Abstract
Mathematical models of cervical cancer have been widely used to evaluate the comparative effectiveness and cost-effectiveness of preventive strategies. Major advances in the understanding of cervical carcinogenesis motivate the creation of a new disease paradigm in such models. To keep pace with the most recent evidence, we updated a previously developed microsimulation model of human papillomavirus (HPV) infection and cervical cancer to reflect 1) a shift towards health states based on HPV rather than poorly reproducible histological diagnoses and 2) HPV clearance and progression to precancer as a function of infection duration and genotype, as derived from the control arm of the Costa Rica Vaccine Trial (2004–2010). The model was calibrated leveraging empirical data from the New Mexico Surveillance, Epidemiology, and End Results Registry (1980–1999) and a state-of-the-art cervical cancer screening registry in New Mexico (2007–2009). The calibrated model had good correspondence with data on genotype- and age-specific HPV prevalence, genotype frequency in precancer and cancer, and age-specific cancer incidence. We present this model in response to a call for new natural history models of cervical cancer intended for decision analysis and economic evaluation at a time when global cervical cancer prevention policy continues to evolve and evidence of the long-term health effects of cervical interventions remains critical.
Keywords: decision analysis, human papillomavirus, mathematical models, uterine cervical neoplasms
Major advances in the biological and clinical understanding of cervical carcinogenesis motivate a new approach to disease simulation models used to inform policy decisions (1). Persistent cervical infection by oncogenic types of human papillomavirus (HPV) is a necessary condition for the development of cervical cancer (2). Although most HPV infections clear rapidly, the probability of persistence increases the longer an infection is observed (3). Persistent infection with one of approximately 15 oncogenic HPV genotypes strongly increases the risk of high-grade precancerous lesions, which, if untreated, may invade surrounding tissues (4).
The discovery of persistent HPV infection as a causal agent for cervical cancer has been accompanied by the development of technologies that aim to improve both the effectiveness and the efficiency of cervical cancer prevention. Reliable assays for detection of oncogenic HPV types and the licensure of 2 prophylactic vaccines that are highly efficacious against HPV types 16 and 18—which together are responsible for up to 70% of invasive cervical cancers (5)—have shifted the landscape for clinical decision-making and health policy, generating critical questions about how to optimize primary and secondary prevention as emerging technologies for vaccination (e.g., nonavalent prophylactic and therapeutic vaccines) and screening (e.g., HPV genotype and related biomarker testing) become available.
While no single empirical study can evaluate all possible prevention strategies, computer-based mathematical models are increasingly being used to assist decision-making. These models are able to integrate the best biological, epidemiologic, and economic data, simulate the burden of disease in populations, and make projections on the long-term harms and benefits of different strategies (6). Because the underlying course of disease is often unobserved, data for model inputs are not fully available, requiring some form of model-fitting, or calibration, to epidemiologic data (7, 8).
Historically, simulation models of cervical carcinogenesis have assumed a sequence of steps through histopathological classifications (e.g., cervical intraepithelial neoplasia (CIN), grades 1–3) (9–13) which are poorly reproducible (14) and reflect flawed diagnostic standards, rather than the actual course of underlying disease as reported in recent longitudinal studies (1, 15). To keep pace with the current understanding of the disease process, health states in simulation models should reflect HPV infection status rather than histopathological or cytological diagnosis (1). In particular, models should incorporate increasingly available data on risk stratification by HPV genotype (16, 17) and the natural course of persistent versus nonpersistent infections. Our objective was to update an existing disease simulation model of cervical cancer to improve model fidelity to the latest data on HPV natural history and to enhance model functionality for evaluations of forthcoming preventive strategies.
METHODS
Model description
We modified a first-order (individual-based) Monte Carlo simulation model of cervical carcinogenesis, in which individual women representative of a single birth cohort enter the model at an early age (e.g., age 9 years) and are followed over their lifetimes (13, 18–21). While the model has been used to evaluate the comparative effectiveness and cost-effectiveness of screening and HPV vaccination strategies (20, 21), here we focus on modifications to the underlying natural history parameters in the absence of preventive interventions. The model tracks disease progression as each woman undergoes monthly transitions between health states that describe underlying true health, including HPV infection status, precancer histological grade (i.e., CIN2 or CIN3), and stage of invasive cancer (i.e., local, regional, or distant) (Figure 1). States are further stratified into 3 categories: oncogenic HPV types (HPV types 16, 18, 31, 33, 45, 52, and 58), each considered separately; other oncogenic types (including HPV types 35, 39, 51, 56, and 59), considered together; and nononcogenic types. Transition probabilities can vary by age, HPV type, duration of infection or lesion status, and a woman's history of prior HPV infection. Cancer detection can occur through symptoms. Each month, death can occur from non-cervical-cancer causes or from cervical cancer after its onset.
Figure 1.
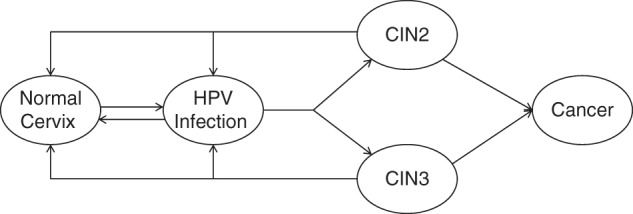
Model schematic for the progression of human papillomavirus (HPV) infection to cervical cancer. HPV infections and precancer (cervical intraepithelial neoplasia (CIN), grade 2 (CIN2) or grade 3 (CIN3)) were stratified by genotype (HPV types 16, 18, 31, 33, 45, 52, and 58; other oncogenic types; and nononcogenic types). Oncogenic HPV clearance and progression rates were based on primary data from the Costa Rica Vaccine Trial (22). Precancerous health states (CIN2 and CIN3) were considered as heterogeneous entities with differential probabilities of regression and progression to cancer. Progression to cancer required infection with an oncogenic type. Cancer could be symptom-detected at either the local stage, the regional stage, or the distant stage.
We made the following model assumptions based on the best available evidence: 1) the natural course of cervical HPV infection is similar regardless of geographical setting, permitting use of high-quality epidemiologic data from diverse settings to derive initial model inputs; 2) type-specific HPV infections are independent, and women infected with multiple types of HPV face independent risks of clearance and disease progression; 3) natural immunity is modeled as a reduction in future type-specific infection after initial acquisition and clearance; 4) in contrast to the historical model structure comprising mandatory progression through sequential precancer states (e.g., from CIN1 to CIN2 to CIN3), CIN2 and CIN3 are modeled as nonsequential precancerous health states with distinct probabilities of progression to cancer, whereas CIN1 is interpreted as a microscopic manifestation of acute HPV infection and is therefore incorporated into the HPV-infected state; and 5) invasive cancer cannot occur in the absence of infection with an oncogenic HPV type.
Overview: model parameterization and calibration
Derivation of natural history model parameter values required an iterative process involving comprehensive literature reviews, data synthesis and analysis (Table 1), consultations with epidemiologists and clinical experts, and explorations of the influence of uncertain parameters and assumptions in the model. We initially estimated baseline model values using data that could be used directly as inputs (e.g., HPV incidence and clearance) from large cohort studies of HPV natural history and the control arm of an HPV vaccine trial (22, 23) to represent the natural history of HPV infection in the absence of vaccination and screening. For parameters with high uncertainty and variability, we used a likelihood-based calibration approach to maximize model fit to corresponding empirical data (e.g., HPV prevalence).
Table 1.
Data Sources Used for Variables and Calibration Targets in a Model of the Natural History of Human Papillomavirus Infection and Cervical Cancer
| First Author, Year (Reference No.) | Data Source | Variable | Location | Sample Size, no. | Population |
|---|---|---|---|---|---|
| Natural History Parameters | |||||
| Muñoz, 2004 (23) | Prospective cohort study | HPV incidence | Bogotá, Colombia | 1,610 | Sexually active women aged 15–85 years |
| Herrero, 2008 (22) | Primary data from the Costa Rica Vaccine Trial | HPV clearance and progression | Guanacaste, Costa Rica | 3,736 | Women aged 18–25 years |
| Keefe, 2001 (27) | Randomized controlled trial | CIN regression | California, United States | 103 | Women with CIN2/3 aged ≥18 years |
| McCredie, 2008 (28) | Retrospective cohort study | CIN progression to invasive cervical cancer | Auckland, New Zealand | 1,063 | Women with CIN3, from 1955–1976 |
| Myers, 2000 (31); Kim, 2002 (47) | Decision analysis modeling studies | Cancer progression; Cancer symptom detection | United States | NA | NA |
| SEER Program, 2011 (33) | Population-based registry data | Cancer survival by stage | United States | By cancer stage: | SEER 18 registries + Hurricane Katrina-impacted Louisiana cases, 2000–2009 |
| Local: 10,240 | |||||
| Regional: 9,119 | |||||
| Distant: 2,122 | |||||
| Calibration Targets | |||||
| New Mexico HPV Pap Registry (34) | Population-based cross-sectional data | HPV prevalence | New Mexico, United States | 47,617 | Women with Pap test specimens, 2007–2009 |
| New Mexico SEER Registry (17) | Cross-sectional data | HPV genotype frequency in CIN | New Mexico, United States | 1,213 | Cases of in situ cancer, New Mexico, 1985–1999 |
| New Mexico SEER Registry (17) | Cross-sectional data | HPV genotype frequency in cervical cancer | New Mexico, United States | 808 | Cases of cervical cancer, New Mexico, 1980–1999 |
Abbreviations: CIN, cervical intraepithelial neoplasia; HPV, human papillomavirus; NA, not applicable; Pap, Papanicolaou; SEER, Surveillance, Epidemiology, and End Results.
Initial model parameterization
HPV incidence
HPV incidence rates, as a function of genotype and age, were derived from published data on a cohort of 1,610 sexually active women aged 15–85 years in Bogotá, Colombia, who were followed every 6 months for 4.1 years, on average (23). Cumulative incidence rates for each genotype and 5-year age group were converted to monthly probabilities for use in the model.
Oncogenic HPV clearance and progression
Probabilities of HPV clearance and progression were derived as a function of genotype and time since infection (see Web Appendix and Web Figures 1 and 2, available at http://aje.oxfordjournals.org/) from analysis of primary data from the control arm of the Costa Rica Vaccine Trial (2004–2010), including 3,736 women aged 18–25 years at enrollment (22). The duration of study follow-up was approximately 4 years, with annual visits, except for women with Papanicolaou (Pap) test results of low-grade squamous intraepithelial lesions (LSIL) or atypical squamous cells of undetermined significance (ASC-US), who were followed every 6 months. For estimations of type-specific HPV clearance and progression to CIN2+, we performed discrete-time survival analysis by oncogenic genotype strata. We considered each HPV infection separately, and thus referred to an index infection (rather than a woman) as the unit of analysis. Both incident and prevalent infections were included to obtain more stable estimates, since previous studies suggested similar persistence in young women (24). Using a competing-risks framework with censoring due to loss to follow-up (25, 26), we calculated cumulative incidence estimates by HPV type for 3 mutually exclusive probabilities: 1) clearance; 2) persistence without progression to CIN2+; and 3) progression to CIN2+. Cumulative incidence was then transformed into monthly hazard rates of clearance and progression to CIN2+, which were converted to monthly probabilities and used as model inputs.
To account for delayed visits and to minimize differences in persistence that could arise from differing follow-up schedules, we collapsed the duration of each infection into approximate yearly intervals that coincided with the timing of study visits. Further details on interval assumptions and data imputations for missed visits are provided in the Web Appendix. Infections were censored following detection of CIN2+ (n = 149).
The relevance of HPV infections for CIN2+ attribution was determined statistically (rather than molecularly), with the key visit being defined as the same visit as the CIN2+ procedure, or the last visit with polymerase chain reaction results before the CIN2+ procedure. Our algorithm for determining relevance prioritized persistent oncogenic infections at and prior to the key visit. Based on determination of multiple relevant HPV types, CIN2+ cases were attributed hierarchically such that if HPV16 was relevant, CIN2+ was attributed fully to HPV16. If multiple oncogenic non-HPV16 types were relevant, CIN2+ was attributed to all relevant types, with the percent attribution set at the proportion of CIN2+ infected with each respective single relevant type divided by the sum of the percentage of CIN2+ attributed to single infections with any relevant types present. Following this type attribution hierarchy, progression rates were calculated based on the cumulative incidence of CIN2+ associated with each type. Further details, as well as methods for deriving nononcogenic HPV clearance and progression rates, are provided in the Web Appendix.
Precancer regression
Precancer regression probabilities were estimated from the placebo arm of a randomized controlled trial of oral β-carotene supplementation for women with CIN2 or CIN3 and were assumed to be constant by HPV type (Web Table 1) (27).
Precancer progression
Due to ethical obligations to provide treatment for women diagnosed with CIN3, the probability of invasion is generally unobserved. However, data from a natural history study of women with carcinoma in situ from whom treatment was withheld in New Zealand from 1965 to 1974 have been retrospectively analyzed to obtain estimates of progression rates from CIN3 to cancer (28). We assumed that the study's 5-year probability of progression to CIN3 was representative of advanced, prevalent lesions, because of limited screening and the prevalence of large lesions at study entry. We adjusted progression rates by allowing progression risk to increase over time since lesion onset to ensure a reasonable visual fit to historical (i.e., prescreening) US cancer incidence rates (Web Table 1) (29).
Cancer progression and mortality
Upon development of invasive cancer, women in the model progress through worsening stages (i.e., local to regional to distant) if their cancer is left undetected (30–32). With each progressive stage, women face an increasing probability of symptom detection. In addition to background mortality, women with cervical cancer face excess mortality, depending on cancer stage, age, and time since diagnosis according to the Surveillance, Epidemiology, and End Results (SEER) Program (Web Table 1) (33).
Model calibration
The purpose of model calibration is to search the bounds of uncertainty of input parameters and identify values that, when used together in the model, achieve a good fit to real-world data (i.e., calibration targets). Following the establishment of baseline input values, as described above, we employed a likelihood-based approach (13, 21) to infer values for key uncertain parameters: 1) age- and type-specific incidence of HPV infection, which varies by setting, and 2) naturally acquired immunity following type-specific HPV infection.
For these calibration parameters, we set plausible search ranges around baseline input values and performed repeated model simulations in the absence of any preventive intervention. For each simulation, we randomly selected a single value for each of the uncertain parameters from the identified plausible range (Table 2), creating a unique natural history parameter set. To ascertain model goodness of fit, we calculated the likelihood of model-projected outcomes from each parameter set against corresponding calibration targets. In order to capture uncertainty in the model parameters, we selected a sample of sets that produced a good fit to the empirical data to use in analyses as a form of probabilistic sensitivity analysis (details on the likelihood-based scoring are provided in the Web Appendix).
Table 2.
Selected Baseline Values, Calibration Ranges, and Best-Fitting Calibrated Values in a Model of the Natural History of Human Papillomavirus Infection and Cervical Cancer
| HPV Type and Age Group, years | Baseline Valuea | Multiplier Search Rangeb | Range of Multiplier Values Among Top 50 Parameter Sets |
|---|---|---|---|
| HPV16 | |||
| <21 | 0.00001–0.00186 | 1–4 | 2.5–4.0 |
| 21–24 | 0.00090–0.00125 | 1–4 | 2.1–3.9 |
| 25–29 | 0.00078–0.00087 | 1–4 | 2.1–3.9 |
| 30–49 | 0.00060–0.00078 | 1–4 | 2.1–3.9 |
| ≥50 | 0.00022–0.00059 | 1–4 | 2.1–3.9 |
| HPV18 | |||
| <21 | 0.000005–0.00082 | 1–4 | 2.5–4.0 |
| 21–24 | 0.00110–0.00117 | 1–4 | 1.0–3.3 |
| 25–29 | 0.00060–0.00100 | 1–4 | 1.0–3.3 |
| 30–49 | 0.00030–0.00058 | 1–4 | 1.0–3.3 |
| ≥50 | 0.00011–0.00030 | 1–4 | 1.0–3.3 |
| HPV31 | |||
| <21 | 0.000005–0.001428 | 1–4 | 1.7–3.8 |
| 21–24 | 0.00090–0.00140 | 1–4 | 1.7–3.8 |
| 25–29 | 0.00060–0.00080 | 1–4 | 1.7–3.8 |
| 30–49 | 0.00030–0.00060 | 1–4 | 1.7–3.8 |
| ≥50 | 0.00010–0.00030 | 1–4 | 1.7–3.8 |
| HPV33 | |||
| <21 | 0.000005–0.000786 | 1–4 | 1.2–3.2 |
| 21–24 | 0.00041–0.00069 | 1–4 | 1.2–3.2 |
| 25–29 | 0.00025–0.00036 | 1–4 | 1.2–3.2 |
| 30–49 | 0.00013–0.00022 | 1–4 | 1.2–3.2 |
| ≥50 | 0.00005–0.00012 | 1–4 | 1.2–3.2 |
| HPV45 | |||
| <21 | 0.000005–0.001000 | 1–4 | 1.1–3.1 |
| 21–24 | 0.00040–0.00080 | 1–4 | 1.1–3.1 |
| 25–29 | 0.00032–0.00037 | 0.5–2 | 0.5–1.6 |
| 30–49 | 0.00016–0.00031 | 0.5–2 | 0.5–1.6 |
| ≥50 | 0.00005–0.00015 | 0.5–2 | 0.5–1.6 |
| HPV52 | |||
| <21 | 0.000005–0.001186 | 1–4 | 3.1–4.0 |
| 21–24 | 0.00030–0.00080 | 1–4 | 3.1–4.0 |
| 25–29 | 0.00018–0.00025 | 1–4 | 3.1–4.0 |
| 30–49 | 0.00015–0.00017 | 1–4 | 2.7–4.0 |
| ≥50 | 0.00005–0.00015 | 1–4 | 2.7–4.0 |
| HPV58 | |||
| <21 | 0.000005–0.001189 | 1–4 | 1.2–3.2 |
| 21–24 | 0.00060–0.00110 | 1–4 | 1.2–3.2 |
| 25–29 | 0.00047–0.00055 | 0.5–2 | 0.5–1.9 |
| 30–49 | 0.00024–0.00045 | 0.5–2 | 0.5–1.9 |
| ≥50 | 0.00005–0.00024 | 0.5–2 | 0.5–1.9 |
| Other oncogenic types | |||
| <21 | 0.000005–0.00247 | 1–4 | 1.0–3.9 |
| 21–24 | 0.00180–0.00230 | 1–4 | 1.0–3.9 |
| 25–29 | 0.00120–0.00170 | 1–4 | 1.0–3.9 |
| 30–49 | 0.00060–0.00110 | 1–4 | 1.0–3.9 |
| ≥50 | 0.00020–0.00060 | 1–4 | 1.0–3.9 |
| Nononcogenic types | |||
| <21 | 0.000005–0.00258 | 1–15 | 1.0–14.8 |
| 21–24 | 0.00255–0.00262 | 1–8 | 1.0–7.8 |
| 25–29 | 0.00200–0.00250 | 1–8 | 1.0–7.8 |
| 30–49 | 0.00085–0.00186 | 1–8 | 1.1–8.0 |
| ≥50 | 0.00022–0.00080 | 1–8 | 1.1–8.0 |
| Natural immunityc | 0.5–1 | 0.5–0.9 |
Abbreviation: HPV, human papillomavirus.
a Monthly prior probabilities of type-specific HPV infection; girls in the model are at risk for HPV infection beginning at age 9 years. Values displayed represent the range of incidence probabilities within each age group.
b Age-specific multipliers were necessary to fit HPV prevalence targets. Multipliers were not unique for each age group within a type but were generally the same among younger women (ages <21 years and 21–24 years) and older women (age ≥25 years).
c Natural immunity represents the reduction in risk of subsequent, type-specific infection after a woman has cleared an oncogenic infection with the same type. Risk reduction is assumed to be constant across age, time, and genotype.
Calibration target data are presented in Web Table 2. We calibrated the model to data targets from the New Mexico SEER Registry and the New Mexico HPV Pap Registry, the only existing statewide registry of cervical cancer screening practice in the United States. The New Mexico HPV Pap Registry was established in 2006 to monitor the impact of HPV vaccine introduction and changes in cervical cancer screening behaviors over time (34). The registry, due to the large volume of linkages between cervical screening, diagnosis, and treatment records across all of New Mexico, serves as one of the largest US population-based resources for information on HPV and cervical cancer-related events. Specifically, the calibration targets from the New Mexico HPV Pap Registry (2007–2009) included type-specific prevalence of HPV infections by age (34). Calibration targets from the New Mexico SEER Registry (1980–1999) included the frequency of oncogenic HPV genotypes in CIN2, CIN3, and cancer (17). Following formal calibration, we used a sample of good-fitting sets to assess the projective validity of the model by visually comparing model-projected cervical cancer incidence rates by age in the absence of any intervention with those reported historically in US SEER cancer registries prior to widespread Pap smear screening (29).
RESULTS
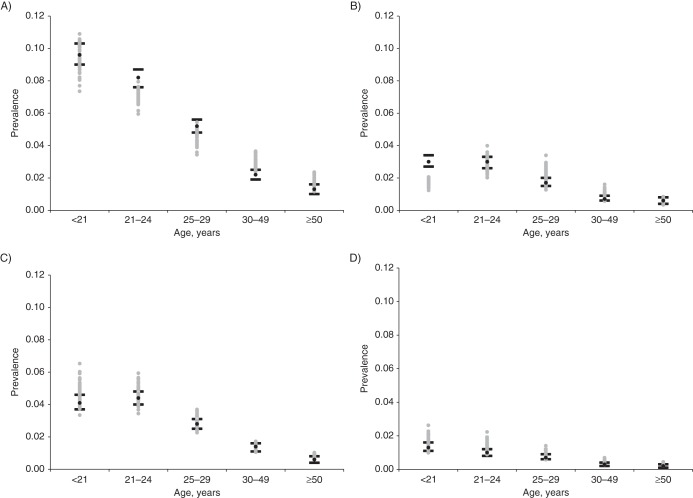
In the calibration process, over 730,000 unique input parameter sets were randomly sampled and used in the simulation model. We selected 50 sets with the highest aggregate likelihood score (i.e., top-fitting sets) to showcase calibration results. Prior to calibration, using the baseline incidence inputs directly from the HPV natural history study in Colombia, we found that the model produced outputs consistent with age-specific HPV prevalence reported in the Colombian cohort, as expected (35); however, when we compared them with the HPV prevalence target data from New Mexico, the model outputs were relatively low. Following the likelihood-based calibration process, we identified parameter values that dramatically improved model fit to HPV prevalence data from the New Mexico HPV Pap Registry (Figure 2) (34). Parameter values for natural immunity following type-specific infection yielded small variation in prevalence over the search range; this impact was more apparent at older ages, when women were more likely to be exposed to subsequent infections with more prevalent types like HPV16.
Figure 2.
Model output relative to calibration targets for age- and type-specific prevalence of human papillomavirus (HPV). Each panel represents a different type of HPV: A) HPV16; B) HPV18; C) HPV31; D) HPV33; E) HPV45; F) HPV52; and G) HPV58. The black circles represent the point estimate from the New Mexico HPV Pap Registry for each age group (34); the black lines represent the 95% confidence intervals. Model output from the top 50 parameter sets following likelihood-based scoring is displayed by gray circles.
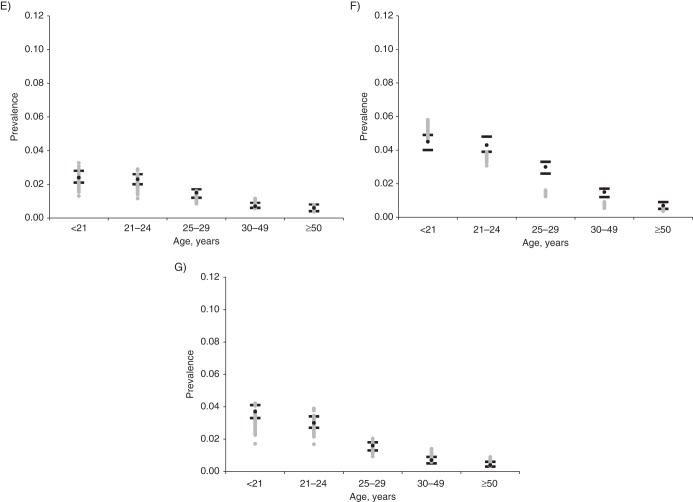
Overall, the calibrated sets produced reasonable fit to the empirical data on genotype frequencies in CIN2, CIN3, and cancer in the New Mexico SEER Registry (Figure 3). As suggested by the data, the relative significance of HPV16 increased with lesion severity.
Figure 3.
Model output relative to calibration targets for human papillomavirus (HPV) type-specific frequency in cervical intraepithelial neoplasia (CIN) grade 2 (CIN2), CIN grade 3 (CIN3), and cancer. Each panel represents a different health state: A) CIN2; B) CIN3; and C) cervical cancer. The black circles represent the point estimate from the New Mexico Surveillance, Epidemiology, and End Results Registry for each HPV type (17); the black lines represent the 95% confidence intervals. Model output from the top 50 parameter sets following likelihood-based scoring is displayed by gray circles.
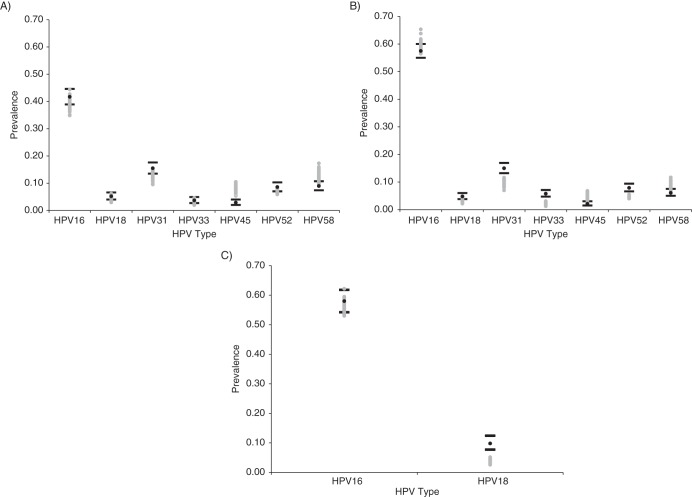
Since cervical cancer incidence data in the prescreening era were from a limited number of cancer registries in the 1950s and 1960s, we elected, a priori, to exclude cervical cancer incidence as a calibration target and rather assess the predictive validity of the calibrated natural history model (i.e., without screening or vaccination). We found that model output was generally consistent with the historic SEER registry data across ages and that it suggested a peak or plateau in cervical cancer incidence from ages 35 to 55 years (Figure 4) (4, 29). In addition, we found that the 50 top-fitting sets were nearly identical regardless of whether or not cancer incidence was included as a calibration target, suggesting the robustness of our good-fitting parameter sets.
Figure 4.
Model output relative to historical cervical cancer incidence data from the Surveillance, Epidemiology, and End Results Program, prior to widespread screening (29). The black lines represent the bounds of the cancer registry data for each age group, and the black circles represent the point estimates. Model output from the top 50 parameter sets following likelihood-based scoring is displayed by gray circles. Cancer incidence was not included in the likelihood-based scoring algorithm, but the comparison was used to assess the predictive validity of the calibrated model.
DISCUSSION
Motivated to address policy questions that require consideration of new screening technologies, the availability of type-specific vaccination, and the potential for individual risk-based management (36), we updated a disease simulation model to more accurately reflect health states defined by underlying HPV infection status and transition probabilities governed by duration of infection. To achieve this goal, we capitalized on recent primary and published longitudinal data to derive baseline model inputs; however, additional adjustments were made through an extensive calibration procedure to adapt the model to the US setting. With access to data from the New Mexico SEER Registry and the New Mexico HPV Pap Registry, the sole population-based registry of cervical cancer screening in the United States, we were able to undertake a rigorous likelihood-based calibration approach, achieving close model correspondence to empirical target data, including age- and genotype-specific HPV prevalence, and genotype frequency in CIN2, CIN3, and invasive cancer in US women. Model projections were also consistent with cancer incidence rates from historic US cancer registries that were not formally used to parameterize or calibrate the model.
A range of modeling techniques have been used to evaluate cervical cancer prevention strategies. Dynamic transmission models have the ability to incorporate the transmission of HPV infections between sexual partners over time; these models can thus directly capture herd immunity benefits associated with HPV vaccination (37–42). One recent dynamic transmission model utilized data from the control arm of a vaccine trial to derive HPV clearance rates dependent on time since infection, but the absence of precancer and cancer health states restricts the model's ability to project the impact of HPV vaccination and/or screening on cancer outcomes (43). Numerous Markov cohort and microsimulation models have been developed and adapted to many settings to evaluate both screening and vaccination strategies (9, 11–13, 18–21, 31). To our knowledge, the model we describe here is the first with capabilities to evaluate complex screening and vaccination strategies without relying on HPV infection and CIN1 as separate health states or requiring sequential progression through CIN1, CIN2, and CIN3. To date, only one other analysis has derived HPV type-specific and time-dependent health state transition probabilities based on large longitudinal studies for the purpose of modeling cervical carcinogenesis (10). The expanded number of individual genotypes represented in the current model will strengthen evaluations of emerging technologies.
There are limitations to our approach relating to the model structure, data inputs, and calibration process. First, although CIN2 is heterogeneous and may be caused by infections destined to regress or progress to CIN3, the stringent definition of precancer (4), we have included it as a distinct health state. Although we derived inputs from adjudicated diagnoses subject to stringent quality control whenever possible, classification of CIN2 versus CIN3 is still subject to error and variation. Because final histological classifications for CIN2 versus CIN3 from the Costa Rica Vaccine Trial were not available at the time of model development, and because of small numbers of CIN3+ lesions in the study population, we were not able to discern differential progression rates for CIN2 versus CIN3 at the time of this writing. Still, model inputs reflect the increased likelihood of regression and the decreased likelihood of progression for CIN2 (relative to CIN3).
Second, although HPV is a sexually transmitted infection, in this particular model we do not explicitly reflect transmission of HPV in the population based on sexual behavior, but rather rely on age-related HPV incidence as a proxy. Our model has the capability to be linked to an accompanying transmission model in which the probability of an individual's acquiring an infection is dependent on sexual contact patterns and the distribution of infection in the population at a given time (18, 19). Although HPV incidence baseline inputs were derived from a Colombian cohort, our calibration process allowed us flexibility in adjusting these estimates to fit HPV prevalence data from a population-based US screening registry. While these data were from New Mexico, comparisons with national surveys (e.g., the National Health and Nutrition Examination Survey) on age-specific HPV16/18 prevalence (44, 45) and type distribution in cancer (5) suggest that the population simulated in the calibrated model may reasonably represent the US population.
The use of longitudinal data from the Costa Rica Vaccine Trial to derive type-specific HPV clearance and progression rates by time since infection is a key strength of the model, but there remain limitations to our analytic approach. Data were based on visits taking place approximately biannually or annually, and because of differences in follow-up schedules according to risk profiles, we collapsed data into approximate yearly intervals and assumed constant hazards within each interval. Thus, our model inputs reflect the average rate over an interval and do not capture high initial clearance rates for very transient infections. This limitation, however, afflicts even longitudinal studies with more frequent follow-up, and all natural history studies are limited by current HPV and CIN2+ detection methods. The study population of the Costa Rica Vaccine Trial comprised young women and results may not be generalizable to older women, but other longitudinal studies have strongly suggested that viral persistence is a function of the duration of infection, irrespective of a woman's age (24).
At the time of model development, cases of CIN2+ in the Costa Rica Vaccine Trial had not been molecularly attributed to a particular HPV type through such methods as laser capture microdissection. The presence of multiple oncogenic types at detection of some CIN2+ lesions required statistical type attribution assumptions. We prioritized HPV16 infections and persistent infections with oncogenic types, determining fractional attribution when multiple infections were present. It is possible that we overestimated progression attributable to HPV16 at the expense of other oncogenic types, but recent microdissection studies of precancerous lesions suggesting the dominance of HPV16 (46) and the close fit of model outcomes on genotype frequency in CIN2, CIN3, and cancer provides reassurance that our hierarchical attribution scheme was reasonable.
We have discussed limitations of our calibration approach elsewhere (13, 21), but we reiterate these briefly here. With multiple parameters being varied simultaneously, it is difficult to know whether the parameter space was searched comprehensively. To address this issue, we substantially reduced the number of parameters subject to searching relative to our previous modeling efforts. This downsizing was possible due to the availability of recent high-quality longitudinal data (22, 28) and the use of visual fitting and model experimentation to refine search ranges prior to initiating parameter searches.
We constructed a revised and enhanced natural history model of cervical cancer that is equipped to evaluate many aspects of cervical cancer prevention policies. By relying on new understanding of the underlying disease process, we structured our model around HPV infection status rather than poorly reproducible cytological or histological classifications. We also analyzed longitudinal data from the Costa Rica Vaccine Trial to derive time- and type-specific transitions from the HPV state. Empirical data on the burden of HPV from a state-of-the-art cervical disease registry in New Mexico provided a rich source of epidemiologic targets for assessing model fit to a US setting. We present this model in response to a call for new natural history models of cervical cancer intended for decision analysis and economic evaluation (1) at a time when global cervical cancer prevention policy continues to evolve and evidence of the long-term health effects of cervical interventions remains critical.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Center for Health Decision Science, Department of Health Policy and Management, Harvard School of Public Health, Boston, Massachusetts (Nicole G. Campos, Emily A. Burger, Stephen Sy, Monisha Sharma, Jane J. Kim); Department of Health Management and Health Economics, Faculty of Medicine, University of Oslo, Oslo, Norway (Emily A. Burger); Department of Epidemiology, School of Public Health, University of Washington, Seattle, Washington (Monisha Sharma); Division of Cancer Epidemiology and Genetics, National Cancer Institute, Bethesda, Maryland (Mark Schiffman, Alan Hildesheim); Proyecto Epidemiológica Guanacaste, Fundación INCIENSA, San José, Costa Rica (Ana Cecilia Rodriguez, Rolando Herrero); and Prevention and Implementation Group, International Agency for Research on Cancer, Lyon, France (Rolando Herrero).
This work was supported by the National Cancer Institute (grant U54CA164336) and in part by the Intramural Research Program of the National Institutes of Health.
Conflict of interest: none declared.
REFERENCES
- 1.Schiffman M. The need for forward-looking decision analyses to guide cervical cancer prevention. Cancer Epidemiol Biomarkers Prev. 2011;20(2):219–220. doi: 10.1158/1055-9965.EPI-10-1130. [DOI] [PubMed] [Google Scholar]
- 2.Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 3.Plummer M, Schiffman M, Castle PE, et al. A 2-year prospective study of human papillomavirus persistence among women with a cytological diagnosis of atypical squamous cells of undetermined significance or low-grade squamous intraepithelial lesion. J Infect Dis. 2007;195(11):1582–1589. doi: 10.1086/516784. [DOI] [PubMed] [Google Scholar]
- 4.Schiffman M, Castle PE, Jeronimo J, et al. Human papillomavirus and cervical cancer. Lancet. 2007;370(9590):890–907. doi: 10.1016/S0140-6736(07)61416-0. [DOI] [PubMed] [Google Scholar]
- 5.Smith JS, Lindsay L, Hoots B, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer. 2007;121(3):621–632. doi: 10.1002/ijc.22527. [DOI] [PubMed] [Google Scholar]
- 6.Goldie SJ, Goldhaber-Fiebert JD, Garnett GP. Chapter 18: public health policy for cervical cancer prevention: the role of decision science, economic evaluation, and mathematical modeling. Vaccine. 2006;24(suppl 3):S3/155–S3/163. doi: 10.1016/j.vaccine.2006.05.112. [DOI] [PubMed] [Google Scholar]
- 7.Stout NK, Knudsen AB, Kong CY, et al. Calibration methods used in cancer simulation models and suggested reporting guidelines. Pharmacoeconomics. 2009;27(7):533–545. doi: 10.2165/11314830-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rutter CM, Miglioretti DL, Savarino JE. Bayesian calibration of microsimulation models. J Am Stat Assoc. 2009;104(488):1338–1350. doi: 10.1198/jasa.2009.ap07466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Insinga RP, Dasbach EJ, Elbasha EH. Epidemiologic natural history and clinical management of human papillomavirus (HPV) disease: a critical and systematic review of the literature in the development of an HPV dynamic transmission model. BMC Infect Dis. 2009;9:119. doi: 10.1186/1471-2334-9-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Insinga RP, Perez G, Wheeler CM, et al. Incident cervical HPV infections in young women: transition probabilities for CIN and infection clearance. Cancer Epidemiol Biomarkers Prev. 2011;20(2):287–296. doi: 10.1158/1055-9965.EPI-10-0791. [DOI] [PubMed] [Google Scholar]
- 11.Goldie SJ, Gaffikin L, Goldhaber-Fiebert JD, et al. Cost-effectiveness of cervical-cancer screening in five developing countries. N Engl J Med. 2005;353(20):2158–2168. doi: 10.1056/NEJMsa044278. [DOI] [PubMed] [Google Scholar]
- 12.Kulasingam SL, Havrilesky L, Ghebre R, et al. Screening for Cervical Cancer: A Decision Analysis for the U.S. Preventive Services Task Force. Rockville, MD: Agency for Healthcare Research and Quality, US Department of Health and Human Services; 2011. [PubMed] [Google Scholar]
- 13.Kim JJ, Kuntz KM, Stout NK, et al. Multiparameter calibration of a natural history model of cervical cancer. Am J Epidemiol. 2007;166(2):137–150. doi: 10.1093/aje/kwm086. [DOI] [PubMed] [Google Scholar]
- 14.Castle PE, Stoler MH, Solomon D, et al. The relationship of community biopsy-diagnosed cervical intraepithelial neoplasia grade 2 to the quality control pathology-reviewed diagnoses: an ALTS report. Am J Clin Pathol. 2007;127(5):805–815. doi: 10.1309/PT3PNC1QL2F4D2VL. [DOI] [PubMed] [Google Scholar]
- 15.Cox JT, Schiffman M, Solomon D, et al. Prospective follow-up suggests similar risk of subsequent cervical intraepithelial neoplasia grade 2 or 3 among women with cervical intraepithelial neoplasia grade 1 or negative colposcopy and directed biopsy. Am J Obstet Gynecol. 2003;188(6):1406–1412. doi: 10.1067/mob.2003.461. [DOI] [PubMed] [Google Scholar]
- 16.Kjær SK, Frederiksen K, Munk C, et al. Long-term absolute risk of cervical intraepithelial neoplasia grade 3 or worse following human papillomavirus infection: role of persistence. J Natl Cancer Inst. 2010;102(19):1478–1488. doi: 10.1093/jnci/djq356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wheeler CM, Hunt WC, Joste NE, et al. Human papillomavirus genotype distributions: implications for vaccination and cancer screening in the United States. J Natl Cancer Inst. 2009;101(7):475–487. doi: 10.1093/jnci/djn510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim JJ, Goldie SJ. Health and economic implications of HPV vaccination in the United States. N Engl J Med. 2008;359(8):821–832. doi: 10.1056/NEJMsa0707052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim JJ, Goldie SJ. Cost effectiveness analysis of including boys in a human papillomavirus vaccination programme in the United States. BMJ. 2009;339:b3884. doi: 10.1136/bmj.b3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldie SJ, Kim JJ, Kobus K, et al. Cost-effectiveness of HPV 16, 18 vaccination in Brazil. Vaccine. 2007;25(33):6257–6270. doi: 10.1016/j.vaccine.2007.05.058. [DOI] [PubMed] [Google Scholar]
- 21.Goldhaber-Fiebert JD, Stout NK, Ortendahl J, et al. Modeling human papillomavirus and cervical cancer in the United States for analyses of screening and vaccination. Popul Health Metr. 2007;5:11. doi: 10.1186/1478-7954-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herrero R, Hildesheim A, Rodríguez AC, et al. Rationale and design of a community-based double-blind randomized clinical trial of an HPV 16 and 18 vaccine in Guanacaste, Costa Rica. Vaccine. 2008;26(37):4795–4808. doi: 10.1016/j.vaccine.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muñoz N, Méndez F, Posso H, et al. Incidence, duration, and determinants of cervical human papillomavirus infection in a cohort of Colombian women with normal cytological results. J Infect Dis. 2004;190(12):2077–2087. doi: 10.1086/425907. [DOI] [PubMed] [Google Scholar]
- 24.Rodríguez AC, Schiffman M, Herrero R, et al. Longitudinal study of human papillomavirus persistence and cervical intraepithelial neoplasia grade 2/3: critical role of duration of infection. J Natl Cancer Inst. 2010;102(5):315–324. doi: 10.1093/jnci/djq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim HT. Cumulative incidence in competing risks data and competing risks regression analysis. Clin Cancer Res. 2007;13(2):559–565. doi: 10.1158/1078-0432.CCR-06-1210. [DOI] [PubMed] [Google Scholar]
- 26.Gooley TA, Leisenring W, Crowley J, et al. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18(6):695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 27.Keefe KA, Schell MJ, Brewer C, et al. A randomized, double blind, Phase III trial using oral beta-carotene supplementation for women with high-grade cervical intraepithelial neoplasia. Cancer Epidemiol Biomarkers Prev. 2001;10(10):1029–1035. [PubMed] [Google Scholar]
- 28.McCredie MR, Sharples KJ, Paul C, et al. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol. 2008;9(5):425–434. doi: 10.1016/S1470-2045(08)70103-7. [DOI] [PubMed] [Google Scholar]
- 29.Parkin DM, Whelen SL, Ferlay J, et al. Cancer Incidence in Five Continents. Vols. I–VIII. Lyon, France: International Association of Cancer Registries; 2005. (IARC Cancer Base no. 7) [Google Scholar]
- 30.Ries LAG, Melbert D, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975–2001. Bethesda, MD: National Cancer Institute; 2004. [Google Scholar]
- 31.Myers ER, McCrory DC, Nanda K, et al. Mathematical model for the natural history of human papillomavirus infection and cervical carcinogenesis. Am J Epidemiol. 2000;151(12):1158–1171. doi: 10.1093/oxfordjournals.aje.a010166. [DOI] [PubMed] [Google Scholar]
- 32.McCrory D, Mather D, Bastian L, et al. Evaluation of Cervical Cytology. Rockville, MD: Agency for Health Care Policy and Research, US Department of Health and Human Services; 1999. (Evidence report/technology assessment no. 5) [Google Scholar]
- 33.Surveillance, Epidemiology, and End Results (SEER) Program, National Cancer Institute, Division of Cancer Control and Population Sciences. SEER*Stat Database: Incidence—SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases Nov 2011 Sub (1973–2009 Varying)—Linked to County Attributes—Total U.S., 1969–2010 Counties. Bethesda, MD: National Cancer Institute, Division of Cancer Control and Population Sciences, Surveillance Research Program, Surveillance Systems Branch; 2012. [Google Scholar]
- 34.Wheeler CM, Hunt WC, Cuzick J, et al. A population-based study of human papillomavirus genotype prevalence in the United States: baseline measures prior to mass human papillomavirus vaccination. Int J Cancer. 2013;132(1):198–207. doi: 10.1002/ijc.27608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Molano M, Posso H, Weiderpass E, et al. Prevalence and determinants of HPV infection among Colombian women with normal cytology. Br J Cancer. 2002;87(3):324–333. doi: 10.1038/sj.bjc.6600442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Castle PE, Katki HA. Benefits and risks of HPV testing in cervical cancer screening. Lancet Oncol. 2010;11(3):214–215. doi: 10.1016/S1470-2045(09)70385-7. [DOI] [PubMed] [Google Scholar]
- 37.Van de Velde N, Boily MC, Drolet M, et al. Population-level impact of the bivalent, quadrivalent, and nonavalent human papillomavirus vaccines: a model-based analysis. J Natl Cancer Inst. 2012;104(22):1712–1723. doi: 10.1093/jnci/djs395. [DOI] [PubMed] [Google Scholar]
- 38.Barnabas RV, Laukkanen P, Koskela P, et al. Epidemiology of HPV 16 and cervical cancer in Finland and the potential impact of vaccination: mathematical modelling analyses. PLoS Med. 2006;3(5):e138. doi: 10.1371/journal.pmed.0030138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.French KM, Barnabas RV, Lehtinen M, et al. Strategies for the introduction of human papillomavirus vaccination: modelling the optimum age- and sex-specific pattern of vaccination in Finland. Br J Cancer. 2007;96(3):514–518. doi: 10.1038/sj.bjc.6603575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elbasha EH, Dasbach EJ, Insinga RP. Model for assessing human papillomavirus vaccination strategies. Emerg Infect Dis. 2007;13(1):28–41. doi: 10.3201/eid1301.060438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taira AV, Neukermans CP, Sanders GD. Evaluating human papillomavirus vaccination programs. Emerg Infect Dis. 2004;10(11):1915–1923. doi: 10.3201/eid1011.040222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hughes JP, Garnett GP, Koutsky L. The theoretical population-level impact of a prophylactic human papilloma virus vaccine. Epidemiology. 2002;13(6):631–639. doi: 10.1097/00001648-200211000-00006. [DOI] [PubMed] [Google Scholar]
- 43.Vänskä S, Auranen K, Leino T, et al. Impact of vaccination on 14 high-risk HPV type infections: a mathematical modelling approach. PLoS One. 2013;8(8):e72088. doi: 10.1371/journal.pone.0072088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dunne EF, Sternberg M, Markowitz LE, et al. Human papillomavirus (HPV) 6, 11, 16, and 18 prevalence among females in the United States—National Health and Nutrition Examination Survey, 2003–2006: opportunity to measure HPV vaccine impact? J Infect Dis. 2011;204(4):562–565. doi: 10.1093/infdis/jir342. [DOI] [PubMed] [Google Scholar]
- 45.Hariri S, Unger ER, Sternberg M, et al. Prevalence of genital human papillomavirus among females in the United States, the National Health and Nutrition Examination Survey, 2003–2006. J Infect Dis. 2011;204(4):566–573. doi: 10.1093/infdis/jir341. [DOI] [PubMed] [Google Scholar]
- 46.van der Marel J, Quint WG, Schiffman M, et al. Molecular mapping of high-grade cervical intraepithelial neoplasia shows etiological dominance of HPV16. Int J Cancer. 2012;131(6):E946–E953. doi: 10.1002/ijc.27532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim JJ, Wright TC, Goldie SJ. Cost-effectiveness of alternative triage strategies for atypical squamous cells of undetermined significance. JAMA. 2002;287(18):2382–2390. doi: 10.1001/jama.287.18.2382. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.