Birth weight and height are correlated and likely to be markers of some aspect of growth that affects cancer risk in adulthood. However, birth weight adds little, if any, additional information to adult height as a predictor of cancer incidence in women.
Keywords: birth weight, cancer, height, growth
Abstract
Background
Most evidence about associations between birth weight and adult cancer risk comes from studies linking birth records to cancer registration data, where information on known risk factors for cancer is generally lacking. Here, we report on associations between birth weight and cause-specific cancer risk in a large cohort of UK women, and investigate how observed associations are affected by other factors.
Methods
A total of 453 023 women, born in the 1930s and 1940s, reported their birth weight, maternal smoking, parental heights, age at menarche, adult height, adult smoking, and many other personal characteristics. They were followed for incident cancer. Using Cox regression, relative risks by birth weight were estimated for cancers with more than 1500 incident cases, adjusting for 17 potential confounding factors, individually and simultaneously.
Results
Birth weight reported in adulthood was strongly correlated with that recorded at birth (correlation coefficient = 0.78, P < 0.0001). Reported birth weight was associated with most of the potential confounding factors examined, the strongest association being with adult height. After 9.2 years follow-up per woman, 39 060 incident cancers were registered (4414 colorectal, 3175 lung, 1795 malignant melanoma, 14 542 breast, 2623 endometrial, 2009 ovarian, 1565 non-Hodgkin lymphoma, and 8937 other cancers). Associations with birth weight were null or weak and reduced after adjustment by adult height (P[trend] > 0.01 for every cancer, after adjustment). In contrast, adult height was strongly related to the risk of every cancer except lung cancer, after adjusting for birth weight and other factors (P[trend] < 0.0001 for most cancers). For lung cancer, adjusting for smoking reduced the association with birth weight. Meta-analyses were dominated by our findings.
Conclusion
Birth weight and adult height are correlated and likely to be markers of some aspect of growth that affects cancer risk in adulthood. However, birth weight adds little, if any, additional information to adult height as a predictor of cancer incidence in women.
introduction
Findings from some studies have suggested an association between birth weight and the incidence of certain cancers, particularly breast cancer, in adulthood [1–17]. However, most of the evidence comes from studies where birth or early childhood records had been linked to adult cancer registration data, and such studies generally lacked information on possible confounding or intermediary pathways by which birth weight may be related to adult cancer risk. Birth weight is associated with many factors, such as adult height and weight, which are known to predict cancer risk in adulthood [18]. In a large cohort of UK women, we describe associations between birth weight and characteristics of the women at around the time of their birth, including maternal smoking, parental heights, and whether or not they had been breast fed as an infant, and during adulthood, such as their height, body mass index, and smoking. Our aim was to study associations between birth weight and cancer incidence and to examine the role of possible confounding or intermediary pathways by adjusting, individually and simultaneously, for 17 different characteristics of the women or their parents.
methods
study population and follow-up
The details of the Million Women Study have been described elsewhere [18]. Briefly, 1.3 million women aged 50–64 years were recruited when they were invited to routine breast cancer screening in England and Scotland between 1996 and 2001, and completed a recruitment questionnaire on health, lifestyle, and reproductive factors and gave consent for follow-up. About 3 years afterwards, they were sent a postal questionnaire which included questions about their birth weight, whether they had been breastfed, maternal smoking, and maternal and paternal height. Study questionnaires can be found at www.millionwomenstudy.org. Good agreement between self-reported and measured birth weight was found among women in this cohort who were also participants in the National Survey of Health and Development, a nationally representative birth cohort of people born in a single week in 1946, for whom birth weight had been recorded at the time of their birth [19]. The Pearson correlation between the measured and self-reported values was 0.78 (P < 0.0001).
All participants are followed for cause-specific deaths and incident cancer by linkage to the National Health Service (NHS) Central Registers, which regularly provide investigators with information coded to the 10th Revision (ICD-10) of the International Classification of Diseases. The study was approved by the Oxford and Anglia Multi-Centre Research and Ethics Committee.
statistical analysis
Analyses were restricted to women who had reported their birth weight and who had not had a prior cancer registration (except non-melanoma skin cancer, ICD-10 C44). Information on birth weight was reported in pounds and ounces and converted to kilograms. Analyses divided women into five categories of birth weight: <2.5, 2.5–2.9, 3.0–3.4, 3.5–3.9, and ≥4.0 kg. We first examined how various known or suspected risk factors for cancer varied by birth weight.
For analyses of cancer risk, women were followed from the date when their birth weight was reported to whichever was earliest of: the date of cancer registration (except for non-melanoma skin cancer), date of death, date of emigration or other loss to follow-up, or the last date of follow-up (31 December 2011). The seven cancer types for which more than 1500 incident cases had accrued during follow-up were studied separately: colorectal cancer (ICD-10 code C18–20), lung cancer (C34), malignant melanoma (C43), breast cancer (C50), endometrial cancer (C54), ovarian cancer (C56), and non-Hodgkin lymphoma (C82–85). All other incident invasive cancers were grouped together (C00-C97, excluding the above seven sites or types and non-melanoma skin cancer).
We used Cox regression with attained age as the underlying time variable to estimate relative risks (RRs) and appropriate confidence intervals (CIs) for cancer incidence, by birth weight, taking birth weight 3.0–3.4 kg as the reference group. To facilitate valid comparisons between any birth weight groups, the variance of the log risk was estimated for each group, and these group-specific variances were used to calculate group-specific CIs [20].
Unless otherwise specified, analyses were routinely stratified by year of birth (before 1939, 1940–1945, 1946–) and 10 regions of residence in Scotland and England, and adjusted by maternal smoking during pregnancy (yes, no), maternal height (<155, 155–159, 160–164, 165–169, 170–174, ≥175 cm), paternal height (<165, 165–169, 170–174, 175–179, 180–184, ≥185 cm), whether the woman had been breast fed as an infant (yes, no), age at menarche (<12, 12, 13, 14, ≥15 years old), adult height (<155, 155–159, 160–164, 165–169, 170–174, ≥175 cm), parity (nulliparous, 1–2 children, 3 or more children), age the woman was when she had her first baby (<25, ≥25 years old), use of hormone therapy for menopause (never, former user having stopped for <5 or ≥5 years, current user at baseline), socio-economic status (quintiles of Townsend deprivation index [21]), body mass index (<22.5, 22.5–27.4, 27.5–32.4, 32.5–35.0, ≥35.0 kg/m2), smoking (never, past smoker who stopped <10 years or ≥10 years ago, current smokers at baseline <15, 15–24, and ≥25 cigarettes per day), strenuous exercise (never, once per week, more than once per week), and alcohol consumption (0, 1–4, 5–14, 15–29, 30 g/day [22]). The small number of women with missing values for any of these adjustment variables were assigned to a separate category for that variable.
Women with a history of hysterectomy or for whom hysterectomy status was unknown were excluded from analyses of endometrial cancer. Women who had a history of bilateral oophorectomy or for whom bilateral oophorectomy status was unknown were excluded from analyses of ovarian cancer.
To correct for regression dilution resulting from imperfect recall of birth weight [23], RRs per 1 kg increase in birth weight were estimated using the mean measured birth weights in each of the five categories of self-reported birth weight (2.63, 2.98, 3.37, 3.68, and 3.94 kg, respectively; Figure 1) [19]. Because of the large number of cancers studied, 99% CIs are presented.
Figure 1.
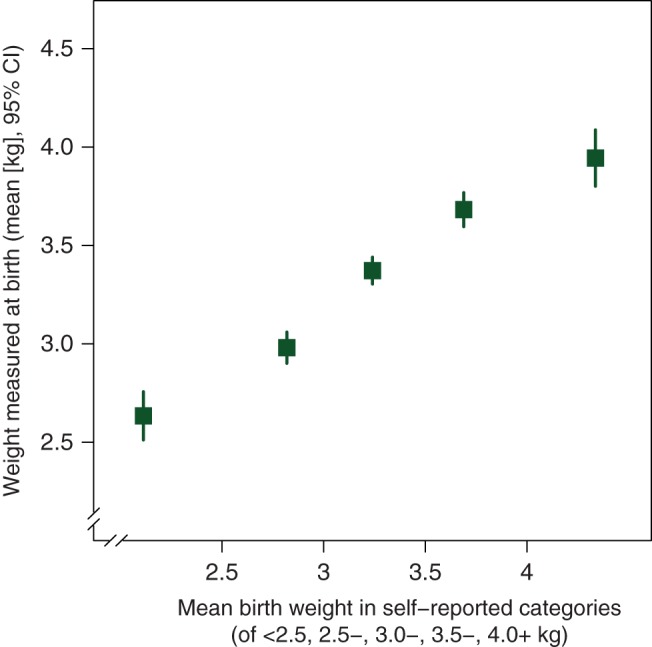
Mean measured birth weight versus mean self-reported birth weight, in each of the five categories of self-reported birth weight. Adapted from Cairns et al. [19] under the terms of the Creative Commons Attribution License 2.0; http://creativecommons.org/licenses/by/2.0.
systematic review and meta-analysis
A systematic review was conducted for published associations between birth weight and the seven specific cancer sites or types examined here. We searched PubMed and EMBase databases and review articles (search terms and further details of the search are given in supplementary Appendix S2 and S3, available at Annals of Oncology online). We sought studies with prospectively recorded information on birth weight and adult cancer risk and, where possible, extracted RR estimates which had been adjusted, and those which had not been adjusted, by adult height and by smoking. We combined the study-specific results to give a RR per 1 kg increase in birth weight for each type of cancer, calculated where necessary by regression across birth weight categories [24], using inverse-variance-weighted meta-analysis. We used χ2 tests to assess heterogeneity across studies.
results
The 453 023 women without prior cancer who reported their birth weight at baseline were followed for a total of 4.2 million person-years (9.2 years per woman, on average). During follow-up, 39 060 incident cancers were registered (4414 colorectal, 3175 lung, 1795 malignant melanoma, 14 542 breast, 2623 endometrial, 2009 ovarian, 1565 non-Hodgkin lymphoma, and 8937 other cancers). Table 1 shows 17 characteristics of the women, at around the time of their birth, in adolescence and early adulthood, and in middle age, by their reported birth weight. Of the 17 variables examined, only 4 were unrelated to birth weight (year of birth, age at baseline, age the woman was when she had her first baby, and use of hormone therapy for the menopause). All the other 13 variables varied significantly by birth weight, although some associations were not particularly strong or linear. Of all the variables examined, women's adult height had the strongest association with birth weight. Further details of the data shown in Table 1 are given in supplementary Appendix S4, available at Annals of Oncology online.
Table 1.
Characteristics of women at different periods of their lives, by their reported birth weight
| Birth weight |
χ2 value for heterogeneitya (all P values <0.001 unless specified) | |||||
|---|---|---|---|---|---|---|
| <2.5 kg (N = 61 308) | 2.5–2.9 kg (N = 86 377) | 3.0–3.4 kg (N = 157 437) | 3.5–3.9 kg (N = 85 256) | ≥4.0 kg (N = 62 645) | ||
| Year of birth, mean (SD) | 1942 | 1942 | 1942 | 1943 | 1942 | 0.8 (P = 0.4) |
| Age at baseline (years), mean (SD) | 59.1 (4.6) | 58.9 (4.5) | 58.7 (4.5) | 58.6 (4.5) | 59.3 (4.7) | 0.0 (P = 0.9) |
| Birth and family | ||||||
| Maternal height (cm), mean (SD) | 159.5 (7.1) | 159.7 (6.9) | 160.5 (6.8) | 160.9 (6.8) | 161.3 (7.0) | 2982 |
| Paternal height (cm), mean (SD) | 173.7 (8.1) | 173.8 (7.7) | 174.4 (7.5) | 174.9 (7.5) | 175.2 (7.7) | 1579 |
| Mother smoked during pregnancy (%) | 34.3 | 31.3 | 30.0 | 28.6 | 27.8 | 659 |
| Was breast fed as an infant (%) | 56.1 | 68.9 | 73.3 | 75.4 | 75.4 | 5749 |
| Mother alive when birth details were collected (%) | 26.5 | 33.8 | 35.4 | 35.8 | 28.8 | 157 |
| Adolescence, early adulthood, and reproductive factors | ||||||
| Age at menarche (years), mean (SD) | 12.8 (1.7) | 12.9 (1.6) | 12.9 (1.5) | 12.9 (1.6) | 12.9 (1.6) | 7.1 (P = 0.008) |
| Adult height (cm), mean (SD) | 160.2 (6.7) | 161.2 (6.5) | 162.6 (6.4) | 163.6 (6.5) | 164.5 (6.6) | 18 590 |
| Nulliparity (%) | 12.7 | 12.3 | 10.1 | 12.1 | 12.3 | 10.2 (P = 0.001) |
| Age the woman was when she had her first baby, mean (SD) | 23.9 (4.3) | 24.1 (4.3) | 24.1 (4.3) | 24.2 (4.3) | 23.9 (4.3) | 0.6 (P = 0.4) |
| Middle age | ||||||
| Lowest fifth of socio-economic status (%) | 21.5 | 18.7 | 17.9 | 18.0 | 20.3 | 47 |
| Ever used hormone therapy for menopause (%) | 55.2 | 55.6 | 56.0 | 55.5 | 54.8 | 0.7 (P = 0.4) |
| Body mass index (kg/m2), mean (SD) | 26.5 (4.9) | 26.0 (4.6) | 26.0 (4.5) | 26.2 (4.7) | 26.7 (4.9) | 180 |
| Current smoker (%) | 12.7 | 11.4 | 11.5 | 11.7 | 13.2 | 8.2 (P = 0.004) |
| Strenuous exercise <1 time per week (%) | 58.7 | 56.6 | 55.0 | 55.4 | 57.9 | 30 |
| Alcohol consumption (g/day), mean (SD) | 5.7 (8.0) | 6.5 (8.5) | 7.0 (8.7) | 7.1 (8.9) | 6.7 (8.8) | 429 |
aχ2 tests of regression models using self-reported birth weight as independent variable.
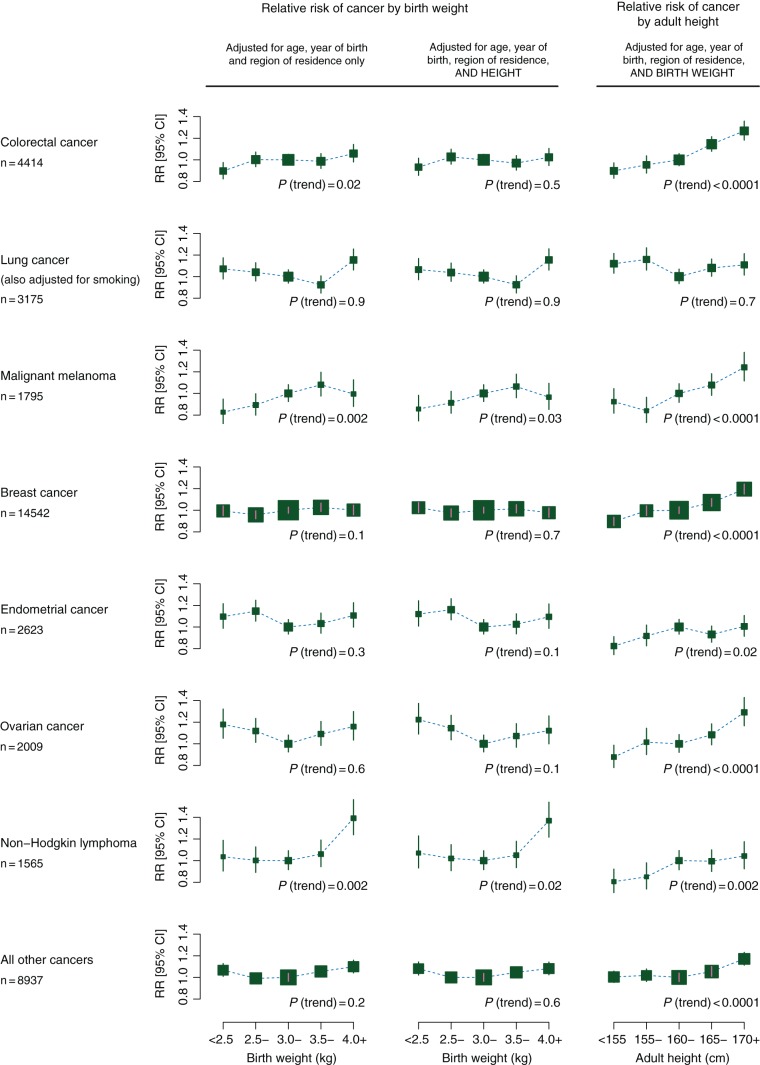
We estimated trends in the RR per 1 kg increase in birth weight for the seven cancer sites and types with more than 1500 incident cases and for all other cancers combined (Table 2). With adjustment only for age, year of birth, and region of residence, increasing birth weight was associated with an slight increase in the risk of malignant melanoma (P = 0.002) and of non-Hodgkin lymphoma (P = 0.002), but not with the risk of the other cancer sites (Table 2). However, when we studied the effect of additional adjustment for 14 other factors, one at a time, adult height had the greatest effect and reduced all associations, except for lung cancer (supplementary Appendix S5, available at Annals of Oncology online). After additional adjustment for height, none of the trends were statistically significant, given the number of tests done (the P value for trend was >0.01 for every cancer; Table 2). Simultaneous adjustment by all 17 potential confounding factors had little effect beyond the separate effects of adjusting just for height and for smoking (Table 2).
Table 2.
Relative risk and 99% confidence intervals for cancer incidence per 1 kg increase in birth weight: effect of adjustment for various factors
| Colorectal cancer | Lung cancer | Malignant melanoma | Breast cancer | Endometrial cancer | Ovarian cancer | Non-Hodgkin lymphoma | All other cancers | |
|---|---|---|---|---|---|---|---|---|
| n = 4414 | n = 3175 | n = 1795 | n = 14 542 | n = 2623 | n = 2009 | n = 1565 | n = 8937 | |
| Adjusted for age, year of birth, and region only | 1.09 (0.99–1.20) | 1.04 (0.93–1.17) | 1.20 (1.03–1.39) | 1.03 (0.98–1.09) | 0.95 (0.84–1.08) | 0.97 (0.84–1.12) | 1.21 (1.03–1.42) | 1.04 (0.97–1.11) |
| Adjusted for age, year of birth, region, and adult height | 1.03 (0.93–1.13) | 1.06 (0.95–1.19) | 1.14 (0.97–1.33) | 0.99 (0.94–1.05) | 0.93 (0.82–1.06) | 0.92 (0.79–1.06) | 1.17 (0.99–1.38) | 1.01 (0.95–1.09) |
| Adjusted for age, year of birth, region, and adult height and smoking | 1.02 (0.93–1.13) | 1.00 (0.90–1.12) | 1.14 (0.98–1.34) | 0.99 (0.94–1.04) | 0.93 (0.82–1.06) | 0.92 (0.79–1.06) | 1.16 (0.99–1.37) | 1.00 (0.94–1.07) |
| Simultaneous adjustment for 17 factorsa | 1.01 (0.92–1.11) | 1.04 (0.93–1.17) | 1.13 (0.97–1.32) | 0.98 (0.92–1.03) | 0.89 (0.79–1.01) | 0.92 (0.79–1.06) | 1.15 (0.98–1.36) | 1.00 (0.93–1.07) |
aRelative risks are adjusted for age, year of birth, region of residence, socio-economic status, having been breast fed as an infant, maternal smoking during pregnancy, maternal height, paternal height, age at menarche, adult height, smoking, parity, age the woman was when she had her first baby, body mass index, use of menopausal hormone therapy, strenuous exercise, and alcohol consumption.
We also estimated RRs per 1 kg increase in birth weight in subgroups of women defined by 15 of their personal characteristics, including their age, socio-economic status, height, and smoking history, but there was little to suggest strong heterogeneity of the findings across the subgroups (supplementary Appendix S6, available at Annals of Oncology online).
Figure 2 shows the RRs for each cancer site in each of the five categories of birth weight studied, after adjustment for age, year of birth, and region of residence only (left-hand column) and after additional adjustment by adult height (middle column). Results for lung cancer were also adjusted by smoking. The weak relationships between birth weight and cancer risk are evident, and it can be seen that they are further weakened by additional adjustment for adult height. RR estimates and CIs for each data point shown in Figure 2 are given in supplementary Appendix S7, available at Annals of Oncology online. After simultaneous adjustment for all 17 potential confounding factors (supplementary Appendix S8, available at Annals of Oncology online), the relationships between birth weight and cancer risk were almost identical to those additionally adjusted only for adult height (Figure 2, middle column).
Figure 2.
Relative risks for cancer by birth weight and by height.
The most extreme RR estimate, after adjustment for all potential confounding factors, was that for non-Hodgkin lymphoma in women who had been the largest size at birth (adjusted RR = 1.33, 95% CI 1.15–1.54, for birth weight 4.0+ versus 3.0–3.4 kg, Figure 2 and supplementary Appendix S7, available at Annals of Oncology online). The P value for this single risk estimate is 0.0002. Given the large number of risk estimates presented, this could well be a chance finding, although others have reported that big babies may be at increased risk of childhood leukaemia [25]. We examined the risk of adult leukaemia (n = 692) in the same subgroup of women, but the findings were not remarkable (adjusted RR = 1.05, 95% CI 0.83–1.34, for birth weight 4.0+ kg or more versus 3.0–3.4 kg).
Adult height is strongly correlated with birth weight, and adjusting for it led to the greatest reduction in associations between birth weight and adult cancer risk. Height is also associated with cancer (without adjustment for birth weight) [18]. As both are markers of growth and adult height is known to be associated with cancer risk, it is important to assess whether the associations between adult height and cancer risk persist after adjusting for birth weight. The right-hand column of Figure 2 shows, for women included in these analyses, height-associated RRs adjusted by age, year of birth, region of residence, and birth weight. Except for lung cancer, there are strong and highly significant trends of increasing cancer risk with increasing height (P < 0.0001 for colorectal cancer, malignant melanoma, breast cancer, ovarian cancer, and other cancers and P = 0.002 for non-Hodgkin lymphoma). The P value of 0.02 for endometrial cancer became highly significant (P < 0.0001) after additional adjustment for other factors, and was most affected by adjustment for body mass index (supplementary Appendix S8 and S9, available at Annals of Oncology online).
systematic review and meta-analysis
We sought published results from studies with prospectively recorded birth weight information on the association between birth weight and risk of cancer of the colorectum, lung, malignant melanoma, breast, ovary, endometrium, and non-Hodgkin lymphoma. Supplementary Appendix S2 and S3, available at Annals of Oncology online, gives the search strategy used and describes the studies with relevant data. Because of the apparent importance of height as a potential explanatory or mediating variable (and of smoking for lung cancer), we sought results from these studies both with and without adjustment for height and with and without adjustment for smoking (for lung cancer).
Most published prospective data come from studies that linked birth records to cancer registration data, with little or no information on other factors beyond those recorded at birth [1–13] (supplementary Appendix S3, available at Annals of Oncology online). For the cancers other than breast cancer, the total number of cancers in all previous publications combined was generally smaller than the numbers included in our study (1571 versus 4414 for colorectal cancer, 2032 versus 3175 for lung cancer, 2205 versus 1795 for malignant melanoma, 1084 versus 2623 for endometrial cancer, 871 versus 2009 for ovarian cancer, and 491 versus 1565 for non-Hodgkin lymphoma). Hence, meta-analyses for these cancers are dominated by our findings (supplementary Appendix S10, available at Annals of Oncology online). Furthermore, almost all published results were unadjusted for height, and no previous study of lung cancer adjusted for smoking.
For breast cancer, a meta-analysis of individual participant data from 32 studies was published in 2008 [14] and three other analyses at about the same time [15–17]. Meta-analyses based on published data are difficult to interpret, as they are hampered by different definitions and different division of birth weight across studies. The individual participant meta-analysis [14] used similar definitions across studies and reported RRs (not adjusted for height) per 0.5 kg increase in birth weight of: 1.06 (95% CI 1.02–1.09) in studies with 4135 cases with prospective recording of birth weight at the time of birth; 1.02 (95% CI 0.99–1.05) in studies with 2887 cases with prospective recording of birth weight during adolescence; and 0.98 (95% CI 0.95–1.01) in studies with 6359 cases where birth weight was self-reported (birth weight was recorded after breast cancer diagnosis for some of these cases). Combined results for all 13 381 women give a RR per 1 kg increase in birth weight of 1.04 (95% CI 1.00–1.07 ), similar to our estimate of 1.03 (95% CI 0.99–1.08) before adjustment by height, based on 14 542 incident cases. Since 2008, one record linkage study based in Denmark has published new results based on 716 women with breast cancer: the RR estimate per 1 kg birth weight (not adjusted by height) was 0.89 (95% CI 0.75–1.06) [26]. It was not possible to derive a summary estimate of breast cancer risk per 1 kg for previous publications since the individual participant meta-analysis [14] included most published studies, some of which were retrospective. Furthermore, almost none of the previously published results from prospective studies had adjusted for height [14–17]. For example, results were presented before and after adjustment for height for just 541 of the 13 381 women in the individual participant meta-analysis [14], and the resulting CIs were wide [RR = 1.02 (95% CI 0.93–1.12), per 0.5 kg increase in birth weight, after adjustment for height].
discussion
It has been proposed that birth weight has a persistent and independent effect on cancer risk in adulthood [1–17]. Birth weight is, however, correlated with other characteristics of women, at the time they were born and in later life, that could themselves affect cancer risk. Such factors include maternal smoking, maternal and paternal heights, age at menarche, adult height, smoking, alcohol consumption, body mass index, and physical activity. All these factors were associated with birth weight in this cohort, and the association with adult height was the strongest.
We found weak associations between birth weight and the risks of malignant melanoma and non-Hodgkin lymphoma before adjusting for height, but these associations became non-significant after adjusting for height. The non-significant associations between birth weight and the risk of colorectal, breast, endometrial, and ovarian cancer before adjusting for height were also further weakened after adjustment for height. In contrast, we found strong associations between adult height and cancer risk for each of these cancers, after adjusting for birth weight (P < 0.0001 for most of the cancer sites).
Lung cancer differs from the six other specific cancers considered here, in that its risk appears to be unrelated to height (as we had reported previously [18]). While there was little association between birth weight and lung cancer risk, adjusting for adult smoking made any association completely null.
We found good agreement between birth weight reported in adulthood and measurements made at birth (Figure 1). Correlation coefficients reported by others have ranged from 0.63 to 0.83 [25, 27, 28, 29, 30] and our estimate of 0.78 [19] is consistent with these. With correlation coefficients of this magnitude, self-reported birth weight can be used as a reasonably reliable variable in epidemiological analyses, although the strength of any association may be underestimated due to regression dilution bias [23]. We allowed for this bias by assigning mean measured birth weight to each self-reported birth weight category when calculating trends [19, 23].
In studies that recorded birth weight retrospectively, recall of birth weight could differ between those with and without cancer. To avoid possible recall bias, our systematic review was restricted to studies with prospectively recorded information on birth weight. Our review revealed that almost all studies with prospectively recorded birth weight had linked birth records with cancer registrations, and that these studies lacked information on other risk factors for adult cancer, particularly height. For example in the meta-analysis of individual data on breast cancer, only 541 of the 13 381 cases appeared to have information recorded on adult height. For other cancers, our findings dominate the worldwide evidence: almost all previous reports did not adjust for height and none adjusted for smoking.
This is the first study with substantial numbers of site-specific cancers, in which it has been possible to examine the effects of separate and simultaneous adjustment for a range of potential confounding and intermediary factors. While we cannot exclude residual confounding and the role of other perinatal and early life correlates of birth weight, such as gestational age or childhood socio-economic status, our findings suggest that birth weight has little or no effect on the incidence of adult cancer.
Birth weight and adult height are both markers of growth, albeit during different periods of life, and can share environmental as well as genetic determinants [31]. Some aspect of growth, measured here as height, has a clear and strong effect on the incidence of most adult cancers [18]. Birth weight appears to add little additional information to adult height as a predictor of cancer risk in women.
funding
The study is funded by Cancer Research UK (C570/A16491) and the UK Medical Research Council (MR/K02700X/1).
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
acknowledgement
We thank all the women who participated in the Million Women Study.
appendix
The co-ordinating staff for the Million Women Study are: Hayley Abbiss, Simon Abbott, Miranda Armstrong, Angela Balkwill, Vicky Benson, Valerie Beral, Judith Black, Kathryn Bradbury, Anna Brown, Andrea Buron, Benjamin Cairns, Dexter Canoy, Andrew Chadwick, Barbara Crossley, Francesca Crowe, Dave Ewart, Sarah Ewart, Lee Fletcher, Sarah Floud, Toral Gathani, Laura Gerrard, Adrian Goodill, Jane Green, Lynden Guiver, Michal Hozak, Sau Wan Kan, Tim Key, Oksana Kirichek, Mary Kroll, Nicky Langston, Isobel Lingard, Maria Jose Luque, Kath Moser, Lynn Pank, Kirstin Pirie, Gillian Reeves, Keith Shaw, Emma Sherman, Evie Sherry-Starmer, Julie Schmidt, Helena Strange, Sian Sweetland, Alison Timadjer, Sarah Tipper, Ruth Travis, Lyndsey Trickett, Lucy Wright, Owen Yang, Heather Young.
references
- 1.Barker DJ, Winter PD, Osmond C, et al. Weight-gain in infancy and cancer of the ovary. Lancet. 1995;345:1087–1088. doi: 10.1016/s0140-6736(95)90821-8. [DOI] [PubMed] [Google Scholar]
- 2.Nilsen TIL, Romundstad PR, Troisi R, et al. Birth size and colorectal cancer risk: a prospective population based study. Gut. 2005;54:1728–1732. doi: 10.1136/gut.2004.060475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCormack VA, Silva ID, Koupil I, et al. Birth characteristics and adult cancer incidence: Swedish cohort of over 11,000 men and women. Int J Cancer. 2005;115:611–617. doi: 10.1002/ijc.20915. [DOI] [PubMed] [Google Scholar]
- 4.Ahlgren M, Wohlfahrt J, Olsen LW, et al. Birth weight and risk of cancer. Cancer. 2007;110:412–419. doi: 10.1002/cncr.22773. [DOI] [PubMed] [Google Scholar]
- 5.Franco-Lie I, Iversen T, Robsahm TE, Abdelnoor M. Birth weight and melanoma risk: a population-based case-control study. Br J Cancer. 2008;98:179–182. doi: 10.1038/sj.bjc.6604159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olesen AV, Parner ET, Mortensen PB, et al. Prenatal risk factors for cutaneous malignant melanoma: follow-up of 2,594,783 Danes born from 1950 to 2002. Cancer Epidemiol Biomarkers Prev. 2009;18:155–161. doi: 10.1158/1055-9965.EPI-08-0294. [DOI] [PubMed] [Google Scholar]
- 7.Eriksson JG, Thornburg KL, Osmond C, et al. The prenatal origins of lung cancer. I. The fetus. Am J Hum Biol. 2010;22:508–511. doi: 10.1002/ajhb.21040. [DOI] [PubMed] [Google Scholar]
- 8.Barker DJP, Osmond C, Thornburg KL, et al. The intrauterine origins of Hodgkin's lymphoma. Cancer Epidemiol. 2013;37:321–323. doi: 10.1016/j.canep.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 9.O'Rorke MA, Black C, Murray LJ, et al. Do perinatal and early life exposures influence the risk of malignant melanoma? A Northern Ireland birth cohort analysis. Eur J Cancer. 2013;49:1109–1116. doi: 10.1016/j.ejca.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 10.Sandhu MS, Luben R, Day NE, Khaw KT. Self-reported birth weight and subsequent risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2002;11:935–938. [PubMed] [Google Scholar]
- 11.Lof M, Sandin S, Hilakivi-Clarke L, Weiderpass E. Birth weight in relation to endometrial and breast cancer risks in Swedish women. Br J Cancer. 2007;96:134–136. doi: 10.1038/sj.bjc.6603504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xue F, Hilakivi-Clarke LA, Maxwell GL, et al. Longitudinal study on birthweight and the incidence of endometrial cancer. Br J Cancer. 2008;98:1288–1291. doi: 10.1038/sj.bjc.6604304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baer HJ, Hankinson SE, Tworoger SS. Body size in early life and risk of epithelial ovarian cancer: results from the Nurses’ Health Studies. Br J Cancer. 2008;99:1916–1922. doi: 10.1038/sj.bjc.6604742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dos Santos Silva I, De Stavola B, McCormack V. Birth size and breast cancer risk: re-analysis of individual participant data from 32 studies. PLoS Med. 2008;5:e193. doi: 10.1371/journal.pmed.0050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xue F, Michels KB. Intrauterine factors and risk of breast cancer: a systematic review and meta-analysis of current evidence. Lancet Oncol. 2007;8:1088–1100. doi: 10.1016/S1470-2045(07)70377-7. [DOI] [PubMed] [Google Scholar]
- 16.Park SK, Kang D, McGlynn KA, et al. Intrauterine environments and breast cancer risk: meta-analysis and systematic review. Breast Cancer Res. 2008;10:R8. doi: 10.1186/bcr1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu XH, Dailey AB, Peoples-Sheps M, et al. Birth weight as a risk factor for breast cancer: a meta-analysis of 18 epidemiological studies. J Womens Health (Larchmt) 2009;18:1169–1178. doi: 10.1089/jwh.2008.1034. [DOI] [PubMed] [Google Scholar]
- 18.Green J, Cairns BJ, Casabonne D, et al. Height and cancer incidence in the Million Women Study: prospective cohort, and meta-analysis of prospective studies of height and total cancer risk. Lancet Oncol. 2011;12:785–794. doi: 10.1016/S1470-2045(11)70154-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cairns BJ, Liu B, Clennell S, et al. Lifetime body size and reproductive factors: comparisons of data recorded prospectively with self reports in middle age. BMC Med Res Methodol. 2011;11:13. doi: 10.1186/1471-2288-11-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Plummer M. Improved estimates of floating absolute risk. Stat Med. 2004;23:93–104. doi: 10.1002/sim.1485. [DOI] [PubMed] [Google Scholar]
- 21.Townsend P, Phillimore P, Beattie A. Health and Deprivation: Inequality and the North. London: Croon Helm; 1988. [Google Scholar]
- 22.Allen NE, Beral V, Casabonne D, et al. Moderate alcohol intake and cancer incidence in women. J Natl Cancer Inst. 2009;101:296–305. doi: 10.1093/jnci/djn514. [DOI] [PubMed] [Google Scholar]
- 23.Macmahon S, Peto R, Cutler J, et al. Blood-pressure, stroke, and coronary heart-disease.1. Prolonged differences in blood-pressure—prospective observational studies corrected for the regression dilution bias. Lancet. 1990;335:765–774. doi: 10.1016/0140-6736(90)90878-9. [DOI] [PubMed] [Google Scholar]
- 24.Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata J. 2006;6:40–57. [Google Scholar]
- 25.Andersson SW, Niklasson A, Lapidus L, et al. Poor agreement between self-reported birth weight and birth weight from original records in adult women. Am J Epidemiol. 2000;152:609–616. doi: 10.1093/aje/152.7.609. [DOI] [PubMed] [Google Scholar]
- 26.Andersen ZJ, Baker JL, Bihrmann K, et al. Birth weight, childhood body mass index, and height in relation to mammographic density and breast cancer: a register-based cohort study. Breast Cancer Res. 2014;16:R4. doi: 10.1186/bcr3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Troy LM, Michels KB, Hunter DJ, et al. Self-reported birthweight and history of having been breastfed among younger women: an assessment of validity. Int J Epidemiol. 1996;25:122–127. doi: 10.1093/ije/25.1.122. [DOI] [PubMed] [Google Scholar]
- 28.Sanderson M, Williams MA, White E, et al. Validity and reliability of subject and mother reporting of perinatal factors. Am J Epidemiol. 1998;147:136–140. doi: 10.1093/oxfordjournals.aje.a009425. [DOI] [PubMed] [Google Scholar]
- 29.Kemp M, Gunnell D, Maynard M, et al. How accurate is self reported birth weight among the elderly? J Epidemiol Community Health. 2000;54:639–640. doi: 10.1136/jech.54.8.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wodskou PM, Hundrup YA, Obel EB, Jorgensen T. Validity of self-reported birthweight among middle-aged and elderly women in the Danish Nurse Cohort Study. Acta Obstet Gynecol Scand. 2010;89:1134–1139. doi: 10.3109/00016349.2010.500370. [DOI] [PubMed] [Google Scholar]
- 31.Patti ME. Intergenerational programming of metabolic disease: evidence from human populations and experimental animal models. Cell Mol Life Sci. 2013;70:1597–1608. doi: 10.1007/s00018-013-1298-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.