Early phase clinical trials have evaluated the nelipepimut-S vaccine administered to breast cancer patients in the adjuvant setting. Final analyses after 60 months follow-up show the vaccine to be safe and capable of stimulating an antigen-specific immune response and suggest the vaccine has clinical efficacy. Results from these trials have informed the design of a phase III registration trial.
Keywords: breast cancer, nelipepimut-S, vaccine, immunotherapy
Abstract
Background
E75 (nelipepimut-S) is a human leukocyte antigen (HLA)-A2/A3-restricted immunogenic peptide derived from the HER2 protein. We have conducted phase I/II clinical trials vaccinating breast cancer patients with nelipepimut-S and granulocyte–macrophage colony-stimulating factor (GM-CSF) in the adjuvant setting to prevent disease recurrence. All patients have completed 60 months follow-up, and here, we report the final analyses.
Patients and methods
The studies were conducted as dose escalation/schedule optimization trials enrolling node-positive and high-risk node-negative patients with tumors expressing any degree of HER2 (immunohistochemistry 1–3+). HLA-A2/3+ patients were vaccinated; others were followed prospectively as controls. Local and systemic toxicity was monitored. Clinical recurrences were documented, and disease-free survival (DFS) was analyzed by Kaplan–Meier curves; groups were compared using log-rank tests.
Results
Of 195 enrolled patients, 187 were assessable: 108 (57.8%) in the vaccinated group (VG) and 79 (42.2%) in the control group (CG). The groups were well matched for clinicopathologic characteristics. Toxicities were minimal. Five-year DFS was 89.7% in the VG versus 80.2% in the CG (P = 0.08). Due to trial design, 65% of patients received less than the optimal vaccine dose. Five-year DFS was 94.6% in optimally dosed patients (P = 0.05 versus the CG) and 87.1% in suboptimally dosed patients. A voluntary booster program was initiated, and among the 21 patients that were optimally boosted, there was only one recurrence (DFS = 95.2%).
Conclusion
The E75 vaccine is safe and appears to have clinical efficacy. A phase III trial evaluating the optimal dose and including booster inoculations has been initiated.
Clinical Trials
NCT00841399, NCT00584789.
introduction
The majority of cancer vaccines target tumor-associated antigens (TAA) to elicit a cytotoxic T-lymphocyte (CTL) response. HER2 is a well-described TAA in breast cancer, and several HER2-derived peptides have been shown to elicit a specific immune response. The most studied HER2-derived peptide is E75 (nelipepimut-S) (reviewed by Mittendorf et al. [1]). Nelipepimut-S has been used in multiple vaccine formulations; it has been loaded on to autologous dendritic cells [2], embedded in longer peptides capable of eliciting both CTL and CD4+ helper T-cell responses [3], and used as a single peptide combined with various immunoadjuvants [4, 5].
All of these formulations are safe with comparable effectiveness in stimulating peptide-specific immunity. Combining nelipepimut-S with an immunoadjuvant is the simplest approach; therefore, our group has investigated nelipepimut-S with granulocyte–macrophage colony-stimulating factor (GM-CSF) administered in the adjuvant setting to disease-free breast cancer patients at high risk for recurrence. We have conducted phase I/II trials to document the safety, immunogenicity, and clinical efficacy of nelipepimut-S. Per protocol design, the primary analysis of the combined trials was initiated at 18-month median follow-up. At this point, the vaccine was safe, capable of stimulating HER2-specific immunity and there was evidence of clinical benefit with a recurrence rate of 5.6% in the vaccinated group compared with 14.2% in the control group (P = 0.04) [6].
Trial follow-up was extended to 60 months and a booster program was initiated. Boosters are safe and effective in stimulating E75-specific immunity in patients with waning levels of E75-specific CTLs [7].
When the length of follow-up was extended, additional analyses were incorporated to include disease-free survival (DFS) evaluation at 24 and 60 months. Sixty-month follow-up has been completed in all patients, and here we report the final trial results.
methods
patient characteristics and clinical protocols
The trials were conducted under an investigational new drug application and were approved by the Institutional Review Board. Trial details have been reported [6, 8, 9]. Patients had histologically confirmed node-positive or high-risk node-negative [≥T2, grade 3, estrogen receptor (ER)- and progesterone receptor (PR)-negative, HER2 3+ by immunohistochemistry (IHC), or having lymphovascular invasion or isolated tumor cells (N0(i+))] breast cancer.
Before enrollment, all patients completed standard-of-care therapy with surgery, chemotherapy, and if indicated, radiation. Patients receiving endocrine therapy continued their prescribed regimen. Because nelipepimut-S binds the HLA-A2 and A3 alleles, patients were enrolled and then HLA-typed. HLA-A2/A3+ patients were vaccinated, whereas HLA-A2/3– patients were observed prospectively for recurrence.
The node-positive trial was initially a phase I two-stage safety trial [9]. The node-negative trial was designed to further delineate optimal dosing (Table 1). Both trials transitioned to phase II with a primary efficacy end point of disease recurrence. The initial protocol called for the primary analysis at a median follow-up of 18 months after which it was amended to allow for follow-up through 60 months. At the time that follow-up was extended, five patients did not sign informed consent for follow-up beyond the initial 18 months. No recurrences were documented among these five patients, and they are included in these analyses. Remaining patients all completed 60 months of follow-up.
Table 1.
Nelipepimut-S dosing regimens for breast cancer node-positive and node-negative patient groups by trial design
| Patient group | No. of patients | Months vaccinated |
|---|---|---|
| Node-positive | ||
| 100.250.6a | 4b | 0, 1, 2, 3, 4, 5 |
| 500.250.4 | 6 | 0, 1, 2, 5 |
| 500.250.6 | 5 | 0, 1, 2, 3, 4, 5 |
| 1000.250.4 | 11 | 0, 1, 2, 5 |
| 1000.250.6 | 27c | 0, 1, 2, 3, 4, 5 |
| Node-negative | ||
| 500.125.3 | 10 | 0, 1, 5 |
| 500.125.4 | 9 | 0, 1, 2, 5 |
| 500.250.4 | 12 | 0, 1, 2, 5 |
| 500.250.6 | 13 | 0, 1, 2, 3, 4, 5 |
| 1000.250.6 | 11 | 0, 1, 2, 3, 4, 5 |
| Total | 108 | |
aNomenclature signifies peptide dose, granulocyte–macrophage colony-stimulating factor (GM-CSF) dose, and number of inoculations (i.e. 100.250.6 describes 100 µg of nelipepimut-S mixed with 250 µg of GM-CSF administered in 6 monthly inoculations).
bOne patient assigned to the 100.250.6 group withdrew before completing the primary vaccination series. She was monitored in the vaccine arm on an intention-to-treat basis.
cOne patient assigned to the optimal dose group received only a single inoculation secondary to a hepatitis C infection. She was monitored in the vaccine arm on an intention-to-treat basis for the overall disease-free survival analysis but was not included in subsequent analyses evaluating optimal dosing.
At the time that the protocol was revised, a voluntary booster program was initiated. Patients who had previously consented and were ≥6 months from completion of their primary vaccination series were offered optional boosters. Patients who enrolled after the booster program gave consent prospectively. Boosters were administered every 6 months until trial completion at 5 years.
vaccine
The nelipepimut-S peptide was produced in good manufacturing practices grade and purified to >95% (NeoMPS, San Diego, CA). Lyophilized peptide was reconstituted at the prescribed dose in 0.5 ml sterile saline. The peptide was mixed with GM-CSF (Seattle, WA) in 0.5 ml, and the 1.0 ml inoculation was split, with 0.5 ml given intradermally at two sites 5 cm apart in the same extremity.
in vivo immune monitoring
A delayed-type hypersensitivity (DTH) reaction was used to assess in vivo immune responses [9]. Briefly, 100 μg of nelipepimut-S in 0.5 ml of normal saline was injected 1 month after vaccination series completion; 0.5 ml of normal saline was injected as a control. The DTH was measured in two dimensions using the sensitive ballpoint pen method. Data were recorded as the orthogonal mean. In the node-negative trial, a DTH reaction was also assessed prevaccination [6].
clinical recurrences of disease
Patients were assessed for breast cancer recurrence per standard screening dictated by their oncologists. Patients were considered to have recurrent disease if recurrence was biopsy-proven or if they were treated for recurrence.
statistical analysis
A prespecified 60-month analysis was conducted. Clinicopathologic data were compared between groups. Continuous data were summarized using the median and range, and the groups were compared using a Wilcoxon rank-sum test. Categorical variables were compared between groups using a χ2 or Fisher's exact test. Data for DTH were presented as means ± standard deviations and compared using Student's t-test. Kaplan–Meier curves were used to quantify DFS, and a simple log-rank test was used to compare between groups. A prespecified subgroup analysis by HER2 status was carried out. A P value of <0.05 was considered statistically significant. Statistical analyses were carried out with use of SPSS (Armonk, NY).
results
patients
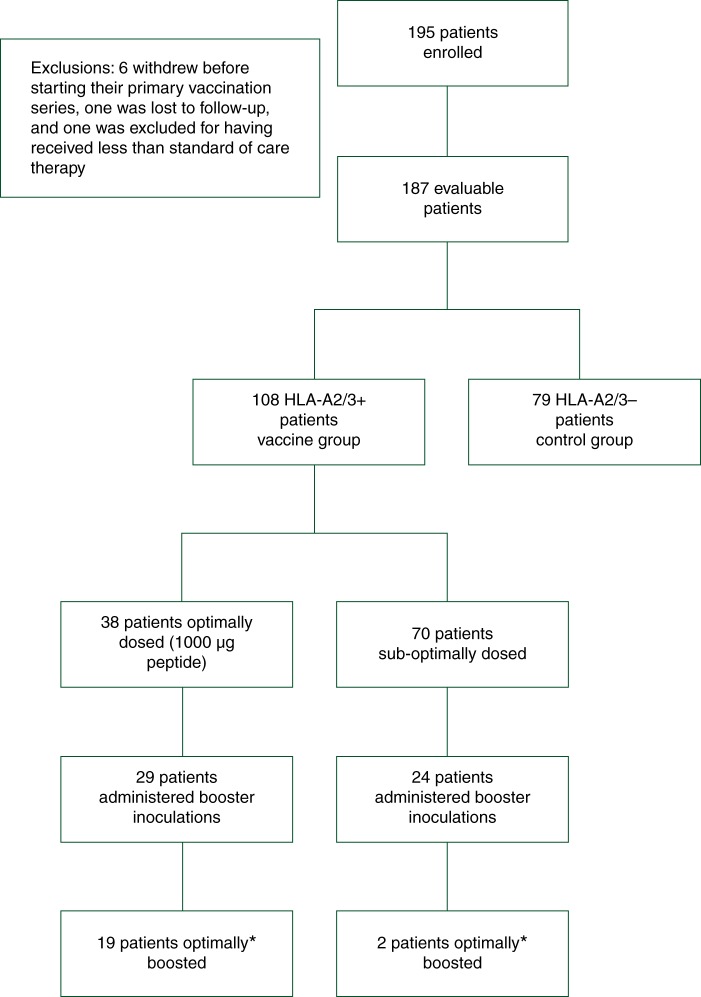
The combined trials enrolled 195 patients (Figure 1). Six patients withdrew before vaccination began, one was lost to follow-up, and one was excluded for failure to receive standard-of-care therapy leaving a cohort of 187 assessable patients. HLA-A2/3+ patients (n = 108) were vaccinated, and HLA-A2/3– patients (n = 79) were observed. Two patients received only a single inoculation and were included in the overall DFS analysis on an intention-to-treat basis. Table 1 shows the dosing regimens. Table 2 details clinicopathologic characteristics by treatment group. The groups were well matched except that vaccinated patients were more likely to be hormone receptor-negative.
Figure 1.
Flow of patients through the study. *Optimally boosted was defined as beginning booster inoculations six months after completion of the primary vaccinations series.
Table 2.
Clinicopathologic characteristics by treatment group
| Characteristics | No. (%) of vaccinated patients (N = 108) | No. (%) of controls (N = 79) | P value |
|---|---|---|---|
| Median age (years) (range) | 57 (28–78) | 53 (32–83) | 0.49 |
| Race | |||
| White | 96 (89) | 66 (84) | 0.40 |
| Non-white | 12 (11) | 13 (16) | |
| T stage | |||
| Tis | 1 (1) | 1 (1) | 0.51 |
| T1 | 75 (69) | 47 (59) | |
| T2 | 21 (19) | 19 (24) | |
| T3 | 8 (8) | 8 (10) | |
| T4 | 2 (2) | 4 (5) | |
| Unknown | 1 (1) | 0 | |
| Nodal status | |||
| Positive | 53 (49) | 44 (56) | 0.37 |
| Negative | 55 (51) | 35 (44) | |
| Histology | |||
| DCIS | 1 (1) | 1 (1) | 1.0 |
| Infiltrating ductal | 95 (88) | 69 (87) | |
| Infiltrating lobular | 12 (11) | 9 (11) | |
| Nuclear grade | |||
| 1 | 21 (19) | 18 (23) | 0.85 |
| 2 | 40 (37) | 29 (37) | |
| 3 | 44 (41) | 30 (38) | |
| Unknown | 3 (3) | 2 (2) | |
| ER/PR status | |||
| Negative | 34 (31) | 14 (18) | 0.03 |
| Positive | 73 (68) | 65 (82) | |
| Unknown | 1 (1) | 0 | |
| HER2 status | |||
| Negative | 68 (63) | 51 (65) | 0.53 |
| Positive | 33 (31) | 20 (25) | |
| Unknown | 7 (6) | 8 (10) | |
| Surgery | |||
| Lumpectomy | 55 (51) | 37 (47) | 0.79 |
| Mastectomy | 48 (44) | 35 (44) | |
| Unknown | 5 (5) | 7 (9) | |
| Postmastectomy radiation | |||
| No | 25 (52) | 12 (34) | 0.11 |
| Yes | 23 (48) | 23 (66) | |
| Chemotherapy | |||
| No | 26 (24) | 21 (27) | 0.70 |
| Yes | 82 (76) | 58 (73) | |
| Endocrine therapy | |||
| No | 33 (31) | 18 (23) | 0.24 |
| Yes | 75 (69) | 61 (77) | |
| Endocrine therapy in hormone receptor-positive patients | |||
| No | 3 (4) | 7 (11) | 0.24 |
| Yes | 70 (96) | 58 (89) | |
| Trastuzumab use in HER2-positive patients | |||
| No | 22 (67) | 18 (90) | 0.10 |
| Yes | 11 (33) | 2 (10) | |
toxicity
Local and systemic toxicities were mild during the primary vaccination series (Figure 2A) and administration of booster inoculations (Figure 2B). Four (7.5%) patients receiving booster inoculations developed delayed urticarial reactions manifested as hives and pruritus 1–2 weeks after the booster. All patients were treated with an antihistamine, two received oral steroids, and two used topical steroids. All patients' symptoms resolved with outpatient treatment.
Figure 2.

Vaccine toxicity. Maximum local and systemic toxicity during the (A) primary vaccination series or (B) booster inoculations. Common local toxicities were injection site erythema or pruritis. Common systemic toxicities were bone pain, influenza-like symptoms, and fatigue (associated with the granulocyte–macrophage colony-stimulating factor).
immunologic response–DTH reactions
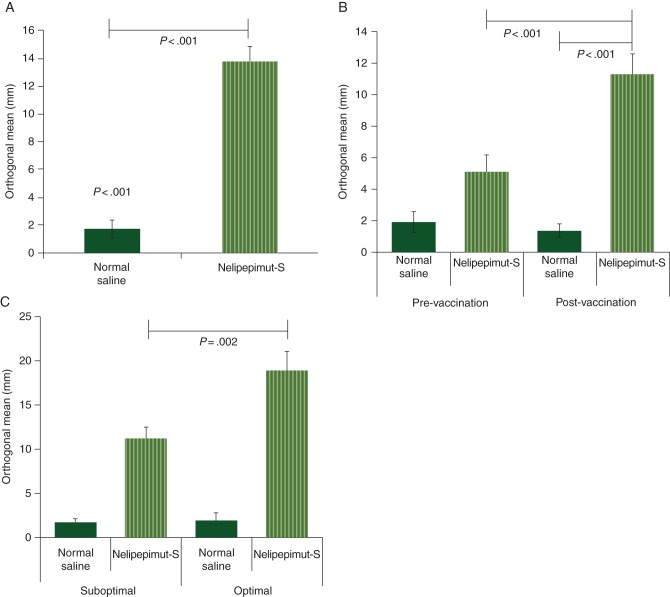
A DTH reaction was assessed 1 month after completion of the primary vaccination series to measure in vivo immunologic effectiveness. The average induration to nelipepimut-S was 13.7 ± 1.2 mm compared with 1.7 ± 0.4 mm to the normal saline control (P < 0.001) (Figure 3A). For node-negative patients, DTH was assessed before and after vaccination (Figure 3B). Before vaccination, the DTH reaction to nelipepimut-S was greater than the reaction to normal saline (5.1 ± 1.1 versus 1.9 ± 0.7 mm; P = 0.01), suggesting some preexisting immunity to the peptide in some individuals. After vaccination, the DTH response to nelipepimut-S was significantly larger than the response to normal saline (11.3 ± 1.3 versus 1.3 ± 0.5 mm; P < 0.001). The response to nelipepimut-S was also significantly larger after vaccination (11.3 ± 1.3 versus 5.1 ± 1.1 mm prevaccination; P < 0.001). When DTH response was assessed as a function of vaccine dose, patients receiving the optimal dose had a significantly larger DTH reaction than did those receiving a less-than-optimal dose (18.9 ± 2.2 versus 11.2 ± 1.4 mm; P = 0.002) (Figure 3C).
Figure 3.
In vivo immune responses. (A) Delayed-type hypersensitivity (DTH) for all patients after vaccination. (B) Pre- and postvaccination DTH for node-negative patients. (C) Postvaccination DTH responses by dosing group.
disease-free survival
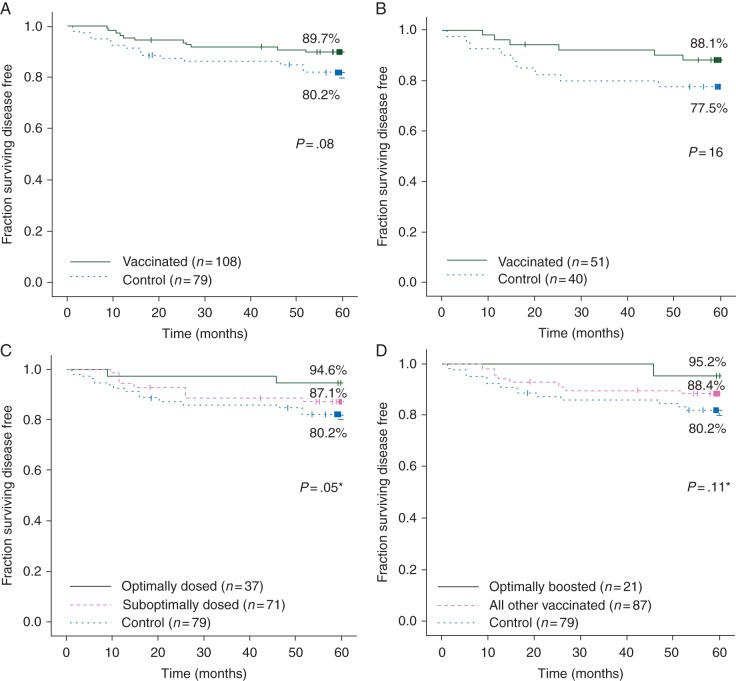
At 5 years, the DFS rate for vaccinated patients was 89.7% versus 80.2% for controls (P = 0.08), a 48.0% reduction in relative recurrence risk (Figure 4A). These trials enrolled patients with tumors expressing HER2 at any level. We had previously determined that patients with low levels of HER2 (IHC 1+ or 2+) had more robust immune responses than patients with tumors overexpressing HER2 [10]. When only low HER2 patients were evaluated, the vaccinated and control groups were well matched with respect to clinicopathologic characteristics (supplementary Table S1, available at Annals of Oncology online). DFS in vaccinated patients was 88.1% versus 77.5% in controls (P = 0.16) (Figure 4B).
Figure 4.
Disease-free survival. (A) All assessable patients; (B) patients with HER2 1+ or 2+ disease. (C) By dosing group (*P value compares optimally dosed group to control group); (D) evaluating optimally boosted patients (*P value compares optimally boosted group to control group).
Because the trials began as dose- and schedule-finding trials, not all patients received the dose that was determined to be optimal (1000 µg nelipepimut-S + 250 µg GM-CSF). When evaluated by dosing, DFS was 94.6% for those who received the optimal dose (n = 37) (P = 0.05 versus controls) (Figure 4C). Patients receiving the optimal dose were more likely to have grade 3 (54% versus 38% for controls; P = 0.30), ER-negative (27% versus 18% for controls; P = 0.25), node-positive (70% versus 56% for controls; P = 0.16) tumors and were, therefore, more likely to have received adjuvant chemotherapy (92% versus 73% for controls; P = 0.03) (supplementary Table S2, available at Annals of Oncology online).
A voluntary booster program was initiated after late recurrences were noted in vaccinated patients. Fifty-three patients (49.0%) received booster inoculations including 21 who were prospectively boosted after initiation of the program and received their initial booster 6 months after completing their primary vaccination series (i.e. ‘optimally boosted’). Optimally boosted and control patients were well matched except optimally boosted patients were more likely to be hormone receptor-negative and, therefore, less likely to receive adjuvant endocrine therapy (supplementary Table S3, available at Annals of Oncology online). The DFS rate for optimally boosted patients was 95.2% (P = 0.11 versus control patients).
discussion
This final report of our clinical trials evaluating nelipepimut-S combined with GM-CSF administered to disease-free, node-positive and high-risk node-negative breast cancer patients shows that the vaccine is safe, effective in stimulating an in vivo immune response, and may reduce the disease recurrence rate.
A critical aspect of these trials is that they were conducted in the adjuvant setting. Previous peptide vaccine trials enrolled patients with metastatic disease. In a review of studies investigating peptide vaccines in 381 patients from 1995–2004, Rosenberg et al. reported a 2.9% objective response rate [11]. All patients in these trials had metastatic disease, the majority having melanoma with diffuse visceral and/or nodal disease. In the metastatic setting, the tumor burden can be immunosuppressive, resulting in difficulties generating an effective immune response [12]. Overcoming the immunosuppressive microenvironment associated with metastatic disease will require additional strategies to augment response to vaccination. In a phase III trial enrolling patients with stage III or IV melanoma randomized to receive the gp100 vaccine followed by IL-2 or IL-2 alone, patients in the vaccine plus IL-2 group had significantly improved progression-free survival [13]. These data suggest that response to vaccines in patients with more advanced disease may be improved if cytokines driving the postvaccination immune response are administered [14]. There may also be a role for vaccines in combination with antibodies blocking the T-cell inhibitory molecules CTLA-4 or PD-1.
We showed an approximate 50% reduction in recurrence risk in high-risk breast cancer patients. Our data suggest the importance of administering the optimal biologic dose as well as booster inoculations. With respect to the optimal biologic dose, all dose levels were well tolerated; therefore, the highest dose, which represented the greatest concentration of peptide that could be solubilized for intradermal inoculation, was determined to be optimal. The in vivo immunologic response to vaccination showed that all patients developed a DTH response to E75 after vaccination, and that DTH reactions were dose dependent.
The DFS data also suggest that optimal dosing is required for greatest clinical benefit, with a 5-year DFS rate of 94.6% for optimally dosed patients versus 87.1% in patients receiving less than the optimal dose and 80.2% for unvaccinated controls. The optimally dosed group included more grade three, ER-negative, node-positive patients, which was offset by a greater percentage of these patients receiving chemotherapy. In addition, although only a third of patients in the optimally dosed group and a quarter of patients in the control group were HER2-positive, a greater percentage of HER2-positive patients in the optimally dosed group received trastuzumab. These trials began before trastuzumab was approved as standard-of-care therapy for HER2-positive breast cancer. Therefore, more optimally dosed patients were enrolled after trastuzumab was approved than were suboptimally dosed patients and controls who were enrolled throughout the entire accrual period. The NSABP B-31 and NCCTG N9831 trials showed that trastuzumab in the adjuvant setting decreased the recurrence rate by 50% [15]. Given that only eight patients in the optimally dosed group received trastuzumab, and the recurrence rate was 20% in our control group, it would be estimated that trastuzumab could be responsible for preventing only one recurrence in the optimally dosed group. Therefore, trastuzumab treatment alone could not account for the differences in recurrence.
After vaccination, antigen-specific CTL levels increase substantially during an expansion phase then markedly decrease during the death phase, when lymphocytes undergo apoptosis. The subsequent memory phase consists primarily of antigen-specific memory CTLs that can persist for a variable amount of time, depending on the antigen and vaccine dose. It is unclear whether antigen-specific CTL persistence requires antigen re-exposure [16]. Our group and others [4, 7] have shown that levels of antigen-specific CTLs decline with time after inoculation with a peptide vaccine, and we have shown that E75-CTLs can be repeatedly boosted without signs of unresponsiveness [7]. This is an important observation because one risk of administering repeated booster inoculations is that repeated antigen encounter might lead to CTL exhaustion or tolerance [17]. The requirement for booster inoculations as a component of an adjuvant vaccination strategy is supported by recent mouse work, in which investigators found that priming with a dendritic cell vaccine induced long-lasting CTL responses in wild-type mice and that boosting sustained the memory T-cell pool associated with protection against challenge with B16F1 melanoma cells [18]. In contrast, in a tumor-bearing mouse, used to mimic a therapeutic vaccination strategy, booster inoculations were not beneficial. The investigators suggested that boosting tumor-free mice, akin to disease-free patients vaccinated in the adjuvant setting, is required for antigen-driven memory T-cell persistence, whereas in tumor-bearing mice, analogous to vaccination of patients with significant tumor burden, boosting may lead to overstimulation. Our data suggest that boosters administered to restimulate the E75-CTL response may contribute to prevention of disease recurrence.
As a result of the encouraging data from these early phase trials, a phase III adjuvant trial is currently enrolling patients with node-positive, HER2 1+ and 2+ tumors [19]. The decision to enroll patients with HER2 1+ and 2+ tumors was based in part on data from these trials showing that patients with low-expressing tumors had the most robust immune responses [10]. In addition, for HER2 1+ and 2+ patients enrolled in the control arm of these trials, 5-year DFS was 76.5%, suggesting a need for additional therapeutic options in these patients.
funding
This work was funded by the United States Military Cancer Institute, Department of Surgery, Uniformed Services University of the Health Sciences; Clinical Breast Care Project; and the Department of Clinical Investigation, Walter Reed Army Medical Center. No NIH grant funding supported this work. Funding sources were not involved with the study design; in the collection, analysis, or interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. E.A.M. is an R. Lee Clark Fellow at the University of Texas MD Anderson Cancer Center supported by the Jeanne F. Shelby Scholarship Fund.
disclosure
GEP and SP have inventor rights to E75. This vaccine has been licensed for commercial development. They are entitled to financial proceeds associated with this license per Federal policy. GEP also consults in the development of the vaccine. All remaining authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
The view(s) expressed herein are those of the author(s) and do not reflect the official policy or position of Brooke Army Medical Center, the US Army Medical Department, the US Army Office of the Surgeon General, the Department of the Army, the Department of Defense, or the US Government.
references
- 1.Mittendorf EA, Holmes JP, Ponniah S, Peoples GE. The E75 HER2/neu peptide vaccine. Cancer Immunol Immunother. 2008;57:1511–1521. doi: 10.1007/s00262-008-0540-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brossart P, Wirths S, Stuhler G, et al. Induction of cytotoxic T-lymphocyte responses in vivo after vaccinations with peptide-pulsed dendritic cells. Blood. 2000;96:3102–3108. [PubMed] [Google Scholar]
- 3.Disis ML, Gooley TA, Rinn K, et al. Generation of T-cell immunity to the HER-2/neu protein after active immunization with HER-2/neu peptide-based vaccines. J Clin Oncol. 2002;20:2624–2632. doi: 10.1200/JCO.2002.06.171. [DOI] [PubMed] [Google Scholar]
- 4.Knutson KL, Schiffman K, Cheever MA, Disis ML. Immunization of cancer patients with a HER-2/neu, HLA-A2 peptide, p369–377, results in short-lived peptide-specific immunity. Clin Cancer Res. 2002;8:1014–1018. [PubMed] [Google Scholar]
- 5.Zaks TZ, Rosenberg SA. Immunization with a peptide epitope (p369–377) from HER-2/neu leads to peptide-specific cytotoxic T lymphocytes that fail to recognize HER-2/neu+ tumors. Cancer Res. 1998;58:4902–4908. [PubMed] [Google Scholar]
- 6.Peoples GE, Holmes JP, Hueman MT, et al. Combined clinical trial results of a HER2/neu (E75) vaccine for the prevention of recurrence in high-risk breast cancer patients. Clin Cancer Res. 2008;14:797–803. doi: 10.1158/1078-0432.CCR-07-1448. [DOI] [PubMed] [Google Scholar]
- 7.Holmes JP, Clifton GT, Patil R, et al. Use of booster inoculations to sustain the clinical effect of an adjuvant breast cancer vaccine. Cancer. 2011;117:463–471. doi: 10.1002/cncr.25586. [DOI] [PubMed] [Google Scholar]
- 8.Mittendorf EA, Clifton GT, Holmes JP, et al. Clinical trial results of the HER-2/neu (E75) vaccine to prevent breast cancer recurrence in high-risk patients. Cancer. 2012;118:2594–2602. doi: 10.1002/cncr.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peoples GE, Gurney JM, Hueman MT, et al. Clinical trial results of a HER2/neu (E75) vaccine to prevent recurrence in high-risk breast cancer patients. J Clin Oncol. 2005;23:7536–7545. doi: 10.1200/JCO.2005.03.047. [DOI] [PubMed] [Google Scholar]
- 10.Benavides LC, Gates JD, Carmichael MG, et al. The impact of HER2/neu expression level on response to the E75 vaccine. Clin Cancer Res. 2009;15:2895–2904. doi: 10.1158/1078-0432.CCR-08-1126. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10:909–915. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morton DL, Ollila DW, Hsueh EC, et al. Cytoreductive surgery and adjuvant immunotherapy: a new management paradigm for metastatic melanoma. CA Cancer J Clin. 1999;49:101–116. doi: 10.3322/canjclin.49.2.101. 165. [DOI] [PubMed] [Google Scholar]
- 13.Schwartzentruber DJ, Lawson DH, Richards JM, et al. gp100 peptide vaccine and interleukin-2 in patients with advanced melanoma. N Engl J Med. 2011;364:2119–2127. doi: 10.1056/NEJMoa1012863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Overwijk WW, Theoret MR, Restifo NP. The future of interleukin-2: enhancing therapeutic anticancer vaccines. Cancer J Sci Am. 2000;6((Suppl 1):S76–S80. [PMC free article] [PubMed] [Google Scholar]
- 15.Perez EA, Romond EH, Suman VJ, et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol. 2011;29:3366–3373. doi: 10.1200/JCO.2011.35.0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lau LL, Jamieson BD, Somasundaram T, Ahmed R. Cytotoxic T-cell memory without antigen. Nature. 1994;369:648–652. [PubMed] [Google Scholar]
- 17.Sallusto F, Lanzavecchia A, Araki K, Ahmed R. From vaccines to memory and back. Immunity. 2010;33:451–463. doi: 10.1016/j.immuni.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ricupito A, Grioni M, Calcinotto A, et al. Booster vaccinations against cancer are critical in prophylactic but detrimental in therapeutic settings. Cancer Res. 2013;73:3545–3554. doi: 10.1158/0008-5472.CAN-12-2449. [DOI] [PubMed] [Google Scholar]
- 19. Efficacy and Safety Study of NeuVax(TM) (Nelipepimut-S or E75) Vaccine to Prevent Breast Cancer Recurrence (PRESENT) http://clinicaltrials.gov/show/NCT01479244. 3 February 2014, date last accessed.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.