/ars.2009.2743. [DOI] [PubMed] [Google Scholar]
- 19.Anthony JR, Warczak KL, Donohue TJ. A transcriptional response to singlet oxygen, a toxic byproduct of photosynthesis. Proc Natl Acad Sci USA. 2005;102(18):6502–6507. doi: 10.1073/pnas.0502225102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nam TW, Ziegelhoffer EC, Lemke RAS, Donohue TJ. Proteins needed to activate a transcriptional response to the reactive oxygen species singlet oxygen. MBio. 2013;4(1):e00541–12. doi: 10.1128/mBio.00541-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dufour YS, Landick R, Donohue TJ. Organization and evolution of the biological response to singlet oxygen stress. J Mol Biol. 2008;383(3):713–730. doi: 10.1016/j.jmb.2008.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anthony JR, Newman JD, Donohue TJ. Interactions between the Rhodobacter sphaeroides ECF sigma factor, σE, and its anti-sigma factor, ChrR. J Mol Biol. 2004;341:345–360. doi: 10.1016/j.jmb.2004.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newman JD, Falkowski MJ, Schilke BA, Anthony LC, Donohue TJ. The Rhodobacter sphaeroides ECF sigma factor, σE, and the target promoters cycA P3 and rpoE P1. J Mol Biol. 1999;294(2):307–320. doi: 10.1006/jmbi.1999.3263. [DOI] [PubMed] [Google Scholar]
- 24.Donohue TJ, Cain BD, Kaplan S. Purification and characterization of an N-acylphosphatidylserine from Rhodopseudomonas sphaeroides. Biochemistry. 1982;21(11):2765–2773. doi: 10.1021/bi00540a029. [DOI] [PubMed] [Google Scholar]
- 25.Hands AR, Bartley W. The fatty acids of Rhodopseudomonas particles. Biochem J. 1962;84:238–239. doi: 10.1042/bj0840238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qureshi N, Honovich JP, Hara H, Cotter RJ, Takayama K. Location of fatty acids in lipid A obtained from lipopolysaccharide of Rhodopseudomonas sphaeroides ATCC 17023. J Biol Chem. 1988;263(12):5502–5504. [PubMed] [Google Scholar]
- 27.Russell NJ, Harwood JL. Changes in the acyl lipid composition of photosynthetic bacteria grown under photosynthetic and non-photosynthetic conditions. Biochem J. 1979;181(2):339–345. doi: 10.1042/bj1810339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Michaud AL, Diau G-Y, Abril R, Brenna JT. Double bond localization in minor homoallylic fatty acid methyl esters using acetonitrile chemical ionization tandem mass spectrometry. Anal Biochem. 2002;307(2):348–360. doi: 10.1016/s0003-2697(02)00037-4. [DOI] [PubMed] [Google Scholar]
- 29.Grogan DW, Cronan JE. Cyclopropane ring formation in membrane lipids of bacteria. Microbiol Mol Biol Rev. 1997;61(4):429–441. doi: 10.1128/mmbr.61.4.429-441.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuan Y, Crane DC, Musser JM, Sreevatsan S, Barry CE. MMAS-1, the branch point between cis- and trans-cyclopropane-containing oxygenated mycolates in Mycobacterium tuberculosis. J Biol Chem. 1997;272(15):10041–10049. doi: 10.1074/jbc.272.15.10041. [DOI] [PubMed] [Google Scholar]
- 31.Guianvarc'h D, Drujon T, Leang TE, Courtois F, Ploux O. Identification of new inhibitors of E. coli cyclopropane fatty acid synthase using a colorimetric assay. Biochim Biophys Acta. 2006;1764(8):1381–1388. doi: 10.1016/j.bbapap.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Iwig DF, Uchida A, Stromberg JA, Booker SJ. The activity of Escherichia coli cyclopropane fatty acid synthase depends on the presence of bicarbonate. J Am Chem Soc. 2005;127(33):11612–11613. doi: 10.1021/ja053899z. [DOI] [PubMed] [Google Scholar]
- 33.Magnuson K, Jackowski S, Rock CO, Cronan JE. Regulation of fatty acid biosynthesis in Escherichia coli. Microbiol Rev. 1993;57(3):522–542. doi: 10.1128/mr.57.3.522-542.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.White DC, et al. Phospholipid furan fatty acids and ubiquinone-8: Lipid biomarkers that may protect dehalococcoides strains from free radicals. Appl Environ Microbiol. 2005;71(12):8426–8433. doi: 10.1128/AEM.71.12.8426-8433.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wakimoto T, et al. Furan fatty acid as an anti-inflammatory component from the green-lipped mussel Perna canaliculus. Proc Natl Acad Sci USA. 2011;108(42):17533–17537. doi: 10.1073/pnas.1110577108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kontur WS, et al. Revised sequence and annotation of the Rhodobacter sphaeroides 2.4.1 genome. J Bacteriol. 2012;194:7016–7017. doi: 10.1128/JB.01214-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mackenzie C, et al. The home stretch, a first analysis of the nearly completed genome of Rhodobacter sphaeroides 2.4.1. Photosynth Res. 2001;70(1):19–41. doi: 10.1023/A:1013831823701. [DOI] [PubMed] [Google Scholar]
- 38.Armstrong G, Hearst J. Carotenoids 2: Genetics and molecular biology of carotenoid pigment biosynthesis. FASEB J. 1996;10(2):228–237. doi: 10.1096/fasebj.10.2.8641556. [DOI] [PubMed] [Google Scholar]
- 39.Cogdell RJ. How carotenoids protect bacterial photosynthesis. Philos Trans R Soc Lond B Biol Sci. 2000;355:1345–1349. doi: 10.1098/rstb.2000.0696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krinsky NI. Antioxidant functions of carotenoids. Free Radic Biol Med. 1989;7(6):617–635. doi: 10.1016/0891-5849(89)90143-3. [DOI] [PubMed] [Google Scholar]
- 41.Nuss AM, et al. DegS and RseP homologous proteases are involved in singlet oxygen dependent activation of RpoE in Rhodobacter sphaeroides. PLoS ONE. 2013;8(11):e79520. doi: 10.1371/journal.pone.0079520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greenwell RS, Nam TW, Donohue TJ. Aspects of the zinc metalloprotein ChrR required for dissociation of σE/ChrR complexes. J Mol Biol. 2011;407:477–491. doi: 10.1016/j.jmb.2011.01.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shirasaka N, Nishi K, Shimizu M. Occurrence of a furan fatty acid in marine bacteria. Biochim Biophys Acta. 1995;1258(3):225–227. doi: 10.1016/0005-2760(95)00126-w. [DOI] [PubMed] [Google Scholar]
- 44.Shirasaka N, Nishi K, Shimizu S. Biosynthesis of furan fatty acids (F-acids) by a marine bacterium, Shewanella putrefaciens. Biochim Biophys Acta. 1997;1346(3):253–260. doi: 10.1016/s0005-2760(97)00042-8. [DOI] [PubMed] [Google Scholar]
- 45.Courtois F, Guerard C, Thomas X, Ploux O. Escherichia coli cyclopropane fatty acid synthase. Eur J Biochem. 2004;271(23-24):4769–4778. doi: 10.1111/j.1432-1033.2004.04441.x. [DOI] [PubMed] [Google Scholar]
- 46.Rouser G, Fkeischer S, Yamamoto A. Two dimensional then layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 1970;5(5):494–496. doi: 10.1007/BF02531316. [DOI] [PubMed] [Google Scholar]
- 47.Christie WW, Han X. 2010. Lipid Analysis (Oily, Bridgwater, UK), 4th Ed, p 148.
- 48.Baba T, et al. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: The Keio collection. Mol Syst Biol. 2006;2:2006 0008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang AY, Grogan DW, Cronan JE. Cyclopropane fatty acid synthase of Escherichia coli: Deduced amino acid sequence, purification, and studies of the enzyme active site. Biochemistry. 1992;31(45):11020–11028. doi: 10.1021/bi00160a011. [DOI] [PubMed] [Google Scholar]
- 50.Montanari C, Sado Kamdem SL, Serrazanetti DI, Etoa FX, Guerzoni ME. Synthesis of cyclopropane fatty acids in Lactobacillus helveticus and Lactobacillus sanfranciscensis and their cellular fatty acids changes following short term acid and cold stresses. Food Microbiol. 2010;27(4):493–502. doi: 10.1016/j.fm.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 51.Tjellström H, Strawsine M, Silva J, Cahoon EB, Ohlrogge JB. Disruption of plastid acyl:acyl carrier protein synthetases increases medium chain fatty acid accumulation in seeds of transgenic Arabidopsis. FEBS Lett. 2013;587(7):936–942. doi: 10.1016/j.febslet.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 52.Bethesda Research Laboratories BRL pUC host: Escherichia coli DH5α competent cells. Bethesda Res Lab Focus. 1986;8:9–10. [Google Scholar]
- 53.Simon R, Priefer U, Puhler A. A broad host range mobilization system for in vitro genetic engineering: Transposon mutagenesis in Gram negative bacteria. Nature Biotech. 1983;1:748–791. [Google Scholar]
- 54.Schilke BA, Donohue TJ. ChrR positively regulates transcription of the Rhodobacter sphaeroides cytochrome c2 gene. J Bacteriol. 1995;177(8):1929–1937. doi: 10.1128/jb.177.8.1929-1937.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.