Abstract
Many clinicians regard tuberculosis as an adult pulmonary disease, but tuberculosis (TB) is a major cause of disease, both pulmonary and extrapulmonary, and death in young children from TB-endemic countries, especially in areas affected by poverty, social disruption, and human immunodeficiency virus (HIV) infection. This article reviews the disease burden and the natural history of disease in children with TB. It also provides guidance regarding the diagnosis, treatment, and prevention of TB in children.
TB is a major cause of disease and death in young children in TB-endemic countries, especially in areas affected by poverty and HIV. Understanding, diagnosing, treating, and preventing TB in children requires special consideration.
DISEASE BURDEN
Tuberculosis (TB) is a major cause of disease, both pulmonary and extrapulmonary, and death in children from TB-endemic areas (Bates et al. 2013; Graham et al. 2014), but is also seen in nonendemic areas because of increased international travel, population migration, and refugee resettlement. Although overdiagnosis does occur in some settings, underdiagnosis is the rule in most TB-endemic areas, where young children can only access TB care via referral hospitals. Table 1 summarizes key differences between adults and children with TB. Poor case ascertainment and incomplete recording and reporting limit the accuracy of disease burden estimates in children (Newton et al. 2008; Du Preez et al. 2011; Rose et al. 2013). According to the most recent estimates, nearly 1 million children develop TB every year (Jenkins et al. 2014); this is nearly double World Health Organization (WHO) estimates of 530,000 cases for 2012, causing 74,000 deaths, which exclude deaths in human immunodeficiency virus (HIV)–infected children (World Health Organization 2013).
Table 1.
Tuberculosis: Differences between adults and children
| Aspect | Adultsa | Childrena |
|---|---|---|
| Epidemiology | Massive global disease burden that is well quantified; excellent awareness | Massive global disease burden that is poorly quantified; minimal awareness |
| TB control | Main focus of TB control programs | Not recognized as a TB control priority |
| Pathogenesis | Usually “adult-type” lung disease | Usually intrathoracic lymph node disease, but extrapulmonary disease common |
| Infection control | Multibacillary; high infection risk | Paucibacillary; low infection risk, unless cavities or extensive lung involvement; epidemiologic marker of transmission |
| Drug resistance | Difficult to differentiate acquired from primary drug resistance | Nearly always primary drug resistance indicating recent transmission |
| Exposure history | Important, but often neglectedb | Essential part of diagnostic work up |
| Risk of progression to disease | Relatively low risk of progression to disease following TB exposure/infection unless immune-compromised | Highly variable risk of progression to disease following TB exposure/infection—greatest in the very young and/or immune-compromised |
| Preventive therapy | Limited value, except in immune-compromised adults | Definite value in young (<5 yr of age) and/or immune-compromised children |
| Imaging studies | Chest radiographs (CXRs) not routinely required, unless sputum negative for mycobacterial investigations | CXRs (with both anteroposterior and lateral views, of good quality, and competently read) are the most informative study to perform |
| Disease classification | Pulmonary versus extrapulmonary distinction Postprimary TB is a confusing conceptc |
Diverse spectrum of pathology that requires accurate classification |
| Microbiological studies | Relatively easy to collect adequate respiratory specimen and confirm presence of mycobacteria | Difficult to collect adequate respiratory specimens (young children cannot expectorate); smear microscopy has very low yield |
| Treatment (drug-susceptible TB) | With four drugs in intensive phase | With three or four drugs depending on likely organism load and severity of disease in intensive phase |
| Prognosis | Excellent outcomes achievable with timely and appropriate treatment | Excellent outcomes achievable; potentially grave outcome with delayed diagnosis of especially tuberculous meningitis |
Data adapted from supplementary material in Perez-Velez and Marais 2012.
aTypical characteristics in the absence of HIV infection and/or severe immune compromise.
bTaking a careful contact history is often neglected in adults, but it has particular relevance to identify drug-resistant TB suspects.
cThe distinction between primary and postprimary TB obscures the fact that adult-type (postprimary) TB frequently results from recent reinfection and may also occur within months of documented primary infection (particularly in adolescents).
HIV coinfection has had a major impact on the epidemiology of TB, especially in sub-Saharan Africa (Källenius 2014). In addition to a massive increase in the absolute number of TB patients, HIV induced a pronounced shift in the age and gender profile of TB patients, with more young adults and women of child-bearing age being affected (Lawn et al. 2006). This demographic shift implies increased TB exposure of young and vulnerable children living in HIV-affected households, as illustrated by high rates of TB exposure within the first 6 months of life in babies born to HIV-infected mothers (irrespective of their HIV status) (Cotton et al. 2008), and high disease rates among HIV-infected infants (Hesseling et al. 2009). Routine HIV testing in pregnancy, together with optimal prevention of mother-to-child transmission and early initiation of antiretroviral therapy (ART) in HIV-infected infants, is essential to reduce overall mortality and TB risk in HIV-infected infants (Violari et al. 2008).
The emergence of drug-resistant (DR)-TB poses a major threat to global TB control (Abubakar et al. 2013; Seung et al. 2014). For a long time, DR-TB strains, especially isoniazid-resistant strains, were considered to be less virulent and therefore less transmissible than drug-susceptible strains, with failure to appreciate the risk for global epidemic spread. However, pediatric TB cases provide a valuable epidemiological perspective because they reflect ongoing transmission within communities. The development of multidrug-resistant (MDR)-TB (i.e., resistance to at least isoniazid and rifampicin) in child contacts of infectious adult cases present confirmation that clinical MDR strains are transmissible and cause disease (Schaaf et al. 2007; Amanullah et al. 2014). This has been corroborated by molecular epidemiology studies that confirmed transmission from adult source cases to child contacts (Schaaf et al. 2000) and by high rates of clustering and multiple clusters with clear transmission chains among newly identified MDR-TB cases (Marais et al. 2013b). In settings with relevant surveillance data, rates of DR-TB in children were found to be similar to those in adults from the same community (Zignol et al. 2013), especially among treatment-naïve patients (Jenkins et al. 2004). Therefore, although the incidence of DR-TB in children is poorly quantified, the fact that WHO estimated MDR-TB to occur in 3.6% of newly diagnosed TB cases in 2012 (World Health Organization 2013) implies a similar burden among children.
NATURAL HISTORY OF DISEASE
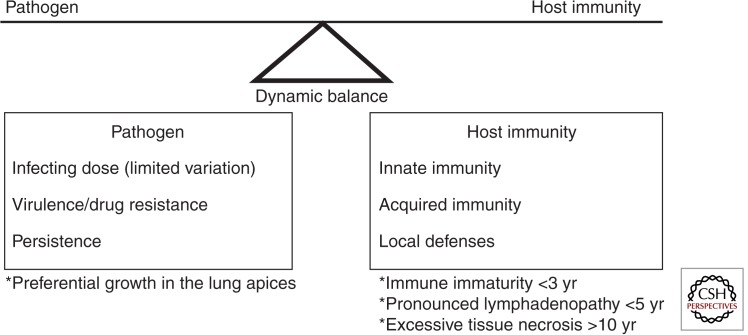
Understanding the natural history of TB is fundamental to appreciate the variable vulnerability and the diverse spectrum of disease observed in children. Meticulous disease descriptions from the prechemotherapy literature provide unique insight into events following primary Mycobacterium tuberculosis infection, summarized as the so-called “timetable of childhood TB” (Fig. 1) (Marais et al. 2004a; Perez-Velez and Marais 2012). Pulmonary infection occurs when a few bacilli reach a terminal airway and establish infection. A localized inflammatory process occurs within the lung, referred to as the primary (Ghon) focus, from where bacilli drain via lymphatics to the regional lymph nodes. The primary focus together with affected regional lymph nodes (with/without overlying pleural reaction) is referred to as the primary (Ghon) complex. Before acquired immune responses contain disease progression, bacilli may enter the systemic circulation via the regional lymph nodes with occult hematogenous spread. Bacilli may survive in target organs for prolonged periods depending on dynamic pathogen–host interactions at the site of deposition; Figure 2 provides an overview of factors that influence disease risk.
Figure 2.
Factors that influence the dynamic balance between M. tuberculosis and host immunity in children. (Adapted from Marais et al. 2005a.)
Figure 1.
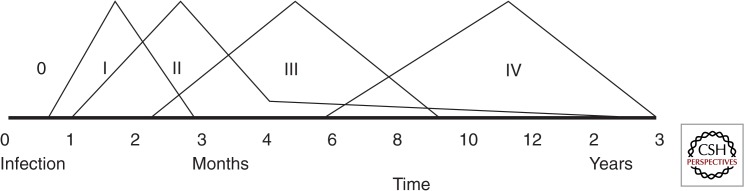
Schematic time line following primary pulmonary infection with M. tuberculosis. Data of time line of tuberculosis is adapted from Marais et al. (2005a), first described by Wallgren (1948). 0, Incubation; I, tuberculin skin test conversion; II, Ghon focus and/or disseminated (miliary) disease; III, lymph node disease (<5 yr of age)/pleural effusion (>5 yr of age); IV, adult-type disease (>10 yr of age).
The rate of progression is highly variable, with the youngest children experiencing the greatest risk and the most rapid disease progression. Table 2 provides an overview of clinical syndromes associated with primary M. tuberculosis infection in children; summarized in the following phases.
Phase 1 occurs 3–8 wk after primary infection and may be heralded by prolonged fever, the formation of a visible primary complex on chest radiograph (CXR), and hypersensitivity reactions such as erythema nodosum and tuberculin skin test (TST) conversion.
Phase 2 occurs 1–3 mo after primary infection and follows occult hematogenous spread. This represents the period of highest risk for the development of tuberculous meningitis (TBM) and disseminated (miliary) TB in young children. However, these disease entities may occur after any time interval; hematogenous dissemination frequently acted as the final terminal pathway following uncontrolled disease progression in the absence of treatment.
Phase 3 occurs 3–7 mo after primary infection and may manifest as airway involvement caused by diseased lymph nodes in young children (<5 yr of age) and reactive pleural effusions in older children.
Phase 4 lasts until the primary complex is calcified, 1–3 yr after primary infection. It represents the period of osteoarticular TB in children of <5 yr of age and adult-type disease in adolescents. As a general rule the risk of disease progression following primary infection had passed by the time calcification appeared, although adult-type disease may have a delayed presentation in adolescent children.
Phase 5 occurs after calcification is complete, >3 yr after primary infection. By this time the highest risk period has passed, although late manifestations of TB, including pulmonary reactivation, may be observed.
Table 2.
Different clinical syndromes associated with tuberculosis in children
| Pathological classification | Disease phase (time period) | Clinical syndromes | Risk groups | Pathogenesis | Imaging manifestations |
|---|---|---|---|---|---|
| Primary Mtb infection | Incubation(0–6 wk) | Asymptomatic | All ages | No adaptive immunity TST(−); IGRA(−) |
None |
| Infection(1–3 mo) | Self-limiting symptoms (mild, viral-like) | Adaptive immunity IGRA(+); TST(+) No test to register reinfection |
Transient hilar or mediastinal lymphadenopathy (50%–70% of cases), rarely visible transient Ghon focus | ||
| Hypersensitivity reactions (fever; erythema nodosum; phlyctenular conjunctivitis) | |||||
|
Early disease progression >90% of disease occur within 12 months of primary infection |
Very early(2–6 mo) | Uncomplicated lymph node (LN) disease | <10 years | Inadequate innate and/or adaptive immunity TST(+); IGRA(+) May be negative with immune compromise or extensive disease, cannot be used as “rule out” tests |
Hilar or mediastinal lymphadenopathy without airway or parenchymal involvement |
| Progressive Ghon focus | <1 yr | Ghon focus with visible cavitation | |||
Disseminated disease:
|
<3 yr |
|
|||
| Early(4–12 mo) | Complicated LN disease
|
<5 yr |
|
||
Pleural disease
|
>3 yr | Effusion usually unilateral; some pleural thickening and loculations (attributable to fibrinous strands) | |||
Lymphadenitis
|
1–10 yr | Usually not needed, matting and edema of adjacent soft tissue | |||
|
Late disease progression Generally rare apart from adult-type disease in adolescents |
Late(1–3 yr) | Adult-type pulmonary disease
|
≥8 yr | “Overagressive” innate and/or adaptive immunity | Apical cavities; may be bilateral; minimal or no LN enlargement (previously referred to as postprimary TB) |
Osteoarticular disease:
|
≥5 yr | Inadequate local control; usually local manifestations only, but can disseminate from any active focus | Periarticular osteopenia, subchondral cystic erosions, joint space narrowing | ||
| Very late(>3 yr) | Urinary tract (renal, ureter, bladder) disease | >5 yr | Renal calcifications; hydronephrosis, calyceal dilation and/or ureter stricture |
Data adapted from Perez-Velez and Marais 2012 and based on detailed disease descriptions provided by Wallgren 1948 and Lincoln and Sewell 1963.
Age ranges, risk groups, and time lines specified provide general guidance only. HIV-infected children are particularly vulnerable and may present with atypical features.
DISEASE SPECTRUM
Intrathoracic Disease
Pulmonary involvement in children presents with a diverse spectrum of pathology that can be visualized on CXR. Appreciating the mechanism of disease is helpful because a CXR is merely a two-dimensional reflection of the underlying pathology. Accurate disease classification has important clinical relevance for prognosis and management; it also facilitates scientific communication (Marais et al. 2004b).
Primary (Ghon) Focus
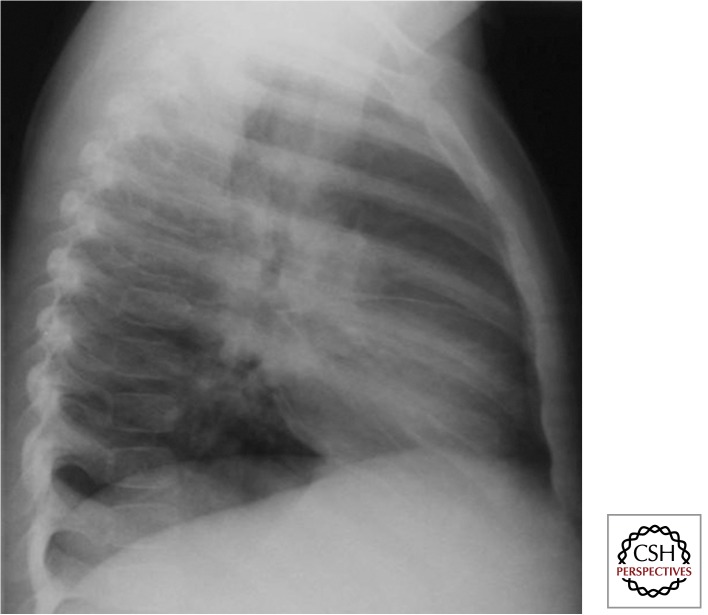
The primary focus describes the parenchymal involvement associated with recent primary infection. It is usually single, transient, and visualized as part of the primary complex. Primary foci are variable in size and may show an overlying pleural reaction. Rarely more than one primary foci may be seen or parenchymal involvement may progress. Complications include local parenchymal destruction with cavity formation, which may result in intrabronchial spread with bronchopneumonic consolidation (Marais et al. 2004b). Figures 3 and 4 illustrate uncomplicated and complicated Ghon foci, respectively.
Figure 3.
Uncomplicated Ghon focus—apex of the left lower lobe (Gie 2003; Marais et al. 2004b). Place where the “eagle has landed,” rarely seen because it is usually transient.
Figure 4.
Complicated Ghon focus (Gie 2003; Marais et al. 2004b). This image reflects poor disease containment at the point of entry; infants and severely immune-compromised individuals are particularly vulnerable.
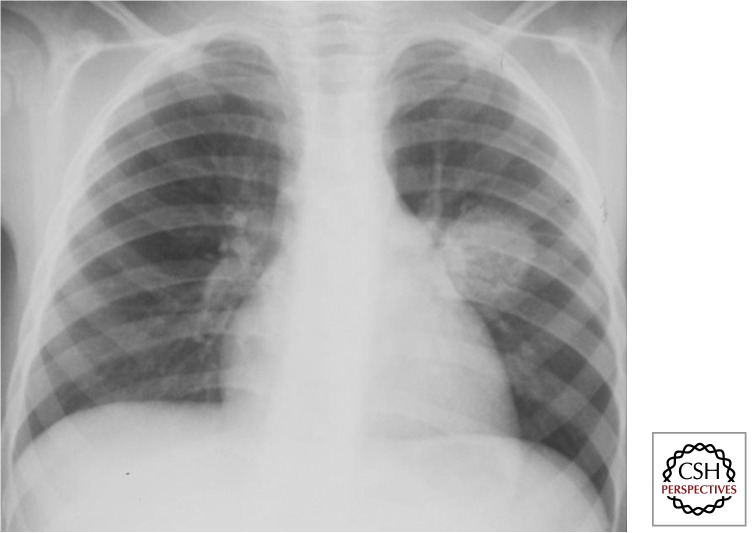
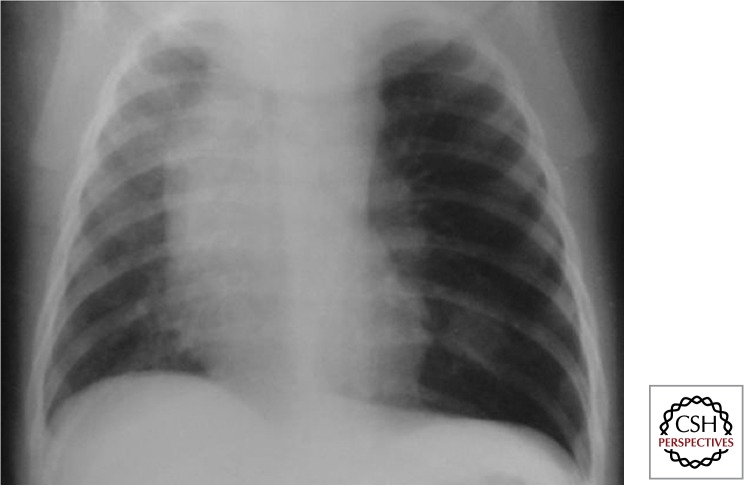
Lymph Node Disease
Involvement of the intrathoracic lymph nodes (perihilar and/or paratracheal) is considered the radiological hallmark of primary infection. Both anteroposterior (AP) and lateral views are required for optimal lymph node visualization (Figs. 5 and 6). Lymph node disease may be complicated by airway involvement and/or penetration into adjacent anatomical structures. Complicated lymph node disease occurs most commonly in children <5 yr of age, probably because of exuberant lymph node responses and the small caliber of their airways.
Figure 5.
Intrathoracic lymph node disease (anteroposterior view) (Gie 2003; Marais et al. 2004b). Lymph nodes are part of the Ghon complex and the main site of disease; however, they are often difficult to visualize with certainty.
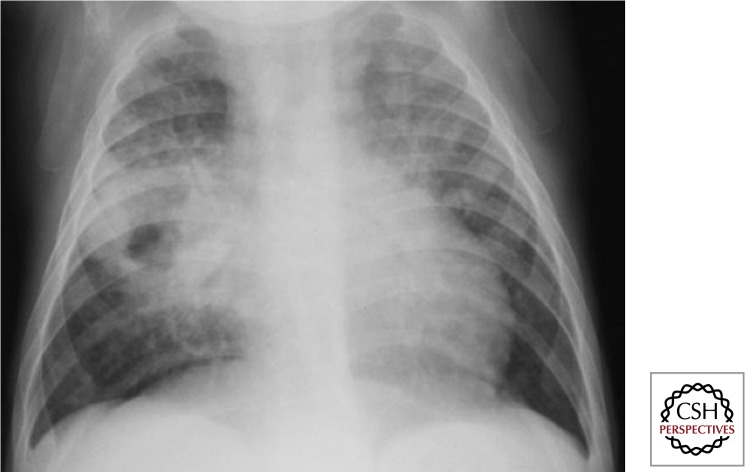
Figure 6.
Intrathoracic lymph node disease (lateral view) (Gie 2003; Marais et al. 2004b). It is essential to perform good quality AP and lateral views. One can see density behind and below the carina, which is not the area where normal physiological structures occur. Also, some fluid in the horizontal and transverse fissures indicates some inflammatory response (not indicative of TB). The dense circular ring around the hilum (so-called doughnut sign) is indicative of perihilar lymph node involvement.
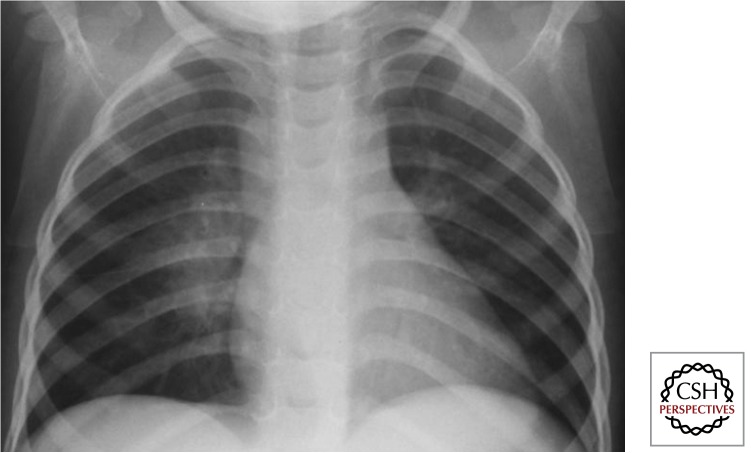
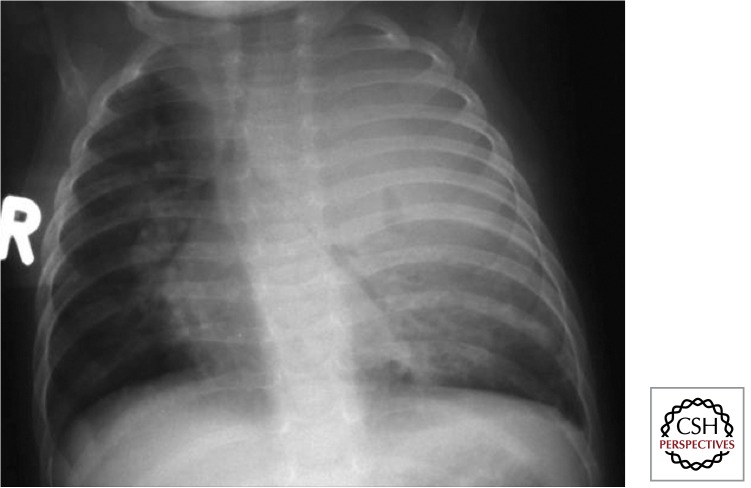
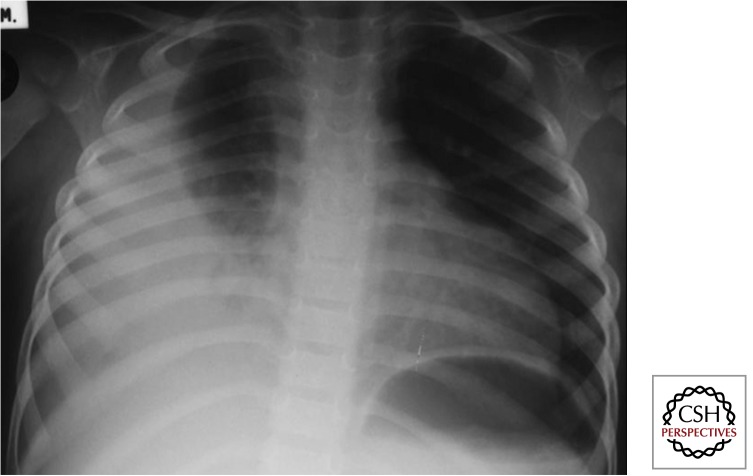
Large airway compression results when the trachea or a main bronchus is surrounded and fixated by diseased lymph nodes and associated inflammation and this may be best visualized on a high-kilovolt (overpenetrated) CXR. Various disease presentations include partial airway obstruction with a check-valve effect and alveolar hyperinflation (Fig. 7) or total airway obstruction with alveolar collapse. When a diseased lymph node erupts into an airway, caseous material may be aspirated, and the resulting pathology may range from pure hypersensitivity-induced inflammation (dead bacilli and/ or “toxins”) to destructive caseating pneumonia (virulent bacilli), depending on the bacterial load and viability of the bacilli aspirated. Caseating pneumonia often causes an expansile (bulging against their anatomical boundaries) pneumonic process with visible parenchymal breakdown (Fig. 8). Penetration into adjacent anatomical structures may involve the phrenic nerve with unilateral diaphragmatic palsy, the esophagus with the formation of a broncho- or tracheoesophageal fistula, and/or the thoracic (lymphatic) duct with the formation of a unilateral chylothorax.
Figure 7.
Airway obstruction with “check valve” effect (Gie et al. 2003; Marais et al. 2004b). The enlarged lymph nodes frequently cause airway complications, especially in young children with small and pliable airways. Hyperinflation (ball valve effect) of left lung. See remnant of Ghon focus and large left-sided perihilar nodes.
Figure 8.
Caseating (expansile) pneumonia (Gie 2003; Marais et al. 2004b). TB lymph nodes cause airway narrowing (almost pathognomonic), because, unlike other mass lesions, it traps and compresses the airway. Lymph nodes can also “rupture” (with sinus formation) into the airway (as occurs with scrofula), which may result in aspiration of caseous material and development of dense (expansile) caseating pneumonia. If this was any other organism, the child would have been in the intensive care unit. Always think of TB when there is this dichotomy between the radiological severity of disease and the clinical signs and symptoms (children often chronically ill, but rarely hyperacute—probably because of reduced V/Q [ventilation/perfusion ratio] mismatch).
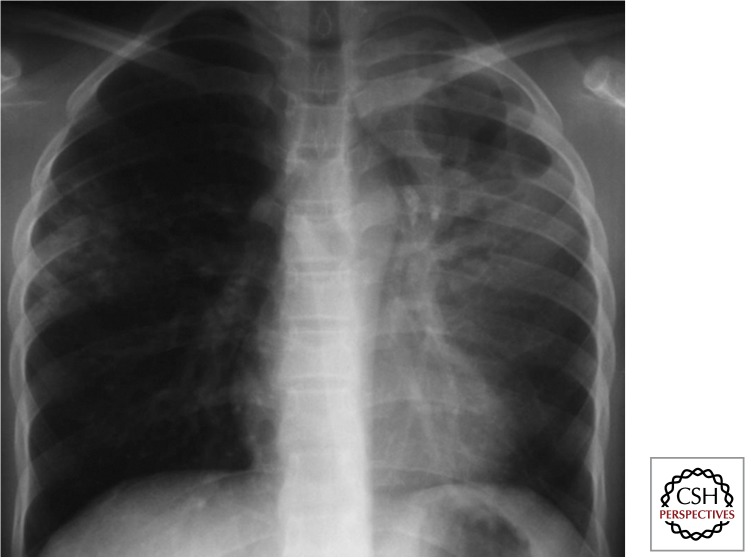
Pleural or Pericardial Effusion
The accumulation of the typical lymphocyte-rich, straw-colored fluid represents a hypersensitivity response (Fig. 9). Isolated pleural effusions are unusual in children <3 yr of age and tend to develop soon (within the first 3–9 mo) after primary infection. A loculated fluid collection may indicate TB empyema that results when bacilli actively multiply within the pleural space. Pericardial effusion usually develops when a subcarinal lymph node erupts into the pericardial space. Cardiac ultrasound is the most sensitive test to confirm or exclude the presence of a pericardial effusion; long-term sequelae include constrictive pericarditis.
Figure 9.
Pleural effusion (Gie 2003; Marais et al. 2004b). Uncommon in children of <6–8 yr. Usually a hypersensitivity response (may develop TB empyema) with typical presentation, well localized chest pain with intermittent fever, and the child otherwise remarkably well. Unilateral dullness on percussion. Fluid characteristically clear with yellow discoloration; TST (tuberculin skin text) very reactive >15–20 mm.
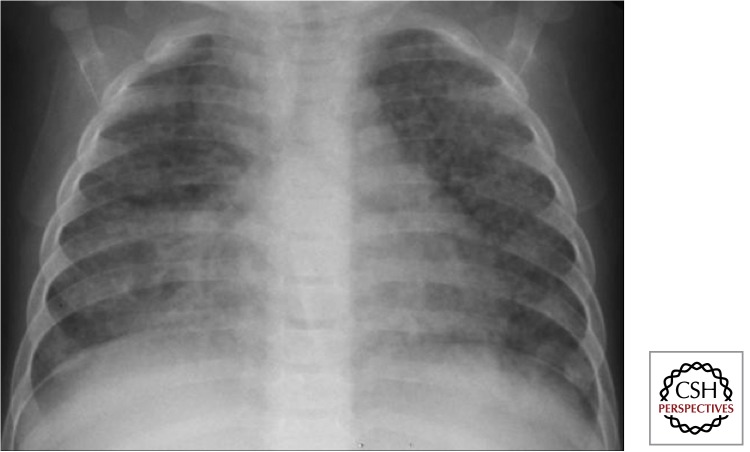
Disseminated (Miliary) Disease
Dissemination represents a condition of infinite gradation. Occult dissemination is common following primary infection; however, it rarely progresses to disseminated disease except in very young (<2–3 yr of age) and immune-compromised children. Typical radiologic signs include the presence of even-sized miliary lesions (<2 mm) that are distributed bilaterally into the very periphery of the lung (Fig. 10). Diagnostic confusion often exists in HIV-infected children in whom lymphocytic interstitial pneumonitis (LIP), malignancies, and opportunistic infections such as Pneumocystis jiroveci pneumonia (PCP) may present with a similar radiological picture. In these instances, response to treatment and/or bacteriologic confirmation may be the only way to establish a definitive diagnosis.
Figure 10.
Disseminated (miliary) disease (Gie 2003; Marais et al. 2004b). Usually in very young children (<2–3 yr of age) or severely immune-compromised within 6 mo after primary infection. Incidence proven to be reduced by BCG vaccination in HIV-uninfected/unexposed children (not in HIV-infected). Mtb spreads via the bloodstream; therefore, tubercles are not restricted to the lungs, but also affect other organs (e.g., liver, spleen) and is frequently associated with TB meningitis (consider lumbar puncture to exclude TBM—management the same).
Adult-Type Disease
Adult-type disease first appears around puberty (8–10 yr of age) and becomes the dominant disease manifestation during adolescence. As with pulmonary TB in adults, the apical and posterior segments of the upper lobes and the apical segment of the lower lobes are most commonly affected (Fig. 11). Complications include progressive cavity formation and bronchial spread.
Figure 11.
Adult-type disease (Gie 2003; Marais et al. 2004b). This type of disease manifestation is not seen in young children, emerges with the onset of puberty at ∼8–10 yr of age, and seems to be associated with an excessive and poorly regulated immune response (excessive hypersensitivity type response). Any child with cavitary disease is infectious (as infectious as an adult sputum smear-positive case) and should be regarded as a potential source case.
Extrathoracic Disease
Cervical Lymphadenitis
The most common extrathoracic manifestation of TB in children is cervical lymphadenitis (Marais et al. 2006a) (Table 3). The cervical mass is usually painless and includes matted nodes with considerable periadenitis. A cold abscess results when the caseous material liquefies. This is signified by a soft fluctuant node with violaceous discoloration of the overlying skin; spontaneous drainage and sinus formation may follow. Untreated, the natural course of TB lymphadenitis follows a prolonged and relapsing course, often interrupted by transient lymph node enlargement, fluctuation, and/or sinus formation.
Table 3.
Disease spectrum documented in children treated for TB in a highly endemic area
| TB manifestation | Total (%) N = 439 |
|---|---|
| Not TB | 85 (19.4) |
| Intrathoracic TB | 307 (69.9) |
| Ghon focus | |
| Uncomplicated (with/without hilar adenopathy) |
16/307 (5.2) |
| Complicated | 3/307 (1.0) |
| Lymph node disease | |
| Uncomplicated | 147/307 (47.9) |
| Complicated | |
| Compression | 25/307 (8.1) |
| Consolidation | 62/307 (20.6) |
| Pleurisy | 24/307 (7.8) |
| Pericarditis | 1/307 (0.3) |
| Disseminated (miliary) disease | 15/307 (4.9) |
| Adult-type disease | 14/307 (4.6) |
| Extrathoracic TB | 72 (16.4) |
| Peripheral lymphadenitis | |
| Cervical | 35/72 (48.6) |
| Other | 1/72 (1.4) |
| Central nervous system TB | |
| Meningitis | 14/72 (19.4) |
| Tuberculoma | 2/72 (2.8) |
| Abdominal TB | 1/72 (1.4) |
| Osteoarticular TB | |
| Vertebral spondylitis | 4/72 (5.6) |
| Other | 7/72 (9.7) |
| Skin | 8/72 (11.1) |
| Intra- + Extrathoracic TB | [25 (5.7)] |
Data adapted from Marais et al. 2006a.TB, tuberculosis; Not TB, chest radiograph not suggestive of TB (confirmed by two independent child TB experts), no bacteriologic or histologic proof and no extrathoracic TB recorded; Intra- + extrathoracic TB, intra- and extrathoracic TB, included in both groups.
Table 4 reflects the clinical characteristics of children diagnosed with TB lymphadenitis (Marais et al. 2006b). A simple clinical algorithm that identifies children with persistent (>4 wk) cervical adenopathy, without a visible local cause or response to first-line antibiotics, and a cervical mass of ≥2 × 2 cm showed excellent diagnostic accuracy, but would be far less accurate in nonendemic areas and in areas where alternative diagnoses, such as Burkitt’s lymphoma, are more common. Establishing a definitive tissues and/or culture diagnosis remains preferable and this can be performed in a minimally invasive fashion using fine needle aspiration biopsy (FNAB). In areas where the control of bovine TB is poor and milk is not routinely pasteurized, Mycobacterium bovis might cause similar disease, whereas nontuberculosis mycobacteria should be considered in areas where both human and bovine TB are well controlled.
Table 4.
Clinical characteristics of children with TB lymphadenitis
| Lymph node characteristics | Total (%) N = 35 |
|---|---|
| Persistence (present for >4 wk, no response to antibiotics) | 35 (100) |
| Size | |
| <2 × 2 cm | 4 (11.4) |
| (2–4) × (2–4) cm | 25 (71.5) |
| >4 × 4 cm | 6 (17.1) |
| Character | |
| Single | 5 (14.3) |
| Multiple –discreet |
14 (40.0) |
| –matted | 16 (45.7) |
| Solid | 28 (80.0) |
| Fluctuant –without secondary bacterial infection |
5 (14.3) |
| –with secondary bacterial infection (red and warm) | 2 (5.7) |
| Associated findings | |
| Tuberculin skin test | |
| 0 mm | 2 (5.7) |
| 1–9 mm | 0 |
| ≥10 mm | 33 (94.3) |
| ≥15 mm | 32 (91.4) |
| Constitutional symptoms | |
| Any symptom | 21 (60.0) |
| Fever | 7 (20.0) |
| Cough | 9 (25.7) |
| Night sweats | 8 (22.8) |
| Fatigue | 19 (54.3) |
| Failure to thrive | 10 (28.6) |
| Chest radiograph | |
| Suggestive of tuberculosis | 13 (37.1) |
| Lymph node disease –uncomplicated |
8 (22.8) |
| – with airway compression | 1 (2.9) |
| – with parenchymal consolidation | 4 (11.4) |
Data adapted from Marais et al. 2006b.
Size, transverse diameter of the largest cervical mass; Fatigue, less playful and active since the mass was first noted; Failure to thrive, crossing at least one weight-for-age centile line in the preceding 3 mo or having lost >10% of body weight (≥1 kg) over any time interval.
Tuberculous Meningitis (TBM)
TBM is the most severe manifestation of childhood TB. Bacille Calmette–Guérin (BCG) vaccination provides some degree of protection against the severe forms of TB (miliary disease and TBM) (Trunz et al. 2006), but despite universal BCG vaccination in most TB-endemic areas, severe disease manifestations still occur. TBM is most common in young children (<3 yr of age) who frequently present with nonspecific symptoms before more advanced disease becomes apparent. This requires clinical vigilance and careful assessment of potential TB exposure, because early diagnosis is essential to ensure an optimal outcome. Symptoms and signs of early disease include fever, listlessness, apathy, anorexia, and/or failure to thrive and headache (in older children). As the disease progresses, localizing neurological signs with/without a suppressed level of consciousness and/or convulsions develop. Most clinical features can be explained by the combined effects of raised intracranial pressure and cerebral vasculitis with brain ischemia/infarction. Together with a dense basal exudate these are the key findings suggestive of TBM on brain imaging (Thwaites et al. 2013).
Perinatal TB
Newborn babies may acquire TB in multiple ways, but generally experience rapid disease progression that requires early diagnosis and treatment (Schaaf et al. 2010). Congenital TB infection is acquired via the placenta, with the primary (Ghon) focus located in the liver or during birth by aspiration of infected amniotic fluid, in which case primary lung involvement usually follows. This occurs when the mother develops hematogenous dissemination during pregnancy, which may be overt (as a result of advanced maternal disease) or occult following recent primary infection of the mother, sometimes signified by a pleural effusion in pregnancy. Alternatively, if mothers or other close contact have infectious pulmonary TB, the baby may inhale the bacillus after delivery (postnatal TB rather than congenital TB).
Other TB Manifestations
TB can affect nearly every organ system as a result of disease progression that occurs at sites where the TB bacillus was deposited during the initial phase of occult dissemination.
Immune Reconstitution
The clinical syndrome was first documented in the prechemotherapy era, following nutritional rehabilitation and/or the termination of high-dose steroid treatment. Recently immune reconstitution inflammatory syndrome (IRIS) has emerged as an important complication to consider shortly after the introduction of combination antiretroviral therapy (cART) in HIV-infected patients with severe immune compromise, usually within the first 6–12 wk. This may manifest as “unmasking IRIS” if mycobacterial disease was undetected before ART initiation or as “paradoxical IRIS” if preexisting disease deteriorates after ART initiation (Meintjes et al. 2009). Radiologic signs may include airway compression caused by increased inflammation surrounding diseased lymph nodes or dense alveolar consolidation caused by excessive inflammation in areas of previous “subclinical” TB infiltration. This temporary exacerbation of TB symptoms and signs is mainly ascribed to the effects of improved immune function, although a “hypersensitivity” reaction to antigens released by killed TB bacilli may also contribute. Although temporary, central nervous system IRIS (resulting from TBM) is associated with rapid clinical deterioration and poor outcomes (Marais et al. 2013c). Care should also be taken to differentiate IRIS from superimposed infections, poor adherence, and drug-resistant disease (Marais et al. 2013c).
DIAGNOSIS
Differentiating infection from disease is critically important because infection is a common event and the management of the two conditions is very different. Disease progression is usually indicated by the onset of persistent nonremitting symptoms. Children may be evaluated for TB following presentation with symptoms or signs suggestive of TB (passive case finding) or as a result of contact investigation or during routine immigrant screening (active case finding). The clinical presentation of children detected by active case finding differs from those detected by passive case finding, with the former often having M. tuberculosis infection only or disease in a very early phase. M. tuberculosis infection detected in young children or following recent TB exposure implies a higher risk of disease progression. In nonendemic areas, the pretest probability is highly dependent on the child’s TB exposure status, which influences the positive predictive value of all subsequent investigations (Perez-Velez and Marais 2012).
Clinical Evaluation
Taking a careful history is essential to explore possible TB exposure and to perform adequate symptom characterization. The variable presentation and the nonspecific nature of most symptoms complicate the diagnosis. Common constitutional symptoms include failure to thrive (documented deviation of the growth curve trajectory) and reduced playfulness; low-grade or intermittent fever is less common (Marais et al. 2004a). With airway involvement, the usual presenting symptom is a persistent nonremitting cough or wheeze, unresponsive to treatment of likely alternative causes (Marais et al. 2006c). However, clinical signs may be fairly acute or more subtle. Scoring systems, of which there are many, often make use of clinical symptoms, signs, contact history, and basic investigations, but no diagnostic scoring system has been adequately validated (Hesseling et al. 2002); sensitivity and specificity is particularly poor in HIV-infected children (Edwards et al. 2007). Despite these limitations a combination of clinical, radiological, and/or laboratory findings consistent with TB disease, together with epidemiological evidence of TB exposure or immunological evidence of M. tuberculosis infection, allow for an accurate diagnosis in most cases (Perez-Velez and Marais 2012).
Imaging Studies
In clinical practice, chest radiography, if adequately performed and read, is one of the most useful studies to perform. Both antero-posterior and lateral views should be taken; lateral views assist assessment of the mediastinum and hilar areas. The International Union Against Tuberculosis and Lung Disease (The Union) compiled an atlas of illustrative cases that provides a wonderful resource for clinicians (Gie 2003). Ultrasound is useful to confirm pericardial or pleural effusions and abdominal lymphadenopathy, solid organ involvement, or ascites. High-resolution computed tomography (CT) offers excellent anatomical visualization (Andronikou et al. 2009), but its use should be reserved for complicated intrathoracic cases caused by high radiation exposure and cost. CT and/or magnetic resonance imaging (MRI) are particularly helpful to visualize intracranial pathology. MRI is more sensitive for detecting brain-stem lesions or early perfusion defects in patients with TBM, and also provides better evaluation of the spine and soft tissues (Pienaar et al. 2009).
Laboratory Studies
Table 5 summarizes available diagnostic investigations. Sputum smear microscopy provides the cornerstone of TB diagnosis in most countries, but it has limited utility in young children with paucibacillary disease who are unable to expectorate. Although sensitivity remains suboptimal in children, the Xpert-MTB/RIF assay is rapid and highly specific. Using two sputum samples. it detects three times more cases than microscopy and ∼70% of the cases detected by liquid culture (Nicol et al. 2011; Rachow et al. 2012). Both immunological assays, the traditional TST and newer interferon-γ release assays (IGRAs), fail to differentiate M. tuberculosis infection from TB disease. WHO recommends that IGRAs should not replace the TST for the detection of M. tuberculosis infection in low- or middle-income countries (World Health Organization 2011a), although they may be complementary by improving sensitivity and/or specificity in specific clinical situations (MMWR 2010).
Table 5.
Summary of investigations to diagnose tuberculosis in children
| Investigation | Uses | Strengths and limitations |
|---|---|---|
| Microbiological studies | ||
| Microscopy | Diagnosis of TB | Strengths: Specificity high; useful in all specimen types; rapid (<1 h) detection; low cost. Limitations: Sensitivity very low, especially in young children; highly operator-dependent; labor intensive; unable to distinguish viable and dead bacilli. |
| Culture | Diagnosis of TB; drug susceptibility testing | Strengths: Specificity high. Limitations: Sensitivity moderate to low in young children; slow turnaround time; need proper laboratory facilities. |
| DNA detection (PCR) | Strengths: Specificity high; fully automated; rapid turnaround. Limitations: Sensitivity moderate to low in young children; unable to distinguish viable from dead bacilli. | |
| Histopathological studies | ||
| Stained tissue samples | Diagnosis of TB | Allows exclusion of other diagnoses (such as malignancy). |
| Immune-based studies | ||
| TST; IGRA (e.g., Quantiferon Gold) | Diagnosis of Mtb infection | Neither test can differentiate Mtb infection from TB disease; careful evaluation of discordant results. TST: Affected by BCG vaccination; requires a second visit after 48–72 h. IGRAs: Unaffected by BCG vaccination; single visit; reduced sensitivity in very young and/or immune-compromised children; collecting adequate volume of blood and indeterminate results problematic. |
| Imaging | ||
| Radiography CT and MRI Ultrasonography |
Diagnosis of TB | Chest radiography (AP and lateral views) most helpful; CT or MRI useful in uncertain or complicated cases; ultrasonography useful to identify intraabdominal/retroperitoneal lymphadenopathy or pleural/pericardial effusions, highly operator-dependent. |
Data adapted from Perez-Velez and Marais 2012.
TST, tuberculin skin test; IGRA, interferon-γ release assay; CT, computed tomography; MRI, magnetic resonance imaging.
Collecting expectorated sputum in young children is problematic; gastric aspirates and induced sputa offer feasible alternatives (Nicol and Zar 2011). Table 6 provides an overview of specimen collection techniques (Marais and Pai 2006). The string test works well in sputum-scarce adults and preliminary results in children seem promising (Perez-Velez et al. 2010), although administration is difficult in young children who are unable to swallow the string-containing capsule. With adequate DNA extraction protocols, stool also offers promise as a diagnostic specimen (Nicol et al. 2013). FNAB has a high utility in children with a peripheral lymph node mass (Wright et al. 2009). The Union developed a pragmatic desk guide-to-guide TB diagnosis and management in resource-limited settings (IUTLD 2010).
Table 6.
Bacteriologic specimen collection methods—perceived problems and/or benefits
| Specimen collection method | Problems/Benefits | Potential clinical application |
|---|---|---|
| Sputum | Not feasible in very young children; assistance and supervision may improve the quality of the specimen | Routine sample to be collected in children >7 yr of age (all children who can produce a good quality specimen) |
| Induced sputum | Comparable yield to gastric aspirate; no age restriction; specialized technique, which requires nebulization and suction facilities; potential transmission risk | To be considered in the hospital setting on an in- or out-patient basis |
| Gastric aspirate | Unpleasant procedure, but not difficult to perform; requires fasting; sample collection advised on 3 consecutive days | Routine sample to be collected in hospitalized who cannot produce a good quality sputum specimen |
| Nasopharyngeal aspiration | Less invasive than gastric aspirate; no fasting required; comparable yield to gastric aspirate | To be considered in primary health care clinics or on an outpatient basis |
| String test | Less invasive than gastric aspirate; tolerated well in children >4 yr; bacteriologic yield and feasibility requires further investigation | Potential to become the routine sample collected in children who can swallow the capsule but cannot produce a good quality sputum specimen |
| Bronchoalveolar lavage | Extremely invasive | Only for use in patients who are intubated or who require diagnostic bronchoscopy |
| Stool | Culture not practical, DNA extraction difficult; not invasive; M. tuberculosis excretion well documented | Reasonable yield using Gene Xpert |
| Urine | Not invasive; excretion of M. tuberculosis components | Lipoarabinomannan (LAM) assay has poor sensitivity; unreliable in children |
| Blood/Bone marrow | Good sample sources to consider in the case of probable disseminated TB | To be considered for the confirmation of probable disseminated TB in hospitalized patients |
| Cerebrospinal fluid (CSF) | Fairly invasive; bacteriologic yield low | To be considered if signs of tuberculous meningitis |
| Fine needle aspiration (FNA) | Minimally invasive using a fine 23G needle; excellent bacteriologic yield; minimal side effects | Procedure of choice in children with superficial lymphadenopathy |
Data adapted from Marais and Pai 2006.
MANAGEMENT
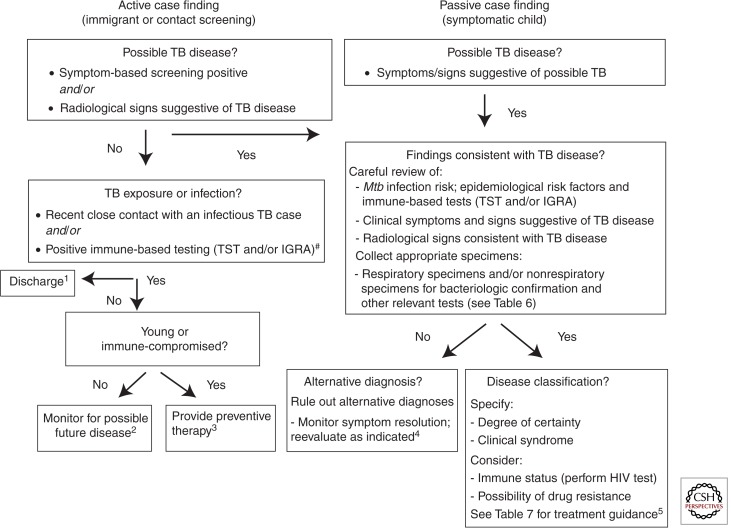
Although bacteriological confirmation of TB remains the ideal, yields are low and treatment initiation is a clinical decision in most instances once all appropriate tests have been completed. Young children rarely experience serious adverse events with first-line TB drugs and are at low risk of acquiring or transmitting DR-TB. TB treatment aims to cure the individual patient, whereas the public health aim is to terminate transmission and prevent the emergence of drug resistance. Actively metabolizing bacilli are rapidly killed by bactericidal drugs, improving clinical symptoms, terminating transmission, and providing protection to companion drugs. Sterilizing drugs are required to eradicate persistent subpopulations of bacilli to establish long-term cure. Pragmatic disease classification should guide individual case management (Fig. 12).
Figure 12.
Algorithm for diagnosis and classification of tuberculosis in children. HIV, human immunodeficiency virus; TST, tuberculin skin test; IGRA, interferon-γ release assay; Mtb—Mycobacterium tuberculosis. #None of the immune-based tests (TST/IGRA) can “rule out” TB disease with confidence and conversion may be delayed for 2–3 mo after documented exposure. Diagnostic labels: 1No TB exposure or infection; 2TB exposure/infection with low risk of progression to disease; 3TB exposure/infection with high risk of progression to disease; 4unlikely TB disease; 5TB disease. (Data adapted from Perez-Velez and Marais 2012.)
The most important disease variables to consider are bacillary load and anatomical location. Drug resistance should be considered in children from settings with a high prevalence of DR-TB or following documented contact with a drug-resistant source case—someone who died while on TB treatment without known drug susceptibility test results, is poorly adherent to therapy, or is a retreatment case. Young children with uncomplicated disease, from settings with a low prevalence of isoniazid resistance, can be treated with three drugs (isoniazid, rifampicin, and pyrazinamide) during the 2-mo intensive phase, followed by isoniazid and rifampicin during the 4-mo continuation phase. However, children with extensive and/or cavitary lung disease (implying a high organism load) or from settings with a high prevalence of isoniazid resistance, should receive a fourth drug (ethambutol, which is safe in children of all ages within the recommended dosage range) during the 2-mo intensive phase (WHO Press 2014). Table 7 summarizes the mechanism of action, main adverse effects, and recommended pediatric dosages of first-line TB drugs.
Table 7.
Summary of first-line TB drugs and dosage recommendations in children
| First-line drugs | Mode and mechanism of action | Main toxicitiesa | Daily dose mg/kg (range) [maximum dose]b |
|---|---|---|---|
| Isoniazid (INH) |
Bactericidal—inhibits cell wall synthesis; most potent early bactericidal activity offering the best protection to companion drugs Contributes mainly by rapidly killing actively metabolizing extracellular bacilli; contributes to sterilization if given for a prolonged period |
Hepatitis; peripheral neuropathy | 10 (7–15) [300 mg] |
| Rifampicin (RMP) |
Bactericidal and sterilizing—inhibits RNA synthesis; contributes by killing extracellular and slower growing intracellular bacilli; important contribution to sterilization | Hepatitis; orange discoloration of secretions; drug–drug interactions | 15 (10–20) [600 mg] |
| Pyrazinamide (PZA) |
Sterilizing—disrupts energy metabolism; contributes by specifically killing bacilli that persist within the acidic centers of caseating granulomas | Hepatitis; arthralgia | 35 (30–40) [2000 mg] |
| Ethambutol (EMB) |
Bacteriostatic—inhibits cell wall synthesis; contributes mainly by offering some additional protection against drug-resistant mutants | Visual disturbance (acuity, color vision) |
20 (15–25) [1200 mg] |
| Suggested treatment regimens | ||
|---|---|---|
| Disease category | Treatment regimen | Rationale |
| Uncomplicated intrathoracic disease | INH, RMP, PZA (2-mo intensive phase) INH, RMP (4-mo continuation phase) |
Organism load low; drug penetration good |
| Extensive lung infiltrates and/or cavities | Add EMB during 2-mo intensive phase | Organism load high; drug penetration good |
| Tuberculous meningitis (TBM)c | Add fourth drug—at least during 2-mo intensive phase. Prolong continuation phase to 10 months (WHO recommendation) Add steroids for 1 mo |
Organism load low; drug penetration variable; risk of severe immune mediated sequelae |
| Severe airway compression | Three- or four-drug regimen depending on extent of lung infiltration/cavities Consider adding steroids for 1 mo |
Organism and drug penetration variabled; inflammation may worsen airway compression |
| Recent exposure/infection No active disease |
Preventive therapy INH (6–9 mo) INH, RMP (3 mo) |
Organism load very low; drug penetration good |
Data adapted from Perez-Velez and Marais 2012.
aHypersensitivity reactions and drug rashes may occur with any drug.
bWHO dosage recommendations for children.
cRecommendations around fourth drug and duration of therapy vary.
dDrug penetration into large cold abscesses may be limited, requiring surgical drainage.
The most likely cause of poor response to treatment, in the absence of drug resistance, is nonadherence. All TB medications should preferably be given as directly observed therapy (DOT). Standard first-line treatment is appropriate in the absence of exposure to a source case with likely DR-TB or clinical and radiological deterioration while receiving adherent first-line therapy. Use of an escalated retreatment regimen that includes only streptomycin is not indicated; TB recurrence >12 mo after treatment completion most likely represents a new disease episode (reinfection) and should be treated as such. Poor clinical response to adherent treatment requires critical reevaluation of the diagnosis, including consideration of IRIS and drug resistance. Practice-based recommendations on the management of children with DR-TB were recently published (Seddon et al. 2012) and excellent treatment outcomes can be achieved with optimal management (Ettehad et al. 2012).
Immune recovery following ART initiation or nutritional rehabilitation may unmask existing subclinical disease or induce paradoxical deterioration despite adequate TB treatment. IRIS does not indicate treatment failure and treatment should usually not be interrupted; severe cases may require a course of corticosteroids. Despite the risk of IRIS, adult data indicate that ART is best initiated within 8 wk of starting TB treatment and within 2–4 wk in the severely immune-compromised (Blanc et al. 2011). The only exception for stopping ART would be patients with central nervous system TB, where IRIS could have devastating consequences (e.g., CSF outflow obstruction due to new tuberculomas or brain abscesses with increasing intracranial pressure) (Török et al. 2011). With HIV-associated TB, treatment should be daily and the total duration may require extension depending on the level of immune compromise and extent of disease (Siberry et al. 2013).
PREVENTION
Transmission within health-care facilities is a particular concern in settings where very young or immune-compromised children may be exposed. Careful consideration should be given to patient flows and air exchange in hospitals and clinics, considering that symptomatic parents or caregivers pose a transmission risk (Muñoz et al. 2002). Although BCG vaccination reduces the risk of disseminated (miliary) disease and TBM in young children, it offers no consistent protection against adult-type TB (Trunz et al. 2006). No benefit has been established in HIV-infected children, in whom BCG vaccination is contraindicated because of the risk of disseminated BCG disease (Hesseling et al. 2007; World Health Organization 2007). However, with good prevention of mother-to-child transmission of HIV (PMTCT) the risk of not giving BCG in the 97% infants of HIV-infected mothers who will be HIV-negative outweighs the benefit of not giving it to the few who might become HIV-infected (Rabie et al. 2011). In addition, early ART initiation prevents disseminated BCG disease and BCG IRIS that has been observed with delayed ART initiation (Rabie et al. 2001). The development of a safe and effective vaccine remains a top global health research priority.
With good adherence, isoniazid preventive therapy (IPT) for 6–9 mo provides excellent protection against TB disease (Marais et al. 2009), but implementation remains poor with a pronounced policy-practice gap (Hill et al. 2011). Parents are often reluctant to provide “treatment” to an otherwise well child and the long duration of preventive therapy provides further discouragement. Isoniazid and rifampicin for 3 mo provides equivalent efficacy and improved adherence compared with 9 mo of isoniazid (Spyridis et al. 2007). A regimen of 12 doses of weekly rifapentine and isoniazid was efficacious in adults (Sterling et al. 2011), but is not yet recommended in children of <12 yr of age awaiting safety data. All rifampicin- and rifapentine-containing regimens interact with protease inhibitor-containing ART; this is less with rifabutin, but its use in preventive therapy regimens has not been evaluated. WHO recommends IPT for 6-36 mo after completion of TB treatment in all HIV-infected individuals, including children, who live in settings with a high TB prevalence (World Health Organization 2011b). However, ongoing TB exposure screening and meticulous postexposure prophylaxis following every documented TB exposure event, together with early ART initiation in all HIV-infected children, seem adequate (Schaaf et al. 2013). Preventive therapy has benefit in vulnerable children following exposure to DR-TB, but this should be tailored to the drug susceptibility test result of the source case or known drug resistance profiles in the region (Seddon et al. 2013).
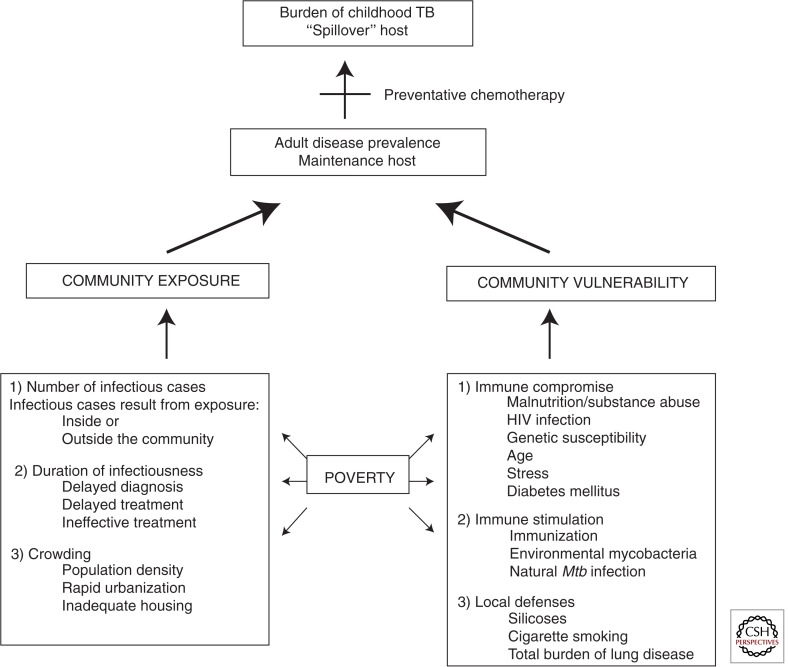
Ultimately, the burden of childhood TB within a community reflects the underlying variables that sustain the TB epidemic, because pediatric cases reflect ongoing M. tuberculosis transmission (Fig. 13) (Marais et al. 2005b). Whereas ultimate epidemic control require these factors to be addressed at a population level, much can be done to alleviate the TB disease burden in children by offering adequate prevention, considering TB in the differential diagnosis, and providing appropriate treatment.
Figure 13.
The main variables that contribute to the burden of childhood TB in a particular community. (Data adapted from Marais et al. 2005b.)
Footnotes
Editors: Stefan H.E. Kaufmann, Eric J. Rubin, and Alimuddin Zumla
Additional Perspectives on Tuberculosis available at www.perspectivesinmedicine.org
REFERENCES
*Reference is also in this subject collection.
- Abubakar I, Ford N, Cox H 2013. The rising tide of drug-resistant tuberculosis—Time for visionary political leadership. Lancet Infect Dis 13: 529–539 [DOI] [PubMed] [Google Scholar]
- Amanullah F, Ashfaq M, Khowaja S, Parekh A, Salahuddin N, Lotia-Farrukh I, Khan A, Becerra MC 2014. High tuberculosis prevalence in children exposed at home to drug-resistant tuberculosis. Int J Tuberc Lung Dis 18: 520–527 [DOI] [PubMed] [Google Scholar]
- Andronikou S, van Hoenacker FM, de Backer AI 2009. Advances in imaging chest tuberculosis: Blurring-of-differences-between-children-and-adults. Clin Chest Med 30: 717–744 [DOI] [PubMed] [Google Scholar]
- Bates M, Mudenda V, Mwaba P, Zumla A 2013. Deaths due to respiratory tract infections in Africa: A review of autopsy studies. Curr Opin Pulm Med 19: 229–237 [DOI] [PubMed] [Google Scholar]
- Blanc FX, Sok T, Laureillard D, Borand L, Rekacewicz C, Nerrienet E, Madec Y, Marcy O, Chan S, Prak N, et al. 2011. Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med 365: 1471–1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton MF, Schaaf HS, Lottering G, Wever HL, Coetzee J, Nachman S 2008. Tuberculosis exposure in HIV-exposed infants in a high-prevalence setting. Int J Tuberc Lung Dis 12: 225–227 [PubMed] [Google Scholar]
- Du Preez K, Schaaf HS, Dunbar R, Swartz A, Bissell K, Enarson DA, Hessling AC 2011. Incomplete registration and reporting of culture-confirmed childhood tuberculosis diagnosed in hospital. Public Health Action 1: 19–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards DJ, Kitelele F, van Rie A 2007. Agreement between clinical scoring systems used for the diagnosis of tuberculosis in the HIV era. Int J Tuberc Lung 11: 263–269 [PubMed] [Google Scholar]
- Ettehad D, Schaaf HS, Seddon JA, Cooke G, Ford N 2012. Treatment outcomes for children with multi-drug resistant tuberculosis: A systematic review and meta-analysis. Lancet Infect Dis 12: 449–456 [DOI] [PubMed] [Google Scholar]
- Gie RP 2003. Diagnostic atlas of intrathoracic tuberculosis in children: A guide for low income countries. IUATLD, Paris: http://www.theunion.org/download/clh/diagnostic_atlas_intrathoraric_tuberculosis_child.pdf [Google Scholar]
- Graham SM, Sismanidis C, Menzies HJ, Marais BJ, Detjen AK, Black RE 2014. Importance of tuberculosis control to address child survival. Lancet 383: 1605–1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesseling AC, Schaaf HS, Gie RP, Starke JR, Beyers N 2002. A critical review of diagnostic approaches used in the diagnosis of childhood tuberculosis. Int J Tuberc Lung Dis 6: 1038–1045 [PubMed] [Google Scholar]
- Hesseling AC, Marais BJ, Gie RP, Schaaf HS, Fine PEM, Godfrey-Faussett P, Beyers N 2007. The risk of disseminated bacilli Calmette–Guérin (BCG) disease in HIV-infected children. Vaccine 25: 14–18 [DOI] [PubMed] [Google Scholar]
- Hesseling AC, Cotton MF, Jennings T, Whitelaw A, Johnson LF, Eley B, Roux P, Godfrey-Faussett P, Schaaf HS 2009. High incidence of tuberculosis among HIV-infected infants: Evidence from a South African population-based study highlights the need for improved tuberculosis control strategies. Clin Infect Dis 48: 108–114 [DOI] [PubMed] [Google Scholar]
- Hill PC, Rutherford ME, Audas R, van Crevel R, Graham SM 2011. Closing the policy-practice gap in the management of child contacts of tuberculosis in developing countries. PLoS Med 8: e10001105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IUATLD. 2010. Desk-guide for the diagnosis and management of TB in children. www.theunion.org/index.php/en/resources/scientific-publications/tuberculosis/item/193-desk-guide-for-diagnosis-and-management-of-tb-in-children
- Jenkins HE, Tolman AW, Yuen CM, Parr JB, Keshavjee S, Perez-Velez CM, Pagano M, Becerra MC, Cohen T 2014. Incidence of multidrug-resistant tuberculosis disease in children: Systematic review and global estimates. Lancet 383: 1572–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Källenius G 2014. Tuberculosis and HIV coinfections. Cold Spring Harb Perspect Med 10.1101/cshperspect.a017871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn SD, Bekker LG, Middelkoop K, Myer L, Wood R 2006. Impact of HIV infection on the epidemiology of tuberculosis in a peri-urban community in South Africa: The need for age-specific interventions. Clin Infect Dis 42: 1040–1047 [DOI] [PubMed] [Google Scholar]
- Lincoln EM, Sewell EM 1963. Tuberculosis in children. McGraw-Hill, New York [Google Scholar]
- Marais BJ, Pai M 2006. Specimen collection methods in the diagnosis of childhood tuberculosis. Indian J Med Microbiol 24: 249–251 [DOI] [PubMed] [Google Scholar]
- Marais BJ, Gie RP, Schaaf HS, Hesseling AC, Obihara CC, Starke JJ, Enarson DA, Donald PR, Beyers N 2004a. The natural history of childhood intra-thoracic tuberculosis: A critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis 8: 392–402 [PubMed] [Google Scholar]
- Marais BJ, Gie RP, Schaaf HS, Starke JR, Hesseling AC, Donald PR, Beyers N 2004b. A proposed radiologic classification of childhood intra-thoracic tuberculosis. Pediatr Rad 33: 886–894 [DOI] [PubMed] [Google Scholar]
- Marais BJ, Donald PR, Gie RP, Schaaf HS, Beyers N 2005a. Diversity of disease manifestations in childhood pulmonary tuberculosis. Ann Trop Paed 25: 79–86 [DOI] [PubMed] [Google Scholar]
- Marais BJ, Obihara CC, Warren RM, Schaaf HS, Gie RP, Donald PR 2005b. The burden of childhood tuberculosis: A public health perspective. Int J Tuberc Lung Dis 9: 1305–1313 [PubMed] [Google Scholar]
- Marais BJ, Gie RP, Schaaf HS, Hesseling AC, Enarson DA, Beyers N 2006a. The spectrum of disease in children treated for tuberculosis in a highly endemic area. Int J Tuberc Lung Dis 10: 732–738 [PubMed] [Google Scholar]
- Marais BJ, Wright CA, Schaaf HS, Gie RP, Hesseling AC, Enarson DA, Beyers N 2006b. Tuberculous lymphadenitis as a cause of persistent cervical lymphadenopathy in children from a tuberculosis-endemic area. Pediatr Inf Dis J 25: 142–146 [DOI] [PubMed] [Google Scholar]
- Marais BJ, Gie RP, Hesseling AC, Schaaf HS, Lombard C, Enarson DA, Beyers N 2006c. A refined symptom-based approach to diagnose pulmonary tuberculosis in children. Pediatrics 118: e1350–e1359 [DOI] [PubMed] [Google Scholar]
- Marais BJ, Ayles H, Graham SM, Godfrey-Faussett P 2009. Screening and preventive therapy for tuberculosis. Chest Clin N Am 30: 827–846 [DOI] [PubMed] [Google Scholar]
- Marais B, Graham S, Maeurer M, Zumla A 2013a. Progress and challenges in childhood tuberculosis. Lancet Infect Dis 13: 287–289 [DOI] [PubMed] [Google Scholar]
- Marais BJ, Mlambo CK, Rastogi N, Zozio T, Duse AG, Victor TC, Marais E, Warren RM 2013b. Epidemic spread of multidrug-resistant (MDR) tuberculosis in Johannesburg, South Africa. J Clin Microbiol 51: 1818–1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marais S, Meintjes G, Pepper DJ, Dodd LE, Schutz C, Ismail Z, Wilkinson KA, Wilkinson RJ 2013c. Frequency, severity, and prediction of tuberculous meningitis immune reconstitution inflammatory syndrome. Clin Infect Dis 56: 450–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meintjes G, Rabie H, Wilkinson RJ, Cotton MF 2009. Tuberculosis-associated immune reconstitution inflammatory syndrome and unmasking of tuberculosis by antiretroviral therapy. Clin Chest Med 30: 797–810 [DOI] [PubMed] [Google Scholar]
- MMWR. 2010. Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection—United States. MMWR 59: 1–25 [PubMed] [Google Scholar]
- Muñoz FM, Ong LT, Seavy D, Medina D, Correa A, Starke JR 2002. Tuberculosis among adult visitors of children with suspected tuberculosis and employees at a children’s hospital. Infect Control Hosp Epidemiol 23: 568–572 [DOI] [PubMed] [Google Scholar]
- Newton SM, Brent AJ, Anderson S, Whittaker E, Kampmann B 2008. Paediatric tuberculosis. Lancet Infect Dis 8: 499–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol MP, Zar HJ 2011. New specimens and laboratory diagnostics for childhood pulmonary TB: Progress and prospects. Paediatr Respir Rev 12: 16–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol MP, Workman L, Isaacs W, Munro J, Eley B, Boehme CC, Zemanay W, Zar HJ 2011. Accuracy of the Xpert MTB/RIF test for the diagnosis of pulmonary tuberculosis in children admitted to hospital in Cape Town, South Africa: A descriptive study. Lancet Infect Dis 11: 819–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol MP, Spiers K, Workman L, Isaacs W, Munro J, Black F, Zemanay W, Zar HJ 2013. Xpert MTB/RIF testing of stool samples for the diagnosis of pulmonary tuberculosis in children. Clin Infect Dis 57: e18–e21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Velez CM, Marais BJ 2012. Tuberculosis in children. N Engl J Med 367: 348–361 [DOI] [PubMed] [Google Scholar]
- Perez-Velez CM, Wilches-Luna EC, Hernández-Sarmiento JM, Casanova-Reynolds AL, Hernández N, Moreno-Ortega SP, Atehortúa-Muñoz SL, Rozo-Anaya JC, Mejía GI, Gómez V, et al. 2010. Preliminary results of a comparative yield study of induced sputum, string test, and gastric aspirate for the microbiological diagnosis of pulmonary tuberculosis in children. Am J Respir Crit Care Med 181: A1775 [Google Scholar]
- Pienaar M, Andronikou S, van Toorn R 2009. MRI to demonstrate diagnostic features and complications of TBM not seen with CT. Child Nerv Syst 25: 941–947 [DOI] [PubMed] [Google Scholar]
- Rabie H, Violari A, Duong T, Madhi SA, Josipovic D, Innes S, Dobbels E, Lazarus E, Panchia R, Babiker AG, et al. 2011. Early antiretroviral treatment reduces risk of bacille Calmette–Guérin immune reconstitution adenitis. Int J Tuberc Lung Dis 15: 1194–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachow A, Clowes P, Saathoff E, Mtafya B, Ntinginya EN, Kowour D, Rojas-Ponce G, Kroidl A, Maboko L, Heinrich N, et al. 2012. Increased and expedited case detection by Xpert MTB/RIF assay in childhood tuberculosis: A prospective cohort study. Clin Infect Dis 54: 1388–1396 [DOI] [PubMed] [Google Scholar]
- Rose PC, Schaaf HS, du Preez K, Seddon JA, Garcia-Prats AJ, Zimri K, Dunbar R, Hesseling AC 2013. Completeness and accuracy of paediatric electronic drug-resistant tuberculosis registration in Cape Town, South Africa. Public Health Action 3: 214–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaf HS, Van Rie A, Gie RP, Beyers N, Victor TC, Van Helden PD, Donald PR 2000. Transmission of multidrug resistant tuberculosis. Pediatr Infect Dis J 19: 695–699 [DOI] [PubMed] [Google Scholar]
- Schaaf HS, Marais BJ, Whitelaw A, Hesseling AC, Eley B, Hussey GD, Donald PR 2007. Culture-confirmed childhood tuberculosis in Cape Town, South Africa: A review of 596 cases. BMC Infect Dis 7: 140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaf HS, Collins A, Bekker A, Davies PDO 2010. Tuberculosis at the extremes of age. Respirology 15: 747–763 [DOI] [PubMed] [Google Scholar]
- Schaaf HS, Cotton MF, Boon GPG, Jeena PM 2013. Isoniazid preventive therapy in HIV-infected and uninfected children (0–14 years). S Afr Med J 103: 714–715 [DOI] [PubMed] [Google Scholar]
- Seddon JA, Furin JJ, Gale M, Del Castillo Barrientos H, Hurtado RM, Amanullah F, Ford N, Starke JR, Schaaf HS, Sentinel Project on Pediatric Drug-Resistant Tuberculosis. 2012. Caring for children with drug-resistant tuberculosis: Practice-based recommendations. Am J Respir Crit Care Med 186: 953–964 [DOI] [PubMed] [Google Scholar]
- Seddon JA, Hesseling AC, Finlayson H, Fielding K, Cox H, Hughes J, Godfrey-Faussett P, Schaaf HS 2013. Preventive therapy for child contacts of multidrug-resistant tuberculosis: A prospective cohort study. Clin Infect Dis 57: 1676–1684 [DOI] [PubMed] [Google Scholar]
- *.Seung KJ, Rich M, Keshavjee S 2014. MDR and XDR tuberculosis. Cold Spring Harb Perspect Med 10.1101/cshperspect.a017863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siberry GK, Abzug MJ, Nachman S, the Panel on Opportunistic Infections in HIV-Exposed and HIV-Infected Children. 2013. Guidelines for the prevention and treatment of opportunistic infections in HIV-exposed and HIV-infected children: Recommendations from the National Institutes of Health, Centers for Disease Control and Prevention, the HIV Medicine Association of the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the American Academy of Pediatrics. Pediatr Infect Dis J 32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spyridis NP, Spyridis PG, Gelesme A, Sypsa V, Valianatou M, Metsou F, Gourgiotis D, Tsolia MN 2007. The effectiveness of a 9-month regimen of isoniazid alone versus 3- and 4-month regimens of isoniazid plus rifampin for treatment of latent tuberculosis infection in children: Results of an 11-year randomized study. Clin Infect Dis 45: 715–722 [DOI] [PubMed] [Google Scholar]
- Sterling TR, Villarino ME, Borisov AS, Shang N, Gordin F, Bliven-Sizemore E, Hackman J, Hamilton CD, Menzies D, et al. 2011. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Eng J Med 365: 2155–2166 [DOI] [PubMed] [Google Scholar]
- Thwaites GE, van Toorn R, Schoeman J 2013. Tuberculous meningitis: More questions, still too few answers. Lancet Neurol 12: 999–1010 [DOI] [PubMed] [Google Scholar]
- Török ME, Yen NT, Chau TT, Mai NT, Phu NH, Mai PP, Dung NT, Chau NV, Bang ND, Tien NA, et al. 2011. Timing of initiation of antiretroviral therapy in human immunodeficiency virus (HIV)-associated tuberculous meningitis. Clin Infect Dis 52: 1374–1383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trunz BB, Fine P, Dye C 2006. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: A meta-analysis and assessment of cost-effectiveness. Lancet 367: 1173–1180 [DOI] [PubMed] [Google Scholar]
- Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA, Jean-Philippe P, McIntyre JA,CHER Study Team. 2008. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med 359: 2233–2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallgren A 1948. The time-table of tuberculosis. Tubercle 29: 245–251 [DOI] [PubMed] [Google Scholar]
- WHO Press. 2014. Guidance for national tuberculosis programmes on the management of tuberculosis in children, 2nd ed. Geneva, Switzerland: (WHO/HTM/TB/2014.03) [PubMed] [Google Scholar]
- World Health Organization. 2007. Revised BCG vaccination guideline for infants at risk of HIV infection. Wkly Edimiol Rev 82: 193. [PubMed] [Google Scholar]
- World Health Organization. 2011a. Use of tuberculosis interferon-γ release assays (IGRAs) in low- and middle-income countries: Policy statement. Geneva, WHO Press; [PubMed] [Google Scholar]
- World Health Organization. 2011b. Guidelines for intensified TB case-finding and INH preventive therapy for people living with HIV in resource-constrained settings: Annexes. WHO Press, Geneva: http://whqlibdoc.who.int/publications/2011/9789241500708_annexes_eng.pdf [Google Scholar]
- World Health Organization. 2013. Global Tuberculosis Report 2013. World Health Organisation, Geneva: WHO/HTM/TB/2013 [Google Scholar]
- Wright CA, Warren RW, Marais BJ 2009. Fine needle aspiration biopsy: An undervalued diagnostic modality in pediatric mycobacterial disease. Int J Tuberc Lung Dis 13: 1467–1475 [PubMed] [Google Scholar]
- Zignol M, Sismanidis C, Falzon D, Glaziou P, Dara M, Floyd K 2013. Multidrug-resistant tuberculosis in children: Evidence from global surveillance. Eur Respir J 42: 701–707 [DOI] [PMC free article] [PubMed] [Google Scholar]