Abstract
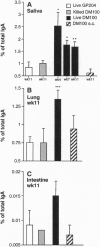
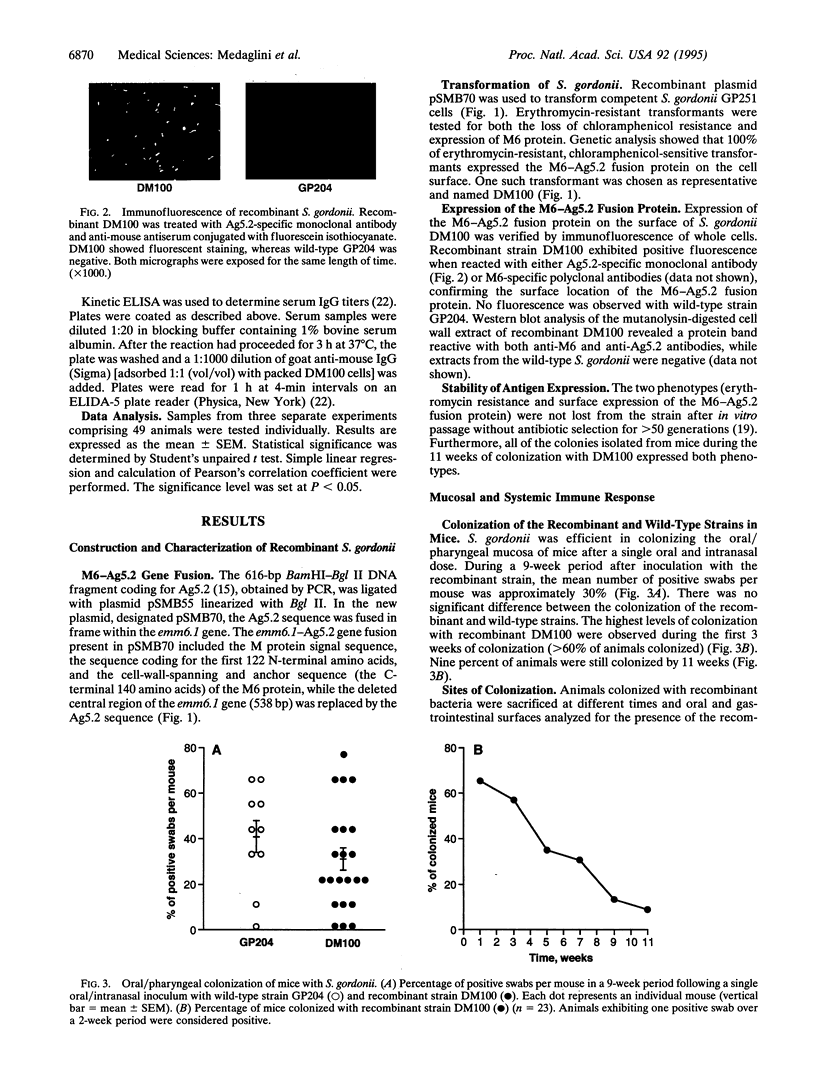
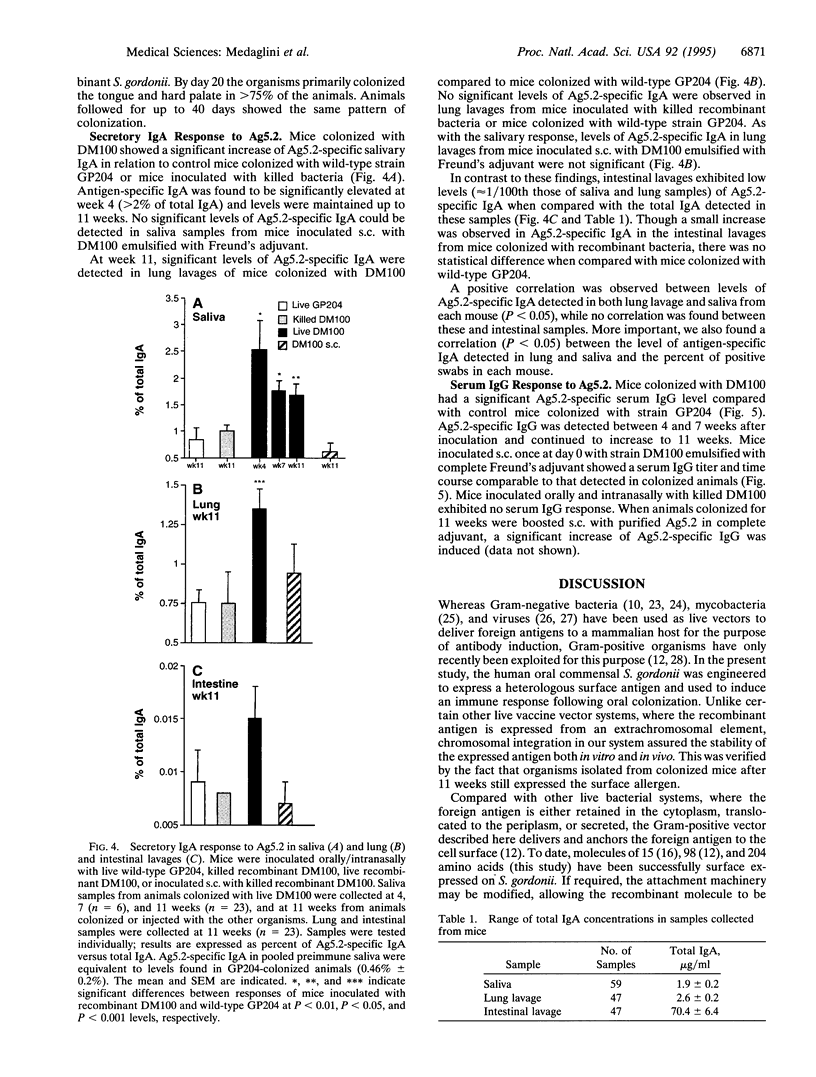
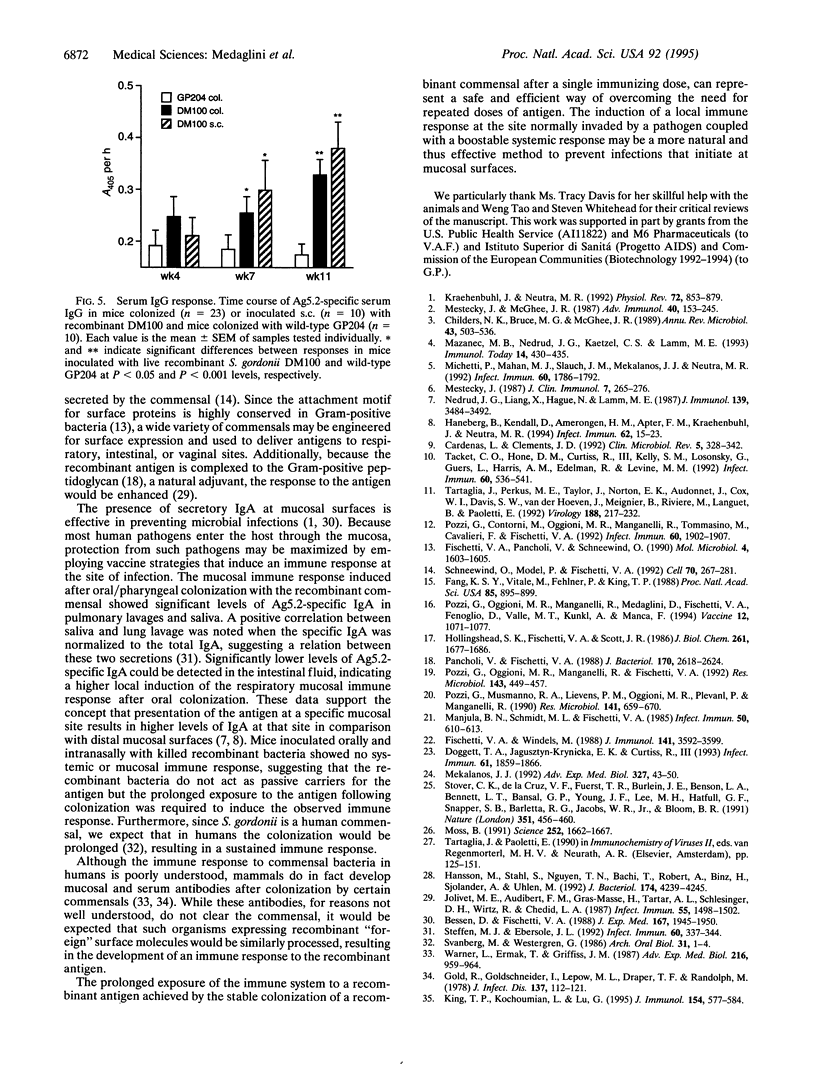
To circumvent the need to engineer pathogenic microorganisms as live vaccine-delivery vehicles, a system was developed which allowed for the stable expression of a wide range of protein antigens on the surface of Gram-positive commensal bacteria. The human oral commensal Streptococcus gordonii was engineered to surface express a 204-amino acid allergen from hornet venom (Ag5.2) as a fusion with the anchor region of the M6 protein of Streptococcus pyogenes. The immunogenicity of the M6-Ag5.2 fusion protein was assessed in mice inoculated orally and intranasally with a single dose of recombinant bacteria, resulting in the colonization of the oral/pharyngeal mucosa for 10-11 weeks. A significant increase of Ag5.2-specific IgA with relation to the total IgA was detected in saliva and lung lavages when compared with mice colonized with wild-type S. gordonii. A systemic IgG response to Ag5.2 was also induced after oral colonization. Thus, recombinant Gram-positive commensal bacteria may be a safe and effective way of inducing a local and systemic immune response.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bessen D., Fischetti V. A. Passive acquired mucosal immunity to group A streptococci by secretory immunoglobulin A. J Exp Med. 1988 Jun 1;167(6):1945–1950. doi: 10.1084/jem.167.6.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childers N. K., Bruce M. G., McGhee J. R. Molecular mechanisms of immunoglobulin A defense. Annu Rev Microbiol. 1989;43:503–536. doi: 10.1146/annurev.mi.43.100189.002443. [DOI] [PubMed] [Google Scholar]
- Cárdenas L., Clements J. D. Oral immunization using live attenuated Salmonella spp. as carriers of foreign antigens. Clin Microbiol Rev. 1992 Jul;5(3):328–342. doi: 10.1128/cmr.5.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doggett T. A., Jagusztyn-Krynicka E. K., Curtiss R., 3rd Immune responses to Streptococcus sobrinus surface protein antigen A expressed by recombinant Salmonella typhimurium. Infect Immun. 1993 May;61(5):1859–1866. doi: 10.1128/iai.61.5.1859-1866.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang K. S., Vitale M., Fehlner P., King T. P. cDNA cloning and primary structure of a white-face hornet venom allergen, antigen 5. Proc Natl Acad Sci U S A. 1988 Feb;85(3):895–899. doi: 10.1073/pnas.85.3.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti V. A., Pancholi V., Schneewind O. Conservation of a hexapeptide sequence in the anchor region of surface proteins from gram-positive cocci. Mol Microbiol. 1990 Sep;4(9):1603–1605. doi: 10.1111/j.1365-2958.1990.tb02072.x. [DOI] [PubMed] [Google Scholar]
- Fischetti V. A., Windels M. Mapping the immunodeterminants of the complete streptococcal M6 protein molecule. Identification of an immunodominant region. J Immunol. 1988 Nov 15;141(10):3592–3599. [PubMed] [Google Scholar]
- Gold R., Goldschneider I., Lepow M. L., Draper T. F., Randolph M. Carriage of Neisseria meningitidis and Neisseria lactamica in infants and children. J Infect Dis. 1978 Feb;137(2):112–121. doi: 10.1093/infdis/137.2.112. [DOI] [PubMed] [Google Scholar]
- Haneberg B., Kendall D., Amerongen H. M., Apter F. M., Kraehenbuhl J. P., Neutra M. R. Induction of specific immunoglobulin A in the small intestine, colon-rectum, and vagina measured by a new method for collection of secretions from local mucosal surfaces. Infect Immun. 1994 Jan;62(1):15–23. doi: 10.1128/iai.62.1.15-23.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson M., Ståhl S., Nguyen T. N., Bächi T., Robert A., Binz H., Sjölander A., Uhlén M. Expression of recombinant proteins on the surface of the coagulase-negative bacterium Staphylococcus xylosus. J Bacteriol. 1992 Jul;174(13):4239–4245. doi: 10.1128/jb.174.13.4239-4245.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead S. K., Fischetti V. A., Scott J. R. Complete nucleotide sequence of type 6 M protein of the group A Streptococcus. Repetitive structure and membrane anchor. J Biol Chem. 1986 Feb 5;261(4):1677–1686. [PubMed] [Google Scholar]
- Jolivet M. E., Audibert F. M., Gras-Masse H., Tartar A. L., Schlesinger D. H., Wirtz R., Chedid L. A. Induction of biologically active antibodies by a polyvalent synthetic vaccine constructed without carrier. Infect Immun. 1987 Jun;55(6):1498–1502. doi: 10.1128/iai.55.6.1498-1502.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King T. P., Kochoumian L., Lu G. Murine T and B cell responses to natural and recombinant hornet venom allergen Dol m 5.02 and its recombinant fragments. J Immunol. 1995 Jan 15;154(2):577–584. [PubMed] [Google Scholar]
- Kraehenbuhl J. P., Neutra M. R. Molecular and cellular basis of immune protection of mucosal surfaces. Physiol Rev. 1992 Oct;72(4):853–879. doi: 10.1152/physrev.1992.72.4.853. [DOI] [PubMed] [Google Scholar]
- Manjula B. N., Schmidt M. L., Fischetti V. A. Unimpaired function of human phagocytes in the presence of phagocytosis-resistant group A streptococci. Infect Immun. 1985 Dec;50(3):610–613. doi: 10.1128/iai.50.3.610-613.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazanec M. B., Nedrud J. G., Kaetzel C. S., Lamm M. E. A three-tiered view of the role of IgA in mucosal defense. Immunol Today. 1993 Sep;14(9):430–435. doi: 10.1016/0167-5699(93)90245-G. [DOI] [PubMed] [Google Scholar]
- Mekalanos J. J. Bacterial mucosal vaccines. Adv Exp Med Biol. 1992;327:43–50. doi: 10.1007/978-1-4615-3410-5_6. [DOI] [PubMed] [Google Scholar]
- Mestecky J., McGhee J. R. Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv Immunol. 1987;40:153–245. doi: 10.1016/s0065-2776(08)60240-0. [DOI] [PubMed] [Google Scholar]
- Mestecky J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J Clin Immunol. 1987 Jul;7(4):265–276. doi: 10.1007/BF00915547. [DOI] [PubMed] [Google Scholar]
- Michetti P., Mahan M. J., Slauch J. M., Mekalanos J. J., Neutra M. R. Monoclonal secretory immunoglobulin A protects mice against oral challenge with the invasive pathogen Salmonella typhimurium. Infect Immun. 1992 May;60(5):1786–1792. doi: 10.1128/iai.60.5.1786-1792.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B. Vaccinia virus: a tool for research and vaccine development. Science. 1991 Jun 21;252(5013):1662–1667. doi: 10.1126/science.2047875. [DOI] [PubMed] [Google Scholar]
- Nedrud J. G., Liang X. P., Hague N., Lamm M. E. Combined oral/nasal immunization protects mice from Sendai virus infection. J Immunol. 1987 Nov 15;139(10):3484–3492. [PubMed] [Google Scholar]
- Pancholi V., Fischetti V. A. Isolation and characterization of the cell-associated region of group A streptococcal M6 protein. J Bacteriol. 1988 Jun;170(6):2618–2624. doi: 10.1128/jb.170.6.2618-2624.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi G., Contorni M., Oggioni M. R., Manganelli R., Tommasino M., Cavalieri F., Fischetti V. A. Delivery and expression of a heterologous antigen on the surface of streptococci. Infect Immun. 1992 May;60(5):1902–1907. doi: 10.1128/iai.60.5.1902-1907.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi G., Musmanno R. A., Lievens P. M., Oggioni M. R., Plevani P., Manganelli R. Method and parameters for genetic transformation of Streptococcus sanguis Challis. Res Microbiol. 1990 Jul-Aug;141(6):659–670. doi: 10.1016/0923-2508(90)90060-4. [DOI] [PubMed] [Google Scholar]
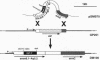
- Pozzi G., Oggioni M. R., Manganelli R., Fischetti V. A. Expression of M6 protein gene of Streptococcus pyogenes in Streptococcus gordonii after chromosomal integration and transcriptional fusion. Res Microbiol. 1992 Jun;143(5):449–457. doi: 10.1016/0923-2508(92)90090-b. [DOI] [PubMed] [Google Scholar]
- Pozzi G., Oggioni M. R., Manganelli R., Medaglini D., Fischetti V. A., Fenoglio D., Valle M. T., Kunkl A., Manca F. Human T-helper cell recognition of an immunodominant epitope of HIV-1 gp120 expressed on the surface of Streptococcus gordonii. Vaccine. 1994 Sep;12(12):1071–1077. doi: 10.1016/0264-410x(94)90175-9. [DOI] [PubMed] [Google Scholar]
- Schneewind O., Model P., Fischetti V. A. Sorting of protein A to the staphylococcal cell wall. Cell. 1992 Jul 24;70(2):267–281. doi: 10.1016/0092-8674(92)90101-h. [DOI] [PubMed] [Google Scholar]
- Steffen M. J., Ebersole J. L. Secretory immune responses to Mycoplasma pulmonis. Infect Immun. 1992 Feb;60(2):337–344. doi: 10.1128/iai.60.2.337-344.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover C. K., de la Cruz V. F., Fuerst T. R., Burlein J. E., Benson L. A., Bennett L. T., Bansal G. P., Young J. F., Lee M. H., Hatfull G. F. New use of BCG for recombinant vaccines. Nature. 1991 Jun 6;351(6326):456–460. doi: 10.1038/351456a0. [DOI] [PubMed] [Google Scholar]
- Svanberg M., Westergren G. Persistence and spread of the orally-implanted bacterium Streptococcus sanguis between persons. Arch Oral Biol. 1986;31(1):1–4. doi: 10.1016/0003-9969(86)90106-8. [DOI] [PubMed] [Google Scholar]
- Tacket C. O., Hone D. M., Curtiss R., 3rd, Kelly S. M., Losonsky G., Guers L., Harris A. M., Edelman R., Levine M. M. Comparison of the safety and immunogenicity of delta aroC delta aroD and delta cya delta crp Salmonella typhi strains in adult volunteers. Infect Immun. 1992 Feb;60(2):536–541. doi: 10.1128/iai.60.2.536-541.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia J., Perkus M. E., Taylor J., Norton E. K., Audonnet J. C., Cox W. I., Davis S. W., van der Hoeven J., Meignier B., Riviere M. NYVAC: a highly attenuated strain of vaccinia virus. Virology. 1992 May;188(1):217–232. doi: 10.1016/0042-6822(92)90752-b. [DOI] [PubMed] [Google Scholar]
- Warner L., Ermak T., Griffiss J. M. Mucosal and serum immunity following commensal enteric colonization. Adv Exp Med Biol. 1987;216B:959–964. [PubMed] [Google Scholar]