Significance
The neuropeptide oxytocin (OXT) is critically involved in mammalian social functioning, and initial clinical research suggests that OXT biology may be altered in individuals with autism spectrum disorder (ASD). Here we provide important evidence that blood OXT concentrations are highly heritable within families, yet also strongly predict social functioning in ASD children, their unaffected siblings, and healthy control children. These findings also extend to OXT receptor genotypes which are significantly associated with differences in social functioning independent of disease status. These findings indicate that dysregulated OXT biology is not uniquely associated with ASD social phenotypes as widely theorized, but instead variation in OXT biology contributes to important individual differences in human social functioning, including the severe social impairments which characterize ASD.
Abstract
The neuropeptide oxytocin (OXT) and its receptor (OXTR) regulate social functioning in animals and humans. Initial clinical research suggests that dysregulated plasma OXT concentrations and/or OXTR SNPs may be biomarkers of social impairments in autism spectrum disorder (ASD). We do not know, however, whether OXT dysregulation is unique to ASD or whether OXT biology influences social functioning more generally, thus contributing to, but not causing, ASD phenotypes. To distinguish between these possibilities, we tested in a child ASD cohort, which included unaffected siblings and unrelated neurotypical controls (ages 3–12 y; n = 193), whether plasma OXT concentrations and OXTR SNPs (i) interact to produce ASD phenotypes, (ii) exert differential phenotypic effects in ASD vs. non-ASD children, or (iii) have similar phenotypic effects independent of disease status. In the largest cohort tested to date, we found no evidence to support the OXT deficit hypothesis of ASD. Rather, OXT concentrations strongly and positively predicted theory of mind and social communication performance in all groups. Furthermore, OXT concentrations showed significant heritability between ASD-discordant siblings (h2 = 85.5%); a heritability estimate on par with that of height in humans. Finally, carriers of the “G” allele of rs53576 showed impaired affect recognition performance and carriers of the “A” allele of rs2254298 exhibited greater global social impairments in all groups. These findings indicate that OXT biology is not uniquely associated with ASD, but instead exerts independent, additive, and highly heritable influences on individual differences in human social functioning, including the severe social impairments which characterize ASD.
The neuropeptide oxytocin (OXT) and its receptor (OXTR) regulate affiliative motivation, social bonding, and social recognition in animals (1, 2). Preclinical research likewise has shown that individual differences in OXT biology (e.g., OXT concentrations, OXTR distribution in key brain regions) are associated with individual differences in social functioning (3–6). At the extreme, experimental manipulations that diminish OXT peptide and/or receptor signaling produce a variety of social deficits in animal models (7, 8).
Translation of this animal research to neurotypical populations has confirmed that OXT administration enhances social functioning in humans (9, 10). Individual differences in endogenous OXT concentrations are also positively correlated with social behavior measures (11, 12), such that lower OXT concentrations are frequently associated with diminished social functioning even within neurotypical cohorts. This research has led to the hypothesis that abnormalities in OXT peptide biology may be directly related to social impairments observed in clinical populations, particularly in people with autism spectrum disorder (ASD), who exhibit core deficits in social interactions and preferences, eye contact, facial recognition, empathy, and social communication.
Several studies have begun to explore the so-called OXT deficit hypothesis of ASD. Three studies reported that plasma OXT concentrations are lower in individuals with ASD compared with control participants (13–15). A fourth study reported no overall group differences (16), and a fifth study reported that plasma OXT concentrations are higher in individuals with ASD compared with control participants (17). Relationships between plasma OXT concentrations and measures of social functioning in these small samples were either not analyzed (14, 15) or not found (17) or were evident only in subsets of individuals (16). Additionally, one study paradoxically reported inverse relationships between plasma OXT concentrations and social competence in ASD (a negative relationship) and control (a positive relationship) children (13). It is important to note that these studies had various limitations, including small study cohorts, use of normative range data for control plasma OXT concentrations instead of individual matched blood samples collected from neurotypical study participants, dilution rather than manufacturer-recommended extraction of plasma samples before OXT assay, use of nonstandard methods or unknown methods by which to diagnose ASD, and/or use of inappropriate statistical methods. The important question as to whether low plasma OXT concentrations are a biomarker of ASD and/or social impairments therefore remains unanswered.
The broader autism phenotype (18, 19), in which unaffected family members of ASD probands show subclinical impairments in social and other aspects of behavioral functioning, is thought to have a strong biological basis. Social impairments in family members (particularly in similar-aged siblings) may therefore reflect the heritability of the underlying physiology (i.e., OXT) critical to social functioning. This physiology may not be unique to ASD, but instead individual differences in OXT concentrations may contribute to individual differences in social functioning, including the milder social impairments documented in unaffected siblings and the more severe social deficits found in ASD probands. Whether or not plasma OXT concentrations are heritable, however, remains unknown, as no prior study has examined plasma OXT concentrations in ASD-discordant siblings.
In addition to functional variability in OXT peptide concentrations, preliminary research suggests that genetic variability in the OXTR gene may be a risk factor for ASD. Association studies have shown that several SNPs and their haplotypes in the OXTR gene increase risk for ASD (20–23). Genome-wide scans likewise indicate that the 3p25 region, which contains the OXTR gene (24), may be associated with ASD (25–28). A separate body of research has repeatedly implicated two intronic OXTR gene SNPs, rs2254298 and rs53576, in social functioning in both neurotypical and neuropsychiatric populations (29–32). These studies provide suggestive evidence that OXTR SNPs may be associated with human social functioning, but it is not clear whether these genetic variants specifically influence ASD social phenotypes or whether they influence social functioning independent of disease status. It is also unknown whether these genetic findings are dependent or independent of OXT peptide concentrations, as no prior study has concomitantly evaluated OXT concentrations and OXTR SNPs in the same study population, inclusive of individuals with and without ASD.
The first aim of this study was to test the prevalent but not well-interrogated OXT deficit hypothesis of ASD by investigating whether plasma OXT concentrations are lower in individuals with ASD and whether this relationship is affected by sex and/or OXTR SNPs. The second aim of this study was to test for the first time, to our knowledge, in the same statistical model whether plasma OXT concentrations and OXTR SNPs (i) interact to produce ASD social phenotypes, (ii) exert differential social phenotypic effects in ASD vs. non-ASD individuals, or (iii) have similar social phenotypic effects for all individuals independent of disease status.
Results
Study participants were 79 children with ASD, 52 unaffected siblings, and 62 unrelated neurotypical control children, ages 3–12 y. Although all cases met criteria for ASD, expert clinical opinion and scores on the diagnostic instruments [i.e., the Autism Diagnostic Interview-Revised (ADI-R) (33, 34) and the Autism Diagnostic Observation Schedule-Generic (ADOS-G)] (35, 36) were also used to test whether OXT biology differed in children with classic autism (n = 47) vs. those with pervasive developmental disorder-not otherwise specified (PDD-NOS) (n = 32)]. A social phenotypic characterization—using the Social Responsiveness Scale (SRS) Total Score (37, 38); NEPSY-II Social and Perception Domain: Affect Recognition and Theory of Mind Tasks (39); and three measures from the Vineland Adaptive Behavior Scales, Second Edition (VABS-2) (40)—was obtained on, and blood samples for OXT quantification and OXTR genotyping were collected from, all participants. As expected, children with ASD exhibited impaired social functioning compared with children without ASD. Participant characteristics are presented in Table S1.
Testing the OXT Deficit Hypothesis of ASD: Are Plasma OXT Concentrations Lower in Participants with ASD and Are They Influenced by Sex, OXTR SNPs, or Their Interactions?
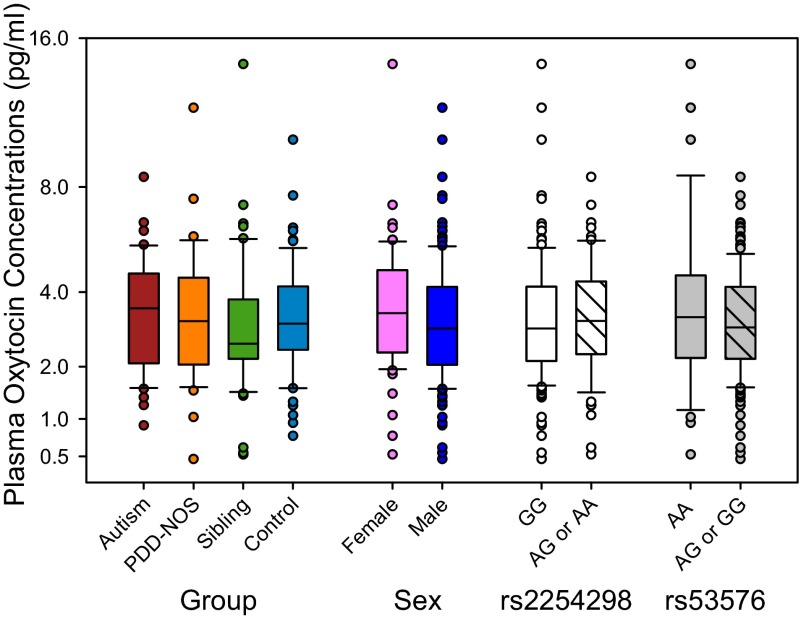
We used a nested design to test for differences between participant groups, and between symptomatic (i.e., autistic and PDD-NOS) and nonsymptomatic (i.e., sibling and neurotypical control) individuals on average in the same analysis. Despite statistically controlling (i.e., blocking) for possible extraneous sources of variability [i.e., age, ethnicity, blood sample collection time, and full-scale intelligence quotient (IQ), none of which were significant themselves], we found no significant main effects (Fig. 1) or interaction effects (i.e., with sex or OXTR SNPs) for plasma OXT concentrations in this model. Thus, these data, collected from the largest cohort tested to date, did not support the OXT deficit hypothesis of ASD, which was previously advanced on the basis of findings from several smaller-scale pilot studies (e.g., refs. 13 and 14).
Fig. 1.
Plasma OXT concentrations do not significantly differ by group, sex, or OXTR SNPs. Group: n = 47 autistic, n = 32 PDD-NOS, n = 52 sibling, and n = 62 neurotypical control children. Sex: n = 62 females and n = 131 males. rs2254928 genotype: n = 127 GG and n = 66 AG or AA. rs53576 genotype: n = 35 AA and n = 158 GG or AG. Box plots are presented as least-squares-mean (LSM) ± SEM and each LSM is partialed for other data in the analysis.
Testing the Role of OXT Biology in Social Impairments: Are Plasma OXT Concentrations and OXTR Allelic SNPs’ Effects on Social Phenotypes Dependent or Independent of Disease Status?
We next tested for the effects of plasma OXT concentrations, the OXTR SNPs, group (nested within symptomatic), and their interactions on social phenotypes (while blocking for sex, age, ethnicity, blood sample collection time, and full-scale IQ). We found no evidence that plasma OXT concentrations and OXTR SNPs either interact directly to produce ASD social phenotypes or exert differential social phenotypic effects in ASD vs. non-ASD individuals. Rather, all findings converged to uniformly support the third hypothesis: Plasma OXT concentrations and OXTR SNPs exert independent, additive, and highly heritable influences on individual differences in human social functioning for children in all groups. (For clarity, we present only the phenotypes with significant findings below.)
Plasma OXT Concentrations and Social Functioning.
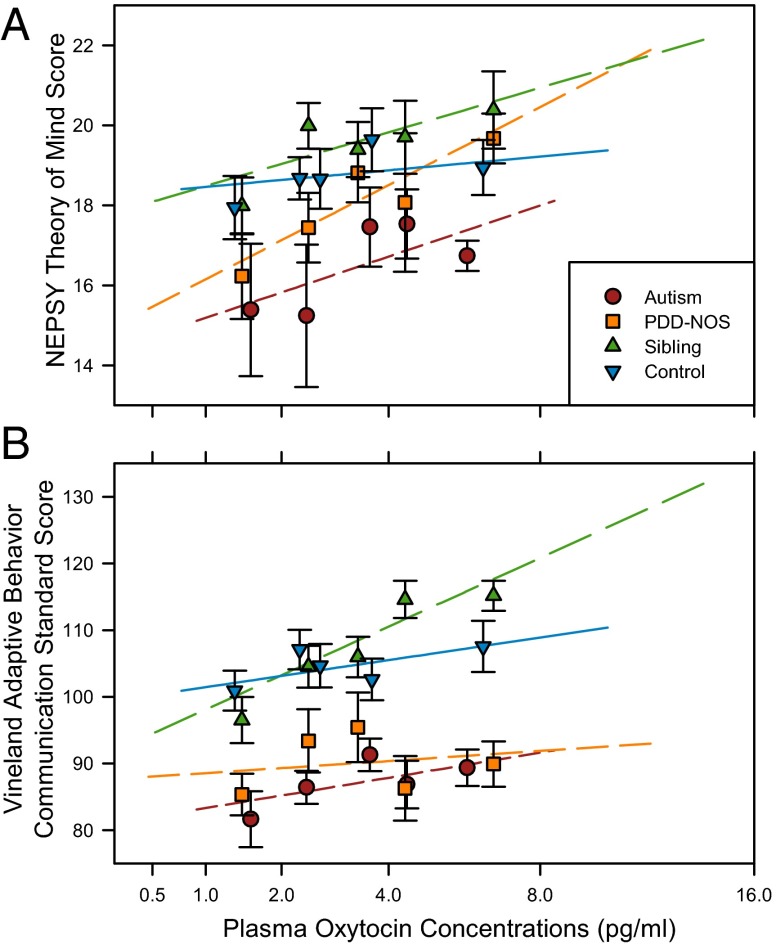
Plasma OXT concentrations positively predicted NEPSY theory of mind scores (F1,168 = 4.6514; P = 0.0327), with lower plasma OXT concentrations associated with impaired theory of mind performance. This effect persisted when children below the age of 5 y (i.e., those that may not have yet achieved theory of mind) were excluded from the analysis (F1,115 = 4.6169; P = 0.0338). This relationship was consistent for all groups, with no significant difference observed in the relationship between the four groups [group within symptomatic × OXT interaction: F2,168 = 0.3910; P = not significant (ns)] or between symptomatic and nonsymptomatic individuals on average (symptomatic × OXT interaction: F1,168 = 0.9846; P = ns). Symptomatic individuals scored worse on average than nonsymptomatic individuals on the theory of mind measure (F1,168 = 7.6402; P = 0.0065), but the subdivision into individual groups did not predict score (i.e., autistic and PDD-NOS individuals did not differ; siblings and neurotypical control individuals did not differ: F2,168 = 1.6248; P = ns) (Fig. 2A).
Fig. 2.
Plasma OXT concentrations predict (A) NEPSY theory of mind and (B) Vineland Communication Domain scores independent of group. Groups and sample sizes are detailed in Fig. 1. In A and B the intercepts (i.e., the mean phenotypic scores) differ significantly by group (P < 0.05), whereas the slopes of the lines do not. The overall regression line averaged across groups is significant. The phenotypic data corrected for other variables in the analysis is shown, and each line is plotted between the full extents of the data. Data points are presented as LSM ± SEM for each quintile of the data, ordered by OXT concentrations within each group.
The same pattern of results was observed for the Vineland Communication Domain. Plasma OXT concentrations positively predicted social communication scores (F1,164 = 7.7567; P = 0.0060), with lower plasma OXT concentrations associated with greater communication impairments. This effect was consistent for all groups, with no significant difference observed in the relationship between the four groups (group within symptomatic × OXT interaction: F2,164 = 1.7381; P = ns) or between ASD and non-ASD individuals on average (symptomatic × OXT interaction: F1,164 = 1.9905; P = ns). Symptomatic individuals scored worse on average than nonsymptomatic individuals (F1,164 = 46.0306; P < 0.0001) but the subdivision into individual groups did not predict score (F2,164 = 1.0683; P = ns) (Fig. 2B).
Testing Heritability in ASD-Discordant Siblings: Are the Observed Individual Differences in Plasma OXT Concentrations and the Two Phenotypic Measures They Predict Heritable?
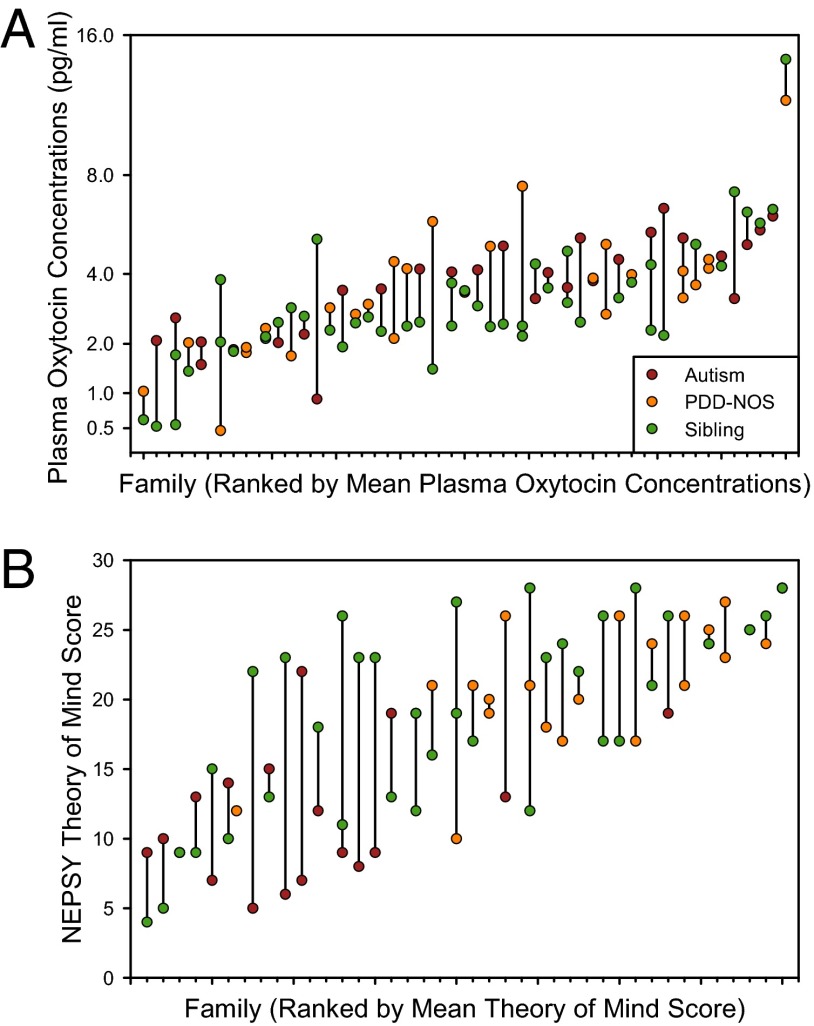
We next calculated narrow-sense heritabilities (h2) (41) for plasma OXT concentrations, NEPSY theory of mind scores, and the Vineland Communication Domain scores for families in which we had data for two individuals. Plasma OXT concentrations were highly heritable (h2 = 85.5%; F44,51 = 2.619; P = 0.0005) (Fig. 3A), corresponding to a mean correlation between family members of 0.427. By comparison, height in humans has a narrow-sense heritability of ∼80% (42). NEPSY theory of mind scores showed moderate heritability (h2 = 55.1%; F34,38 = 1.812; P = 0.0382) (Fig. 3B), whereas Vineland Communication Domain scores did not show significant heritability (h2 = 1.1%; F44,51 = 1.012; P = ns).
Fig. 3.
Narrow-sense heritability is estimated as a function of within-family consistency. Individual values are shown for (A) plasma OXT concentrations (h2 = 85.5%; n = 47 families) and (B) the NEPSY theory of mind score (h2 = 55.1%; n = 37 families). To illustrate consistency of values within families, members of the same family are plotted at the same point on the x axis and joined by a vertical line.
OXTR SNPs and Social Functioning.
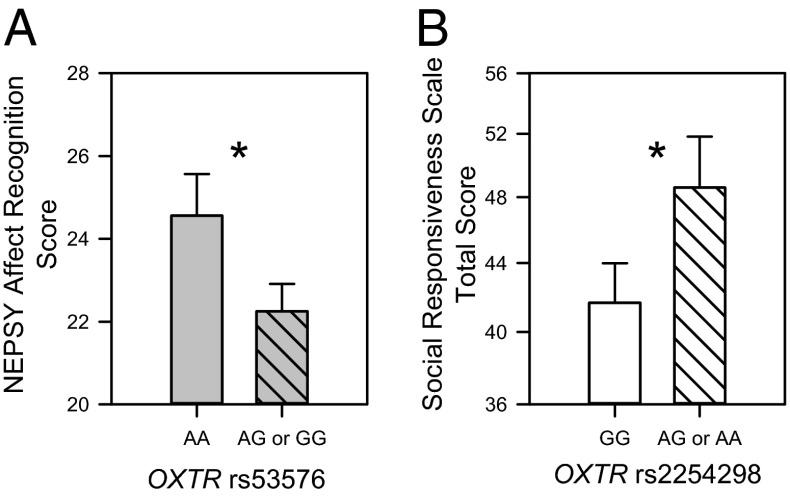
Carriers of the “G” allele (i.e., AG or GG) of rs53576 performed worse on the NEPSY affect recognition task (F1,170 = 4.8577; P = 0.0291) than those with two “A” alleles (Fig. 4A). This effect was consistent for all groups, with no significant difference observed in the relationship between groups (group within symptomatic × rs53576 interaction: F2,170 = 2.5277; P = ns) or between symptomatic and nonsymptomatic individuals on average (symptomatic × rs53576 interaction: F1,170 = 3.3463; P = ns). Further, symptomatic individuals did not score worse on average than nonsymptomatic individuals (F1,170 = 0.1105; P = ns) nor did the subdivision into the individual groups predict score (F2,170 = 0.6376; P = ns).
Fig. 4.
OXTR SNPs predict social functioning independent of group. Groups and sample sizes are detailed in Fig. 1. For each genotype, the study risk allele is shown as a hatched bar. (A) Carriers with the G allele (n = 158) vs. those with the AA genotype (n = 35) of rs53576 show diminished affect recognition. (B) Carriers with the A allele (n = 66) vs. those with the GG genotype (n = 127) of rs2254928 show greater social impairments on the reverse-scored SRS. Data points are presented as LSM ± SEM, with the LSM partialed for all nondepicted variables. The asterisk represents Bonferroni-corrected post hoc comparison with P < 0.05.
Carriers of the A allele (i.e., AG or AA) of rs2254298 exhibited greater global social impairments as measured by the SRS Total Score (F1,167 = 5.2313; P = 0.0234) compared with those with two G alleles (Fig. 4B). This effect was consistent for all groups, with no significant difference observed in the relationship between the four groups (group within symptomatic × rs2254298 interaction: F2,167 = 1.5253; P = ns) or between symptomatic and nonsymptomatic individuals on average (symptomatic × rs2254298 interaction: F2,167 = 0.9676; P = ns). Symptomatic individuals scored worse on average than nonsymptomatic individuals (F1,167 = 220.9639; P < 0.0001) but the subdivision into individual groups did not predict score (F2,167 = 1.8836; P = ns).
OXT Biology and ASD Social Phenotypes.
ADI-R scores were only determined for participants with ASD. Carriers with the A allele of rs2254298 scored worse on the Social A1 total score (failure to use nonverbal behaviors to regulate social interaction) (F1,61 = 4.9899; P = 0.0292) than those with two G alleles. This effect was consistent for both ASD groups (group × rs2254298 interaction: F1,61 = 0.0004; P = ns), and ASD subgroup did not further predict the A1 total score (F1,61 = 1.0614; P = ns). Finally, the relationship between this OXTR genotype and the ADI-R score remains significant even in a simple two-sample t test (i.e., AA/AG vs. GG) in which all of the blocking factors (i.e., sex, age, ethnicity, blood sample collection time, and full-scale IQ) are removed (t = 2.25; n = 77; P = 0.0276 df = 75). There were no significant effects for plasma OXT concentrations or the rs53576 SNP.
Discussion
Recent studies (reviewed in refs. 1 and 2) have suggested a potentially important role for OXT biology in the social phenotypes of ASD, but they also present a challenge for the field. This is because existing data cannot distinguish between two critically different scenarios: Plasma OXT concentrations and/or OXTR SNPs are either biomarkers of social impairments unique to individuals with ASD or exert similar influences on social functioning, contributing to but not causing, ASD social phenotypes. To resolve this issue we collected the first dataset, to our knowledge, in which plasma OXT concentrations and OXTR SNPs were concomitantly investigated in a cohort of children with ASD, their unaffected siblings, and unrelated neurotypical control children using gold standard diagnostic methods and state-of-the-art quantification techniques.
Our study found no empirical evidence to support the prevalent but previously not well-interrogated OXT deficit hypothesis of ASD (study aim 1). Rather, we found that independent of group, that (i) plasma OXT concentrations strongly and positively predicted theory of mind and social communication performance, (ii) carriers of the G allele of rs53576 showed impaired affect recognition performance, and (iii) carriers of the A allele of rs2254298 exhibited greater global social impairments. Together these results paint an intriguing and novel picture with respect to the three hypotheses delineated in study aim 2. First, diminished plasma OXT concentrations and these two OXTR SNPs are not specific risk factors for ASD. Second, these biological measures do not exert differential effects in ASD vs. non-ASD individuals. Third, these OXT measures instead exert similar effects on social functioning for all children, such that they contribute to the variation in severity of ASD phenotypes, but in the same way they contribute to variation in social functioning in neurotypical individuals or unaffected siblings. [A very recent report lends support that these findings may even extend to other OXTR genotypes (i.e., rs237887) (43).] These OXT measures therefore appear to be biomarkers of social functioning in a universal sense, and at the lower tail of the distribution we find evidence for impaired social functioning, which is evident in clinical diagnostic assessments (i.e., ASD individuals who are carriers of the A allele of rs2254298 showed greater impairments on measures of reciprocal social interactions on the ADI-R).
The pattern of narrow-sense heritabilities is also consistent with the interpretation of our findings. Although there were no differences between ASD-discordant siblings in plasma OXT concentrations, plasma OXT concentrations were highly heritable (h2 = 85.5%), on par with height in the general population (42). We also examined heritability for the two behavioral measures that plasma OXT concentrations positively predicted. Theory of mind ability was moderately heritable (h2 = 55.1%), whereas social communication ability did not show evidence of heritability (h2 = 1.1%). This pattern of results suggests the intriguing idea that the heritability of plasma OXT concentrations may be driving, at least in part, social functioning within families. Thus, as plasma OXT concentrations are highly heritable, OXT-associated traits should be inherently correlated within families. The degree to which this is the case will depend on the degree to which nonfamilial environmental effects mitigate the impact of endogenous OXT signaling. Thus, whereas theory of mind is a more innate perceptual cognitive skill, but communication skills can be learned, the moderate heritability of theory of mind and the lack of heritability for social communication are consistent with this notion.
Our study also investigated the two OXTR SNPs (i.e., rs2254298 and rs53576) most frequently implicated in human social functioning (44, 45). Of particular interest is our finding that the minor allele of rs2254298 predicted global social impairments on the SRS and diagnostic severity on the ADI-R. In contrast, the major allele of rs53576 predicted impaired affect recognition performance on the NEPSY. The scientific literature has yielded inconsistent evidence regarding the risk alleles for each OXTR SNP (at least as defined by participant performance on various behavioral and/or biological assessments; see refs. 44 and 45 for reviews) and their associations with autism (21, 22, 46). These variable findings are likely influenced by numerous study-specific differences in sample size and dependent variable measurements. The demographic composition of the study participants may also play an important role (47, 48), as different genetic or ethnic backgrounds may have other functionally significant SNPs in linkage disequilibrium with these two OXTR SNPs. This range of findings also underscores an important point: The functional significance of these two intronic variants remains unknown. A better understanding of how these SNPs influence OXTR transcription or translation might explain the observed study-to-study variability.
This increased scientific interest in endogenous OXT biology in humans has been accompanied by numerous pharmacological reports demonstrating that intranasal OXT administration enhances social functioning in both neurotypical participants as well as those with ASD (9, 10, 14, 49). The fact that individuals with and without ASD showed the same relationship between plasma OXT concentrations and social phenotypes in our study gives credence to the idea that OXT treatment might be an effective pharmacotherapeutic for the core social features of ASD. If so, Fig. 2A hints at an exciting possibility: The ASD individuals with the highest plasma OXT concentrations scored as well on the theory of mind task as the neurotypical control individuals with the lowest plasma OXT concentrations. OXT dysregulation may not be the underlying cause of ASD, but OXT circuitry could be a promising therapeutic target nonetheless. Similar to the serotonin deficiency hypothesis of major depression (50), dysregulation in the OXT system might merely be a manifestation of other upstream neural deficiencies that are more prevalent in ASD, but that may also affect nonsymptomatic individuals to impair social functioning. If intranasal OXT treatment could enhance social functioning to the highest level seen in the ASD individuals we studied, then it might be able to restore social functioning to within (at least the lower end of) the range observed in the neurotypical control participants.
As with all studies, ours is not without limitations. Due to the invasive nature of sample collection, we were able to draw only one blood sample per participant and were unable to assess OXT concentrations in a matrix more proximal to the brain: cerebrospinal fluid (CSF). Although it remains unclear how blood measures of OXT are related to release of OXT in the brain, it is conceivable that individuals with ASD may differ in their relative allocation of OXT to these two compartments, as has been seen in a monkey model of impaired social functioning (5). If so, CSF OXT concentrations might be a more specific biomarker of ASD, whereas (as we found here) plasma OXT concentrations might be a general biomarker of social functioning (perhaps as an indirect measure of tonic hypothalamic OXT release). With regard to our plasma OXT heritability analysis, it should be noted that as every sibling dyad contained one ASD individual, these findings merit replication in sibling dyads from the general population. We were also unable to extend our heritability analysis to assess transmission of the two OXTR SNPs investigated in this study, as parental DNA samples were not available to perform this analysis. Finally, our study included an unequal distribution of males and females as well as a mixed ethnic sample. We controlled (i.e., blocked) for both potential extraneous sources of variability in our model. This conservative statistical approach ensured that any significant effects for sex or an allele could not be driven by either of these variables, and therefore were consistent across the sexes and different ethnic groups represented in the study.
In summary, we found no empirical support for the OXT deficit hypothesis of ASD, nor did plasma OXT concentrations differ by sex, OXTR SNPs, or their interactions. Plasma OXT concentrations did, however, strongly and positively predict theory of mind and social communication performance for children in all groups. Furthermore, plasma OXT concentrations and theory of mind performance were significantly heritable for ASD-discordant siblings, suggesting the intriguing idea that plasma OXT concentrations may contribute to similar social functioning abilities in families. Carriers of the G allele of rs53576 showed impaired affect recognition performance and carriers of the A allele of rs2254298 exhibited greater global social impairments for children in all groups. Taken together, these findings indicate that plasma OXT concentrations and OXTR SNPs are not uniquely associated with ASD, but instead exert independent, additive, and highly heritable influences on individual differences in human social functioning, including the severe social impairments which characterize ASD. Impairments in social cognition are also recognized as core features of many neuropsychiatric disorders (e.g., schizophrenia, bipolar disorder, borderline personality disorder), and for which OXT deficits have been hypothesized, but not yet well interrogated. Research is now required to test whether OXT dysregulation occurs in these patient populations, or whether, like ASD, OXT biology contributes to but does not cause the social features of these disorders.
Materials and Methods
Participants.
This study was approved by the Stanford University Institutional Review Board and all participants and their families provided informed consent before initiation of study procedures. Assent was also obtained from children 7 y of age and older when appropriate. Seventy-nine children with ASD (N = 17 female, 62 male), 52 unaffected siblings (N = 23 female, 29 male), and 62 neurotypical control children (N = 22 female, 40 male) between the ages of 3 and 12 y participated in this study. Participant characteristics are presented in Table S1. Children with ASD and their siblings were primarily recruited through the Autism and Developmental Disorders Research Registry and by flyers posted in the Autism and Developmental Disorders Clinic at Stanford University. Unrelated control participants were recruited through advertisements posted online (e.g., Parent Listservs, www.craigslist.org) or hardcopy in the surrounding community (e.g., pediatrician offices, shopping malls). All participants were (i) prepubertal, (ii) in good medical health, and (iii) willing to provide a blood sample. Participants with ASD were included if they had a full-scale IQ of 50 and above. Control participants and siblings were included if they had an IQ in the average range. Cognitive functioning was determined using the Stanford-Binet manual (51). Diagnostic methods and exclusion criteria are described in SI Materials and Methods.
Social Phenotyping.
Social phenotyping included the following three instruments. The SRS is a norm-referenced questionnaire that measures social behavior (e.g., social awareness, social cognition, social communication, social motivation, and autistic mannerisms) in both clinical and nonclinical populations. This parent-report measure includes the SRS Total Score, which was used in this study. The psychometric properties of the Total Score have been tested in younger and older participants, and are continuously distributed within each group (37, 38). The NEPSY-II (39) is a widely used and validated norm-referenced measure of child neurocognitive abilities and includes affect recognition and theory of mind, the two measures tested here. The VABS-2 (40) is a well-validated parent interview that measures adaptive behavioral functioning, particularly in socially relevant domains. We assessed socialization, communication, and daily living skills in this study. We also examined ADI-R Social Domain Scores in children with ASD.
Blood Sampling and Plasma OXT Quantification.
Descriptions of whole blood collection and plasma OXT quantification are provided in SI Materials and Methods.
OXTR Genotyping.
DNA was extracted from whole blood using standard laboratory procedures. OXTR genotyping is described in SI Materials and Methods. In the study population, the OXTR SNP rs2254298 allelic frequencies were 19.4% for the A variant and 80.6% for the G variant. The OXTR SNP rs53576 allelic frequencies were 40.9% for the A variant and 59.1% for the G variant. Tables S2 and S3 provide OXTR allelic information. Allelic frequencies for both OXTR SNPs were in Hardy–Weinberg equilibrium: rs2254298 (χ2 = 0.621; df = 2; n = 193; P = 0.733) and rs53576 (χ2 = 0.629; df = 2; n = 193; P = 0.731). Initial statistical analyses testing for allelic dosing effects for rs2254298 [AA (n = 9; 4.7%), AG (n = 57; 29.5%), GG (n = 127; 65.8%)] and rs53576 [AA (n = 35; 18.1%), AG (n = 88; 45.6%), GG (n = 70; 36.3%)] revealed the A allele to be the risk allele for rs2254298 and G to be the risk allele for rs53576 for the phenotypic variables of interest. We therefore combined the AA and AG groups for rs2254298 and the AG and GG groups for rs53576 in subsequent analyses. Finally, our San Francisco Bay Area sample was of mixed ethnicity. Given reports of ethnic differences in OXTR allelic frequency and effects in several studies (47, 48), we included ethnicity as a statistical covariate for all analyses. This conservative approach ensured that any significant effects for an allele could not be driven by ethnicity, and that any observed effects would be consistent across the different ethnic groups represented in the study.
Statistical Analyses.
All analyses were performed as general linear models (GLMs) in JMP Version 10 (SAS Institute Inc.). The first study aim used a GLM to test for differences in mean plasma OXT concentrations by group, sex, and the two OXTR SNPs (while blocking for age, ethnicity, blood sample collection time, and full-scale IQ). We represented groups by subdividing (nesting) this variable into symptomatic (autistic and PDD-NOS) vs. nonsymptomatic (sibling and neurotypical control) individuals. This allowed us to test explicitly whether any overall difference was due to differences between symptomatic and nonsymptomatic individuals in general or a particular group. (This approach is far more powerful than resorting to post hoc multiple comparisons between groups.) Second-order interactions between group, sex, and the two OXTR genotypes were also tested. Even so, no influences of any of these experimental factors on plasma OXT concentrations were found, and therefore did not require follow-up comparisons. This lack of colinearity with plasma OXT concentrations is optimal for the approach taken for the second study aim, as confounding explanations due to colinearity can be ruled out. To test the second aim, we adopted an epidemiological approach to these data, analyzing each social behavior measure in a GLM that examined all of the experimental factors at the same time, thereby controlling for noise from both the experimental and blocking factors. The same model was used for each behavioral measure. Experimental factors were plasma OXT concentrations, OXTR rs53576 genotype, OXTR rs2254298 genotype, and group, represented as group-nested-within-symptomatic. Second order interactions between all experimental factors were included. Sex, age, ethnicity, blood sample collection time, and full-scale IQ were included as blocking factors. A major advantage of this approach is that it further maximizes power by avoiding issues of multiple comparisons: The individual terms within a factorial analysis of this kind represent distinct causal hypotheses (and so we limited the model to include only experimental factors about which we had causal hypotheses). Similarly, we limited the phenotypes examined and picked one to test each of our distinct phenotypic hypotheses.
The assumptions of GLM (normality of error, homogeneity of variance, and linearity) were examined graphically and suitable transformations applied as required (52). Plasma OXT concentrations were square-root transformed for all analyses to correct for a skewed distribution and associated nonlinearity in the analyses. Only the NEPSY theory of mind score showed evidence of nonlinearity after these corrections, which was resolved by including a quadratic effect of age (a quadratic age effect was then tested for all phenotypes and excluded, as it was not significant). Significant interactions were examined with post hoc Bonferroni-corrected orthogonal planned contrasts as required to understand the significance of a factor made up of several levels or when comparing the mean value of groups (52).
Narrow-sense heritability was calculated using the intraclass correlation method (41). Ideally one would calculate heritability from multigenerational data. However, given the considerable changes in autism prevalence in the current generation, this was not a practical approach here. Narrow-sense heritability, in contrast, uses phenotypic data from one generation. This method is the most conservative available for estimating heritability from sibling data, as it excludes dominance, epistatic interactions, and other components which would inflate heritability estimates. Only families where data were available for two individuals were included. A restricted maximum likelihood mixed model was constructed, similar to that used for the main analyses. Thus, the environmental or epistatic effects of sex, age, ethnicity, blood sample collection time, full-scale IQ, and group within symptomatic were controlled by including these factors in the model and estimating the variance components for within-family error and between-family additive genetic effects. These variance components lead directly to an intraclass correlation estimate for family members, and thus to estimates of heritability, and also an approximate F ratio test. Confidence intervals for narrow-sense heritability can be approximated but are typically excessively inflated. The intraclass correlation estimate therefore was tested here instead.
Supplementary Material
Acknowledgments
We thank Wendy Kalkus, Serena Tanaka, Kaeli Yuen, and Katy Brewster for help with blood sample collection and processing as well as data entry. We also thank Dr. Carl Feinstein (Director of the Stanford Autism Center) for his unwavering support of this research program. Additionally, we thank Dr. Toni Zeigler and Dan Wittwer (University of Wisconsin National Primate Research Center Assay Services) for conducting the OXT assays which were made possible by National Institutes of Health Grant RR000167. This research program was supported by grants from the Simons Foundation Autism Research Initiative (to K.J.P.), the Mosbacher Family Fund for Autism Research (to K.J.P.), the Escher Fund at the Silicon Valley Community Foundation (to A.Y.H.), and Stanford University’s Child Health Research Institute (to K.J.P. and A.Y.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1402236111/-/DCSupplemental.
References
- 1.Carter CS, Grippo AJ, Pournajafi-Nazarloo H, Ruscio MG, Porges SW. Oxytocin, vasopressin and sociality. Prog Brain Res. 2008;170:331–336. doi: 10.1016/S0079-6123(08)00427-5. [DOI] [PubMed] [Google Scholar]
- 2.Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: Social neuropeptides for translational medicine. Nat Rev Neurosci. 2011;12(9):524–538. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- 3.Insel TR, Shapiro LE. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc Natl Acad Sci USA. 1992;89(13):5981–5985. doi: 10.1073/pnas.89.13.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parker KJ, Kinney LF, Phillips KM, Lee TM. Paternal behavior is associated with central neurohormone receptor binding patterns in meadow voles (Microtus pennsylvanicus) Behav Neurosci. 2001;115(6):1341–1348. doi: 10.1037//0735-7044.115.6.1341. [DOI] [PubMed] [Google Scholar]
- 5.Winslow JT, Noble PL, Lyons CK, Sterk SM, Insel TR. Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacology. 2003;28(5):910–918. doi: 10.1038/sj.npp.1300128. [DOI] [PubMed] [Google Scholar]
- 6.Maestripieri D, Hoffman CL, Anderson GM, Carter CS, Higley JD. Mother-infant interactions in free-ranging rhesus macaques: Relationships between physiological and behavioral variables. Physiol Behav. 2009;96(4-5):613–619. doi: 10.1016/j.physbeh.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim MM, Bielsky IF, Young LJ. Neuropeptides and the social brain: Potential rodent models of autism. Int J Dev Neurosci. 2005;23(2-3):235–243. doi: 10.1016/j.ijdevneu.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 8.Ferguson JN, et al. Social amnesia in mice lacking the oxytocin gene. Nat Genet. 2000;25(3):284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- 9.Domes G, Heinrichs M, Michel A, Berger C, Herpertz SC. Oxytocin improves “mind-reading” in humans. Biol Psychiatry. 2007;61(6):731–733. doi: 10.1016/j.biopsych.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 10.Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435(7042):673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- 11.Clark CL, et al. Neonatal CSF oxytocin levels are associated with parent report of infant soothability and sociability. Psychoneuroendocrinology. 2013;38(7):1208–1212. doi: 10.1016/j.psyneuen.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feldman R, Gordon I, Zagoory-Sharon O. Maternal and paternal plasma, salivary, and urinary oxytocin and parent-infant synchrony: Considering stress and affiliation components of human bonding. Dev Sci. 2011;14(4):752–761. doi: 10.1111/j.1467-7687.2010.01021.x. [DOI] [PubMed] [Google Scholar]
- 13.Modahl C, et al. Plasma oxytocin levels in autistic children. Biol Psychiatry. 1998;43(4):270–277. doi: 10.1016/s0006-3223(97)00439-3. [DOI] [PubMed] [Google Scholar]
- 14.Andari E, et al. Promoting social behavior with oxytocin in high-functioning autism spectrum disorders. Proc Natl Acad Sci USA. 2010;107(9):4389–4394. doi: 10.1073/pnas.0910249107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Ayadhi LY. Altered oxytocin and vasopressin levels in autistic children in Central Saudi Arabia. Neurosciences (Riyadh) 2005;10(1):47–50. [PubMed] [Google Scholar]
- 16.Miller M, et al. Oxytocin and vasopressin in children and adolescents with autism spectrum disorders: Sex differences and associations with symptoms. Autism Res. 2013;6(2):91–102. doi: 10.1002/aur.1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jansen LM, et al. Autonomic and neuroendocrine responses to a psychosocial stressor in adults with autistic spectrum disorder. J Autism Dev Disord. 2006;36(7):891–899. doi: 10.1007/s10803-006-0124-z. [DOI] [PubMed] [Google Scholar]
- 18.Bishop DV, Maybery M, Wong D, Maley A, Hallmayer J. Characteristics of the broader phenotype in autism: A study of siblings using the children’s communication checklist-2. Am J Med Genet B Neuropsychiatr Genet. 2006;141B(2):117–122. doi: 10.1002/ajmg.b.30267. [DOI] [PubMed] [Google Scholar]
- 19.Constantino JN, et al. Autistic social impairment in the siblings of children with pervasive developmental disorders. Am J Psychiatry. 2006;163(2):294–296. doi: 10.1176/appi.ajp.163.2.294. [DOI] [PubMed] [Google Scholar]
- 20.Lerer E, et al. Association between the oxytocin receptor (OXTR) gene and autism: Relationship to Vineland Adaptive Behavior Scales and cognition. Mol Psychiatry. 2008;13(10):980–988. doi: 10.1038/sj.mp.4002087. [DOI] [PubMed] [Google Scholar]
- 21.Jacob S, et al. Association of the oxytocin receptor gene (OXTR) in Caucasian children and adolescents with autism. Neurosci Lett. 2007;417(1):6–9. doi: 10.1016/j.neulet.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu S, et al. Positive association of the oxytocin receptor gene (OXTR) with autism in the Chinese Han population. Biol Psychiatry. 2005;58(1):74–77. doi: 10.1016/j.biopsych.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 23.Yrigollen CM, et al. Genes controlling affiliative behavior as candidate genes for autism. Biol Psychiatry. 2008;63(10):911–916. doi: 10.1016/j.biopsych.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simmons CF, Jr, Clancy TE, Quan R, Knoll JH. The oxytocin receptor gene (OXTR) localizes to human chromosome 3p25 by fluorescence in situ hybridization and PCR analysis of somatic cell hybrids. Genomics. 1995;26(3):623–625. doi: 10.1016/0888-7543(95)80188-r. [DOI] [PubMed] [Google Scholar]
- 25.McCauley JL, et al. Genome-wide and Ordered-Subset linkage analyses provide support for autism loci on 17q and 19p with evidence of phenotypic and interlocus genetic correlates. BMC Med Genet. 2005;6:1. doi: 10.1186/1471-2350-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lauritsen MB, et al. A genome-wide search for alleles and haplotypes associated with autism and related pervasive developmental disorders on the Faroe Islands. Mol Psychiatry. 2006;11(1):37–46. doi: 10.1038/sj.mp.4001754. [DOI] [PubMed] [Google Scholar]
- 27.Shao Y, et al. Genomic screen and follow-up analysis for autistic disorder. Am J Med Genet. 2002;114(1):99–105. doi: 10.1002/ajmg.10153. [DOI] [PubMed] [Google Scholar]
- 28.Ylisaukko-oja T, et al. Search for autism loci by combined analysis of Autism Genetic Resource Exchange and Finnish families. Ann Neurol. 2006;59(1):145–155. doi: 10.1002/ana.20722. [DOI] [PubMed] [Google Scholar]
- 29.Thompson RJ, Parker KJ, Hallmayer JF, Waugh CE, Gotlib IH. Oxytocin receptor gene polymorphism (rs2254298) interacts with familial risk for psychopathology to predict symptoms of depression and anxiety in adolescent girls. Psychoneuroendocrinology. 2011;36(1):144–147. doi: 10.1016/j.psyneuen.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Costa B, et al. Oxytocin receptor polymorphisms and adult attachment style in patients with depression. Psychoneuroendocrinology. 2009;34(10):1506–1514. doi: 10.1016/j.psyneuen.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 31.Park J, et al. Evidence that genetic variation in the oxytocin receptor (OXTR) gene influences social cognition in ADHD. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(4):697–702. doi: 10.1016/j.pnpbp.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 32.Rodrigues SM, Saslow LR, Garcia N, John OP, Keltner D. Oxytocin receptor genetic variation relates to empathy and stress reactivity in humans. Proc Natl Acad Sci USA. 2009;106(50):21437–21441. doi: 10.1073/pnas.0909579106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: A revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- 34.Le Couteur A, et al. Autism diagnostic interview: A standardized investigatorbased instrument. J Autism Dev Disord. 1989;19(3):363–387. doi: 10.1007/BF02212936. [DOI] [PubMed] [Google Scholar]
- 35.Lord C, et al. The autism diagnostic observation schedule-generic: A standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30(3):205–223. [PubMed] [Google Scholar]
- 36.Lord C, Rutter M, DiLavore PC, Risi S. Autism Diagnostic Observation Schedule - WPS. Los Angeles: Western Psychological Services; 1999. [Google Scholar]
- 37.Pine E, Luby J, Abbacchi A, Constantino JN. Quantitative assessment of autistic symptomatology in preschoolers. Autism. 2006;10(4):344–352. doi: 10.1177/1362361306064434. [DOI] [PubMed] [Google Scholar]
- 38.Constantino JN, et al. Validation of a brief quantitative measure of autistic traits: Comparison of the social responsiveness scale with the autism diagnostic interview-revised. J Autism Dev Disord. 2003;33(4):427–433. doi: 10.1023/a:1025014929212. [DOI] [PubMed] [Google Scholar]
- 39.Korkman M, Kirk U, Kemp S. NEPSY-II Clinical and Interpretive Manual. San Antonio: Harcourt Assessment Inc.; 2007. [Google Scholar]
- 40.Sparrow S, Balla DA, Cichetti D. Vineland Adaptive Behavior Scales. 2nd Ed. Bloomington, IN: Pearson Education, Inc.; 2005. [Google Scholar]
- 41.Van Vleck LD. Selection Index and Introduction to Mixed Model Methods. Boca Raton, FL: CRC; 1993. [Google Scholar]
- 42.Macgregor S, Cornes BK, Martin NG, Visscher PM. Bias, precision and heritability of self-reported and clinically measured height in Australian twins. Hum Genet. 2006;120(4):571–580. doi: 10.1007/s00439-006-0240-z. [DOI] [PubMed] [Google Scholar]
- 43.Skuse DH, et al. Common polymorphism in the oxytocin receptor gene (OXTR) is associated with human social recognition skills. Proc Natl Acad Sci USA. 2014;111(5):1987–1992. doi: 10.1073/pnas.1302985111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamasue H. Function and structure in social brain regions can link oxytocin-receptor genes with autistic social behavior. Brain Dev. 2013;35(2):111–118. doi: 10.1016/j.braindev.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 45.Bakermans-Kranenburg MJ, van Ijzendoorn MH. A sociability gene? Meta-analysis of oxytocin receptor genotype effects in humans. Psychiatr Genet. 2014;24(2):45–51. doi: 10.1097/YPG.0b013e3283643684. [DOI] [PubMed] [Google Scholar]
- 46.Liu X, et al. Association of the oxytocin receptor (OXTR) gene polymorphisms with autism spectrum disorder (ASD) in the Japanese population. J Hum Genet. 2010;55(3):137–141. doi: 10.1038/jhg.2009.140. [DOI] [PubMed] [Google Scholar]
- 47.Kim HS, et al. Culture, distress, and oxytocin receptor polymorphism (OXTR) interact to influence emotional support seeking. Proc Natl Acad Sci USA. 2010;107(36):15717–15721. doi: 10.1073/pnas.1010830107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen FS, Barth ME, Johnson SL, Gotlib IH, Johnson SC. Oxytocin receptor (OXTR) polymorphisms and attachment in human infants. Front Psychol. 2011;2:200. doi: 10.3389/fpsyg.2011.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gordon I, et al. Oxytocin enhances brain function in children with autism. Proc Natl Acad Sci USA. 2013;110(52):20953–20958. doi: 10.1073/pnas.1312857110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oved K, et al. Genome-wide expression profiling of human lymphoblastoid cell lines implicates integrin beta-3 in the mode of action of antidepressants. Transl Psychiatr. 2013;3:e313. doi: 10.1038/tp.2013.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Roid GH. Stanford-Binet’s Intelligence Scales. 5th Ed. Itasca, IL: Riverside Publishing; 2003. [Google Scholar]
- 52.Grafen A, Hails R. Modern Statistics for the Life Sciences. Oxford: Oxford Univ Press; 2002. pp xv, 351. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.