Abstract
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) is a persistent environmental contaminant. Although experimental evidence suggests that TCDD alters thyroid hormone levels in rodents, human data are inconsistent. In 1976, a trichlorophenol plant exploded in Seveso, Italy. Women living in highly exposed areas were followed through the Seveso Women's Health Study. TCDD concentrations were measured in 1976 (n = 981) and 1996 (n = 260), and levels of total thyroxine, free thyroxine, free triiodothyronine, and thyroid-stimulating hormone were measured in 1996 (n = 909) and 2008 (n = 724). We used conditional multiple linear regression and marginal structural models with inverse-probability-of-treatment weights to evaluate associations and causal effects. TCDD concentration in 1976 was inversely associated with total thyroxine level in 1996 but not in 2008. Associations were stronger among women who had been exposed before menarche. Among these women, associations between total thyroxine and concurrent 1996 TCDD were slightly weaker than those with 1976 TCDD. A model including both 1976 and 1996 measurements strengthened the relationship between 1976 TCDD and total thyroxine but drove the association with 1996 TCDD to the null. TCDD exposure was not associated with levels of other thyroid hormones. TCDD exposure, particularly exposure before menarche, may have enduring impacts on women's total thyroxine levels. Initial exposure appears to be more influential than remaining body burden.
Keywords: accidents, dioxin, endocrine disruptors, environmental exposure, TCDD, thyroid hormones
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD), a persistent lipophilic environmental contaminant produced as an unwanted by-product of various combustion and chemical processes (1), is a known human carcinogen and endocrine disruptor (2, 3). Experimental animal data suggest that TCDD causes decreases in total and free thyroxine (T4) levels, with some studies suggesting increases in thyroid-stimulating hormone (TSH) levels (4–6).
Only 4 human studies have investigated associations between serum TCDD concentrations and thyroid hormone levels in adults, and results have been inconsistent. Ott et al. (7) reported positive associations between T4 and whole-blood TCDD concentrations measured in 131 trichlorophenol production plant workers more than 45 years after an accidental exposure to TCDD. Chloracne status and TCDD levels estimated by back-extrapolation to the time of the accident were also positively associated with T4 levels. There were no associations with TSH. Similarly, Calvert et al. (8) found that high serum TCDD concentrations (≥1,860 pg/g lipids) measured more than 15 years after the last exposure were associated with an elevated free T4 index among 278 trichlorophenol plant workers relative to 257 referents with lower exposure (mean serum TCDD concentration = 7 pg/g lipids), although no clear exposure-response relationship was demonstrated. No association was found between TCDD and total T4 or TSH. In 32 Australian herbicide spray workers, back-extrapolated serum TCDD concentrations (but not concurrent levels) were negatively correlated with triiodothyronine (T3) but not T4 (9). Finally, in a large (n = 1,009 exposed and 1,429 referents) prospective study of US Air Force veterans who sprayed TCDD-contaminated Agent Orange in Vietnam between 1962 and 1971, Pavuk et al. (10) reported positive associations between serum TCDD concentrations in 1982–1997 and concomitantly measured TSH levels (but not total T4 levels, free T4 index, or T3% uptake). Except for the study by Calvert et al. (8), which included only 31 women, these studies were exclusively conducted among males.
Although few data are available on TCDD's potential for thyroid hormone disruption in women, a number of studies of other polychlorinated dibenzodioxins, dibenzofurans, and biphenyls whose dioxin-like activity was evaluated using toxic equivalents (TEQs) have been conducted, with similarly inconsistent results (11–18).
On July 10, 1976, an explosion at a trichlorophenol manufacturing plant in Seveso, Italy, released approximately 30 kg of TCDD over the surrounding area, resulting in the highest known exposure to TCDD in a residential population (19). The Seveso Women's Health Study, initiated in 1996, is a historical cohort study of the female population residing around Seveso at the time of the explosion (20). As part of the Seveso Women's Health Study, TCDD was measured in blood samples collected shortly after the accident, creating a unique opportunity to study the long-term health effects of TCDD exposure with limited potential for reverse causality, a bias that may have affected prior studies. Here, we examined the relationship between 1976 serum TCDD levels and thyroid hormone concentrations measured in 1996 and 2008. In a subset, we also examined the relationship between 1996 serum TCDD/TEQ levels and thyroid hormone concentrations measured in 1996 and 2008.
METHODS
Participants
Details on the design of the Seveso Women's Health Study are presented elsewhere (20). Women who were aged 40 years or less in 1976, resided in the most highly contaminated areas (zone A or B, based on surface soil measurements (21)) at the time of the explosion, and had sufficient serum collected soon after the explosion were eligible to participate in the study. Enrollment in the Seveso Women's Health Study cohort took place between March 1996 and July 1998 (referred to hereafter as “the 1996 visit”), and 981 women (80% of those eligible) participated. The cohort was followed up between April 2008 and December 2009 (referred to hereafter as “the 2008 visit”); of those women who were still alive and could be located (n = 929), 833 (90%) participated in this second wave.
To study associations between 1976 TCDD concentrations and 1996 thyroid hormone levels, we excluded women who did not provide a blood sample in 1996 (n = 25), had a history of thyroid disease before the explosion (n = 20), had Turner's syndrome (n = 1), or reported current treatment for thyroid disease (n = 26), leaving a final sample of 909. For the analysis of 1996 TCDD/TEQ and 1996 thyroid hormone levels, the sample was further limited to those with 1996 serum TCDD/TEQ measurements (n = 260).
To evaluate associations between 1976 TCDD and 2008 thyroid hormone levels, we excluded women who did not provide a blood sample in 2008 (n = 27), had a history of thyroid disease before the explosion (n = 16) or reported treatment for thyroid disease in 2008 (n = 61), had Turner's syndrome (n = 1), or were pregnant (n = 4), leaving a final sample of 724. For the analysis of 1996 TCDD/TEQ and 2008 thyroid hormone levels, the sample was further limited to those with 1996 serum TCDD/TEQ measurements (n = 237).
This study was approved by the University of California, Berkeley, Committee for the Protection of Human Subjects, and written informed consent was obtained from all women before participation.
Procedure
At both the 1996 and 2008 study visits, women underwent a fasting blood draw, anthropometric measurements, and a structured personal interview by a nurse who was blinded to zone of residence and to TCDD and serum thyroid hormone concentrations. Information collected included demographic and lifestyle characteristics and reproductive and medical histories.
Measurement of TCDD
We measured TCDD in archived sera collected soon after the explosion by high-resolution gas chromatography/high-resolution mass spectrometry; coefficients of variation were 13%–15% (22). Details of serum sample selection and TCDD concentrations are presented elsewhere (20, 23). Values are reported on a lipid-weight basis in parts per trillion (ppt) (24). For values below the limit of detection (9.4% in 1976 and 7.7% in 1996), a serum TCDD level of one-half the detection limit was assigned. The median serum sample weight was 0.65 g, and the median limit of detection was 18.8 ppt, lipid-adjusted. TEQ was not assessed in the 1976 samples because of limited sample volume.
For a subset of participants (n = 260), we measured levels of 17 polychlorinated dibenzodioxins/dibenzofurans, including TCDD, 4 coplanar polychlorinated biphenyls, and 8 mono-ortho-substituted polychlorinated biphenyls, in serum samples collected during the 1996 study by high-resolution gas chromatography/isotope-dilution high-resolution mass spectrometry (25, 26). Total TEQ concentration was calculated on the basis of the 2005 WHO Toxicity Equivalency Factors (27). Details on TCDD and total TEQ concentrations in 1996 serum are presented elsewhere (28).
Measurement of thyroid hormone levels
Thyroid hormone levels were measured at the Hospital of Desio laboratory in serum samples collected in 1996 and 2008 using sandwich (TSH) or competitive (free T3, free T4, and total T4) electrochemiluminescence immunoassays (Roche Diagnostics, Mannheim, Germany). The limits of detection for TSH, free T3, free T4, and total T4 were 0.01 mIU/L, 0.04 ng/dL, 0.02 ng/dL, and 0.4 µg/dL, respectively. Coefficients of variation ranged between 1.5% and 6.2%.
Statistical analysis
Serum TCDD concentrations were heavily right-skewed, with a wide distribution, and thus the data were log10-transformed to reduce the influence of outliers. We also log10-transformed TSH values in order to normalize residuals. Free T3, free T4, and total T4 values were approximately normally distributed and were thus expressed on the arithmetic scale. We used analysis of variance to compare serum TCDD concentrations across demographic categories and Pearson's correlations to assess bivariate associations of continuous variables. We used multiple linear regression to evaluate the relationship between serum TCDD concentrations and thyroid hormone levels. We first fitted generalized additive models with a 3–degrees of freedom cubic spline to evaluate the shape of exposure-response curves. None of the digression from linearity tests was significant when a liberal P value (P < 0.15) was used. We therefore expressed TCDD linearly (on the log10 scale) in regression models. Regression coefficients represent the mean (free T3, free T4, and total T4) or percent (TSH) change for each 10-fold increase in serum TCDD concentration.
We fitted separate models for associations between TCDD and thyroid hormone levels at the different time points. In order to identify the conditionally independent associations of 1976 and 1996 TCDD with thyroid hormone levels, we also fitted models including TCDD measures at both time points. In addition, we used multiple logistic regression to evaluate whether exposure was associated with increased odds of hypothyroidism or hyperthyroidism, based on reference ranges provided by the laboratory or physician diagnosis. Women who had TSH levels greater than 4.5 mIU/L, had free T4 levels less than 0.9 ng/dL, or were taking thyroid hormone supplements were classified as hypothyroidic (n = 101 in 1996 and n = 111 in 2008); those who had TSH levels less than 0.1 mIU/L, had free T4 levels greater than 1.9 ng/dL, or were being treated for hyperthyroidism were considered hyperthyroidic (n = 31 in 1996 and n = 30 in 2008).
Based on the current literature and directed acyclic graphs, we considered the following variables as potential confounders: age, education, marital status, and history of cigarette smoking and alcohol consumption at the time of thyroid hormone measurement. We included in the models covariates that were associated with both thyroid hormone levels and TCDD/TEQ at P < 0.2 in bivariate analyses. Final models included age at thyroid hormone measurement and age at thyroid hormone measurement squared. We considered effect modification by age and menarcheal status at the time of the explosion based on previous results in this population by including cross-product terms in the models and performing Wald tests (29, 30).
In sensitivity analyses, we refitted the models after excluding outliers with studentized residuals greater than 3. Excluding 2 outliers with suppressed TSH concentrations (<0.01 mIU/L) substantially altered results for the associations with 2008 TSH levels, so we report results for this model without them. To investigate whether the lipid adjustment method that we chose affected our results, we refitted the 1996 TCDD models with wet-weight TCDD (ng/g serum) concentrations, including total lipids as a covariate. In an attempt to adjust for selection bias due to loss to follow-up, we applied stabilized inverse probability weights to the regression models (31). We determined weights using multiple logistic regression with independent variables selected using a “Super Learner” algorithm with V-fold cross-validation (32). To examine the possibility of reverse causality, we also repeated analyses after restricting the data to the 724 participants for whom serum samples had been collected within 90 days of the explosion. Lastly, we reran the analyses with values below the limits of detection randomly imputed based on a lognormal probability distribution whose parameters were determined by maximum likelihood estimation (33).
We repeated the above analyses to estimate causal effects using marginal structural models (34) for continuous exposure following the procedure used by Cerda et al. (35). Inverse-probability-of-treatment weights were determined on the basis of probability density functions whose parameters were estimated using linear regression with TCDD as the dependent variable. To improve the precision of estimates, we used stabilized weights equal to the marginal probability densities divided by probability densities conditional on covariates measured at or before the time of thyroid hormone measurements. When assessing effect modification, both the numerator and the denominator of the weights were conditioned on the effect modifier to further improve precision (34). Conditional probability densities were determined by using the “Super Learner” prediction algorithm described above (32). Weights greater than 10 were truncated at this value. Note that marginal structural and conditional models estimate different quantities. Estimates from these methods may not be directly compared, but conclusions reached using each method may be contrasted. Statistical significance was set at P < 0.05 for main effects and P < 0.10 for effect modification using 2-tailed tests. Analyses were conducted using STATA, version 11.2 (StataCorp LP, College Station, Texas) and R, version 3.15.2 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Population
The average age of participants at the time of the explosion was 19.7 years (standard deviation (SD), 11.2). In 1996, participants had a mean age of 40.3 years (SD, 11.5), 46% had completed high school, and 30% were overweight or obese (Table 1). Most never consumed alcohol or smoked cigarettes (64% and 65%, respectively), and 52% were multiparous. In 2008, the average age of participants was 51.6 years (SD, 11.0); 55% were overweight or obese; 64% and 63%, respectively, abstained from alcohol and cigarettes; and 65% were multiparous.
Table 1.
Demographic Characteristics of Participants at the Time of TCDD Measurement, Seveso Women's Health Study, Italy, 1976–2009
| Characteristic | 1976 (n = 909) |
1996 (n = 260) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| At Time of Explosion (1976) | ||||
| Age, years | ||||
| 0–10 | 223 | 24.5 | 131 | 50.4 |
| 11–20 | 268 | 29.5 | 117 | 45.0 |
| 21–30 | 220 | 24.2 | 10 | 3.9 |
| 31–40 | 198 | 21.8 | 2 | 0.8 |
| Menarcheal status | ||||
| Premenarche | 272 | 29.9 | 161 | 61.9 |
| Postmenarche | 637 | 70.1 | 99 | 38.1 |
| At Time of 1996 Interview | ||||
| Age, years | ||||
| 20–30 | 207 | 22.8 | 121 | 46.5 |
| 31–40 | 272 | 29.9 | 125 | 48.1 |
| 41–50 | 223 | 24.5 | 12 | 4.6 |
| 51–62 | 207 | 22.8 | 2 | 0.8 |
| Parity | ||||
| 0 | 253 | 27.8 | 108 | 41.5 |
| 1 | 184 | 20.2 | 82 | 31.5 |
| ≥2 | 472 | 51.9 | 70 | 26.9 |
| Educational level | ||||
| Less than high school | 494 | 54.4 | 67 | 25.8 |
| High school | 372 | 40.9 | 172 | 66.2 |
| More than high school | 43 | 4.7 | 21 | 8.0 |
| Body mass indexa category | ||||
| <18.5 | 52 | 5.7 | 25 | 9.6 |
| 18.5–24.9 | 582 | 64.0 | 192 | 73.9 |
| 25.0–29.9 | 202 | 22.2 | 31 | 11.9 |
| ≥30.0 | 73 | 8.0 | 12 | 4.6 |
| Alcohol consumption | ||||
| Never drinker | 580 | 63.8 | 186 | 71.5 |
| Current drinker | ||||
| <1 drink/day | 194 | 21.3 | 56 | 21.5 |
| ≥1 drink/day | 135 | 14.9 | 18 | 7.0 |
| Cigarette smoking | ||||
| Never smoker | 592 | 65.1 | 157 | 60.4 |
| Former smoker | 128 | 14.1 | 44 | 16.9 |
| Current smoker | ||||
| ≤10 cigarettes/day | 119 | 13.1 | 44 | 16.9 |
| >10 cigarettes/day | 70 | 7.7 | 15 | 5.8 |
| Marital status | ||||
| Never married | 173 | 19.0 | 78 | 30.0 |
| Ever married | 736 | 81.0 | 182 | 70.0 |
Abbreviation: TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin.
a Weight (kg)/height (m)2.
TCDD/TEQ serum concentrations
Median serum TCDD concentrations were 60.2 ppt (interquartile range, 28.5–163.0) and 7.0 ppt (interquartile range, 4.2–12.9) in 1976 and 1996, respectively, and the measures were moderately correlated (r = 0.59, P < 0.001). The median total TEQ in 1996 was 25.6 ppt (interquartile range, 19.7–35.8). As previously reported (23), 1976 TCDD concentrations were inversely correlated with age at the time of the explosion (r = −0.25, P < 0.001) and were substantially higher among participants who were aged 10 years or less (median, 165.0 ppt) than among those aged more than 10 years (median, 46.5 ppt). Likewise, 1976 TCDD concentrations were higher among women who were premenarcheal at the time of the explosion and women who were nulliparous or had never been married in 1996, all of which are age-related (23). TCDD levels in 1976 and 1996 were substantially elevated relative to females of similar ages with background exposure, though levels of other dioxin-like chemicals were not (28).
Thyroid hormone concentrations
Mean free T3, free T4, and total T4 concentrations were 0.4 ng/dL (SD, 0.1), 1.2 ng/dL (SD, 0.5), and 8.1 µg/dL (SD, 1.9) in 1996 and 0.3 ng/dL (SD, 0.1), 1.2 ng/dL (SD, 0.2), and 8.2 µg/dL (SD, 1.7) in 2008, respectively. Geometric mean values for TSH were 1.9 mIU/L (geometric SD, 1.3) in 1996 and 1.8 mIU/L (geometric SD, 2.4) in 2008. Age was inversely associated with free T4 (r = −0.17, P < 0.001), total T4 (r = −0.11, P < 0.01), and TSH (r = −0.10, P < 0.01) levels in 1996 but associated only with TSH level (r = −0.07, P = 0.05) in 2008. No associations were observed between covariates and free T3 at either time point. Women who were postmenarcheal at the time of the explosion or who were married or multiparous in 1996 had lower serum concentrations of free T4, total T4, and TSH (see Web Table 1, available at http://aje.oxfordjournals.org/). Education was positively associated with free T4 and appeared to have an inverse U-shaped relationship with TSH. Alcohol consumption was inversely associated with total T4, while cigarette smoking was positively related to free T4.
Associations between TCDD and thyroid hormone levels
We found that 1976 TCDD was inversely associated with total T4 level in 1996 (β = −0.27, 95% confidence interval (CI): −0.49, −0.05) (Table 2). The association was stronger among participants who were premenarche at the time of the explosion (β = −0.63, 95% CI: −1.11, −0.15) relative to those who were postmenarche (β = −0.13, 95% CI: −0.37, 0.11; P for interaction = 0.05). We found a weak, nonsignificant association between 1976 TCDD and 2008 total T4 levels (β = −0.11, 95% CI: −0.32, 0.12) and little evidence of effect modification by menarcheal status at explosion. Including 1976 and 1996 TCDD levels in the same model (Figure 1) resulted in stronger associations between 1976 TCDD and 1996 total T4 overall (β = −0.42, 95% CI: −0.96, 0.12) and for women who were premenarcheal at the time of the explosion (β = −1.09, 95% CI: −1.77, −0.41), but it drove the associations with 1996 TCDD towards the null (β = −0.04, 95% CI: −0.72, 0.64).
Table 2.
Associations Between 1976 Log10 TCDD Concentration (Parts per Trillion) and Thyroid Hormone Levels by Menarcheal Status at the Time of the Explosion (1976), Seveso Women's Health Study, Italy, 1976–2009
| Thyroid Hormone | No. of Participantsa | Adjustedb βc | 95% CI | Menarcheal Status at Explosion |
P for Interaction | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Premenarche |
Postmenarche |
|||||||||
| No. | Adjustedb βc | 95% CI | No. | Adjustedb βc | 95% CI | |||||
| 1996 Thyroid Hormone Measurements | ||||||||||
| TSHd | 909 | 6.61 | −0.87, 14.66 | 272 | 0.38 | −9.34, 11.13 | 637 | 9.27 | −0.76, 20.32 | 0.35 |
| Total T4 | 889 | −0.27 | −0.49, −0.05 | 260 | −0.63 | −1.11, −0.15 | 629 | −0.13 | −0.37, 0.11 | 0.05 |
| Free T4 | 894 | 0.01 | −0.04, 0.06 | 260 | 0.02 | −0.07, 0.12 | 634 | 0.01 | −0.05, 0.07 | 0.72 |
| Free T3 | 895 | 0.00 | −0.02, 0.01 | 260 | 0.00 | −0.02, 0.02 | 635 | −0.01 | −0.03, 0.02 | 0.70 |
| 2008 Thyroid Hormone Measurements | ||||||||||
| TSHd | 722 | 5.44 | −3.39, 15.08 | 225 | 4.82 | −8.69, 20.33 | 497 | 6.41 | −4.94, 19.10 | 0.42 |
| Total T4 | 722 | −0.11 | −0.32, 0.12 | 225 | 0.09 | −0.31, 0.48 | 497 | −0.24 | −0.48, 0.00 | 0.13 |
| Free T4 | 724 | 0.00 | −0.02, 0.03 | 225 | 0.01 | −0.03, 0.05 | 499 | 0.00 | −0.03, 0.03 | 0.52 |
| Free T3 | 724 | 0.00 | −0.02, 0.01 | 225 | 0.00 | −0.01, 0.02 | 499 | −0.01 | −0.03, 0.01 | 0.30 |
Abbreviations: CI, confidence interval; T3, triiodothyronine; T4, thyroxine; TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin; TSH, thyroid-stimulating hormone.
a Some participants were missing thyroid hormone measurements due to insufficient sample volume.
b All models adjusted for age at thyroid hormone measurement and age at thyroid hormone measurement squared.
c Coefficients represent the mean (free T3, free T4, and total T4) or percent (TSH) change in thyroid hormone level for each 10-fold increase in serum TCDD concentration.
d Percent change in serum TSH concentration was calculated using the following formula: (10β − 1) × 100.
Figure 1.
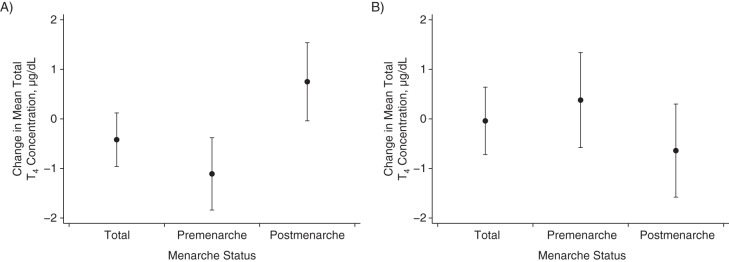
Associations between 1976 (A) and 1996 (B) serum 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) concentrations and 1996 serum total thyroxine (T4) concentrations by menarcheal status at the time of the explosion (n = 251), Seveso Women's Health Study, Italy, 1976–2009. Results were based on multiple linear regression models including exposure at both time points. Coefficients represent the mean change in total T4 concentration for each 10-fold increase in TCDD. Models adjusted for age at thyroid hormone measurement and age at thyroid hormone measurement squared.
There was no clear evidence of a relationship between 1976 TCDD and TSH, free T4, or free T3 measured in 1996 or 2008. We also found no clear evidence of associations between 1996 TCDD and any of the thyroid hormones in 1996 or 2008, regardless of menarcheal status at explosion (Table 3). Although we did observe a weak inverse association between 1996 TCDD and 1996 free T3 (β = −0.02, 95% CI: −0.04, 0.00), the association was not statistically significant (P = 0.13). Thyroid hormone levels in 1996 or 2008 were not associated with 1996 TEQ (Table 4). A large and imprecise inverse association was found between 1996 TEQ and 2008 TSH (β = 19.2, 95% CI: −16.4, 69.9), which was particularly strong among women who were premenarche at the time of the explosion (β = 28.4, 95% CI: −29.6, 134.2; P for interaction = 0.12), but effect modification was not significant.
Table 3.
Associations Between 1996 Log10 TCDD Concentration (Parts per Trillion) and Thyroid Hormone Levels by Menarcheal Status at the Time of the Explosion (1976), Seveso Women's Health Study, Italy, 1976–2009
| Thyroid Hormone | No. of Participantsa | Adjustedb βc | 95% CI | Menarcheal Status at Explosion |
P for Interaction | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Premenarche |
Postmenarche |
|||||||||
| No. | Adjustedb βc | 95% CI | No. | Adjustedb βc | 95% CI | |||||
| 1996 Thyroid Hormone Measurements | ||||||||||
| TSHd | 260 | −4.46 | −18.89, 12.54 | 161 | −8.94 | −25.14, 10.75 | 99 | −1.12 | −26.48, 32.94 | 0.62 |
| Total T4 | 251 | −0.37 | −0.90, 0.17 | 153 | −0.54 | −1.32, 0.20 | 98 | −0.11 | −0.85, 0.64 | 0.42 |
| Free T4 | 251 | −0.03 | −0.16, 0.10 | 153 | 0.00 | −0.14, 0.13 | 98 | −0.04 | −0.31, 0.22 | 0.74 |
| Free T3 | 252 | −0.02 | −0.04, 0.00 | 153 | −0.01 | −0.04, 0.01 | 99 | −0.02 | −0.05, 0.02 | 0.85 |
| 2008 Thyroid Hormone Measurements | ||||||||||
| TSHd | 235 | 11.89 | −4.28, 30.32 | 146 | 14.66 | −10.09, 46.21 | 89 | 2.71 | −17.58, 28.00 | 0.17 |
| Total T4 | 236 | −0.09 | −0.63, 0.45 | 146 | 0.16 | −0.54, 0.87 | 90 | −0.44 | −1.28, 0.39 | 0.26 |
| Free T4 | 237 | 0.01 | −0.05, 0.06 | 146 | 0.02 | −0.05, 0.09 | 91 | −0.01 | −0.10, 0.07 | 0.52 |
| Free T3 | 237 | 0.01 | −0.01, 0.02 | 146 | 0.01 | −0.01, 0.03 | 91 | 0.00 | −0.02, 0.02 | 0.84 |
Abbreviations: CI, confidence interval; T3, triiodothyronine; T4, thyroxine; TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin; TSH, thyroid-stimulating hormone.
a Some participants were missing thyroid hormone measurements due to insufficient sample volume.
b All models adjusted for age at thyroid hormone measurement and age at thyroid hormone measurement squared.
c Coefficients represent the mean (free T3, free T4, and total T4) or percent (TSH) change in thyroid hormone level for each 10-fold increase in serum TCDD concentration.
d Percent change in serum TSH concentration was calculated using the following formula: (10β − 1) × 100.
Table 4.
Associations Between 1996 Log10 Total TCDD Toxic Equivalent (Parts per Trillion) and Thyroid Hormone Levels by Menarcheal Status at the Time of the Explosion (1976), Seveso Women's Health Study, Italy, 1976–2009
| Thyroid Hormone | No. of Participantsa | Adjustedb βc | 95% CI | Menarcheal Status at Explosion |
P for Interaction | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Premenarche |
Postmenarche |
|||||||||
| No. | Adjustedb βc | 95% CI | No. | Adjustedb βc | 95% CI | |||||
| 1996 Thyroid Hormone Measurements | ||||||||||
| TSHd | 260 | −7.71 | −36.09, 33.27 | 161 | −19.64 | −49.56, 28.02 | 99 | −2.35 | −47.64, 82.12 | 0.65 |
| Total T4 | 251 | −0.57 | −1.77, 0.64 | 153 | −1.01 | −2.81, 0.80 | 98 | −0.23 | −1.80, 1.34 | 0.56 |
| Free T4 | 251 | −0.08 | −0.37, 0.22 | 153 | −0.07 | −0.39, 0.25 | 98 | −0.05 | −0.60, 0.50 | 0.93 |
| Free T3 | 252 | −0.04 | −0.08, 0.01 | 153 | −0.03 | −0.09, 0.04 | 99 | −0.04 | −0.11, 0.03 | 0.85 |
| 2008 Thyroid Hormone Measurements | ||||||||||
| TSHd | 235 | 19.17 | −16.39, 69.86 | 146 | 28.42 | −29.58, 134.21 | 89 | −2.53 | −339.84, 55.34 | 0.12 |
| Total T4 | 236 | −0.28 | −1.49, 0.92 | 146 | 0.24 | −1.50, 1.98 | 90 | −0.73 | −2.39, 0.94 | 0.37 |
| Free T4 | 237 | 0.06 | −0.07, 0.18 | 146 | 0.08 | −0.10, 0.26 | 91 | 0.03 | −0.14, 0.21 | 0.65 |
| Free T3 | 237 | 0.02 | −0.02, 0.05 | 146 | 0.02 | −0.03, 0.07 | 91 | 0.01 | −0.03, 0.06 | 0.83 |
Abbreviations: CI, confidence interval; T3, triiodothyronine; T4, thyroxine; TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin; TSH, thyroid-stimulating hormone.
a Some participants were missing thyroid hormone measurements due to insufficient sample volume.
b All models adjusted for age at thyroid hormone measurement and age at thyroid hormone measurement squared.
c Coefficients represent the mean (free T3, free T4, and total T4) or percent (TSH) change in thyroid hormone level for each 10-fold increase in serum TCDD concentration.
d Percent change in serum TSH concentration was calculated using the following formula: (10β − 1) × 100.
Estimates of causal effects using marginal structural models led to similar conclusions as those drawn from multiple linear regression (see Web Tables 2 and 3). A 0.43-µg/dL decrease in 1996 total T4 (95% CI: −0.82, −0.05) was observed for every 10-fold increase in 1976 TCDD concentrations; this relationship was stronger among women who were premenarche at the time of the explosion (β = −0.89, 95% CI: −1.54, −0.24; P for interaction = 0.02) relative to those who were postmenarche (β = −0.10, 95% CI: −0.32, 0.11). Estimated effects of 1996 TCDD on 1996 total T4 (β = −0.76, 95% CI: −1.42, −0.11) and free T3 (β = −0.02, 95% CI: −0.04, −0.01) were significant in marginal models (Web Table 3); inclusion of 1976 TCDD in the model drove estimates towards the null for total T4 (β = −0.45, 95% CI: −1.37, 0.46) but not for free T3 (β = −0.03, 95% CI: −0.06, 0.00). Other estimates were nonsignificant.
Odds ratios for associations between any TCDD/TEQ measures and hyperthyroidism and hypothyroidism in 1996 or 2008 were generally weak, imprecise, and not statistically significant (data not shown). None of the sensitivity analyses substantially altered our results.
DISCUSSION
In this study, we found inverse associations between TCDD concentrations in serum collected shortly after the explosion of a trichlorophenol manufacturing plant and total serum T4 levels in women approximately 20 years later. This association was limited to women who were premenarcheal at the time of the explosion. Among these women, the cross-sectional association between 1996 total T4 and concurrently measured TCDD was slightly weaker than the longitudinal association with 1976 TCDD and was not statistically significant, possibly due to reduced sample size. This association was substantially weakened when both 1976 and 1996 TCDD were included in the same model, while the association with 1976 TCDD was strengthened. There was some evidence of an association between 1996 TCDD and free T3, but the estimate was weak. Marginal structural models yielded similar results, confirming these findings. The difference between the longitudinal and cross-sectional associations did not appear to be caused by selection bias due to loss to follow-up, since adjustment for censoring using inverse probability weighting did not materially affect the results. Taken together, these results suggest that serum TCDD concentrations were inversely associated with total T4 levels in women exposed before menarche.
As previously discussed, studies investigating associations between lipophilic chemicals and thyroid hormones may be affected by reverse causality (36). TCDD and other dioxin-like compounds are highly nonpolar and thus sequester primarily in lipids (37). Thyroid hormones, on the other hand, influence lipid metabolism and serum lipid concentrations (38). Serum TCDD concentrations may therefore be affected by thyroid hormone levels. Inconsistent results from previous studies of TCDD and other dioxin-like compounds may be due to reverse causality, since they all either were cross-sectional, relied on TCDD concentrations in serum collected several years postexposure, or considered ongoing exposure for which the time ordering of exposure and outcome could not be clearly established. The present study was characterized by unique features in that the timing of exposure was precisely known (allowing us to exclude participants who had thyroid disease before the explosion), serum TCDD concentrations were measured shortly after exposure, and the magnitude of exposure would not be expected to have been related to thyroid status when it occurred, thereby limiting the possibility of reverse causation. In this context, our finding of an inverse longitudinal association between TCDD and total T4 provides a new form of support for the effects of this chemical on thyroid function.
The fact that the cross-sectional association of 1996 TCDD and total T4 approached the null when 1976 TCDD was included in the models suggests that the initial exposure, rather than continuously elevated serum TCDD concentrations, was primarily associated with total T4 levels. This has implications for the understanding of the toxicological mechanism of action through which TCDD may affect total T4 concentrations. TCDD is generally believed to reduce serum T4 levels through the induction of uridine diphosphate glucuronosyltransferase in response to binding to the aryl hydrocarbon receptor, resulting in accelerated T4 clearance (39). Under this mechanism, continuous exposure would be expected to primarily affect T4 levels. The fact that we found no cross-sectional association may thus suggest that another mechanism of action is at play, perhaps resulting in long-term injury. This possibility of an alternative mechanism is supported by evidence that TCDD affects T4 levels in aryl hydrocarbon-receptor-null mice (40). In addition, our finding of an inverse association with total T4 but not free T4 suggests that the concentration of transport proteins may be affected by TCDD. Experimental animal studies lend some support to this hypothesis. Decreases in total T4 levels have been consistently reported in laboratory studies conducted in rodents, although those were also generally accompanied by reduction in free T4 levels (4–6). In addition, TCDD has been shown to competitively displace T4 from transthyretin, a T4 transport protein, possibly resulting in increased elimination (41).
Of particular interest is the fact that we found stronger evidence of associations with 1996 thyroid hormone among women who were premenarche at the time of the Seveso explosion, suggesting a specific window of susceptibility. The hypothalamic-pituitary-thyroid axis is known to be particularly sensitive to endocrine modulation before puberty. For instance, the prepubertal axis is estimated to be 6–15 times more sensitive to estrogen than the adult feedback mechanism (42). It is therefore conceivable that TCDD, which has antiestrogenic properties (43), may have a greater effect on thyroid function before puberty. Similar to our findings, serum TCDD was reported to be associated with altered menstrual cycle characteristics among those who were premenarche, but not among those who were postmenarche, at the time of the Seveso explosion (30). It is not clear, however, why this same pattern was not apparent in 2008.
This study had several strengths, including its unique design, which allowed us to investigate both longitudinal and cross-sectional associations. We also had data that allowed us to exclude study participants who had thyroid disease before the explosion, thereby precluding the possibility of reverse causation at the onset of follow-up. In addition, we were able to conduct a number of sensitivity analyses, including an investigation of possible selection bias due to loss to follow-up using inverse probability weighting, and investigated relationships using both standard conditional linear regression and marginal structural models, confirming the robustness of our results. To our knowledge, this is also the first study to have specifically investigated associations between exposure to TCDD and thyroid hormone levels in women.
Limitations include the lack of data on iodine intake, which is an important determinant of thyroid function, and the lack of data on thyroid autoantibody levels, since autoimmune disease is the primary cause of hypothyroidism. However, in the context of the Seveso accident, iodine intake would not be expected to be associated with exposure to TCDD, and we had data on diagnosed thyroid disease. Finally, since only free (unbound) T4 is bioavailable to bind to the thyroid receptor, the health consequences of a reduction in total T4 with no change in free T4 are unclear.
In summary, we report an inverse association between serum TCDD concentrations measured shortly after the Seveso trichlorophenol plant explosion and total T4 (but not free T4, free T3, or TSH) levels measured approximately 20 (but not 30) years later among women who were premenarche at the time of the accident. However, we found no clear associations between 1996 serum TCDD concentrations and any 1996 or 2008 thyroid hormone measures, suggesting an effect of initial exposure rather than of later body burden.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, Biostatistics and Occupational Health, Faculty of Medicine, McGill University, Montréal, Québec, Canada (Jonathan Chevrier); Center for Environmental Research and Children's Health, School of Public Health, University of California, Berkeley, Berkeley, California (Jonathan Chevrier, Marcella Warner, Robert B. Gunier, Brenda Eskenazi); and Department of Laboratory Medicine, School of Medicine, University of Milano-Bicocca and Hospital of Desio, Desio-Milano, Italy (Paolo Brambilla, Paolo Mocarelli).
This study was supported by grant F06 TW02075-01 from the US National Institutes of Health, grants R01 ES07171 and 2P30-ESO01896-17 from the US National Institute of Environmental Health Sciences, grant R82471 from the US Environmental Protection Agency, and grant 2896 from the Regione Lombardia and the Fondazione Lombardia per l'Ambiente, Milan, Italy.
We thank Drs. Don Patterson, Larry Needham, and Wayman Turner for their significant contributions to exposure assessment and sample analysis in the Seveso Women's Health Study, Amelia K. Wesselink for help with the literature review, Aliza Parigi for coordinating data collection at the Hospital of Desio, and Katherine Kogut for editing the manuscript.
Conflict of interest: none declared.
REFERENCES
- 1.Agency for Toxic Substances and Disease Registry, Centers for Disease Control and Prevention. Toxicological Profile for Chlorinated Dibenzo-p-Dioxins. Atlanta, GA: Agency for Toxic Substances and Disease Registry; 1998. [PubMed] [Google Scholar]
- 2.International Agency for Research on Cancer. Chemical Agents and Related Occupations. Lyon, France: International Agency for Research on Cancer; 2012. [Google Scholar]
- 3.Institute of Medicine, National Academy of Sciences. Veterans and Agent Orange: Update 2010. Washington, DC: The National Academies Press; 2012. [Google Scholar]
- 4.Gorski JR, Rozman K. Dose-response and time course of hypothyroxinemia and hypoinsulinemia and characterization of insulin hypersensitivity in 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-treated rats. Toxicology. 1987;44(3):297–307. doi: 10.1016/0300-483x(87)90031-x. [DOI] [PubMed] [Google Scholar]
- 5.Henry EC, Gasiewicz TA. Changes in thyroid hormones and thyroxine glucuronidation in hamsters compared with rats following treatment with 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Appl Pharmacol. 1987;89(2):165–174. doi: 10.1016/0041-008x(87)90037-8. [DOI] [PubMed] [Google Scholar]
- 6.Nishimura N, Miyabara Y, Sato M, et al. Immunohistochemical localization of thyroid stimulating hormone induced by a low oral dose of 2,3,7,8-tetrachlorodibenzo-p-dioxin in female Sprague-Dawley rats. Toxicology. 2002;171(2-3):73–82. doi: 10.1016/s0300-483x(01)00559-5. [DOI] [PubMed] [Google Scholar]
- 7.Ott MG, Zober A, Germann C. Laboratory results for selected target organs in 138 individuals occupationally exposed to TCDD. Chemosphere. 1994;29(9–11):2423–2437. doi: 10.1016/0045-6535(94)90411-1. [DOI] [PubMed] [Google Scholar]
- 8.Calvert GM, Sweeney MH, Deddens J, et al. Evaluation of diabetes mellitus, serum glucose, and thyroid function among United States workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Occup Environ Med. 1999;56(4):270–276. doi: 10.1136/oem.56.4.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson E, Shorter C, Bestervelt L, et al. Serum hormone levels in humans with low serum concentrations of 2,3,7,8-TCDD. Toxicol Ind Health. 2001;17(4):105–112. doi: 10.1191/0748233701th096oa. [DOI] [PubMed] [Google Scholar]
- 10.Pavuk M, Schecter AJ, Akhtar FZ, et al. Serum 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) levels and thyroid function in Air Force veterans of the Vietnam War. Ann Epidemiol. 2003;13(5):335–343. doi: 10.1016/s1047-2797(02)00422-2. [DOI] [PubMed] [Google Scholar]
- 11.Foster WG, Holloway AC, Hughes CL., Jr Dioxin-like activity and maternal thyroid hormone levels in second trimester maternal serum. Am J Obstet Gynecol. 2005;193(6):1900–1907. doi: 10.1016/j.ajog.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 12.Wang SL, Su PH, Jong SB, et al. In utero exposure to dioxins and polychlorinated biphenyls and its relations to thyroid function and growth hormone in newborns. Environ Health Perspect. 2005;113(11):1645–1650. doi: 10.1289/ehp.7994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chevrier J, Eskenazi B, Holland N, et al. Effects of exposure to polychlorinated biphenyls and organochlorine pesticides on thyroid function during pregnancy. Am J Epidemiol. 2008;168(3):298–310. doi: 10.1093/aje/kwn136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Darnerud PO, Lignell S, Glynn A, et al. POP levels in breast milk and maternal serum and thyroid hormone levels in mother-child pairs from Uppsala, Sweden. Environ Int. 2010;36(2):180–187. doi: 10.1016/j.envint.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 15.Zhang J, Jiang Y, Zhou J, et al. Elevated body burdens of PBDEs, dioxins, and PCBs on thyroid hormone homeostasis at an electronic waste recycling site in China. Environ Sci Technol. 2010;44(10):3956–3962. doi: 10.1021/es902883a. [DOI] [PubMed] [Google Scholar]
- 16.Turyk ME, Anderson HA, Persky VW. Relationships of thyroid hormones with polychlorinated biphenyls, dioxins, furans, and DDE in adults. Environ Health Perspect. 2007;115(8):1197–1203. doi: 10.1289/ehp.10179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdelouahab N, Mergler D, Takser L, et al. Gender differences in the effects of organochlorines, mercury, and lead on thyroid hormone levels in lakeside communities of Quebec (Canada) Environ Res. 2008;107(3):380–392. doi: 10.1016/j.envres.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 18.Dallaire R, Muckle G, Dewailly E, et al. Thyroid hormone levels of pregnant Inuit women and their infants exposed to environmental contaminants. Environ Health Perspect. 2009;117(6):1014–1020. doi: 10.1289/ehp.0800219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mocarelli P, Pocchiari F, Nelson N. Preliminary report: 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure to humans—Seveso, Italy. MMWR Morb Mortal Wkly Rep. 1988;37(48):733–736. [PubMed] [Google Scholar]
- 20.Eskenazi B, Mocarelli P, Warner M, et al. Seveso Women's Health Study: a study of the effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on reproductive health. Chemosphere. 2000;40(9-11):1247–1253. doi: 10.1016/s0045-6535(99)00376-8. [DOI] [PubMed] [Google Scholar]
- 21.di Domenico A, Silano V, Viviano G, et al. Accidental release of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) at Sèveso, Italy: II. TCDD distribution in the soil surface layer. Ecotoxicol Environ Saf. 1980;4(3):298–320. doi: 10.1016/0147-6513(80)90032-9. [DOI] [PubMed] [Google Scholar]
- 22.Patterson DG, Jr, Hampton L, Lapeza CR, Jr, et al. High-resolution gas chromatographic/high-resolution mass spectrometric analysis of human serum on a whole-weight and lipid basis for 2,3,7,8-tetrachlorodibenzo-p-dioxin. Anal Chem. 1987;59(15):2000–2005. doi: 10.1021/ac00142a023. [DOI] [PubMed] [Google Scholar]
- 23.Eskenazi B, Mocarelli P, Warner M, et al. Relationship of serum TCDD concentrations and age at exposure of female residents of Seveso, Italy. Environ Health Perspect. 2004;112(1):22–27. doi: 10.1289/ehp.6573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akins JR, Waldrep K, Bernert JT., Jr. The estimation of total serum lipids by a completely enzymatic ‘summation’ method. Clin Chim Acta. 1989;184(3):219–226. doi: 10.1016/0009-8981(89)90054-5. [DOI] [PubMed] [Google Scholar]
- 25.Patterson DG, Turner WE. Method 28: Measurement of PCDDs, PCDFs, and Coplanar PCBs in Serum by HRGC/ID-HRMS. Atlanta, GA: National Center for Environmental Health, Centers for Disease Control and Prevention; 2005. [Google Scholar]
- 26.Patterson DG, Turner WE. Method 28: Measurement of PCBs and Persistent Pesticides in Serum by HRGC/ID-HRMS. Atlanta, GA: National Center for Environmental Health, Centers for Disease Control and Prevention; 2005. [Google Scholar]
- 27.Van den Berg M, Birnbaum LS, Denison M, et al. The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol Sci. 2006;93(2):223–241. doi: 10.1093/toxsci/kfl055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Warner M, Mocarelli P, Brambilla P, et al. Serum TCDD and TEQ concentrations among Seveso women, 20 years after the explosion. J Expo Sci Environ Epidemiol. doi: 10.1038/jes.2013.70. [published online ahead of print October 23, 2013] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Warner M, Mocarelli P, Brambilla P, et al. Diabetes, metabolic syndrome, and obesity in relation to serum dioxin concentrations: the Seveso Women's Health Study. Environ Health Perspect. 2013;121(8):906–911. doi: 10.1289/ehp.1206113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eskenazi B, Warner M, Mocarelli P, et al. Serum dioxin concentrations and menstrual cycle characteristics. Am J Epidemiol. 2002;156(4):383–392. doi: 10.1093/aje/kwf046. [DOI] [PubMed] [Google Scholar]
- 31.Hernán MA, Hernández-Díaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15(5):615–625. doi: 10.1097/01.ede.0000135174.63482.43. [DOI] [PubMed] [Google Scholar]
- 32.van der Laan MJ, Polley EC, Hubbard AE. Berkeley, CA: Division of Biostatistics, University of California, Berkeley;; 2007. Super Learner. (UC Berkeley Division of Biostatistics working paper 222). http://biostats.bepress.com/ucbbiostat/paper222. ) (Accessed May 9, 2014) [Google Scholar]
- 33.Lubin JH, Colt JS, Camann D, et al. Epidemiologic evaluation of measurement data in the presence of detection limits. Environ Health Perspect. 2004;112(17):1691–1696. doi: 10.1289/ehp.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 35.Cerdá M, Diez-Roux AV, Tchetgen ET, et al. The relationship between neighborhood poverty and alcohol use: estimation by marginal structural models. Epidemiology. 2010;21(4):482–489. doi: 10.1097/EDE.0b013e3181e13539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chevrier J. Invited commentary: maternal plasma polybrominated diphenyl ethers and thyroid hormones—challenges and opportunities. Am J Epidemiol. 2013;178(5):714–719. doi: 10.1093/aje/kwt138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saçan MT, Ozkul M, Erdem SS. Physico-chemical properties of PCDD/PCDFs and phthalate esters. SAR QSAR Environ Res. 2005;16(5):443–459. doi: 10.1080/10659360500320602. [DOI] [PubMed] [Google Scholar]
- 38.Yen PM, Brent GA. Genomic and nongenomic actions of thyroid hormones. In: Braveman LE, Cooper DS, editors. Werner & Ingbar's The Thyroid: A Fundamental and Clinical Text. Philadelphia, PA: Lippincott Williams & Wilkins; 2013. pp. 127–138. [Google Scholar]
- 39.Visser TJ, Kaptein E, van Toor H, et al. Glucuronidation of thyroid hormone in rat liver: effects of in vivo treatment with microsomal enzyme inducers and in vitro assay conditions. Endocrinology. 1993;133(5):2177–2186. doi: 10.1210/endo.133.5.8404669. [DOI] [PubMed] [Google Scholar]
- 40.Nishimura N, Yonemoto J, Miyabara Y, et al. Altered thyroxin and retinoid metabolic response to 2,3,7,8-tetrachlorodibenzo-p-dioxin in aryl hydrocarbon receptor-null mice. Arch Toxicol. 2005;79(5):260–267. doi: 10.1007/s00204-004-0626-4. [DOI] [PubMed] [Google Scholar]
- 41.Lans MC, Spiertz C, Brouwer A, et al. Different competition of thyroxine binding to transthyretin and thyroxine-binding globulin by hydroxy-PCBs, PCDDs and PCDFs. Eur J Pharmacol. 1994;270(2-3):129–136. doi: 10.1016/0926-6917(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 42.Grumbach MM, Styne DM. Puberty: ontogeny, neuroendocrinology, physiology, and disorders. In: Larsen PR, Kronenberg HM, Melmed S, et al., editors. Williams Textbook of Endocrinology. 10th ed. Philadelphia, PA: WB Saunders Company; 2003. pp. 1115–1239. [Google Scholar]
- 43.Boverhof DR, Kwekel JC, Humes DG, et al. Dioxin induces an estrogen-like, estrogen receptor-dependent gene expression response in the murine uterus. Mol Pharmacol. 2006;69(5):1599–1606. doi: 10.1124/mol.105.019638. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


