Abstract
We investigated associations between ambient air pollution and microvessel function measured by peripheral arterial tonometry between 2003 and 2008 in the Framingham Heart Study Offspring and Third Generation Cohorts. We measured particulate matter with aerodynamic diameter ≤2.5 µm (PM2.5), black carbon, sulfates, particle number, nitrogen oxides, and ozone by using fixed monitors, and we determined moving averages for 1–7 days preceding vascular testing. We examined associations between these exposures and hyperemic response to ischemia and baseline pulse amplitude, a measure of arterial tone (n = 2,369). Higher short-term exposure to air pollutants, including PM2.5, black carbon, and particle number was associated with higher baseline pulse amplitude. For example, higher 3-day average PM2.5 exposure was associated with 6.3% higher baseline pulse amplitude (95% confidence interval: 2.0, 10.9). However, there were no consistent associations between the air pollution exposures assessed and hyperemic response. Our findings in a community-based sample exposed to relatively low pollution levels suggest that short-term exposure to ambient particulate pollution is not associated with vasodilator response, but that particulate air pollution is associated with baseline pulse amplitude, suggesting potentially adverse alterations in baseline vascular tone or compliance.
Keywords: air pollutants; endothelium, vascular; particulate matter; vascular diseases
Short-term exposure to ambient air pollutants has been associated with death (1, 2) and cardiovascular morbidity, including myocardial infarction (3, 4), heart failure (5), and stroke (5, 6). It has been postulated that the short-term adverse effects of particulate matter exposure may be mediated by induction of abnormal vascular responses characterized by reduced endothelium-mediated vasodilation and vessel constriction. In some (7, 8), but not all (9–11) controlled human exposure experiments, acute exposure to air pollution has been associated with impaired endothelial vasodilator function in the brachial artery. Prior observational studies evaluating short-term air pollution exposure and endothelial function have had mixed findings. In a previous study in the Boston, Massachusetts, area, short-term exposure to air pollution was associated with lower brachial artery flow–mediated dilation only in patients with type 2 diabetes (12). In the Multi-Ethnic Study of Atherosclerosis, short-term exposure to particulate matter was not associated with resting brachial artery diameter or flow-mediated vasodilation (13).
Peripheral arterial tonometry (PAT) is a novel measure of microvascular vasodilator function that depends in part on endothelial-derived nitric oxide. The PAT hyperemic response has demonstrated predictive value for ischemic heart disease (14) and adverse cardiac events (15). Several prior studies have investigated how modulating air pollution exposure might affect the hyperemic response as measured by PAT. Improved PAT hyperemic response was observed after air filtration for 7 days in a study of individuals exposed to wood smoke (16) and after 48 hours in a second study of elderly participants in an urban environment (17). Experimental studies using human exposure chambers reported no effect of particulate matter, wood smoke, or ozone exposure on the PAT hyperemic response (18–20). In contrast to the null results seen for experimental exposure, Pope et al. (20) reported associations between the preceding 2 days of ambient PM2.5 exposure and decreased digital vasodilator responses. In the present study, we aimed to investigate whether short-term ambient air pollutant exposures are associated with vascular function as measured by PAT in a large community-based cohort.
METHODS
Study sample
The study included participants enrolled in the Offspring and Third Generation Cohorts of the Framingham Heart Study. The study design has been described previously (21, 22). PAT examinations began in 2003 and were performed in the eighth cycle of data collection in the Offspring Cohort (in 2005–2008) and were added partway through the first cycle of the Third Generation Cohort (in 2003–2005). All participants provided written informed consent for the Framingham Heart Study examinations, and both the committee on clinical investigation at Beth Israel Deaconess Medical Center and the institutional review board at Boston University Medical Center approved this work. Of the eligible study participants in the Offspring Cohort (n = 2,915) and Third Generation Cohort (n = 2,217), 685 participants were excluded because of participant refusal, anatomical reasons such as mastectomy or limb abnormality, Raynaud's disease, latex allergy, technically inadequate studies, and other reasons, leaving a total of 4,447 PAT examinations. Six observations were excluded because the digital vascular measurements were performed on different dates than the study visits. We further excluded 2,072 study participants with primary residential addresses located more than 50 km from the Harvard Air Pollution Monitoring Supersite, located approximately 1 mile (1.6 km) from downtown Boston, Massachusetts. As a result, 2,369 participants were included for analysis.
Digital vascular function measurements
Digital pulse amplitude was measured as previously described (23). In short, by using a PAT device (Endo-PAT2000, Itamar Medical. Ltd., Caesarea, Israel) on the tip of each index finger, we electronically measured pulse volume changes and expressed them as pulse amplitude. Baseline pulse amplitude was measured in the fingertips of both hands for 2 minutes and 20 seconds. Ischemia was induced by an inflated forearm cuff at 250 mm Hg on 1 arm for 5 minutes. The ratio of the postdeflation pulse amplitude (90–120 seconds after deflation) to the baseline pulse amplitude of the same finger was calculated and divided by the ratio of the pulse amplitude measured in the control finger during the same interval to the baseline pulse amplitude of the control finger, producing the “PAT ratio.” For analyses, we used the natural logarithm of both the PAT ratio and the mean baseline pulse amplitudes from each finger. A lower PAT ratio indicates impaired hyperemic response to ischemia.
Air pollution and meteorological variables
Levels of ambient air pollutants were measured continuously at the Harvard Air Pollution Monitoring Supersite to reflect urban background levels. This site is on the rooftop of the Francis A. Countway Library of Medicine, 5 stories above ground level and 50 m from the nearest street. Daily means were computed using hourly data from 9 am to 9 am. PM2.5 was measured using a tapered-element oscillating microbalance (Model 1400A, Rupprecht & Patashnick Co. Inc., Albany, New York), and black carbon was measured using an Aethalometer (model AE-16, Magee Scientific Corp., Berkeley, California) on the basis of optical transmittance at a single wavelength (λ = 880 nm). Daily sulfate concentrations were calculated from elemental sulfur measured by x-ray fluorescence analysis of the PM2.5 filter samples. On days when sulfur measurements were not available, daily sulfate concentrations were determined by using a sulfate analyzer (Model 5020, Thermo Electron Corp., Franklin, Massachusetts). Hourly particle number concentrations (number of particles per cm3 on filter sample) were measured with a condensation particle counter (Model 3022A, TSI, Inc., Shoreview, Minnesota). Hourly concentrations of the gaseous pollutants ozone and nitrogen oxides were measured by local state monitors located within the Greater Boston area, and exposures were estimated by averaging data from the available sites (24). Finally, hourly temperature and relative humidity data were obtained from the Logan International Airport (Boston, Massachusetts) weather station, located 12 km from the central monitoring site.
Statistical methods
We assessed 1-, 2-, 3-, 5-, and 7-day moving averages of PM2.5 mass, black carbon, particle number, sulfate, nitrogen oxides, and ozone prior to PAT examinations. We fit multivariable linear regression models for each pollutant's moving average as a linear term. Prevalent individual-level covariates that were expected to confound or be associated with the outcome were added to the model, including age (linear and quadratic term); sex; body mass index (weight (kg)/height (m)2); cohort (Offspring or Third Generation); smoking status (current, former, or never smoker); systolic blood pressure (mean of 2 measurements taken by the physician administering the clinical examination); diabetes mellitus (defined as having a fasting blood glucose level ≥126 mg/dL, reporting antidiabetic medication use at an examination, or having any previous history of diabetes (excluding gestational diabetes); triglyceride level; ratio of total cholesterol to high-density lipoprotein; educational level (no high school degree, high school degree, some college education, or college degree); and quartile of median household income in the participants' residential census tracts from the 2000 US Census. Because regular uses of antihypertensive and statin medications were highly collinear, we used a combined indicator variable for these. We also adjusted for season by modeling the sine and cosine of the day of year, linear time trend using date of examination, day of week, temperature, relative humidity, and the cross-product term of ambient temperature and relative humidity.
Recognizing that systolic blood pressure could also be a mediator, we ran models without adjustment for systolic blood pressure in sensitivity analyses. Furthermore, to explore associations within current US Environmental Protection Agency (EPA) standards, we excluded days exceeding the EPA National Ambient Air Quality Standard for 24-hour PM2.5 of 35 μg/m3. Additional sensitivity analyses included exclusion of current smokers and exploration of nonlinear associations by fitting restricted natural cubic splines of 2- and 3-day moving average pollutant exposures using Harrell's cut-points for 5 knots (5%, 27.5%, 50%, 72.5%, and 95%). Visual representation was used to assess departure from linearity. Furthermore, we compared the results after restricting analysis to the participants who resided 40 km or less from the Harvard Air Pollution Monitoring Supersite monitor.
Previous studies have indicated differences in cardiovascular susceptibility to air pollution exposures by sex and diabetes status (13, 25) and differences in associations with air pollution exposure for other vascular outcomes by use of antihypertensive (26) and statin (27) medications. These medications have demonstrated associations with microvascular function as measured by PAT (23, 28, 29). In secondary analyses, we explored interactions by age, sex, diagnosis of diabetes, and regular use of antihypertensive or statin medication. We used cross-product terms of a continuous variable for 2-day moving averages of PM2.5, our primary air pollutant, and a binary indicator variable for age (≤65 vs. >65 years), sex, diabetes diagnosis, and antihypertensive or statin medication use.
For comparability with other studies, we expressed the associations of air pollution with baseline pulse amplitude and PAT ratio as the percent change in outcome per 5 µg/m3 for PM2.5, 0.4 µg/m3 for black carbon, 15,000 particles/cm3 for particle number, 2 µg/m3 for sulfate, and 0.01 ppm for nitrogen oxides and ozone. Incremental units approximated the interquartile range of each pollutant. We used an estimation approach in our analyses of correlated pollutants and moving averages and did not use a prespecified α level to conduct formal hypothesis testing. All estimates are, therefore, presented with 95% confidence intervals. We present P values for tests of homogeneity (the cross-product terms) and consider P values of less than 0.05 as suggestive of interaction. The GLM procedure in SAS, version 9.3, software (SAS Institute, Inc., Cary, North Carolina) was used for all main analyses. Nonlinear analyses (using splines) were performed using Stata, version 13, software (StataCorp LP, College Station, Texas).
RESULTS
Study participants
There were similar numbers of men and women. The mean body mass index value was in the overweight category (Table 1). Average systolic blood pressure level was normal, although 39% of the participants used antihypertensive medication. The majority of participants were either previous or current smokers and were highly educated, with more than 70% having attended college.
Table 1.
Characteristics of 2,369 Participants in the Framingham Heart Study Third Generation and Offspring Cohorts,a Greater Boston Area, Massachusetts, 2003–2008
| Characteristic | No. | % | Mean (SD) |
|---|---|---|---|
| Female sex | 1,197 | 51 | |
| Age, years | 56 (16) | ||
| Body mass indexb | 28.0 (5.5) | ||
| Total cholesterol:high-density lipoprotein | 3.6 (1.2) | ||
| Triglycerides, mg/dL | 119 (79) | ||
| Systolic blood pressure, mm Hg | 124 (17) | ||
| Diabetes mellitus | 278 | 12 | |
| Smoking status | |||
| Missing | 42 | 2 | |
| Current smoker | 327 | 14 | |
| Former smoker | 996 | 42 | |
| Never smoker | 1,004 | 42 | |
| Educational level | |||
| No high school degree | 66 | 3 | |
| High school degree | 593 | 25 | |
| Some college | 790 | 33 | |
| College degree | 920 | 39 | |
| Medication use | |||
| Antihypertensives | 912 | 39 | |
| Statins | 698 | 29 | |
| Both antihypertensives and statins | 510 | 22 |
Abbreviation: SD, standard deviation.
a There were 1,458 (62%) participants in the Offspring Cohort.
b Weight (kg)/height (m)2.
Air pollution and meteorological variables
Mean air pollutant concentrations 1 day preceding PAT examinations were relatively low with a fair amount of variability (Table 2). During the entire study period, only 3 of the 7 days prior to vascular testing exceeded the EPA National Ambient Air Quality Standard for 24-hour PM2.5 (affecting 21 observations). Sulfate had the largest portion of missing data (15% missing 1-day averages). Spearman's rank correlation coefficients (Table 3) showed that PM2.5 was most strongly correlated with sulfate and black carbon. Slightly weaker correlations were observed between black carbon and nitrogen oxides. Particle number was negatively correlated with all pollutants except nitrogen oxides. Temperature showed weak correlation with all pollutants, with the exception of particle number, to which it was strongly negatively correlated. The correlation coefficients between 2-day moving averages of PM2.5 and other averaging periods (from 1 to 7 days) ranged from 0.63 to 0.91. There was very little variation in the interquartile ranges among averaging times.
Table 2.
Daily Concentrations of Pollutants and Meteorological Variables for the Framingham Heart Study Third Generation and Offspring Cohorts, Greater Boston Area, Massachusetts, 2003–2008
| Pollutant or Meteorological Variable | No. of Observations | Mean (SD) | Range | IQR |
|---|---|---|---|---|
| PM2.5, µg/m3 | 2,366 | 9.6 (5.3) | 0.9–36.3 | 6.3 |
| Black carbon, µg/m3 | 2,365 | 0.7 (0.4) | 0.1–2.7 | 0.5 |
| Particle number, particles/cm3 | 2,098 | 20,560 (10,886) | 3,791–63,886 | 15,299 |
| Sulfate, µg/m3 | 2,072 | 3.2 (2.5) | 0.0–14.7 | 2.6 |
| Nitrogen oxides, ppm | 2,369 | 0.034 (0.016) | 0.006–0.135 | 0.016 |
| Ozone, ppm | 2,369 | 0.023 (0.011) | 0.001–0.064 | 0.015 |
| Temperature, °C | 2,369 | 10.7 (9.5) | −18.3–31.0 | 15.0 |
| Relative humidity, % | 2,369 | 66.4 (16.0) | 20.7–99.3 | 25.6 |
Abbreviations: IQR, interquartile range; PM2.5, particulate matter with an aerodynamic diameter ≤2.5 μm; SD, standard deviation.
Table 3.
Daily Spearman's Rank Correlation Coefficients for Pollutants and Meteorological Variables From the Framingham Heart Study Third Generation and Offspring Cohorts, Greater Boston Area, Massachusetts, 2003–2008
| Pollutant or Meteorological Variable | PM2.5 | Black Carbon | Particle No. | Sulfate | Nitrogen Oxides | Ozone | Temperature |
|---|---|---|---|---|---|---|---|
| PM2.5 | 1.00 | ||||||
| Black carbon | 0.69 | 1.00 | |||||
| Particle number | −0.16 | −0.12 | 1.00 | ||||
| Sulfate | 0.86 | 0.52 | −0.23 | 1.00 | |||
| Nitrogen oxides | 0.37 | 0.56 | 0.31 | 0.12 | 1.00 | ||
| Ozone | 0.20 | −0.13 | −0.22 | 0.37 | −0.57 | 1.00 | |
| Temperature | 0.31 | 0.36 | −0.71 | 0.37 | −0.28 | 0.37 | 1.00 |
| Relative humidity | 0.20 | 0.41 | −0.25 | 0.17 | 0.02 | −0.16 | 0.32 |
Abbreviation: PM2.5, particulate matter with aerodynamic diameter ≤2.5 μm.
Associations between air pollutants and PAT ratio
The mean PAT ratios were 0.57 (standard deviation (SD), 0.38) for men and 0.83 (SD, 0.40) for women. No consistent pattern of association was evident between averaging periods of PM2.5, black carbon, particle number, sulfate, and nitrogen oxides and hyperemic response (Figure 1A). Ozone showed a weak pattern of associations with lower PAT ratio for 1- to 3-day moving averages. For longer moving averages of PM2.5, sulfate, and ozone, we observed unexpected higher PAT ratios with higher exposure.
Figure 1.
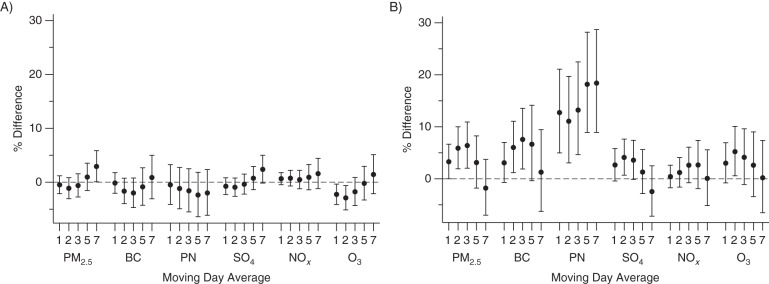
Associations of moving day averages in air pollutants with measurements of microvascular function (n = 2,369) in the Framingham Heart Study Offspring and Third Generation Cohorts, Greater Boston Area, Massachusetts, 2003–2008. A) Peripheral arterial tonometry ratio. B) Baseline pulse amplitude. Results expressed as percent difference in measurement per increment in air pollution. Unit changes in air pollutants are as follows: particulate matter with aerodynamic diameter ≤2.5 µm (PM2.5), 5 µg/m3; black carbon (BC), 0.4 µg/m3; particle number (PN), 15,000/cm3; sulfate (SO4), 2 µg/m3; nitrogen oxides (NOx), 0.01 ppm; and ozone (O3), 0.01 ppm. Models are adjusted for age, age2, sex, cohort, presence of diabetes, body mass index (weight (kg)/height (m)2), triglyceride level, ratio of total cholesterol to high-density lipoprotein, mean systolic blood pressure, median household income of US census tract in 2000, educational level, smoking status, day of week, season (sine and cosine of day of year), time trend, temperature, relative humidity, temperature × relative humidity, and use of statin or antihypertensive medication. Bars, 95% confidence intervals.
Associations between air pollutants and baseline pulse amplitude
The mean logs of baseline pulse amplitude in the full sample were 6.1 (SD, 0.76) for men and 5.2 (SD, 0.81) for women. Overall, we observed patterns of association suggesting that higher air pollutant exposure in the 2–5 days preceding vascular testing was associated with higher baseline pulse amplitude. A 5-μg/m3 higher 2- or 3-day average of PM2.5 was associated with 5.9% (95% confidence interval (CI): 1.9%, 10.0%) and 6.4% (95% CI: 2.0%, 10.9%) higher baseline pulse amplitudes, respectively. The magnitude of the associations decreased after 3-day moving averages of PM2.5 (Figure 1B). Higher 2- and 3-day exposures to black carbon demonstrated similar patterns of association as for PM2.5. Higher particle numbers were consistently associated with higher baseline pulse amplitude for several moving averages, with the strongest estimates for 5-day (18.2% higher per 15,000 particles/cm3, 95% CI: 8.9%, 28.2%) and 7-day (18.4% higher, 95% CI: 8.9%, 28.7%) moving averages.
Sensitivity analyses
None of the results was materially altered in models that either excluded the 3 days when air pollution levels exceeded the EPA National Ambient Air Quality Standard for 24-hour PM2.5 (Web Figure 1, available at http://aje.oxfordjournals.org/) or did not adjust for systolic blood pressure (Web Figure 2), a potential intermediate variable; nor were the results altered in models that excluded participants who were current smokers (Web Figure 3). There was no consistent evidence of nonlinear associations between air pollution exposures and PAT ratio (Web Figure 4). Associations between particle number and ozone and baseline pulse amplitude were linear. Other pollutants demonstrated steeper linear slopes with lower levels of these pollutants, where the majority of the observations lay, and less steep slopes with higher levels of these pollutants (Web Figure 5). Restriction to a 40-km catchment area did not materially change associations.
Secondary analyses
We found no evidence that the associations between 2-day moving averages of PM2.5 and PAT ratio and baseline pulse amplitude varied by participant sex or diabetes status (all P values for interaction > 0.10). Lower PAT ratios were observed in participants 65 years of age and younger, as well as participants who were not taking antihypertensive or statin medication (difference in PAT ratio per 5-μg/m3 of PM2.5 for participants ≤65 years of age = −2.4% (95% CI: −4.5%, −0.16%); difference in PAT ratio per 5-μg/m3 of PM2.5 for nonmedicated participants = −2.5% (95% CI: −4.9%, −0.06%), P for interaction = 0.02 and 0.07, respectively). No association was observed in participants over age 65 years or in those taking antihypertensive or statin medication (1.8%, 95% CI: −1.4%, 5.1% and 0.5%, 95% CI: −2.1%, 3.2%, respectively). Both variables were highly correlated (Spearman's ρ = 0.57). Age, sex, diabetes diagnosis, and use of antihypertensive or statin medication did not modify associations between 2-day moving average of PM2.5 and baseline pulse amplitude (all P values for interaction > 0.10).
DISCUSSION
In our large community-based study, short-term exposure to particulate air pollution was not associated with PAT ratio, a measure of hyperemic response to shear stress in the digital vascular bed that is partly endothelium dependent (30). Interestingly, short-term exposures to several pollutants were associated with higher baseline pulse amplitude in the finger circulation. These findings suggest that short-term air pollutant exposure does not impair microvessel vasodilator responses. The observations with baseline pulse amplitude indicate the possibility that higher air pollution exposure over a period of days increases resting pulsatility at air pollution levels within EPA standards.
In larger conduit arteries such as the brachial artery, short-term exposures to PM2.5, sulfate, and black carbon have been associated with vasodilatory response in some (12, 31) but not all studies (13). PAT ratio, however, is a measure of small-vessel vasodilator response that also relates to clinical outcomes (14, 15, 32). Prior studies investigating associations between short-term exposure to particulate pollution and PAT have been limited to small, controlled-exposure studies or air filtration intervention studies and focused solely on the hyperemic response (i.e., PAT ratio) (16–20). Improvements in PAT ratio have been demonstrated in interventional crossover studies by reducing household particulates with filters (16, 17). In contrast, controlled exposure to particulate matter from traffic (18), coal (20), or wood smoke (19, 20) was not associated with PAT ratio.
The lack of associations observed between air pollutant exposures and small-vessel vasodilator response in our study may reflect a true null effect. Several other explanations are also possible. Small vessels may have different physiological properties than brachial arteries (28) and may be less affected by air pollution exposure than has been previously reported for brachial arteries in some (12, 31) but not all studies (13). Exposure distributions and sources may also differ among studies. However, the mean levels observed in our study are only slightly lower than those of other studies investigating PAT ratio, and we did not account for potentially different associations by mixtures or sources of air pollution. We observed unexpectedly higher PAT ratios with higher PM2.5 and sulfate for longer moving averages. It is unclear whether this represents a compensatory mechanism and should be confirmed in other data sets. Results from subgroup analyses suggested that the associations with PAT ratio were not explained by factors such as current smoking status, sex, or diagnosis of diabetes, although there was some indication that younger individuals and participants not taking antihypertensive or statin medication were more susceptible. Age and medication status were highly correlated and, in the absence of randomization, we cannot disentangle medication effects from different baseline characteristics associated with medication use or age.
Our results with acute air pollutant exposure and baseline pulse amplitude are intriguing. Resting pulse amplitude reflects digital blood flow and sympathetic tone. Treatment with phenylephrine, a vasoconstrictive agent, has been shown to reduce baseline pulse amplitude (30). Acute increases in sympathetic tone during ischemia also reduce baseline pulse amplitude. In cross-sectional studies, several cardiovascular risk factors relate to higher baseline pulse amplitude (23, 28). Prior short-term air pollution studies have not reported the effects on baseline pulse amplitude or blood flow. We found that several measures of air pollution, including particle number, PM2.5, and black carbon, showed a time-dependent association with higher baseline pulse amplitude, suggesting that air pollutants may induce altered small-vessel tone or increase peripheral blood flow. The time-dependent patterns of association were similar for PM2.5 and black carbon but different for particle number, possibly reflecting different sources and dispersion patterns. Ultrafine particles, the main determinant of particle number, are predominately freshly generated combustion particles with a high ratio of surface area to volume, increasing their potential for reactivity (33), and they are predominantly emitted from local traffic. Therefore, this metric may be a marker of mixtures of air pollution containing local emissions from traffic and/or oil or wood burning or specifically implicate ultrafine particles, constituting the majority of the particle numbers. These small particles, which are distributed to the alveoli, have been hypothesized to be especially toxic because of their high ratio of surface area to volume (34). PM2.5 consists of primary and secondary species associated with local and regional sources. Black carbon is a light-absorbing component of particulate matter formed by the incomplete combustion of carbon-containing fuels. Both pollutants are markers of local emissions but with a sizable contribution of regional emissions, particularly for PM2.5. Previous studies have also reported associations between short-term exposure to black carbon and related vascular outcomes such as retinal microvascular dysfunction (35) and decreased brachial artery diameter (12).
Associations between other pollutants and baseline pulse amplitude demonstrated less consistent patterns. This may indicate that regional particulate pollution, represented by sulfate, is not strongly associated with microvascular tone. Various components of local traffic pollution, such as particle number and nitrogen oxides, may also have different associations with baseline pulse amplitude. Nitrogen dioxide, 1 of the 2 gases measured as nitrogen oxides (nitrogen oxides = nitrogen dioxide + nitric oxide), did not demonstrate effects on forearm blood flow in a study of controlled exposure, which is consistent with our results (36).
Finally, we found that the associations between PM2.5 and baseline pulse amplitude were similar for men and women regardless of age or diabetes diagnosis. Although participants not taking antihypertensive or statin medication showed stronger associations with greater baseline pulse amplitude than those taking these medications, the interaction was not significant.
Our study has several limitations. The use of stationary monitors to estimate daily exposure to air pollutants for participants with primary addresses within 50 km of the central monitoring site likely introduces some degree of Berkson and classical measurement errors, which will result in wider confidence intervals, reduced statistical power, and some bias toward the null, especially for particle number, which shows more spatial heterogeneity (37). However, the majority of the short-term variation in the pollutants of interest in this study was contributed by temporal variation rather than spatial variation in the study region, and results were similar when restricting the catchment area to within 40 km of the central site. Earlier studies in Boston have shown that PM2.5 concentrations measured at the central site are strong proxies for personal exposure to ambient PM2.5 (38). In sensitivity analyses, we observed a degree of nonlinear association between PM2.5, black carbon, sulfate, and nitrogen oxides and baseline pulse amplitude with steeper slopes at low ranges of exposure and less steep slopes at higher ranges, possibly indicating a log-linear relationship. However, we had few data for higher levels of these pollutants. The study participants were almost all of non-Hispanic white origin, which limits the generalizability of the results to other races or ethnic groups. In addition, the observational and descriptive design of the study included a number of comparisons and precludes us from establishing causal associations.
Our study has several notable strengths. The Framingham Heart Study Cohorts offer the advantage of a large-scale, community-based observational study with a standardized protocol. Our results were adjusted for a robust set of potential confounders and predictors of microvascular function using the rich data incorporated in the Framingham Heart Study. PAT also has the advantage of yielding noninvasive measurements of microvessel function with little operator dependence. Finally, the outcome assessment data were collected independently and by investigators who were blinded to the air pollution measurements.
We found no associations between short-term exposure to ambient particulate pollution and PAT ratio. Our findings suggest that short-term air pollution is not associated with microvessel vasodilator function. However, we observed associations between PM2.5, black carbon, and particle number levels and baseline pulse amplitude. Local combustion sources, such as traffic or domestic heating, rather than regional particulate pollution, may be more important exposures for resting microvascular tone, possibly modulating blood flow, compliance, and sympathetic tone in the digital vascular circulation.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Cardiovascular Epidemiology Research Unit, Department of Cardiology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, Massachusetts (Petter L. Ljungman, Elissa H. Wilker, Mary B. Rice, Murray A. Mittleman); Institute of Environmental Medicine, Karolinska Institute, Stockholm, Sweden (Petter L. Ljungman); Department of Environmental Health, Harvard School of Public Health, Boston, Massachusetts (Joel Schwartz, Diane R. Gold, Petros Koutrakis); Pulmonary and Critical Care Unit, Massachusetts General Hospital, Boston, Massachusetts (Mary B. Rice); National Heart, Lung, and Blood Institute, Bethesda, Maryland (Joseph A. Vita, Ramachandran S. Vasan, Emelia J. Benjamin, Naomi M. Hamburg); Boston University's Framingham Heart Study, Framingham, Massachusetts (Joseph A. Vita, Ramachandran S. Vasan, Emelia J. Benjamin, Naomi M. Hamburg); Whitaker Cardiovascular Institute, Boston University School of Medicine, Boston, Massachusetts (Joseph A. Vita, Ramachandran S. Vasan, Emelia J. Benjamin, Naomi M. Hamburg); and Cardiovascular Engineering, Inc., Norwood, Massachusetts (Gary F. Mitchell).
This work was supported by the National Heart, Lung, and Blood Institute (grants N01HC 25195, 1R01HL60040, and 1RO1HL70100), the US Environmental Protection Agency (grants R832416 and RD83479801), the National Institute of Environmental Health Sciences (grants ES009825, K99ES022243, 1F32ES023352-01, and P30ES000002), the Swedish Council for Working Life and Social Research Marie Curie International Postdoctoral Fellowship Programme, the Swedish Heart-Lung Foundation, the Swedish Society of Cardiology, and the Swedish Society for Medical Research.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Environmental Protection Agency.
Conflict of interest: G.F.M. is the owner of Cardiovascular Engineering, Inc., a company that develops and manufactures devices to measure vascular stiffness, and he serves as a consultant to and receives honoraria from Merck & Co. and Novartis AG.
REFERENCES
- 1.Kinney PL, Ozkaynak H. Associations of daily mortality and air pollution in Los Angeles County. Environ Res. 1991;54(2):99–120. doi: 10.1016/s0013-9351(05)80094-5. [DOI] [PubMed] [Google Scholar]
- 2.Brook RD, Rajagopalan S, Pope CA, 3rd, et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121(21):2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 3.Peters A, Dockery DW, Muller JE, et al. Increased particulate air pollution and the triggering of myocardial infarction. Circulation. 2001;103(23):2810–2815. doi: 10.1161/01.cir.103.23.2810. [DOI] [PubMed] [Google Scholar]
- 4.Peters A, von Klot S, Heier M, et al. Exposure to traffic and the onset of myocardial infarction. N Engl J Med. 2004;351(17):1721–1730. doi: 10.1056/NEJMoa040203. [DOI] [PubMed] [Google Scholar]
- 5.Dominici F, Peng RD, Bell ML, et al. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA. 2006;295(10):1127–1134. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wellenius GA, Burger MR, Coull BA, et al. Ambient air pollution and the risk of acute ischemic stroke. Arch Intern Med. 2012;172(3):229–234. doi: 10.1001/archinternmed.2011.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barath S, Mills NL, Lundbäck M, et al. Impaired vascular function after exposure to diesel exhaust generated at urban transient running conditions. Part Fibre Toxicol. 2010;7:19. doi: 10.1186/1743-8977-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mills NL, Törnqvist H, Robinson SD, et al. Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation. 2005;112(25):3930–3936. doi: 10.1161/CIRCULATIONAHA.105.588962. [DOI] [PubMed] [Google Scholar]
- 9.Brook RD, Brook JR, Urch B, et al. Inhalation of fine particulate air pollution and ozone causes acute arterial vasoconstriction in healthy adults. Circulation. 2002;105(13):1534–1536. doi: 10.1161/01.cir.0000013838.94747.64. [DOI] [PubMed] [Google Scholar]
- 10.Peretz A, Sullivan JH, Leotta DF, et al. Diesel exhaust inhalation elicits acute vasoconstriction in vivo. Environ Health Perspect. 2008;116(7):937–942. doi: 10.1289/ehp.11027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Urch B, Brook JR, Wasserstein D, et al. Relative contributions of PM2.5 chemical constituents to acute arterial vasoconstriction in humans. Inhal Toxicol. 2004;16(6–7):345–352. doi: 10.1080/08958370490439489. [DOI] [PubMed] [Google Scholar]
- 12.O'Neill MS, Veves A, Zanobetti A, et al. Diabetes enhances vulnerability to particulate air pollution–associated impairment in vascular reactivity and endothelial function. Circulation. 2005;111(22):2913–2920. doi: 10.1161/CIRCULATIONAHA.104.517110. [DOI] [PubMed] [Google Scholar]
- 13.Krishnan RM, Adar SD, Szpiro AA, et al. Vascular responses to long- and short-term exposure to fine particulate matter: MESA Air (Multi-Ethnic Study of Atherosclerosis and Air Pollution) J Am Coll Cardiol. 2012;60(21):2158–2166. doi: 10.1016/j.jacc.2012.08.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuzawa Y, Sugiyama S, Sugamura K, et al. Digital assessment of endothelial function and ischemic heart disease in women. J Am Coll Cardiol. 2010;55(16):1688–1696. doi: 10.1016/j.jacc.2009.10.073. [DOI] [PubMed] [Google Scholar]
- 15.Rubinshtein R, Kuvin JT, Soffler M, et al. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur Heart J. 2010;31(9):1142–1148. doi: 10.1093/eurheartj/ehq010. [DOI] [PubMed] [Google Scholar]
- 16.Allen RW, Carlsten C, Karlen B, et al. An air filter intervention study of endothelial function among healthy adults in a woodsmoke-impacted community. Am J Respir Crit Care Med. 2011;183(9):1222–1230. doi: 10.1164/rccm.201010-1572OC. [DOI] [PubMed] [Google Scholar]
- 17.Bräuner EV, Forchhammer L, Møller P, et al. Indoor particles affect vascular function in the aged: an air filtration-based intervention study. Am J Respir Crit Care Med. 2008;177(4):419–425. doi: 10.1164/rccm.200704-632OC. [DOI] [PubMed] [Google Scholar]
- 18.Bräuner EV, Møller P, Barregard L, et al. Exposure to ambient concentrations of particulate air pollution does not influence vascular function or inflammatory pathways in young healthy individuals. Part Fibre Toxicol. 2008;5:13. doi: 10.1186/1743-8977-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forchhammer L, Møller P, Riddervold IS, et al. Controlled human wood smoke exposure: oxidative stress, inflammation and microvascular function. Part Fibre Toxicol. 2012;9:7. doi: 10.1186/1743-8977-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pope CA, 3rd, Hansen JC, Kuprov R, et al. Vascular function and short-term exposure to fine particulate air pollution. J Air Waste Manag Assoc. 2011;61(8):858–863. doi: 10.3155/1047-3289.61.8.858. [DOI] [PubMed] [Google Scholar]
- 21.Splansky GL, Corey D, Yang Q, et al. The Third Generation Cohort of the National Heart, Lung, and Blood Institute's Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165(11):1328–1335. doi: 10.1093/aje/kwm021. [DOI] [PubMed] [Google Scholar]
- 22.Kannel WB, Feinleib M, McNamara PM, et al. An investigation of coronary heart disease in families. The Framingham Offspring Study. Am J Epidemiol. 1979;110(3):281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 23.Hamburg NM, Keyes MJ, Larson MG, et al. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation. 2008;117(19):2467–2474. doi: 10.1161/CIRCULATIONAHA.107.748574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz J. The distributed lag between air pollution and daily deaths. Epidemiology. 2000;11(3):320–326. doi: 10.1097/00001648-200005000-00016. [DOI] [PubMed] [Google Scholar]
- 25.Adar SD, Sheppard L, Vedal S, et al. Fine particulate air pollution and the progression of carotid intima-medial thickness: a prospective cohort study from the Multi-Ethnic Study of Atherosclerosis and Air Pollution. PLoS Med. 2013;10(4):e1001430. doi: 10.1371/journal.pmed.1001430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dvonch JT, Kannan S, Schulz AJ, et al. Acute effects of ambient particulate matter on blood pressure: differential effects across urban communities. Hypertension. 2009;53(5):853–859. doi: 10.1161/HYPERTENSIONAHA.108.123877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allen RW, Criqui MH, Diez Roux AV, et al. Fine particulate matter air pollution, proximity to traffic, and aortic atherosclerosis. Epidemiology. 2009;20(2):254–264. doi: 10.1097/EDE.0b013e31819644cc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schnabel RB, Schulz A, Wild PS, et al. Noninvasive vascular function measurement in the community: cross-sectional relations and comparison of methods. Circ Cardiovasc Imaging. 2011;4(4):371–380. doi: 10.1161/CIRCIMAGING.110.961557. [DOI] [PubMed] [Google Scholar]
- 29.Hamburg NM, Palmisano J, Larson MG, et al. Relation of brachial and digital measures of vascular function in the community: the Framingham Heart Study. Hypertension. 2011;57(3):390–396. doi: 10.1161/HYPERTENSIONAHA.110.160812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nohria A, Gerhard-Herman M, Creager MA, et al. Role of nitric oxide in the regulation of digital pulse volume amplitude in humans. J Appl Physiol (1985) 2006;101(2):545–548. doi: 10.1152/japplphysiol.01285.2005. [DOI] [PubMed] [Google Scholar]
- 31.Dales R, Liu L, Szyszkowicz M, et al. Particulate air pollution and vascular reactivity: the Bus Stop Study. Int Arch Occup Environ Health. 2007;81(2):159–164. doi: 10.1007/s00420-007-0199-7. [DOI] [PubMed] [Google Scholar]
- 32.Bonetti PO, Pumper GM, Higano ST, et al. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J Am Coll Cardiol. 2004;44(11):2137–2141. doi: 10.1016/j.jacc.2004.08.062. [DOI] [PubMed] [Google Scholar]
- 33.Buzea C, Pacheco II, Robbie K. Nanomaterials and nanoparticles: sources and toxicity. Biointerphases. 2007;2(4):MR17–MR71. doi: 10.1116/1.2815690. [DOI] [PubMed] [Google Scholar]
- 34.Nemmar A, Holme JA, Rosas I, et al. Recent advances in particulate matter and nanoparticle toxicology: a review of the in vivo and in vitro studies. BioMed Res Int. 2013;2013 doi: 10.1155/2013/279371. Article ID 279371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Louwies T, Panis LI, Kicinski M, et al. Retinal microvascular responses to short-term changes in particulate air pollution in healthy adults. Environ Health Perspect. 2013;121(9):1011–1016. doi: 10.1289/ehp.1205721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Langrish JP, Lundbäck M, Barath S, et al. Exposure to nitrogen dioxide is not associated with vascular dysfunction in man. Inhal Toxicol. 2010;22(3):192–198. doi: 10.3109/08958370903144105. [DOI] [PubMed] [Google Scholar]
- 37.Durant JL, Ash CA, Wood EC, et al. Short-term variation in near-highway air pollutant gradients on a winter morning. Atmos Chem Phys. 2010;10(2):5599–5626. doi: 10.5194/acpd-10-5599-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown KW, Sarnat JA, Suh HH, et al. Factors influencing relationships between personal and ambient concentrations of gaseous and particulate pollutants. Sci Total Environ. 2009;407(12):3754–3765. doi: 10.1016/j.scitotenv.2009.02.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


