It has taken three decades from the first report of microbial polychlorinated biphenyl (PCB) dechlorination to identify even one of the enzymes responsible. By combining conventional techniques with their own ingenuity, the latest technologies, and a bit of luck, Wang et al., in PNAS, have identified not one, but three distinct enzymes that can reductively dechlorinate PCBs (1). This finding is important because, despite being banned in the 1970s, PCBs still contaminate the sediments of rivers, lakes, and harbors worldwide. PCBs are notorious for their ability to bioaccumulate and biomagnify in the food chain, and for their multiple suspected health effects.
Commercial mixtures of PCBs, known in the United States as Aroclors, are complex mixtures of 60–90 types, congeners, of PCB molecule that differ in the number (1–10) and position of chlorines on the phenyl rings. PCBs were used for decades as dielectric fluids in capacitors and transformers, and as hydraulic fluids, heat transfer fluids, lubricants and cutting oils, and additives in a variety of products (2). For example, Aroclor 1260 is a mixture of PCBs with five to eight chlorines that was used as transformer dielectric fluid.
Thirty years ago it was discovered that PCBs in the anaerobic sediments of rivers were being dechlorinated by unknown agents, presumably anaerobic bacteria (3, 4). This discovery offered the best hope for an effective means of dealing with the notoriously persistent PCBs. PCB dechlorination helps to reduce the toxicity and bioaccumulation potential of PCBs and makes them more susceptible to oxidation and destruction by many organisms. However, despite years of research in multiple laboratories, the PCB-dechlorinating agents were not identified until 2007. In that year two different laboratories identified Dehalococcoides mccartyi as the bacterium responsible for dechlorinating Aroclor 1260 in aquatic sediments (5, 6).
The lifestyle of D. mccartyi explains why it was so hard to identify; it is a tiny, strictly anaerobic bacterium that must derive its energy for growth by removing chlorines from chlorinated organic molecules and using them as electron acceptors for respiration, a process known as organohalide respiration (7). These organisms have a tiny genome, yet each encodes a suite of 10–36 different reductive dehalogenase enzymes (RDases) to assist in its highly restricted way of life (7, 8). At this point, hundreds of different D. mccartyi RDases have been identified and sequenced. However, the difficulty of growing these organisms and low biomass yields have prevented researchers from identifying the substrates of all but a handful of these enzymes. Those that have been identified include several tetrachloroethene (PCE) dehalogenases, a trichloroethene (TCE) dehalogenase, two vinyl chloride dehalogenases, and a chlorinated benzene dehalogenase (8).
The isolation of D. mccartyi strains that can grow using highly chlorinated PCBs for respiration has been severely hampered by the inability to grow these organisms to high cell density because of the extreme insolubility of PCBs. Wang et al., in Jianzhong He’s laboratory, have overcome this problem by using a more soluble alternative substrate, tetrachloroethene (PCE), to grow, isolate, and characterize the genome of three new strains of D. mccartyi that can respire the highly chlorinated commercial PCB mixture Aroclor 1260 (1). It has long been known that many strains of this species can respire PCE, and the authors reasoned that PCE might offer a more rapid means of growing and further enriching PCB-respiring bacteria from cultures that had already been enriched with PCBs. Indeed, the authors showed that the three new strains grew to a 12.5- to 22-fold higher cell density in 30 days with PCE as the electron acceptor, versus 150 days with PCBs in the Aroclor mixture as the electron acceptors. To avoid loss of PCB RDases while growing with PCE, the authors first used shotgun metagenomics to identify and then monitor all RDase genes during the enrichment process.
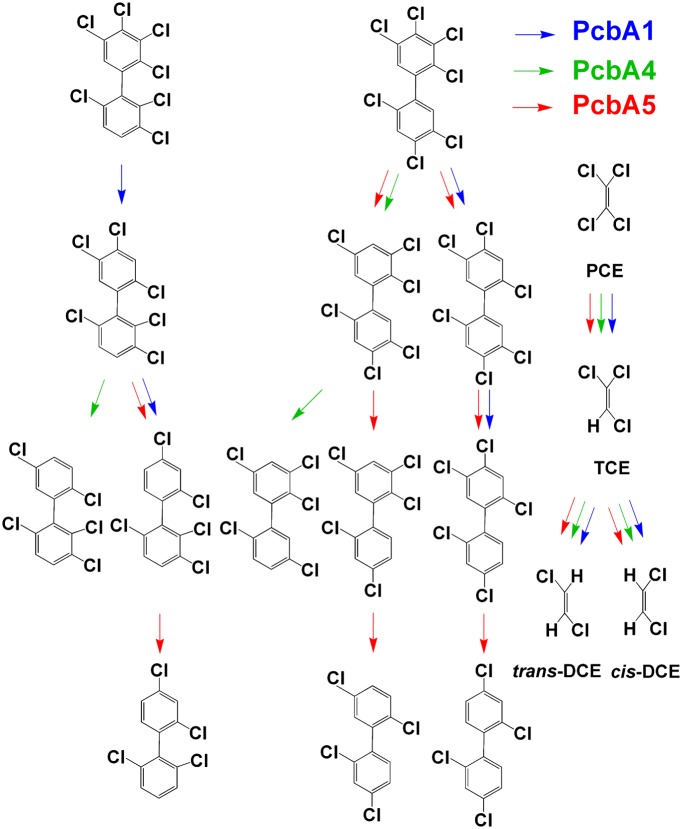
Wang et al., in He’s laboratory, identified and sequenced the genes for three PCB RDases, pcbA1, pcbA4, and pcbA5, one in each of the three new strains (1). Each of the corresponding enzymes attacks dozens of PCB substrates, but each exhibits distinct specificity, removing different chlorines and leading to different terminal products (Fig. 1). All three PCB RDases also dechlorinate PCE to trichloroethene (TCE) and to both cis- and trans-dichloroethene (DCE) (1). This finding was completely unexpected because: (i) several different PCE RDases have already been identified in D. mccartyi, and (ii) the PCE molecule looks nothing like a PCB (Fig. 1). However, the experimental evidence is undeniable. In each case the same RDase gene is the most highly transcribed, whether growing with PCE or PCBs, and each of the three gel-purified PCB RDases dechlorinates both PCE and PCBs (1).
Fig. 1.
Dechlorination of PCE and several PCBs by three different PCB dechlorinases. Note the different specificities for PCB dechlorination.
The discovery that PCE and PCB dechlorinase capabilities are linked on a single enzyme (1) has enormous implications for PCB remediation. The ability to grow these PCB dechlorinators with PCE as the electron acceptor suddenly makes the possibility of bioaugmentation for PCB remediation much more feasible. It should be possible to grow large amounts of PCB dechlorinators using PCE as the electron acceptor. The chlorinated ethene substrates and products can then be removed by purging before using the cells to treat a PCB-contaminated site. This process, called bioaugmentation, is already widely used with great success for remediation of chlorinated ethenes by D. mccartyi (9).
So what do these three PCB RDases tell us? First, that each enzyme can catalyze the dechlorination of dozens of PCB congeners as well as PCE (1). It was previously established that single pure strains of D. mccartyi could replicate complex patterns of Aroclor 1260 dechlorination that occur in the environment (10, 11). However, it was not known whether this dechlorination resulted from a single RDase enzyme or from multiple RDases. Wang et al. establish that the multiplex dechlorination of Aroclor 1260 is carried out by a single PCB RDase in each new PCB-dechlorinating strain. (1).
Second, the amino acid sequences of PcbA4 and PcbA5 are 97% identical, yet the 3% difference, 14 amino acids out of 482, is sufficient to completely change the dechlorination specificity (1). It turns out that three other D. mccartyi strains, 195 (12), GT (13), and JNA (11), each have an RDase that is also nearly identical to PcbA4 and PcbA5. Strains 195 and JNA also have the ability to dechlorinate Aroclor 1260 (11, 14). Are these as yet uncharacterized RDases in fact PCB RDases? If so, it would appear that there is a whole cluster of PCB RDases, each perhaps with different specificity.
Third, at least several different genetic lineages of PCB dechlorinases exist in D. mccartyi. PcbA1 is not closely related to PcbA4 or PcbA5; it shares only 38% amino acid sequence identity, yet it too can dechlorinate PCE as well as multiple highly chlorinated PCBs (1). So far only one other isolate, D. mccartyi GY50, which couples its growth to polybrominated diphenyl ether (or PBDE), shares this PCB RDase. And at least one more entirely distinct PCB RDase must also exist, because D. mccartyi CBDB1 can extensively dechlorinate Aroclor 1260 but it does not have any close relatives of PcbA1, PcbA4, or PcbA5 (10, 13).
Why do several PCB RDases also dechlorinate PCE? Perhaps the more appropriate question is, why does a PCE RDase dechlorinate PCBs? PCE and TCE are not strictly anthropogenic but can be produced by some bacteria (15), so perhaps these compounds have been substrates for D. mccartyi for millennia. This would explain why these organisms have several different enzymes that catalyze the same PCE dechlorination reaction. PCBs, on the other hand, have only been around since 1929. It may just be that the RDases in D. mccartyi have flexible regions that permit rapid adaptation when potential new substrates become available. Or it could be that the PCB RDases actually evolved to dehalogenate some of the thousands of naturally chlorinated aromatic compounds that exist (16) and just happen to work on PCBs.
That 14 amino acid differences of 482 can make such a difference in the specificity
Wang et al., in PNAS, have identified not one, but three distinct enzymes that can reductively dechlorinate PCBs.
of PcbA4 and PcbA5 (1) is intriguing, and is reminiscent of the situation with the large subunits of biphenyl dioxygenase in PCB-degrading strains LB400 and KF707. These enzymes differ by only 21 amino acids of 459 but have completely different PCB specificity (17). A change of four amino acids of the LB400 sequence to conform to those of the KF707 sequence yielded a new enzyme with the combined substrate specificity of both strains (17). These biphenyl dioxygenases also attacked dozens of PCB substrates—albeit they were less chlorinated than the substrates of the PCB dechlorinases—and they were transformed with molecular oxygen, not hydrogen. This finding suggests that it might be possible to genetically modify the PCB RDases to increase the range of congeners that they can dechlorinate and which chlorines they can remove. The implications of such designer PCB RDases could be enormous, and the timing is right. RDases are complicated molecules that require the insertion of two iron sulfur clusters and a cobamide coenzyme—a biochemically active form of vitamin B12—as cofactors, followed by proper folding to be catalytically active. These events occur within the cell and likely require the assistance of special molecules, known as chaperones. Because of these difficulties, the first successful cloning and expression of an active RDase was achieved only very recently (18), but this success and the discovery of three PCB RDases with broad substrate specificity herald a new era, where it will be possible to grow and study these enzymes in more depth.
Supplementary Material
Footnotes
The author declares no conflict of interest.
See companion article on page 12103.
References
- 1.Wang S, et al. Genomic characterization of three unique Dehalococcoides that respire on persistent polychlorinated biphenyls. Proc Natl Acad Sci USA. 2014;111:12103–12108. doi: 10.1073/pnas.1404845111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erickson MD. Analytical Chemistry of PCBs. 2nd Ed. New York: Lewis Publishers; 1997. [Google Scholar]
- 3.Brown JF, Jr, et al. PCB transformations in upper Hudson River, USA sediments. Northeast Environ Sci. 1984;3:167–179. [Google Scholar]
- 4.Brown JF, Jr, et al. Polychlorinated biphenyl dechlorination in aquatic sediments. Science. 1987;236(4802):709–712. doi: 10.1126/science.236.4802.709. [DOI] [PubMed] [Google Scholar]
- 5.Bedard DL, Ritalahti KM, Löffler FE. The Dehalococcoides population in sediment-free mixed cultures metabolically dechlorinates the commercial polychlorinated biphenyl mixture Aroclor 1260. Appl Environ Microbiol. 2007;73(8):2513–2521. doi: 10.1128/AEM.02909-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fagervold SK, May HD, Sowers KR. Microbial reductive dechlorination of Aroclor 1260 in Baltimore Harbor sediment microcosms is catalyzed by three phylotypes within the phylum Chloroflexi. Appl Environ Microbiol. 2007;73(9):3009–3018. doi: 10.1128/AEM.02958-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Löffler FE, et al. Dehalococcoides mccartyi gen. nov., sp. nov., obligately organohalide-respiring anaerobic bacteria relevant to halogen cycling and bioremediation, belong to a novel bacterial class, Dehalococcoidia classis nov., order Dehalococcoidales ord. nov. and family Dehalococcoidaceae fam. nov., within the phylum Chloroflexi. Int J Syst Evol Microbiol. 2013;63(Pt 2):625–635. doi: 10.1099/ijs.0.034926-0. [DOI] [PubMed] [Google Scholar]
- 8.Hug LA, et al. Overview of organohalide-respiring bacteria and a proposal for a classification system for reductive dehalogenases. Philos Trans R Soc Lond B Biol Sci. 2013;368(1616):20120322. doi: 10.1098/rstb.2012.0322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Löffler FE, Ritalahti KM, Zinder SH. Dehalococcoides and reductive dechlorination. In: Stroo HF, Leeson A, Ward CH, editors. Bioaugmentation for Groundwater Remediation. Vol 5. New York: Springer; 2012. [Google Scholar]
- 10.Adrian L, Dudková V, Demnerová K, Bedard DL. “Dehalococcoides” sp. strain CBDB1 extensively dechlorinates the commercial polychlorinated biphenyl mixture Aroclor 1260. Appl Environ Microbiol. 2009;75(13):4516–4524. doi: 10.1128/AEM.00102-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LaRoe SL, Fricker AD, Bedard DL. Dehalococcoides mccartyi strain JNA in pure culture extensively dechlorinates Aroclor 1260 according to polychlorinated biphenyl (PCB) Dechlorination Process N. Environ Sci Technol. 2014 doi: 10.1021/es500872t. [DOI] [PubMed] [Google Scholar]
- 12.Seshadri R, et al. Genome sequence of the PCE-dechlorinating bacterium Dehalococcoides ethenogenes. Science. 2005;307(5706):105–108. doi: 10.1126/science.1102226. [DOI] [PubMed] [Google Scholar]
- 13.Kube M, et al. Genome sequence of the chlorinated compound-respiring bacterium Dehalococcoides species strain CBDB1. Nat Biotechnol. 2005;23(10):1269–1273. doi: 10.1038/nbt1131. [DOI] [PubMed] [Google Scholar]
- 14.Zhen H, Du S, Rodenburg LA, Mainelis G, Fennell DE. Reductive dechlorination of 1,2,3,7,8-pentachlorodibenzo-p-dioxin and Aroclor 1260, 1254 and 1242 by a mixed culture containing Dehalococcoides mccartyi strain 195. Water Res. 2014;52:51–62. doi: 10.1016/j.watres.2013.12.038. [DOI] [PubMed] [Google Scholar]
- 15.Weissflog L, et al. Sediments of salt lakes as a new source of volatile highly chlorinated C1/C2 hydrocarbons. Geophys Res Lett. 2005;32:L01401. [Google Scholar]
- 16.Gribble GW. 2010. Naturally Occurring Organohalogen Compounds—A Comprehensive Update. Progress in the Chemistry of Organic Natural Products (Springer, Wein), Vol 91.
- 17.Erickson BD, Mondello FJ. Enhanced biodegradation of polychlorinated biphenyls after site-directed mutagenesis of a biphenyl dioxygenase gene. Appl Environ Microbiol. 1993;59(11):3858–3862. doi: 10.1128/aem.59.11.3858-3862.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mac Nelly A, Kai M, Svatoš A, Diekert G, Schubert T. Functional heterologous production of reductive dehalogenases from Desulfitobacterium hafniense strains. Appl Environ Microbiol. 2014;80(14):4313–4322. doi: 10.1128/AEM.00881-14. [DOI] [PMC free article] [PubMed] [Google Scholar]