Abstract
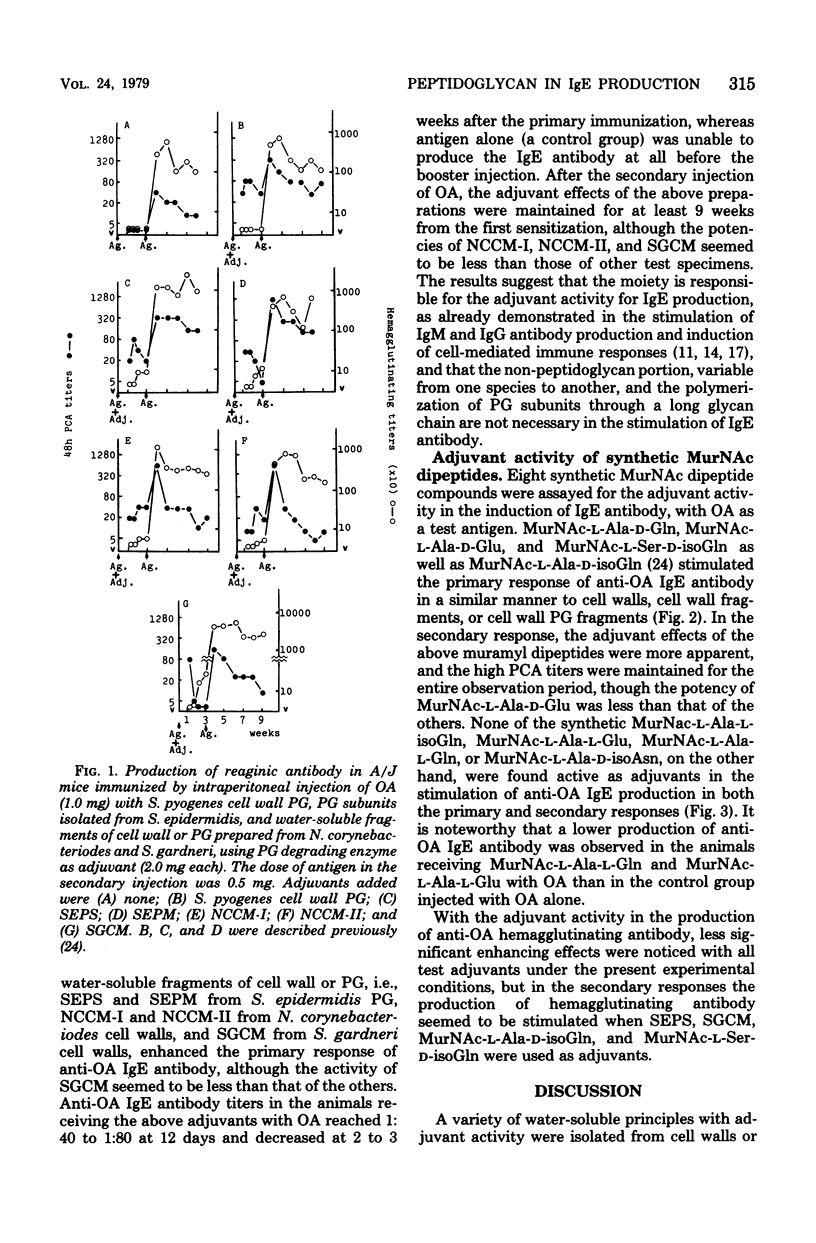
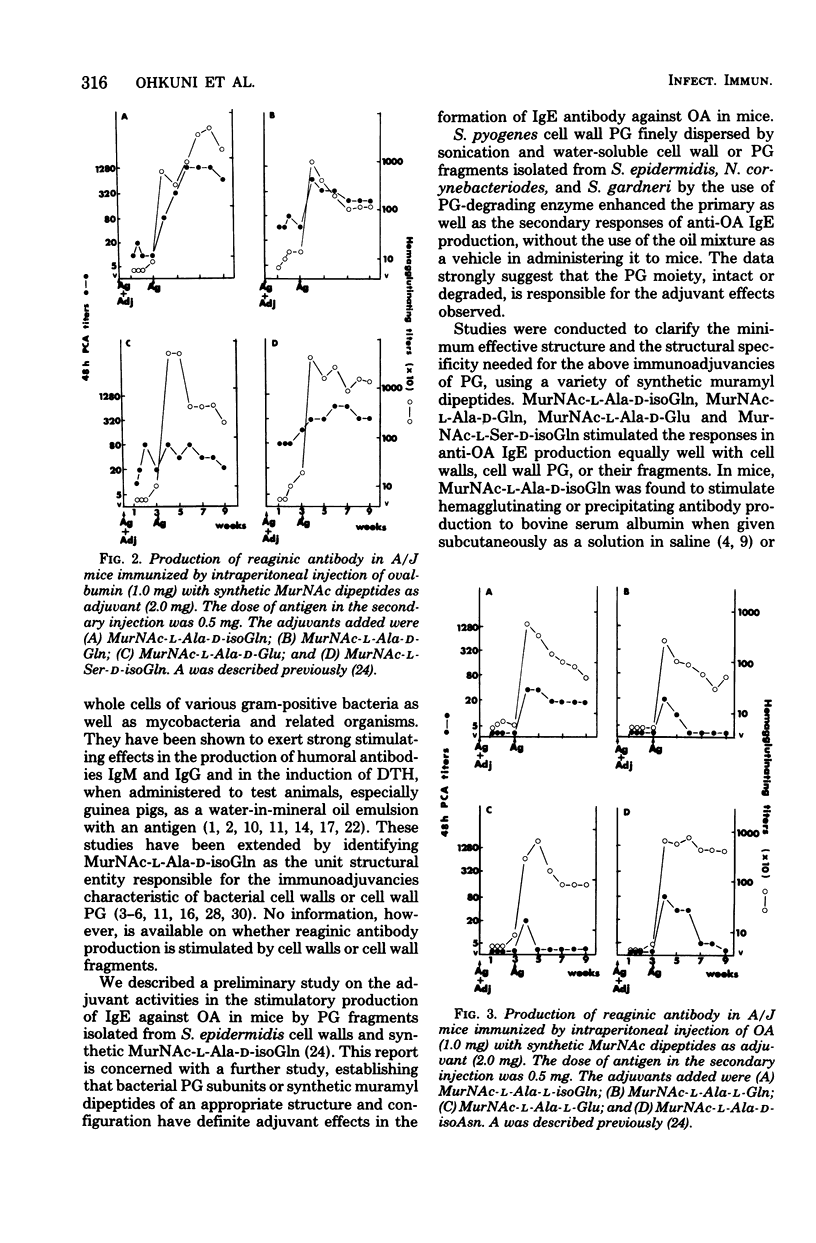
This paper is concerned with the adjuvant activity in stimulatory immunoglobulin E production against ovalbumin (OA) by bacterial cell walls, cell wall peptidoglycan (PG), and their PG fragments and synthetic N-acetylmuramyl (MurNAc) dipeptides in A/J mice. A PG isolated from Streptococcus pyogenes, PG subunit polymer and dimer obtained from Staphylococcus epidermidis, and water-soluble fragments of cell walls or PG prepared from Nocardia corynebacteriodes and Streptomyces gardneri were found to enhance both the primary and secondary responses of anti-OA immunoglobulin E antibody production. It was suggested that the PG portion, either intact or highly degraded, was capable of enhancing the immunoglobulin E antibody production, and there was no need for the non-PG moiety or intactness of PG structure for the adjuvant activity. This finding was confirmed and extended by the use of synthetic MurNAc dipeptides. Among eight MurNAc dipeptides tested, MurNAc-l-Ala-d-isoGln, MurNAc-l-Ala-d-Gln, MurNAc-l-Ala-d-Glu, and MurNAc-l-Ser-d-isoGln were found active as an adjuvant in the stimulation of the primary and secondary reaginic anti-OA antibody production in a similar way to the cell wall PG and their fragments. None of the synthetic MurNAc-l-Ala-l-isoGln, MurNAc-l-Ala-l-Gln, MurNAc-l-Ala-l-Glu, and MurNAc-l-Ala-d-isoAsn, on the other hand, stimulated the anti-OA immunoglobulin E antibody production in either primary or secondary response, indicating the importance for the adjuvancy in immunoglobulin E production of the configuration of the glutamic acid residues adjacent to the l-Ala (or l-Ser) in muramyl dipeptides.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam A., Ciorbaru R., Petit J. F., Lederer E., Chedid L., Lamensans A., Parant F., Parant M., Rosselet J. P., Berger F. M. Preparation and biological properties of water-soluble adjuvant fractions from delipidated cells of Mycobacterium smegmatis and Nocardia opaca. Infect Immun. 1973 Jun;7(6):855–861. doi: 10.1128/iai.7.6.855-861.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam A., Ciorbaru R., Petit J. F., Lederer E. Isolation and properties of a macromolecular, water-soluble, immuno-adjuvant fraction from the cell wall of Mycobacterium smegmatis. Proc Natl Acad Sci U S A. 1972 Apr;69(4):851–854. doi: 10.1073/pnas.69.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam A., Devys M., Souvannavong V., Lefrancier P., Choay J., Lederer E. Correlation of structure and adjuvant activity of N-acetyl muramyl-L-alanyl-D-isoglutamine (MDP), its derivatives and analogues. Anti-adjuvant and competition properties of stereoisomers. Biochem Biophys Res Commun. 1976 Sep 7;72(1):339–346. doi: 10.1016/0006-291x(76)90999-2. [DOI] [PubMed] [Google Scholar]
- Audibert F., Chédid L., Lefrancier P., Choay J. Distinctive adjuvanticity of synthetic analogs of mycobacterial water-soluble components. Cell Immunol. 1976 Feb;21(2):243–249. doi: 10.1016/0008-8749(76)90053-8. [DOI] [PubMed] [Google Scholar]
- Azuma I., Sugimura K., Taniyama T., Yamawaki M., Yamamura Y. Adjuvant activity of mycobacterial fractions: adjuvant activity of synthetic N-acetylmuramyl-dipeptide and the related compounds. Infect Immun. 1976 Jul;14(1):18–27. doi: 10.1128/iai.14.1.18-27.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azuma I., Sugimura K., Yamamura Y., Kusumoto S., Tarumi Y. Adjuvant activity of synthetic cell-wall peptidoglycan subunits on monoazobenzenearsonate-N-acetyl-L-tyrosine and bacterial alpha-amylase in guinea pigs. Jpn J Microbiol. 1976 Feb;20(1):63–66. doi: 10.1111/j.1348-0421.1976.tb00909.x. [DOI] [PubMed] [Google Scholar]
- BOYDEN S. V. The adsorption of proteins on erythrocytes treated with tannic acid and subsequent hemagglutination by antiprotein sera. J Exp Med. 1951 Feb;93(2):107–120. doi: 10.1084/jem.93.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chedid L., Audibert F., Johnson A. G. Biological activities of muramyl dipeptide, a synthetic glycopeptide analogous to bacterial immunoregulating agents. Prog Allergy. 1978;25:63–105. [PubMed] [Google Scholar]
- Chedid L., Audibert F., Lefrancier P., Choay J., Lederer E. Modulation of the immune response by a synthetic adjuvant and analogs. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2472–2475. doi: 10.1073/pnas.73.7.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chedid L., Parant M., Parant F., Gustafson R. H., Berger F. M. Biological study of a nontoxic, water-soluble immunoadjuvant from mycobacterial cell walls. Proc Natl Acad Sci U S A. 1972 Apr;69(4):855–858. doi: 10.1073/pnas.69.4.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellouz F., Adam A., Ciorbaru R., Lederer E. Minimal structural requirements for adjuvant activity of bacterial peptidoglycan derivatives. Biochem Biophys Res Commun. 1974 Aug 19;59(4):1317–1325. doi: 10.1016/0006-291x(74)90458-6. [DOI] [PubMed] [Google Scholar]
- Ishizaka K., Okudaira H. Reaginic antibody formation in the mouse. I. Antibody-mediated suppression of reaginic antibody formation. J Immunol. 1972 Jul;109(1):84–89. [PubMed] [Google Scholar]
- Kotani S., Narita T., Stewart-Tull D. E., Shimono T., Watanabe Y. Immunoadjuvant activities of cell walls and their water-soluble fractions prepared from various gram-positive bacteria. Biken J. 1975 Jun;18(2):77–92. [PubMed] [Google Scholar]
- Kotani S., Watanabe Y., Kinoshita F., Shimono T., Morisaki I. Immunoadjuvant activities of synthetic N-acetyl-muramyl-peptides or -amino acids. Biken J. 1975 Jun;18(2):105–111. [PubMed] [Google Scholar]
- Kotani S., Watanabe Y., Shimono T., Kinoshita F., Narita T. Immunoadjuvant activities of peptidoglycan subunits from the cell walls of Staphyloccus aureus and Lactobacillus plantarum. Biken J. 1975 Jun;18(2):93–103. [PubMed] [Google Scholar]
- Lehrer S. B., Vaughn J. H., Tan E. M. Enhancement of reaginic and hemagglutinating antibody production by an extract of Bordetella pertussis containing histamine sensitizing factor. J Immunol. 1976 Jan;116(1):178–183. [PubMed] [Google Scholar]
- Levine B. B., Vaz N. M. Effect of combinations of inbred strain, antigen, and antigen dose on immune responsiveness and reagin production in the mouse. A potential mouse model for immune aspects of human atopic allergy. Int Arch Allergy Appl Immunol. 1970;39(2-3):156–171. doi: 10.1159/000230343. [DOI] [PubMed] [Google Scholar]
- Nagai Y., Akiyama K., Kotani S., Watanabe Y., Shimono T., Shiba T., Kusumoto S. Structural specificity of synthetic peptide adjuvant for induction of experimental allergic encephalomyelitis. Cell Immunol. 1978 Jan;35(1):168–172. doi: 10.1016/0008-8749(78)90136-3. [DOI] [PubMed] [Google Scholar]
- Nauciel C., Fleck J., Martin J. P., Mock M., Nguyen-Huy H. Adjuvant activity of bacterial peptidoglycans on the production of delayed hypersensitivity and on antibody response. Eur J Immunol. 1974 May;4(5):352–356. doi: 10.1002/eji.1830040509. [DOI] [PubMed] [Google Scholar]
- Ohkuni H., Kimura Y. Increased capillary permeability in guinea pigs and rats by peptidoglycan fraction extracted from Group A streptococcal cell walls. Exp Cell Biol. 1976;44(2):83–94. doi: 10.1159/000163102. [DOI] [PubMed] [Google Scholar]
- Ohkuni H., Norose Y., Hayama M., Kinura Y., Kotani S., Shiba T., Kusumoto S., Yologawa K., Kawata S. Adjuvant activities in production of reaginic antibody in mice of bacterial cell wall peptidoglycans or peptidoglycan subunits and of synthetic N-acetylmuramyl dipeptides. Biken J. 1977 Dec;20(3-4):131–136. [PubMed] [Google Scholar]
- Ovary Z., Caiazza S. S., Kojima S. PCA reactions with mouse antibodies in mice and rats. Int Arch Allergy Appl Immunol. 1975;48(1):16–21. doi: 10.1159/000231289. [DOI] [PubMed] [Google Scholar]
- Souvannavong V., Adam A., Lederer E. Kinetics of the humoral and cellular immune response of guinea pigs after injection of the synthetic adjuvant N-acetylmuramyl L-alanyl-D-isoglutamine: comparison with Freund complete adjuvant. Infect Immun. 1978 Mar;19(3):966–971. doi: 10.1128/iai.19.3.966-971.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada T., Okumura K. Regulation of homocytotropic antibody formation in the rat. I. Feed-back regulation by passively administered antibody. J Immunol. 1971 Apr;106(4):1002–1011. [PubMed] [Google Scholar]
- Tanaka A., Saito R., Sugiyama K., Morisaki I., Kotani S. Adjuvant activity of synthetic N-acetylmuramyl peptides in rats. Infect Immun. 1977 Jan;15(1):332–334. doi: 10.1128/iai.15.1.332-334.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaz E. M., Vaz N. M., Levine B. B. Persistent formation of reagins in mice injected with low doses of ovalbuminl. Immunology. 1971 Jul;21(1):11–15. [PMC free article] [PubMed] [Google Scholar]



