Abstract
Gene identification in human aortic aneurysm conditions is proceeding at a rapid pace and the integration of pathogenesis-based management strategies in clinical practice is an emerging reality. Human genetic alterations causing aneurysm involve diverse gene products including constituents of the extracellular matrix, cell surface receptors, intracellular signaling molecules, and elements of the contractile cytoskeleton. Animal modeling experiments and human genetic discoveries have extensively implicated the transforming growth factor-β (TGF-β) cytokine-signaling cascade in aneurysm progression, but mechanistic links between many gene products remain obscure. This chapter will integrate human genetic alterations associated with aortic aneurysm with current basic research findings in an attempt to form a reconciling if not unifying model for hereditary aortic aneurysm.
Aortic aneurysm can be caused by genetic mutations that alter components of the extracellular matrix, cell surface receptors, intracellular signaling pathways (e.g., TGF-β), and the contractile cytoskeleton.
Among the myriad blood vessels within the body, the large capacitance arteries adjacent to the heart are unique in structure, function, and in their capacity for derangement in disease states. The clear enrichment of involvement of the proximal ascending thoracic aorta, and more specifically the sinuses of Valsalva, in inherited forms of thoracic aortic aneurysm (TAA) has been historically attributed to the unique functional demands and hemodynamic environment of this aortic segment. The aorta is a relatively unique organ in that its critical functional components are located in the extracellular space, whereas the cellular components of the aorta may act primarily to support the development and homeostasis of the supporting matrix. Cardiovascular surgeons show this interesting biology in common surgical practice. Large aortic segments are routinely replaced entirely with synthetic materials such as Dacron, demonstrating the dispensability of aortic cellular components for minimal critical function (to serve as a conduit and distribution network for blood flow). Despite its seemingly simple nature, the aorta has evolved into a complex organ with spatial variation in structure. During cardiac systole, the proximal ascending aorta has the ability to accept pressure and volume loads (capacitance) and to transmit them distally during diastole through recoil (elastance) with the least loss of energy. These requirements are reflected by variation in the major components of the extracellular matrix, collagen (predominantly types I and III) and elastin. Collagens provide tensile strength and stiffness to the tissue to resist rupture and efficiently propagate pressure waves, respectively. Elastic fibers show dynamic lengthening and contraction in response to pressure and volume loads, accounting for the capacitance and recoil shown by large arteries. In keeping with engineering requirements, the proximal ascending aorta shows enrichment of elastic fibers and a relative paucity of collagen, when compared with more distal aortic segments. Despite the intuitive appeal, a number of findings challenge the hypothesis that the proximal ascending aorta shows high predisposition for aneurysm wholly on the basis of its unique structure and hemodynamic environment. First, many inherited forms of TAA commonly associate with a strong predisposition for enlargement of the pulmonary root on the low-pressure side of the circulation. Second, disorders associated with a primary and profound deficiency of types I or III collagen (osteogenesis imperfecta [OI], and vascular Ehlers–Danlos syndrome [vEDS], respectively) only rarely show aortic root enlargement. Although the risk of aneurysm and dissection is high in vEDS, lesions are focal and can occur in any medium-to-large muscular artery without apparent association with sites of enhanced hemodynamic stress. Finally, many disorders associated with failed elastogenesis or impaired elastic fiber homeostasis, such as cutis laxa caused by mutations in FBLN5 encoding fibulin-5 (Loeys et al. 2002) or pseudoxanthoma elasticum (PXE) caused by mutations in ABCC6 (Bergen et al. 2000) (an ATP-binding cassette transporter), respectively, do not show a predisposition for ascending aortic aneurysm, or, for that matter, aneurysm in general.
Multiple models of aneurysm pathogenesis have centered on failure of elastic fiber homeostasis. In support of these models, most presentations of aortic aneurysm associate with increased expression and activity of proteases with elastolytic potential, prominently including matrix metalloproteinases (MMPs) 2 and 9 that can be expressed by resident cells (e.g., vascular smooth muscle cells [VSMCs]) or inflammatory infiltrates. It is notable, however, that these proteases have wide substrate specificity that includes other structural matrix elements including collagens, fibronectin, and proteoglycans. They also recognize (and often activate) bioactive substrates including cytokines (e.g., TGF-β) and other proteases (Newby 2012). It also seems informative that blood vessels that, at least transiently, lack an intact arterial media (e.g., after complete stripping during carotid endarterectomy) show the capacity to withstand hemodynamic stress and avoid aneurysm, whereas other acute chemical or physical insults initiate rapid aneurysm progression. In this light, does the aortic adventitia (which remains intact after endarterectomy) deserve at least equal attention? More broadly, what additional determinants are required for aortic aneurysm progression beyond failure of elastic fiber homeostasis?
Marfan syndrome (MFS) is the most common and in many ways the archetype of the familial connective tissue disorders associated with TAA. Patients with MFS show a high predisposition for aortic aneurysm centered anatomically at the sinuses of Valsalva. Before the molecular era, hypotheses regarding the cause of connective tissue disorders such as MFS tended to emphasize deficits in structural protein quantity or integrity, especially collagens and elastin (McKusick 1973). The identification of mutations in genes encoding structural components of the extracellular matrix (COL3A1 causing vEDS in 1988 [Superti-Furga et al. 1988], FBN1 causing MFS in 1991 [Dietz et al. 1991], and Table 1 containing a list of genes mutated in human aneurysm conditions) seemed to validate early pathogenic theories that singularly invoked structural tissue failure as the driving force for aneurysm. This view of pathogenesis was bleak, requiring the ability to alter the structural composition of tissues for clinical benefit. Fortunately, new discoveries have highlighted the importance of altered cellular performance, either in response to altered matricellular interactions or as a primary event, in altered homeostasis of the aortic wall. Work in animal models has shown that extracellular matrix components not only provide structural integrity to the vessel wall, but also contribute to regulation of the bioavailability of extracellular signaling ligands. A prime example of this phenomenon relates to the fibrillin family of proteins, exemplified by fibrillin-1 (discussed below). The study of fibrillin-1 deficiency in the context of MFS has highlighted the importance of the TGF-β signaling pathway in arterial homeostasis. More recent findings of genetic disturbances in the cytoskeletal apparatus of vascular smooth muscle cells have made it evident that vascular homeostasis can also be perturbed by primary cellular dysfunction. It remains to be determined whether disease gene discovery will reveal strictly parallel pathogenic sequences or a common final pathway for aneurysm progression. In either event, the diversity of gene loci and function in hereditary aneurysmal conditions will no doubt lead to the discovery of new therapeutic targets. This review will discuss current and emerging pathogenic models, focusing on attempts at mechanistic integration, and opportunities for therapeutic intervention.
Table 1.
Human hereditary aneurysm conditions
| Gene (protein) | Human aneurysmal syndrome |
|---|---|
| Extracellular matrix proteins | |
| FBN1 (fibrillin-1) | Marfan syndrome—Fully penetrant ascending aortic aneurysm (Dietz et al. 1991) |
| EFEMP2 (fibulin-4) | Cutis laxa with aneurysm—Ascending aortic aneurysm and tortuosity (Dasouki et al. 2007) |
| ELN (elastin) | Supravalvular aortic stenosis (Curran et al. 1993) Cutis laxa with aneurysm—Low penetrance–ascending aortic aneurysm and dissection (Szabo et al. 2006) |
| COL1A1 (collagen 1 α-1) | Osteogenesis imperfecta—Extremely rare aortic aneurysm and dissection (Isotalo et al. 1999) Ehlers–Danlos syndrome, type 7A—Dissection of medium-sized arteries (Malfait et al. 2007) |
| COL1A2 (collagen 1 α-2) | Osteogenesis imperfecta—Extremely rare aortic aneurysm and dissection (Isotalo et al. 1999) Ehlers–Danlos syndrome—Cardiac valvulodystrophy type 7B borderline aortic root enlargement with aortic regurgitation (Schwarze et al. 2004) |
| COL3A1 (collagen 3 α-1) | Ehlers–Danlos, type 4—Frequent arterial dissection with infrequent aneurysm (Superti-Furga et al. 1988) |
| COL4A1 (collagen 4 α-1) | Hereditary angiopathy, nephropathy, aneurysms, and muscle cramps—Infrequent aneurysms (Plaisier et al. 2007) |
| COL4A5 (collagen 4 α-5) | X-linked Alport syndrome—Ascending aortic and abdominal aneurysms and dissections (Kashtan et al. 2010) |
| PLOD1 (lysyl hydroxylase 1) | Ehlers–Danlos, type 6—Rare aneurysm, arterial rupture (Wenstrup et al. 1989) |
| PLOD3 (lysyl hydroxlase 3) | Bone fragility with contractures, arterial rupture, and deafness—Frequent medium-sized arterial aneurysms (Salo et al. 2008) |
| TGF-β pathway | |
| TGFBR1 (tgfbr1) | Loeys–Dietz syndrome—Highly penetrant root and diffuse large and medium arterial aneurysm (Loeys et al. 2005, 2006) |
| TGFBR2 (tgfbr2) | Loeys–Dietz syndrome—Highly penetrant root and diffuse large and medium arterial aneurysm (Mizuguchi et al. 2004; Loeys et al. 2005, 2006) FTAAD—Highly penetrant root and medium arterial aneurysm |
| TGFB2 (tgfb2) | Loeys–Dietz syndrome—Aortic root aneurysm, club foot, arterial tortuosity (Boileau et al. 2012; Lindsay et al. 2012) |
| SMAD3 (SMAD family member 3) | Loeys–Dietz syndrome with osteoarthritis (van de Laar et al. 2010) |
| SMAD4 (SMAD family member 4) | Juvenile polyposis, aortic aneurysm, mitral valve dysfunction (Andrabi et al. 2011) HHT—Hereditary hemorrhagic telangectasia with intestinal polyposis |
| SKI (v-SKI sarcoma oncogene homolog) | Shprintzen–Goldberg syndrome—Chest wall deformities, aranchnodactly, cleft palate, craniosynostosis, ascending aortic aneurysm (Doyle et al. 2012) |
| SLC2A10 (glucose transporter 10) | Arterial tortuosity syndrome—Diffuse arterial tortuosity, stenoses, aneurysms (Coucke et al. 2006) |
| Cytoskeletal/smooth muscle contraction apparatus proteins | |
| ACTA2 (α-smooth muscle actin) | Familial aortic aneurysm with levido reticularis and iris flocculi (Guo et al. 2007) |
| MYH11 (smooth muscle myosin) | Familial aortic aneurysm with patent ductus arteriosis (Zhu et al. 2006; Pannu et al. 2007) |
| FLNA (filamin A) | Periventricular nodular heterotopia with Ehlers–Danlos features—Ascending aortic aneurysm and valvular dystrophy (Sheen et al. 2005) Familial cardiac valvular dystrophy (Kyndt et al. 2007) |
| MYLK (myosin light chain kinase) PRKG1 (protein kinase, cGMP-dependent, type I) | Familial aortic aneurysm and dissection (Wang et al. 2011) Familial aortic aneurysm and dissection (Guo et al. 2013) |
| RAS-related disorder | |
| NF1 (neurofibromin 1) | Neurofibromatosis—Medium-sized arterial aneurysm and stenosis (Friedman et al. 2002) |
| PTPN11 (SH2 domain-containing protein tyrosine phosphatase-2) | Noonan and LEOPARD syndrome—Coronary artery aneurysms and rare ascending aortic aneurysm (Purnell et al. 2005; Iwasaki et al. 2009) |
| Notch/jagged | |
| NOTCH1 (notch1) | Bicuspid valve with ascending aortic aneurysm (Garg et al. 2005) |
| JAG1 (jagged1) | Alagille syndrome—Intracranial aneurysms, coarctation of the aorta, aortic aneurysm (Kamath et al. 2004) |
| Miscellaneous/unknown | |
| TSC2 (tuberin) | Tuberous sclerosis—Diffuse thorocoabdominal aneurysms (Cao et al. 2010) |
| PKD1 (polycystin-1) | Polycystic kidney disease with intracranial aneurysms |
| PKD2 (polycystin-2) | Polycystic kidney disease with intracranial aneurysms |
| Chromosomal anomaly | |
| 45 XO | Turner syndrome—Bicuspid aortic valve, coarctation of the aorta, ascending aortic aneurysm (Lopez et al. 2008) |
For an up-to-date compendium of human aortic aneurysm genes and mutations, please see OMIM (www.omim.org).
EXTRACELLULAR MATRIX: FIBRILLINS, FIBULINS, AND ELASTIN
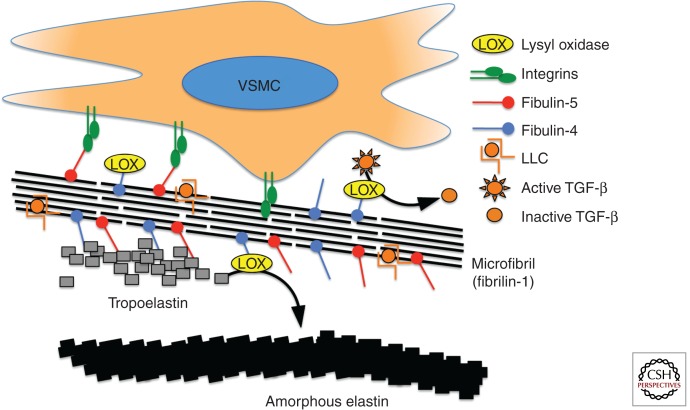
Several human conditions are associated with profound failure of elastogenesis and/or elastic fiber homeostasis. Despite the emphasis placed on the quality and quantity of elastic fibers in aneurysm conditions, elastin deficiency does not necessarily lead to aneurysm. Aneurysm is an infrequent finding in cutis laxa syndromes cause by mutations in the ELN gene (Szabo et al. 2006), which encodes elastin. In fact, ELN haploinsufficiency causes either syndromic (Williams syndrome) or nonsyndromic supravalvular aortic stenosis (SVAS), coronary ostial stenosis, and diffuse pulmonary arterial stenoses without aneurysm (Curran et al. 1993), depending on whether there is or is not concomitant deletion of contiguous genes, respectively. Unlike aneurysm tissue, aortic tissue from patients with Williams syndrome or SVAS shows an increased number of elastic lamella and VSMCs, indicating a role for elastin in the regulation of arterial organization and suppression of VSMC proliferation (Li et al. 1998). Pseudoxanthoma elasticum causes a severe failure of elastic fiber homeostasis, but patients do not typically show aneurysm. Examination of arterial tissue from patients with PXE shows elastic fiber fragmentation and disarray (Miki et al. 2007). Similar histopathology is seen in aortic root aneurysms in MFS caused by mutations in FBN1 encoding fibrillin-1 (Dietz et al. 1991). Mutations in fibulin-4 cause cutis laxa with highly penetrant arterial tortuosity and ascending aortic aneurysm (Dasouki et al. 2007; Renard et al. 2010), whereas mutations in fibulin-5 cause cutis laxa with arterial tortuosity, but not aneurysm (Loeys et al. 2002). Both conditions are associated with a profound failure of elastogenesis and both classes of patients show multiple systemic connective tissue findings. What then accounts for the difference in clinical expression of fibulin-4 versus fibulin-5 deficiency? Both fibulin-4 and fibulin-5 bind to fibrillin-1-containing microfibrils and elastin. Although both fibulin-4 and fibulin-5 act to recruit tropoelastin to developing microfibrils, fibulin-4 additionally recruits lysyl oxidase (LOX, a copper-containing amine oxidase responsible for elastin and collagen cross-linking) (Horiguchi et al. 2009). Fibulin-4 deficiency decreases LOX recruitment to tropoelastin in experimental cutis laxa in mice, and targeted inactivation of fibulin-4 in vascular smooth muscle causes ascending aortic aneurysm (Horiguchi et al. 2009). Consistent with these data, LOX-deficient mice show aortic aneurysm and perinatal death owing to cardiac dysfunction (Maki et al. 2002).
THE TGF-β PATHWAY IN MARFAN SYNDROME
To date, more than 1000 different mutations in the FBN1 gene have been associated with MFS. This includes missense, frame-shift, nonsense and splice-site mutations and indels including whole-exon or even whole-gene deletions (Faivre et al. 2009; Turner et al. 2009). This repertoire strongly suggests loss of function of the fibrillin-1 protein as the relevant mechanism; a prediction confirmed using mouse models (Judge et al. 2004). Despite the appeal of a “common sense” pathogenic model focusing on structural tissue weakness, it was recognized that some clinical manifestations of MFS (such as bone overgrowth, craniofacial dysmorphism and skeletal muscle and fat hypoplasia) are difficult or impossible to reconcile on this basis.
Insight into the pathogenesis of MFS progressed most rapidly on the development of animal models that fully recapitulate the multisystem disease manifestations including aortic aneurysm (Pereira et al. 1997; Judge et al. 2004). About 10%–15% of patients with MFS show a predisposition for pulmonary emphysema, often presenting with upper lobe bullae and spontaneous pneumothorax. In keeping with structural deficiency of the tissues, early explanations posited accelerated destructive and inflammatory emphysema. However, fibrillin-1-deficient mice showed homogeneous widening of the distal airspace at birth without any attendant inflammation. This finding was most consistent with primary developmental failure of distal alveolar septation (Neptune et al. 2003). In consideration of how a defect in an extracellular matrix protein might influence developmental events, it was recognized that fibrillin-1 shows structural homology with the latent transforming growth factor-β–binding protein family (LTBPs). LTBPs 1, 3, and perhaps 4 bind the small latent complex (SLC) of TGF-β (constituting the large latent complex or LLC). They also bind to extracellular matrix proteins including fibrillins and fibronectin. This led to the hypothesis that LTBP interactions serve to sequester latent TGF-β within the matrix, and that this event regulates proximity to or the activity of cell-associated TGF-β activators such as selected integrins or thrombospondin-1. In this view, fibrillin-1 deficiency would be expected to cause promiscuous TGF-β activation and signaling. In keeping with this hypothesis, prenatal or perinatal antagonism of TGF-β activity through systemic delivery of a panspecific TGF-β-neutralizing antibody was able to normalize distal alveolar septation in MFS mice, despite an ongoing deficiency of fibrillin-1. Evidence for increased TGF-β signaling, including increased phosphorylation and nuclear translocation of Smad2 (pSmad2) and increased output of TGF-β-responsive gene products (e.g., types I and III collagen and connective tissue growth factor or CTGF) was evident in other tissues involved in MFS, and TGF-β-neutralizing antibody also mitigated important disease phenotypes including myxomatous degeneration of the mitral valve, skeletal muscle myopathy, and aortic root aneurysm (Ng et al. 2004; Habashi et al. 2006; Cohn et al. 2007). The involvement of the TGF-β signaling cascade in the development of fibrillin-1-dependent aortic aneurysm also provides insight into fibulin-4-mediated vascular disease. As mentioned previously, fibulin-4 recruits the enzyme LOX to microfibrils. Most interestingly, in addition to its matrix cross-linking function, LOX shows negative regulatory activity toward TGF-β, which it binds and inactivates through its amine oxidase activity (Atsawasuwan et al. 2008), and TGF-β pathways have been shown to be perturbed in the ascending aorta of fibulin-4-deficient mice (Hanada et al. 2007). Consistent with these data, LOX has been shown to suppress expression of the TGF-β inducible factor MCP-1 (Onoda et al. 2010), a cytokine thought to contribute to aneurysm progression (Tieu et al. 2009). Therefore, among the pathologic mechanistic possibilities shared by deficiency of fibulin-4 and fibrillin-1, a prominent candidate includes suppression of TGF-β activity (Fig. 1), either by a lack of sequestration (fibrillin-1) or through failed recruitment of a negative regulator (fibulin-4).
Figure 1.
Extracellular matrix: fibrillins, fibulins, and elastin. Vascular smooth muscle cells interact with multiple components of the extracellular matrix including fibrillins, fibulins, and elastin proteins. One function of fibulin proteins is to localize lysyl oxidase (LOX) to fibrillin microfibrils. LOX functions both to catalyze the formation of amorphous elastin from tropoelastin as well as to enzymatically inactivate free TGF-β. As expected, deficiency of either fibulins-4 or fibulins-5 causes a profound failure of elastogenesis. However, only deficiency of fibulin-4 causes aortic aneurysm, perhaps related to its ability to recruit LOX to microfibrils.
A new report has used a genome-wide association study (GWAS) to link FBN1 to a risk for thoracic aneurysm and dissection in nonsyndromic patients (Lemaire et al. 2011). A large cohort of patients with sporadic thoracic aneurysm and/or dissection was subjected to GWAS analysis and a single susceptibility locus was identified at 15q21.1 spanning FBN1. Five independent SNPs closely associated with FBN1 were identified, which reached whole genome significance. The combinatorial nature of the SNPs was not analyzed so that a dominant haplotype associated with aortic disease could be identified. These findings raise interesting questions. As insufficiency of fibrillin-1 expression is the operative mechanism of Marfan aneurysmal disease, presumably, the SNPs are associated with a decrement in fibrillin-1 expression unable to cause MFS, but able to induce arterial dysfunction. Unfortunately, fibrillin-1 expression from patient samples with pathologic SNPs was not analyzed leaving such a mechanism to speculation. Several of the identified SNPs also conferred susceptibility toward aortic aneurysm in a cohort with sporadic bicuspid aortic valve (BAV) with aneurysm. Are these ancestral FBN1 alleles acting primarily to cause BAV or do they act as a modifier of other loci responsible for BAV? Although patients with MFS have been described to have a rate of BAV slightly above the general population, it is not a common feature of the syndrome. Because the gene dysregulation associated with these SNPs would be expected to be subtle (so as not to cause overt syndromic phenotypes), it seems unlikely that they would primarily cause BAV. If these alleles act as modifier loci to worsen intrinsic aortic disease they would be expected to segregate with aneurysm in familial BAV/TAA cohorts; however, this type of analysis was not performed. In summary, these results raise interesting questions regarding the role of fibrillin in nonsyndromic aortic disease, but much work remains to be performed to uncover a plausible mechanism of action.
TGF-β PATHWAY MUTATIONS: LOEYS–DIETZ SYNDROME (LDS)
The involvement of TGF-β in the pathogenesis of aortic aneurysm was highlighted by the description of a systemic disorder of connective tissue, Loeys–Dietz syndrome, caused by mutations in the receptors for TGF-β (TGFBR1 and TGFBR2) (Loeys et al. 2005, 2006). Patients with LDS show many phenotypic features that overlap with those of MFS such as scoliosis, arachnodactyly, pectus deformity and importantly aortic aneurysm centered at the sinuses of Valsalva. Although LDS has a wider clinical phenotypic severity within families than MFS, in general vascular disease in LDS is noted to be more severe with frequent aneurysms outside the proximal aorta and dissections at younger ages and with smaller arterial diameters (Loeys et al. 2006). More recently, additional mutations have been described causing LDS in two other genes encoding positive effectors of TGF-β signaling, SMAD3 (van de Laar et al. 2010), encoding a downstream modulator of TGF-β signaling and encoding a TGF-β ligand, TGFB2 (Boileau et al. 2012; Lindsay et al. 2012).
The majority of mutations in the TGFBR1 and TGFBR2 genes encode missense changes that tend to cluster around the receptor kinase domains. Expression of mutated receptors in cell culture models shows signaling incompetence, a finding that suggested that a simple lack of TGF-β signaling could be causal in pathogenesis. However, tissue samples taken from patients at the time of aortic surgery show paradoxical up-regulation of TGF-β signaling as assessed by phosphorylation of SMAD2, a finding in opposition to a model of simple haploinsufficiency (Loeys et al. 2005). In fact, the mutational repertoire is skewed away from chromosomal deletions, truncations, or nonsense mutations that would be predicted to cause receptor haploinsufficiency by nonsense-mediated decay (NMD) but toward mutations that impair kinase activity, but allow impaired receptors to traffic to the cell surface. In support of this concept, single allele loss of function mutations in TGFBR1 and TGFBR2 have been described in humans, but do not cause LDS. For instance, loss of function mutations in TGFBR1 causes Ferguson–Smith disease (FSD). Patients with FSD experience multiple spontaneous self-healing epitheliomas, but do not show craniofacial dysmorphism or vascular disease (Goudie et al. 2011). Additionally, a deletion of TGFBR2 has been described in a 20 mo old with microcephaly and developmental delay, but without features of LDS (Campbell et al. 2011). In this light, receptor mutations in LDS seemingly select for receptors that must manifest a function separable from simple kinase-mediated signal transduction. It may also be plausible, if not likely, that the presence of the kinase-impaired receptor is a necessary prerequisite for disease expression in LDS.
CANONICAL (SMAD) AND NONCANONICAL (MAPK) TGF-β SIGNALING
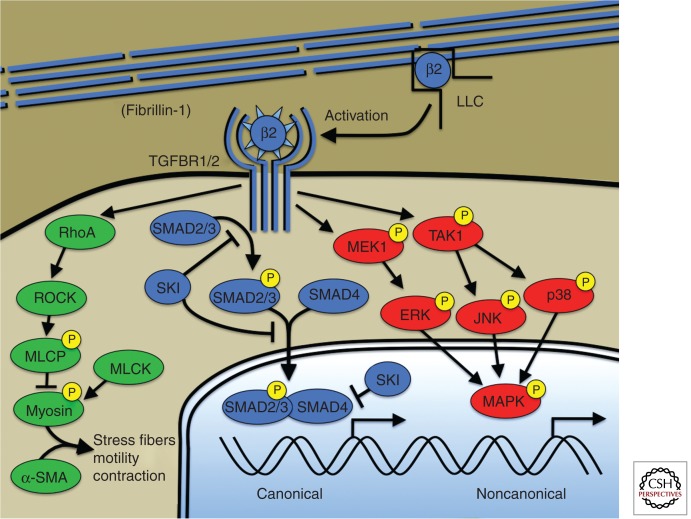
Recent genetic and experimental research on the Smad signaling cascade in aortic aneurysm may begin to offer an explanation of these paradoxical findings. The receptors for TGF-β are transmembrane serine/theonine kinases that work in tandem to transduce signal across the membrane. Ligand is recognized by a type 2 receptor dimer, which recruits type 1 receptors to form a heterotetrameric receptor-ligand complex (Fig. 2). The intracellular kinase domains are brought into close proximity allowing the phosphorylation of regulatory serines on the type 1 receptor by the type 2 receptor kinase. This, in turn, activates the kinase domain of the type 1 receptor allowing the productive phosphorylation and activation of receptor smad proteins such as smad2 or smad3. Activated smad proteins can translocate to the nucleus and direct transcriptional events. The term “canonical signaling” has been used to describe signal transduction by smad proteins downstream from the TGF-β receptor complex and represents historical, but not necessarily functional, precedence. Although increased TGF-β activity as assayed by increased phosphorylation of Smad2 or Smad3 proteins has been seen in diverse aneurysmal conditions, including MFS, it is not clear whether Smad proteins transduce information necessary for aneurysmal progression, or whether this activity is merely correlative. Recent human genetic evidence directly implicates Smad proteins as negative modulators of aneurysmal pathology. Patients with loss of function mutations in SMAD3 were recently described to show the complete phenotypic spectrum of LDS, including vascular disease (van de Laar et al. 2010). Similarly, SMAD4 was recently implicated in human aortic disease. A pedigree was described with the combination of juvenile polyposis, aortopathy, and mitral valve disease (Andrabi et al. 2011). Aortic aneurysm with pathologic evidence of cystic medial necrosis was noted in aortic tissue from a mutation carrier. SMAD4 mutations have previously been implicated in patients with hereditary hemorrhagic telangectasia (HHT) with intestinal polyposis (Gallione et al. 2004), and aortic aneurysms have been described in patients with HHT (Hsi et al. 2003). These data indicate that patients with HHT caused by SMAD4 mutation have a risk of aortic aneurysm, and that Smad3 and Smad4 insufficiency contributes to progression rather than amelioration of aneurysm.
Figure 2.
Canonical and noncanonical TGF-β signaling. Microfibrils composed of fibrillin-1 bind and sequester the large latent complex (LLC) of TGF-β. Activation of TGF-β by proteases (e.g., thrombospondin-1) and/or integrin-mediated mechanisms, allows ligand binding to the TGF-β receptor. Canonical TGF-β signaling is mediated by receptor phosphorylation of R-Smads including Smad2 and Smad3. Phosphorylated R-Smads bind to the cosmad, Smad4, and translocate to the nucleus where the complex directs transcriptional events. The TGF-β repressor SKI acts at multiple levels to suppress canonical TGF-β signaling including R-Smad phosphorylation, nuclear translocation, and direct suppression of the transcriptional complex through recruitment of transcriptional corepressors. TGF-β receptors also activate so-called noncanonical signaling such as extracellular regulated kinases or ERKs. TGF-β signaling also activates RhoA activity, a known activator of smooth muscle cell contraction through myosin-actin interaction (green). Human genetic mutations have been described in all depicted members of the canonical TGF-β pathway (blue) but have not been described in noncanonical pathways required for aneurysm progression (red). Mutations causing aneurysm have been described in genes encoding smooth muscle myosin (MYH11), α-smooth muscle actin (ACTA2), and myosin light chain kinase (MYLK) but mechanistic explanation and/or the interaction between these pathways and TGF-β signaling are lacking. β2, TGF-β2; LLC, large latent complex of TGF-β; ROCK, RhoA-kinase; MLCP, myosin light chain phosphatase; MLCK, myosin light chain kinase; α-SMA, α-smooth muscle actin; ERK1, extracellular regulated protein kinase; JNK, c-Jun amino-terminal kinase; MAPK, any MAP kinase: ERK1, JNK, or p38.
Similar to TGF-β receptor mutations, the finding of loss of function mutations in SMAD3 or TGFB2 that encode positive regulators of the TGF-β canonical signaling cascade, could lead to simple explanations of hereditary aneurysmal pathology from loss of TGF-β signaling (Wang et al. 2010). However, several observations challenge this view. Firstly, tissue obtained from patients with SMAD3 or TGFB2 mutations obtained at the time of aortic surgery show the characteristic signature of increased, rather than decreased TGF-β signaling, as assessed by phosphorylation of smad proteins and increased expression of TGF-β ligands (van de Laar et al. 2010; Boileau et al. 2012; Lindsay et al. 2012). Additional insight was recently obtained by investigation of so-called noncanonical signaling cascades in MFS mice. Although engagement of TGF-β receptors with ligand activates canonical Smad pathways, multiple additional noncanonical pathways are activated such as mitogen-activated protein kinase (MAPK), phosphatidylinositol-3-kinase (PI3K), and RhoA-ROCK signaling cascades. If canonical TGF-β pathways represent positive modulators of aneurysmal pathology, then inhibition of Smad regulators (such as Smad2, Smad3, or Smad4) would be expected to ameliorate disease severity. Contrary to expectation, Smad4 haploinsufficiency introduced into a murine model of MFS (Fbn1C1039G/+) produced significant worsening of aneurysm with aggressive ascending aortic dilation and synthetic lethality owing to dissection (Holm et al. 2011). Molecular analysis of causative pathways in the aorta of experimental animals revealed no difference in canonical TGF-β activity as assayed by phosphorylation of smad2, but up-regulation of noncanonical signaling. The MAP Kinase family members c-Jun amino-terminal kinase (JNK1) and ERK1/2 were found to be aberrantly up-regulated in Fbn1C1039G/+Smad4+/− mice and pharmacological targeting of JNK1 activity resulted in improvement of aortic performance (Holm et al. 2011). So how does loss of smad signaling activity exacerbate aneurysm? One possibility is through an augmentation of TGF-β signaling from the loss of smad-dependent regulatory feedback inhibition. In this scenario, receptor mutations in LDS fail to stimulate canonical signaling, whereas noncanonical signaling remains intact (Lindsay and Dietz 2011). Homeostatic mechanisms aimed at maintaining functional levels of canonical TGF-β signaling would then augment noncanonical signaling to pathogenic levels. This situation would be simulated by decreased Smad activity, as seen in patients with SMAD3 mutations. This type of model would explain experimental findings such as exacerbation of phenotype in Fbn1C1039G/+ Smad4+/− mice (Holm et al. 2011) as well as aortopathy in humans caused by loss of function mutations in SMAD3 and SMAD4 (van de Laar et al. 2010; Andrabi et al. 2011). It could also help explain the skewed mutational repertoire in TGF-β receptor mutations (LDS) favoring intact receptor complexes unable to transduce canonical signaling (Loeys et al. 2006).
Control of noncanonical TGF-β signaling also helps explain the therapeutic benefit of angiotensin 2 type 1 receptor (AT1) blockers (ARBs) in experimental aortic aneurysm. ERK1/2 was the major MAPK member activated in the aortas of Marfan syndrome mice (Fbn1C1039G/+) with intact smad signaling, and treatment with losartan controlled aortic aneurysmal growth and down-regulated ERK1/2 activity (Habashi et al. 2011). Interestingly, intact signaling through the angiotensin 2 type 2 receptor (AT2) was required for the protective effect of losartan. Fbn1C1039G/+At2+/− mice showed worsened aneurysmal pathology and unlike their Fbn1C1039G/+ littermates, showed no clinical response to treatment with ARBs. Although both smad and ERK1/2 signaling pathways were activated in the aortas of MFS mice, targeting of ERK alone was sufficient to inhibit aneurysmal progression (Habashi et al. 2011). Other genetic data in humans support the role of ERK1 signaling in aneurysm formation. A recent article implicating mutations in IGFBP7 as causal in an autosomal recessive syndrome of retinal aneurysm formation and pulmonary stenosis further support these findings (Abu-Safieh et al. 2012). IGFBP7 plays a role in the negative regulation of the BRAF/MEK/ERK pathway and patients with homozygous mutation in this locus show up-regulation of ERK1 signaling. Furthermore, patients with Noonan’s syndrome, now known to be caused by mutations directing dysregulation of ERK1 signaling, can show aortic aneurysm (Purnell et al. 2005). In summary, these data illuminate new targets for pharmacologic intervention in aortic aneurysm and represent a possible common pathway for diverse aneurysm conditions.
SMOOTH MUSCLE VASCULOPATHIES (SMVs)
An emerging theme in aortic aneurysmal disorders is the identification of primary mutations in genes encoding components of the smooth muscle contraction apparatus. In vascular smooth muscle cells a nodal point for activation of contractile force is regulated by the phosphorylation status of the regulatory subunit of the myosin complex, myosin light chain (MLC). The phosphostate of MLC is controlled by the opposing activities of myosin light chain kinase (MLCK) and myosin light chain phosphatase (MLCP). MLCK is positive regulated by calcium-calmodulin, whereas MLCP is negatively regulated by ligand-dependent activation of the Rho GTPase, and productive phosphorylation by of MLCP by ROCK (Fig. 2). Either or both of these activities are required for force generation through productive cross-bridge cycling of actin and myosin. Although the mechanistics of smooth muscle contraction have been of interest for decades, the relevance of this pathway for aneurysmal disease was unsuspected until the description of mutations in MYH11, coding for smooth muscle myosin heavy chain (smMHC), as a causative locus in isolated familial thoracic aortic aneurysm (IFTAA) (Zhu et al. 2006; Pannu et al. 2007). Patients do not seem to show phenotypes outside the cardiovascular system. Ascending aortic aneurysm in patients with MYH11 mutations is accompanied by a high rate of patent ductus arteriosis (PDA). Postnatal ductal closure is a process mediated by active smooth muscle contraction and PDA is seen in other connective tissue disorders such as LDS (Loeys et al. 2005). Despite the high rate of PDA in patients with MYH11 mutations, disruption of this locus appears to be responsible for only a small fraction of sporadic PDA (Zhu et al. 2007a). The binding partner of smMHC, α smooth muscle actin (α-SMA) was next implicated in aneurismal disease. Unlike MYH11 mutations, patients with mutations in ACTA2 (encoding α-SMA) show diverse findings outside the cardiovascular system including livedo reticularis (a purplish reticular discoloration of the skin) and iris flocculi (pigmented epithelial cysts of the iris). Within the arterial system, disease is also more diverse with ascending aortic aneurysm, coarctation of the aorta, bicuspid aortic valve, coronary artery disease, and a neurovascular disorder similar to moya-moya. The importance of this signaling cascade was emphasized with the recent description of loss of function mutations in the activating kinase, MLCK encoded by the gene MYLK (Wang et al. 2011).
Although mutations in proteins encoding extracellular matrix elements and elements of the TGF-β signaling cascade would be expected to affect multiple cell classes and tissues, smMHC is one of a limited number of absolute lineage markers of smooth muscle (Owens et al. 2004), leaving little doubt that the aortic manifestations in SMVs originate from the VSMC compartment. Each of these mutations encode loss of function mutations in the contractile apparatus of VSMCs, which would be predicted to impair contraction, leading to models invoking deficiency of vascular tone through insufficient force generation (Milewicz et al. 2008). However, the role smooth muscle contraction in the VSMCs of large conductance arteries is unclear. The primary role of these cells is to produce and maintain extracellular matrix rather than to modulate blood pressure, the primary role of VSMCs in resistance arterioles. In fact, the contractile apparatus in conductance artery VSMCs is likely primarily involved in managing matricellular interactions and sensing the extracellular environment to control appropriate elaboration and maintenance of extracellular matrix. Defects in this function could lead to dysregulation of matrix control and changes in VSMC differentiation and proliferation. This type of model may explain the involvement of the noncontractile cytoskeletal protein Filamin A in aneurysm. Mutations in FLNA, encoding Filamin A on the X chromosome have been implicated in aortic aneurysm and connective tissue phenotypes in women with the neurologic condition periventricular nodular heterotopia (PVNH) (Sheen et al. 2005). Filamin A serves to cross-link actin polymers and integrates multiple signaling pathways with the cytoskeleton including canonical TGF-β, Ras-ERK1, and integrin signaling (Sasaki et al. 2001; Zhu et al. 2007b; Kim et al. 2010).
Although the nature of cellular dysfunction induced by loss of function mutations in elements of the smooth muscle contractile apparatus is not currently known, insight may be gained by analogy to cardiomyopathic disease. Interestingly, mutations in the contractile apparatus of the cardiomyocyte are well described to cause the human cardiovascular disorder hypertrophic cardiomyopathy (HCM). Mutations at multiple loci involved in cardiac contraction have been shown to be causative in HCM, identifying it as a “disease of the sarcomere” (Thierfelder et al. 1994). The initial hemodynamic dysfunction detected in patients with HCM is an inability of the heart to relax, so-called diastolic dysfunction. Histologically, diastolic dysfunction in HCM corresponds to changes including cardiomyocyte disarray and distinct areas of fibrosis. Recent work in a mouse model of HCM has shown TGF-β is a key cytokine driving pathologic fibrosis and proliferation of nonmyocyte cell populations within HCM hearts, and pathologic benefit could be obtained through the use of TGF-β-neutralizing antibodies (Teekakirikul et al. 2010). Similar clinical benefit, including the prevention of pathologic hypertrophy, could be achieved through treatment of animals with losartan, presumably through its ability to antagonize TGF-β. The therapeutic analogy to murine models of MFS aneurysm allows for speculation regarding the seeming overlap of causative pathologic pathways in smooth muscle contraction vasculopathies (SMCVs) and syndromic aneurysm.
TGF-β SIGNALING, HIGH OR LOW?
Up-regulation of TGF-β signaling is now a well-accepted mechanism of pathogenesis in MFS aortic disease. Controversy is primarily engendered by the nature of mutations encountered in LDS. Mutations in the TGF-β receptors, sparing expression of mutant RNA, typically predict the expression of receptors with impaired kinase function. Similarly, deletions as well as loss of function mutations in the positive TGF-β effectors SMAD3 and TGFB2 causing a human disorder closely resembling classical LDS, strongly infer a loss of function mechanism. Furthermore, experimental data are incontrovertible that receptor mutations causing LDS impair canonical TGF-β signaling when studied in cell-based systems on TGF-β stimulation (Mizuguchi et al. 2004; Loeys et al. 2005; Horbelt et al. 2010). In addition, animal models of developmental modulation of TGF-β receptor components, typically by complete deletion have been shown to create aortic and conotruncal pathology equated to aneurysm. For instance, lineage specific deletion of Tgfbr2 in the second heart field creates conotruncal congenital heart disease and arterial pathology similar to aneurysm (Choudhary et al. 2009). The combination of genetic mutations and embryonic experiments has led to simple pathogenic theories invoking low TGF-β signaling as causal for aneurysm. Despite the intuitive appeal of this type of model, the unattractive reality remains that low TGF-β signaling has yet to be shown in any aneurysm patient sample or any model of progressive aneurysm.
How are we to rectify these seemingly conflicting data? Failure of signal propagation through mutant LDS receptors could be consistent with either a simple loss of function or dominant negative biochemical mechanism. Recent biochemical analysis has shown that TGFR1:TGFR2 proteins within the TGF-β receptor heterotetramer signal as autonomous pairs, rendering a dominant negative mechanism biochemically intractable (Huang et al. 2011). Similarly, a simple loss of function model may explain cell culture findings, but as a pathogenic explanation for aneurysm it fails to incorporate multiple observations of tissue up-regulation of TGF-β signaling (Loeys et al. 2005; Lindsay and Dietz 2011). We have argued that impairment of canonical TGF-β signaling as seen in LDS may induce compensatory autocrine- or paracrine-mediated events that may result in the up-regulation of global TGF-β signaling and/or effect the balance of canonical and noncanonical TGF-β signaling (Lindsay and Dietz 2011). How would this type of complex gain of function mechanism operate? An example has recently been described in TGF-β regulated palatal development. Lineage specific ablation of Tgfbr2 in the murine palate creates a cleft phenotype, but interestingly, rescue of cleft palate rather than exacerbation was seen with synthetic ablation of the TGF-β ligand, Tgfb2 (Iwata et al. 2012). The investigators discovered that in the absence of Tgfbr2, signaling is still propagated through the creation of a disease specific Tgfbr1:Tgfbr3 dimer, which mediates up-regulation of a Tgfb2-dependent noncanonical TGF-β signaling cascade involving TAK1. Analogously, we have reported accentuated noncanonical TGF-β signaling in Fbn1C1039G/+ mice through the introduction of Smad4 haploinsufficiency, an intervention intuitively predicted to inhibit TGF-β signaling capacity (Holm et al. 2011). There is currently insufficient experimental evidence to determine if a complex gain of function mechanism is operant in LDS arterial disease; however, what is abundantly clear is that proposed models of syndromic aneurysm must incorporate all relevant data and have the ability to resolve dichotomous and intuitively conflicting observations.
The identification of new gene causal for aortic aneurysm may help shed light on the role of TGF-β signaling in aneurysm. Shprintzen–Goldberg syndrome (SGS) is a multisystem connective tissue disorder, which shares phenotypic overlap with MFS and LDS including arachnodactyly, chest wall deformity, cleft palate, craniosynostosis, and aortic aneurysm. Through the use of next-generation sequencing, mutations in the prototypical TGF-β repressor SKI have been identified as causal in SGS (Doyle et al. 2012). Identified missense mutations cause residue substitutions that cluster in the amino-terminus of the gene product within the R-smad or the Dachshund-homology (DHD)-binding domains, implicating loss of intermolecular partner binding as a possible disease mechanism. Consistent with a loss of function mechanism, patient fibroblasts show a signature of increased responsiveness to TGF-β with increased Smad and ERK1 phosphorylation as well as increased expression of TGF-β-responsive gene products. Morpholino knockout targeting of the two SKI paralogs in zebrafish (Skia and Skib) phenocopied craniofacial defects and induced cardiac defects, providing evidence for the loss of function nature of the SGS genetic perturbation. The identification of mutations in a prototypical TGF-β repressor (expected to cause increased TGF-β activity) contrasts with LDS mutations in positive modulators of TGF-β such as TGFB2 or SMAD3 (expected to associate with lower TGF-β signaling) expands the range of mutational causality in the canonical TGF-β pathway for aortic aneurysm. Whether high or low TGF-β signaling can recapitulate similar phenotypes and disease states, or alternatively whether compensatory signaling events lead to a common high TGF-β pathologic pathway driving postnatal progression of aortic aneurysm (Dietz 2010; Lindsay and Dietz 2011) represents an experimental challenge for aneurysm researchers in the coming years.
CONCLUDING REMARKS
The next 5 to 10 years will likely represent an exciting time in the genetics of aortic disease as the acceleration of gene discovery continues. The decreased cost of next-generation sequencing will no doubt unleash a deluge of new gene loci implicated as causal for aortic aneurysm and arterial dissection. Whole genome sequencing will no doubt identify more complex genetic situations involving both primary syndromic and nonsyndromic aneurysmal mutations and multiple modifying loci, perhaps affecting TGF-β signaling or smooth muscle contractile elements. Over time, the major challenge in aortic aneurysmal research will likely shift from gene identification to the assessment of gene product function in large vessel homeostasis. Although animal models have been truly revolutionary in this field, significant need exists for the development of cell-based models of aortic aneurysm. Embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs) may be a useful technology in this regard and indeed is a topic under investigation in many laboratories. Hopefully, the development of these models will aid in the analysis of new genes, new biologic pathways, and novel therapeutic targets for patients affected by these life-threatening conditions.
Footnotes
Editors: Margaret Buckingham, Christine L. Mummery, and Kenneth R. Chien
Additional Perspectives on The Biology of Heart Disease available at www.perspectivesinmedicine.org
REFERENCES
- Abu-Safieh L, Abboud EB, Alkuraya H, Shamseldin H, Al-Enzi S, Al-Abdi L, Hashem M, Colak D, Jarallah A, Ahmad H, et al. 2012. Mutation of IGFBP7 causes upregulation of BRAF/MEK/ERK pathway and familial retinal arterial macroaneurysms. Am J Hum Genet 89: 313–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrabi S, Bekheirnia MR, Robbins-Furman P, Lewis RA, Prior TW, Potocki L 2011. SMAD4 mutation segregating in a family with juvenile polyposis, aortopathy, and mitral valve dysfunction. Am J Med Genet A 155A: 1165–1169 [DOI] [PubMed] [Google Scholar]
- Atsawasuwan P, Mochida Y, Katafuchi M, Kaku M, Fong KS, Csiszar K, Yamauchi M 2008. Lysyl oxidase binds transforming growth factor-β and regulates its signaling via amine oxidase activity. J Biol Chem 283: 34229–34240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergen AA, Plomp AS, Schuurman EJ, Terry S, Breuning M, Dauwerse H, Swart J, Kool M, van Soest S, Baas F, et al. 2000. Mutations in ABCC6 cause pseudoxanthoma elasticum. Nat Genet 25: 228–231 [DOI] [PubMed] [Google Scholar]
- Boileau C, Guo DC, Hanna N, Regalado ES, Detaint D, Gong L, Varret M, Prakash SK, Li AH, d’Indy H, et al. 2012. TGFB2 mutations cause familial thoracic aortic aneurysms and dissections associated with mild systemic features of Marfan syndrome. Nat Genet 44: 916–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell IM, Kolodziejska KE, Quach MM, Wolf VL, Cheung SW, Lalani SR, Ramocki MB, Stankiewicz P 2011. TGFBR2 deletion in a 20-month-old female with developmental delay and microcephaly. Am J Med Genet A 155A: 1442–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Gong L, Guo DC, Mietzsch U, Kuang SQ, Kwartler CS, Safi H, Estrera A, Gambello MJ, Milewicz DM 2010. Thoracic aortic disease in tuberous sclerosis complex: Molecular pathogenesis and potential therapies in Tsc2+/− mice. Hum Mol Genet 19: 1908–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary B, Zhou J, Li P, Thomas S, Kaartinen V, Sucov HM 2009. Absence of TGFβ signaling in embryonic vascular smooth muscle leads to reduced lysyl oxidase expression, impaired elastogenesis, and aneurysm. Genesis 47: 115–121 [DOI] [PubMed] [Google Scholar]
- Cohn RD, van Erp C, Habashi JP, Soleimani AA, Klein EC, Lisi MT, Gamradt M, ap Rhys CM, Holm TM, Loeys BL, et al. 2007. Angiotensin II type 1 receptor blockade attenuates TGF-β-induced failure of muscle regeneration in multiple myopathic states. Nat Med 13: 204–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coucke PJ, Willaert A, Wessels MW, Callewaert B, Zoppi N, De Backer J, Fox JE, Mancini GM, Kambouris M, Gardella R, et al. 2006. Mutations in the facilitative glucose transporter GLUT10 alter angiogenesis and cause arterial tortuosity syndrome. Nat Genet 38: 452–457 [DOI] [PubMed] [Google Scholar]
- Curran ME, Atkinson DL, Ewart AK, Morris CA, Leppert MF, Keating MT 1993. The elastin gene is disrupted by a translocation associated with supravalvular aortic stenosis. Cell 73: 159–168 [DOI] [PubMed] [Google Scholar]
- Dasouki M, Markova D, Garola R, Sasaki T, Charbonneau NL, Sakai LY, Chu ML 2007. Compound heterozygous mutations in fibulin-4 causing neonatal lethal pulmonary artery occlusion, aortic aneurysm, arachnodactyly, and mild cutis laxa. Am J Med Genet A 143A: 2635–2641 [DOI] [PubMed] [Google Scholar]
- Dietz HC 2010. TGF-β in the pathogenesis and prevention of disease: A matter of aneurysmic proportions. J Clin Invest 120: 403–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz HC, Cutting GR, Pyeritz RE, Maslen CL, Sakai LY, Corson GM, Puffenberger EG, Hamosh A, Nanthakumar EJ, Curristin SM, et al. 1991. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature 352: 337–339 [DOI] [PubMed] [Google Scholar]
- Doyle AJ, Doyle JJ, Bessling SL, Maragh S, Lindsay ME, Schepers D, Gillis E, Mortier G, Homfray T, Sauls K, et al. 2012. Mutations in the TGF-β repressor SKI cause Shprintzen–Goldberg syndrome with aortic aneurysm. Nat Genet 44: 1249–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faivre L, Masurel-Paulet A, Collod-Beroud G, Callewaert BL, Child AH, Stheneur C, Binquet C, Gautier E, Chevallier B, Huet F, et al. 2009. Clinical and molecular study of 320 children with Marfan syndrome and related type I fibrillinopathies in a series of 1009 probands with pathogenic FBN1 mutations. Pediatrics 123: 391–398 [DOI] [PubMed] [Google Scholar]
- Friedman JM, Arbiser J, Epstein JA, Gutmann DH, Huot SJ, Lin AE, McManus B, Korf BR 2002. Cardiovascular disease in neurofibromatosis 1: Report of the NF1 Cardiovascular Task Force. Genet Med 4: 105–111 [DOI] [PubMed] [Google Scholar]
- Gallione CJ, Repetto GM, Legius E, Rustgi AK, Schelley SL, Tejpar S, Mitchell G, Drouin E, Westermann CJ, Marchuk DA 2004. A combined syndrome of juvenile polyposis and hereditary haemorrhagic telangiectasia associated with mutations in MADH4 (SMAD4). Lancet 363: 852–859 [DOI] [PubMed] [Google Scholar]
- Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, King IN, Grossfeld PD, Srivastava D 2005. Mutations in NOTCH1 cause aortic valve disease. Nature 437: 270–274 [DOI] [PubMed] [Google Scholar]
- Goudie DR, D’Alessandro M, Merriman B, Lee H, Szeverenyi I, Avery S, O’Connor BD, Nelson SF, Coats SE, Stewart A, et al. 2011. Multiple self-healing squamous epithelioma is caused by a disease-specific spectrum of mutations in TGFBR1. Nat Genet 43: 365–369 [DOI] [PubMed] [Google Scholar]
- Guo DC, Pannu H, Tran-Fadulu V, Papke CL, Yu RK, Avidan N, Bourgeois S, Estrera AL, Safi HJ, Sparks E, et al. 2007. Mutations in smooth muscle α-actin (ACTA2) lead to thoracic aortic aneurysms and dissections. Nat Genet 39: 1488–1493 [DOI] [PubMed] [Google Scholar]
- Guo DC, Regalado E, Casteel DE, Santos-Cortez RL, Gong L, Kim JJ, Dyack S, Horne SG, Chang G, Jondeau G, et al. 2013. Recurrent gain-of-function mutation in PRKG1 causes thoracic aortic aneurysms and acute aortic dissections. Am J Hum Genet 93: 398–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habashi JP, Judge DP, Holm TM, Cohn RD, Loeys BL, Cooper TK, Myers L, Klein EC, Liu G, Calvi C, et al. 2006. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science 312: 117–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habashi JP, Doyle JJ, Holm TM, Aziz H, Schoenhoff F, Bedja D, Chen Y, Modiri AN, Judge DP, Dietz HC 2011. Angiotensin II type 2 receptor signaling attenuates aortic aneurysm in mice through ERK antagonism. Science 332: 361–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanada K, Vermeij M, Garinis GA, de Waard MC, Kunen MG, Myers L, Maas A, Duncker DJ, Meijers C, Dietz HC, et al. 2007. Perturbations of vascular homeostasis and aortic valve abnormalities in fibulin-4 deficient mice. Circ Res 100: 738–746 [DOI] [PubMed] [Google Scholar]
- Holm TM, Habashi JP, Doyle JJ, Bedja D, Chen Y, van Erp C, Lindsay ME, Kim D, Schoenhoff F, Cohn RD, et al. 2011. Noncanonical TGFβ signaling contributes to aortic aneurysm progression in Marfan syndrome mice. Science 332: 358–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horbelt D, Guo G, Robinson PN, Knaus P 2010. Quantitative analysis of TGFBR2 mutations in Marfan-syndrome-related disorders suggests a correlation between phenotypic severity and Smad signaling activity. J Cell Sci 123: 4340–4350 [DOI] [PubMed] [Google Scholar]
- Horiguchi M, Inoue T, Ohbayashi T, Hirai M, Noda K, Marmorstein LY, Yabe D, Takagi K, Akama TO, Kita T, et al. 2009. Fibulin-4 conducts proper elastogenesis via interaction with cross-linking enzyme lysyl oxidase. Proc Natl Acad Sci 106: 19029–19034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsi DH, Ryan GF, Hellems SO, Cheeran DC, Sheils LA 2003. Large aneurysms of the ascending aorta and major coronary arteries in a patient with hereditary hemorrhagic telangiectasia. Mayo Clin Proc 78: 774–776 [DOI] [PubMed] [Google Scholar]
- Huang T, David L, Mendoza V, Yang Y, Villarreal M, De K, Sun L, Fang X, Lopez-Casillas F, Wrana JL, et al. 2011. TGF-β signalling is mediated by two autonomously functioning TβRI:TβRII pairs. EMBO J 30: 1263–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isotalo PA, Guindi MM, Bedard P, Brais MP, Veinot JP 1999. Aortic dissection: A rare complication of osteogenesis imperfecta. Can J Cardiol 15: 1139–1142 [PubMed] [Google Scholar]
- Iwasaki Y, Horigome H, Takahashi-Igari M, Kato Y, Razzaque MA, Matsuoka R 2009. Coronary artery dilatation in LEOPARD syndrome. A child case and literature review. Congenit Heart Dis 4: 38–41 [DOI] [PubMed] [Google Scholar]
- Iwata J, Hacia JG, Suzuki A, Sanchez-Lara PA, Urata M, Chai Y 2012. Modulation of noncanonical TGF-β signaling prevents cleft palate in Tgfbr2 mutant mice. J Clin Invest 122: 873–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judge DP, Biery NJ, Keene DR, Geubtner J, Myers L, Huso DL, Sakai LY, Dietz HC 2004. Evidence for a critical contribution of haploinsufficiency in the complex pathogenesis of Marfan syndrome. J Clin Invest 114: 172–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath BM, Spinner NB, Emerick KM, Chudley AE, Booth C, Piccoli DA, Krantz ID 2004. Vascular anomalies in Alagille syndrome: A significant cause of morbidity and mortality. Circulation 109: 1354–1358 [DOI] [PubMed] [Google Scholar]
- Kashtan CE, Segal Y, Flinter F, Makanjuola D, Gan JS, Watnick T 2010. Aortic abnormalities in males with Alport syndrome. Nephrol Dial Transplant 25: 3554–3560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Nakamura F, Lee W, Hong C, Perez-Sala D, McCulloch CA 2010. Regulation of cell adhesion to collagen via β1 integrins is dependent on interactions of filamin A with vimentin and protein kinase C epsilon. Exp Cell Res 316: 1829–1844 [DOI] [PubMed] [Google Scholar]
- Kyndt F, Gueffet JP, Probst V, Jaafar P, Legendre A, Le Bouffant F, Toquet C, Roy E, McGregor L, Lynch SA, et al. 2007. Mutations in the gene encoding filamin A as a cause for familial cardiac valvular dystrophy. Circulation 115: 40–49 [DOI] [PubMed] [Google Scholar]
- Lemaire SA, McDonald ML, Guo DC, Russell L, Miller CC 3rd, Johnson RJ, Bekheirnia MR, Franco LM, Nguyen M, Pyeritz RE, et al. 2011. Genome-wide association study identifies a susceptibility locus for thoracic aortic aneurysms and aortic dissections spanning FBN1 at 15q21.1. Nat Genet 43: 996–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DY, Faury G, Taylor DG, Davis EC, Boyle WA, Mecham RP, Stenzel P, Boak B, Keating MT 1998. Novel arterial pathology in mice and humans hemizygous for elastin. J Clin Invest 102: 1783–1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay ME, Dietz HC 2011. Lessons on the pathogenesis of aneurysm from heritable conditions. Nature 473: 308–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay ME, Schepers D, Bolar NA, Doyle JJ, Gallo E, Fert-Bober J, Kempers MJ, Fishman EK, Chen Y, Myers L, et al. 2012. Loss-of-function mutations in TGFB2 cause a syndromic presentation of thoracic aortic aneurysm. Nat Genet 44: 922–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeys B, Van Maldergem L, Mortier G, Coucke P, Gerniers S, Naeyaert JM, De Paepe A 2002. Homozygosity for a missense mutation in fibulin-5 (FBLN5) results in a severe form of cutis laxa. Hum Mol Genet 11: 2113–2118 [DOI] [PubMed] [Google Scholar]
- Loeys BL, Chen J, Neptune ER, Judge DP, Podowski M, Holm T, Meyers J, Leitch CC, Katsanis N, Sharifi N, et al. 2005. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat Genet 37: 275–281 [DOI] [PubMed] [Google Scholar]
- Loeys BL, Schwarze U, Holm T, Callewaert BL, Thomas GH, Pannu H, De Backer JF, Oswald GL, Symoens S, Manouvrier S, et al. 2006. Aneurysm syndromes caused by mutations in the TGF-β receptor. N Engl J Med 355: 788–798 [DOI] [PubMed] [Google Scholar]
- Lopez L, Arheart KL, Colan SD, Stein NS, Lopez-Mitnik G, Lin AE, Reller MD, Ventura R, Silberbach M 2008. Turner syndrome is an independent risk factor for aortic dilation in the young. Pediatrics 121: e1622–e1627 [DOI] [PubMed] [Google Scholar]
- Maki JM, Rasanen J, Tikkanen H, Sormunen R, Makikallio K, Kivirikko KI, Soininen R 2002. Inactivation of the lysyl oxidase gene Lox leads to aortic aneurysms, cardiovascular dysfunction, and perinatal death in mice. Circulation 106: 2503–2509 [DOI] [PubMed] [Google Scholar]
- Malfait F, Symoens S, De Backer J, Hermanns-Le T, Sakalihasan N, Lapiere CM, Coucke P, De Paepe A 2007. Three arginine to cysteine substitutions in the pro-α(I)-collagen chain cause Ehlers–Danlos syndrome with a propensity to arterial rupture in early adulthood. Hum Mutat 28: 387–395 [DOI] [PubMed] [Google Scholar]
- McKusick V 1973. Heritable disorders of connective tissue. C.V. Mosby, St. Louis [Google Scholar]
- Miki K, Yuri T, Takeda N, Takehana K, Iwasaka T, Tsubura A 2007. An autopsy case of pseudoxanthoma elasticum: Histochemical characteristics. Med Mol Morphol 40: 172–177 [DOI] [PubMed] [Google Scholar]
- Milewicz DM, Guo DC, Tran-Fadulu V, Lafont AL, Papke CL, Inamoto S, Kwartler CS, Pannu H 2008. Genetic basis of thoracic aortic aneurysms and dissections: Focus on smooth muscle cell contractile dysfunction. Annu Rev Genomics Hum Genet 9: 283–302 [DOI] [PubMed] [Google Scholar]
- Mizuguchi T, Collod-Beroud G, Akiyama T, Abifadel M, Harada N, Morisaki T, Allard D, Varret M, Claustres M, Morisaki H, et al. 2004. Heterozygous TGFBR2 mutations in Marfan syndrome. Nat Genet 36: 855–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neptune ER, Frischmeyer PA, Arking DE, Myers L, Bunton TE, Gayraud B, Ramirez F, Sakai LY, Dietz HC 2003. Dysregulation of TGF-β activation contributes to pathogenesis in Marfan syndrome. Nat Genet 33: 407–411 [DOI] [PubMed] [Google Scholar]
- Newby AC 2012. Matrix metalloproteinase inhibition therapy for vascular diseases. Vascul Pharmacol 56: 232–244 [DOI] [PubMed] [Google Scholar]
- Ng CM, Cheng A, Myers LA, Martinez-Murillo F, Jie C, Bedja D, Gabrielson KL, Hausladen JM, Mecham RP, Judge DP, et al. 2004. TGF-β-dependent pathogenesis of mitral valve prolapse in a mouse model of Marfan syndrome. J Clin Invest 114: 1586–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoda M, Yoshimura K, Aoki H, Ikeda Y, Morikage N, Furutani A, Matsuzaki M, Hamano K 2010. Lysyl oxidase resolves inflammation by reducing monocyte chemoattractant protein-1 in abdominal aortic aneurysm. Atherosclerosis 208: 366–369 [DOI] [PubMed] [Google Scholar]
- Owens GK, Kumar MS, Wamhoff BR 2004. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev 84: 767–801 [DOI] [PubMed] [Google Scholar]
- Pannu H, Tran-Fadulu V, Papke CL, Scherer S, Liu Y, Presley C, Guo D, Estrera AL, Safi HJ, Brasier AR, et al. 2007. MYH11 mutations result in a distinct vascular pathology driven by insulin-like growth factor 1 and angiotensin II. Hum Mol Genet 16: 2453–2462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L, Andrikopoulos K, Tian J, Lee SY, Keene DR, Ono R, Reinhardt DP, Sakai LY, Biery NJ, Bunton T, et al. 1997. Targetting of the gene encoding fibrillin-1 recapitulates the vascular aspect of Marfan syndrome. Nat Genet 17: 218–222 [DOI] [PubMed] [Google Scholar]
- Plaisier E, Gribouval O, Alamowitch S, Mougenot B, Prost C, Verpont MC, Marro B, Desmettre T, Cohen SY, Roullet E, et al. 2007. COL4A1 mutations and hereditary angiopathy, nephropathy, aneurysms, and muscle cramps. N Engl J Med 357: 2687–2695 [DOI] [PubMed] [Google Scholar]
- Purnell R, Williams I, Von Oppell U, Wood A 2005. Giant aneurysms of the sinuses of Valsalva and aortic regurgitation in a patient with Noonan’s syndrome. Eur J Cardiothorac Surg 28: 346–348 [DOI] [PubMed] [Google Scholar]
- Renard M, Holm T, Veith R, Callewaert BL, Ades LC, Baspinar O, Pickart A, Dasouki M, Hoyer J, Rauch A, et al. 2010. Altered TGFβ signaling and cardiovascular manifestations in patients with autosomal recessive cutis laxa type I caused by fibulin-4 deficiency. Eur J Hum Genet 18: 895–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo AM, Cox H, Farndon P, Moss C, Grindulis H, Risteli M, Robins SP, Myllyla R 2008. A connective tissue disorder caused by mutations of the lysyl hydroxylase 3 gene. Am J Hum Genet 83: 495–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki A, Masuda Y, Ohta Y, Ikeda K, Watanabe K 2001. Filamin associates with Smads and regulates transforming growth factor-β signaling. J Biol Chem 276: 17871–17877 [DOI] [PubMed] [Google Scholar]
- Schwarze U, Hata R, McKusick VA, Shinkai H, Hoyme HE, Pyeritz RE, Byers PH 2004. Rare autosomal recessive cardiac valvular form of Ehlers–Danlos syndrome results from mutations in the COL1A2 gene that activate the nonsense-mediated RNA decay pathway. Am J Hum Genet 74: 917–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen VL, Jansen A, Chen MH, Parrini E, Morgan T, Ravenscroft R, Ganesh V, Underwood T, Wiley J, Leventer R, et al. 2005. Filamin A mutations cause periventricular heterotopia with Ehlers–Danlos syndrome. Neurology 64: 254–262 [DOI] [PubMed] [Google Scholar]
- Superti-Furga A, Gugler E, Gitzelmann R, Steinmann B 1988. Ehlers–Danlos syndrome type IV: A multi-exon deletion in one of the two COL3A1 alleles affecting structure, stability, and processing of type III procollagen. J Biol Chem 263: 6226–6232 [PubMed] [Google Scholar]
- Szabo Z, Crepeau MW, Mitchell AL, Stephan MJ, Puntel RA, Yin Loke K, Kirk RC, Urban Z 2006. Aortic aneurysmal disease and cutis laxa caused by defects in the elastin gene. J Med Genet 43: 255–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teekakirikul P, Eminaga S, Toka O, Alcalai R, Wang L, Wakimoto H, Nayor M, Konno T, Gorham JM, Wolf CM, et al. 2010. Cardiac fibrosis in mice with hypertrophic cardiomyopathy is mediated by non-myocyte proliferation and requires Tgf-β. J Clin Invest 120: 3520–3529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thierfelder L, Watkins H, MacRae C, Lamas R, McKenna W, Vosberg HP, Seidman JG, Seidman CE 1994. α-Tropomyosin and cardiac troponin T mutations cause familial hypertrophic cardiomyopathy: A disease of the sarcomere. Cell 77: 701–712 [DOI] [PubMed] [Google Scholar]
- Tieu BC, Lee C, Sun H, Lejeune W, Recinos A III, Ju X, Spratt H, Guo DC, Milewicz D, Tilton RG, et al. 2009. An adventitial IL-6/MCP1 amplification loop accelerates macrophage-mediated vascular inflammation leading to aortic dissection in mice. J Clin Invest 119: 3637–3651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CL, Emery H, Collins AL, Howarth RJ, Yearwood CM, Cross E, Duncan PJ, Bunyan DJ, Harvey JF, Foulds NC 2009. Detection of 53 FBN1 mutations (41 novel and 12 recurrent) and genotype-phenotype correlations in 113 unrelated probands referred with Marfan syndrome, or a related fibrillinopathy. Am J Med Genet A 149A: 161–170 [DOI] [PubMed] [Google Scholar]
- van de Laar IM, Oldenburg RA, Pals G, Roos-Hesselink JW, de Graaf BM, Verhagen JM, Hoedemaekers YM, Willemsen R, Severijnen LA, Venselaar H, et al. 2010. Mutations in SMAD3 cause a syndromic form of aortic aneurysms and dissections with early-onset osteoarthritis. Nat Genet 43: 121–126 [DOI] [PubMed] [Google Scholar]
- Wang Y, Ait-Oufella H, Herbin O, Bonnin P, Ramkhelawon B, Taleb S, Huang J, Offenstadt G, Combadiere C, Renia L, et al. 2010. TGF-β activity protects against inflammatory aortic aneurysm progression and complications in angiotensin II-infused mice. J Clin Invest 120: 422–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Guo DC, Cao J, Gong L, Kamm KE, Regalado E, Li L, Shete S, He WQ, Zhu MS, et al. 2011. Mutations in myosin light chain kinase cause familial aortic dissections. Am J Hum Genet 87: 701–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenstrup RJ, Murad S, Pinnell SR 1989. Ehlers–Danlos syndrome type VI: Clinical manifestations of collagen lysyl hydroxylase deficiency. J Pediatr 115: 405–409 [DOI] [PubMed] [Google Scholar]
- Zhu L, Vranckx R, Khau Van Kien P, Lalande A, Boisset N, Mathieu F, Wegman M, Glancy L, Gasc JM, Brunotte F, et al. 2006. Mutations in myosin heavy chain 11 cause a syndrome associating thoracic aortic aneurysm/aortic dissection and patent ductus arteriosus. Nat Genet 38: 343–349 [DOI] [PubMed] [Google Scholar]
- Zhu L, Bonnet D, Boussion M, Vedie B, Sidi D, Jeunemaitre X 2007a. Investigation of the MYH11 gene in sporadic patients with an isolated persistently patent arterial duct. Cardiol Young 17: 666–672 [DOI] [PubMed] [Google Scholar]
- Zhu TN, He HJ, Kole S, D’Souza T, Agarwal R, Morin PJ, Bernier M 2007b. Filamin A–mediated down-regulation of the exchange factor Ras-GRF1 correlates with decreased matrix metalloproteinase-9 expression in human melanoma cells. J Biol Chem 282: 14816–14826 [DOI] [PubMed] [Google Scholar]