Abstract
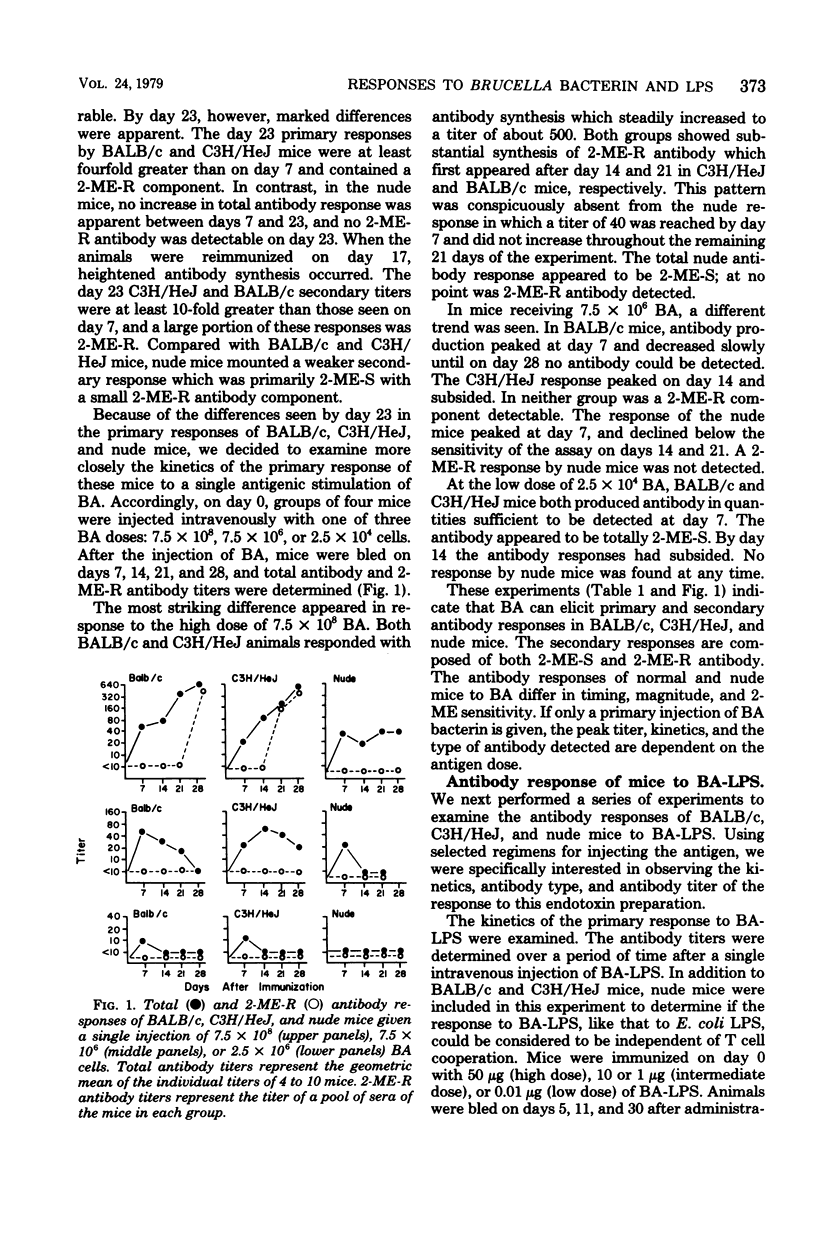
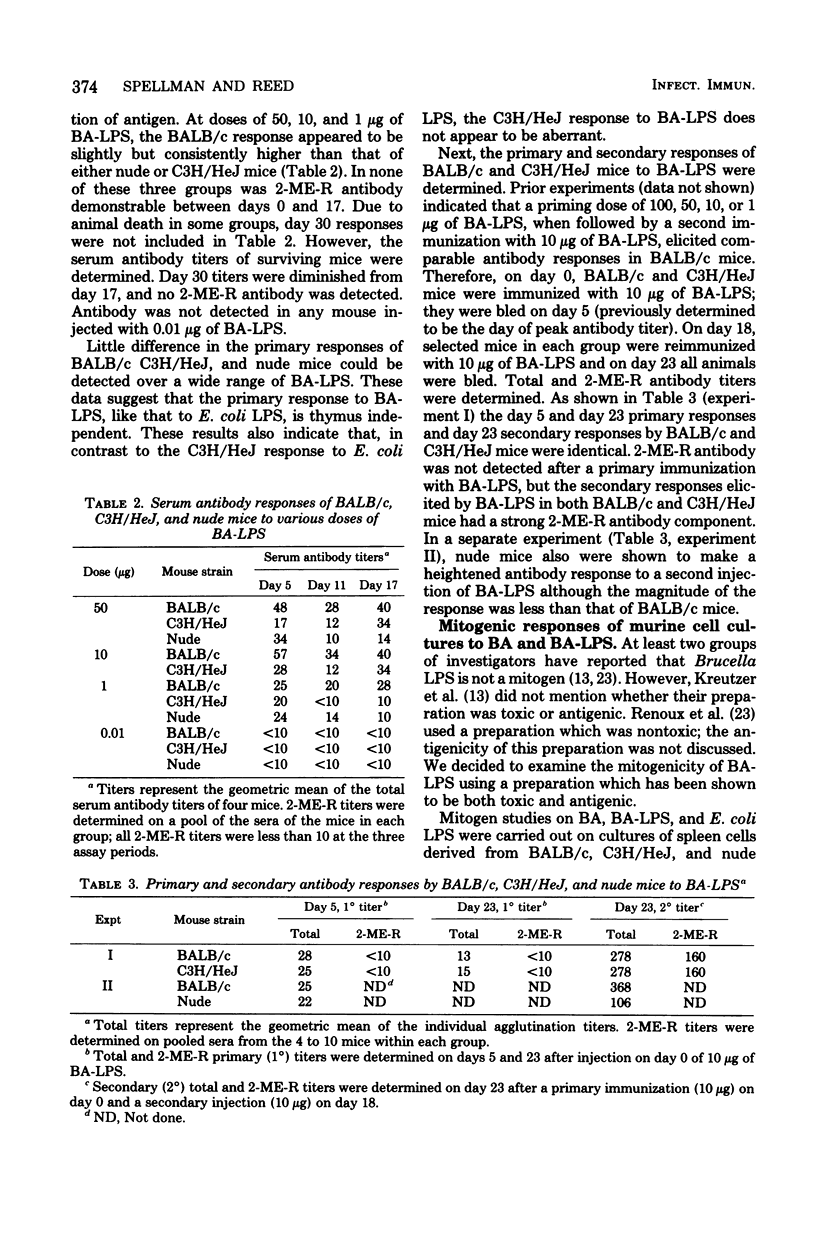
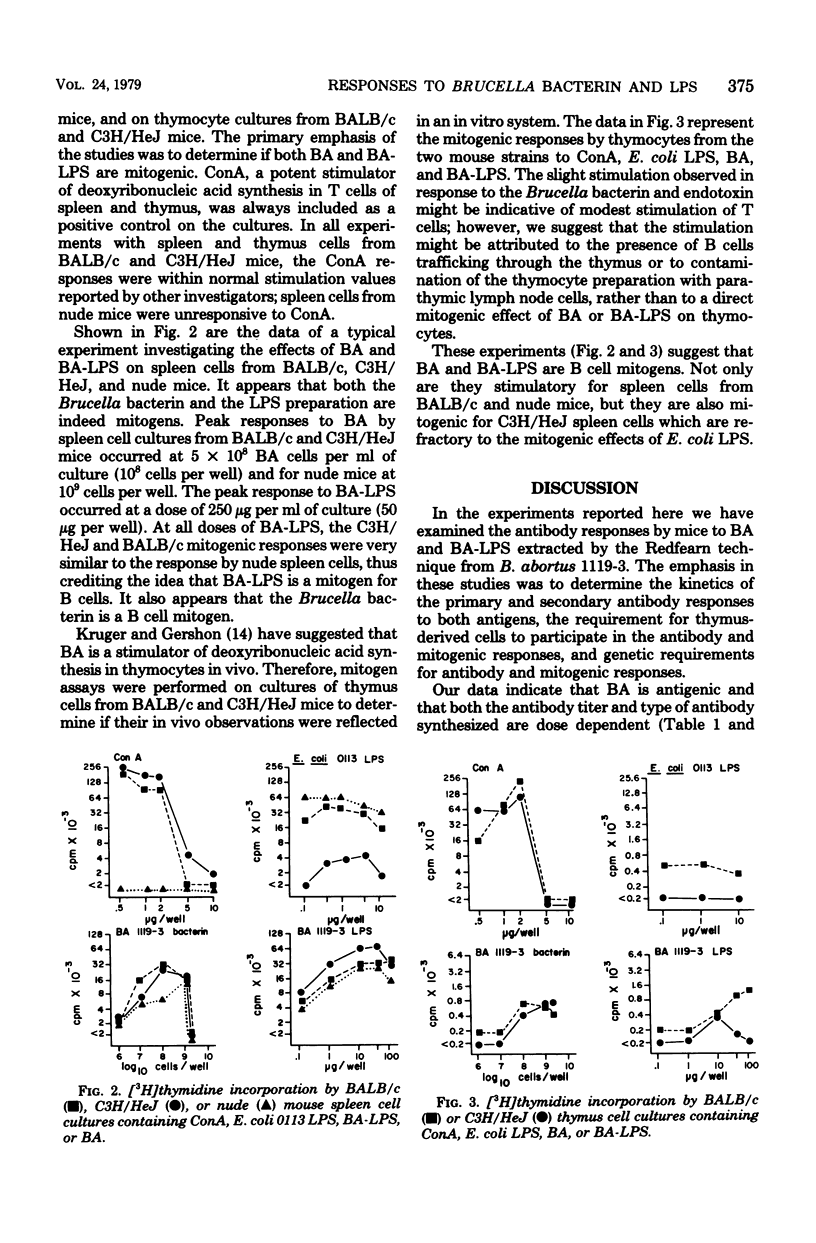
The immunogenic and mitogenic properties of Brucella abortus 1119-3 bacterin (BA) and biologically active B. abortus lipopolysaccharide (BA-LPS) were studied using normal and athymic (nude) BALB/c and C3H/HeJ mice. Although BA stimulated 2-mercaptoethanol-sensitive (2-ME-S) primary and secondary antibody responses in all mice, nude mice, in contrast to normal BALB/c and C3H/HeJ mice, did not make substantial 2-mercaptoethanol-resistant (2-ME-R) antibody responses. Similarly, all mice injected with BA-LPS made 2-ME-S primary responses, and the secondary response of thymus-bearing mice contained a substantial 2-ME-R component. Collectively, these observations suggest that although both BA and BA-LPS can stimulate thymus-independent 2-ME-S antibody synthesis, thymus-derived cells are required for optimal immune responses containing a 2-ME-R component. The antibody responses of normal BALB/c and C3H/HeJ mice to BA and BA-LPS were qualitatively and quantitatively similar. Both BA and BA-LPS were mitogenic for spleen cells from normal and nude BALB/c and C3H/HeJ mice but not for thymus cells from normal BALB/c or C3H/HeJ mice, suggesting that both preparations are B-cell mitogens.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADLER F. L. STUDIES ON MOUSE ANTIBODIES. I. THE RESPONSE TO SHEEP RED CELLS. J Immunol. 1965 Jul;95:26–38. [PubMed] [Google Scholar]
- Andersson B., Blomgren H. Evidence for thymus-independent humoral antibody production in mice against polyvinylpyrrolidone and E. coli lipopolysaccharide. Cell Immunol. 1971 Oct;2(5):411–424. doi: 10.1016/0008-8749(71)90052-9. [DOI] [PubMed] [Google Scholar]
- Andersson J., Melchers F., Galanos C., Lüderitz O. The mitogenic effect of lipopolysaccharide on bone marrow-derived mouse lymphocytes. Lipid A as the mitogenic part of the molecule. J Exp Med. 1973 Apr 1;137(4):943–953. doi: 10.1084/jem.137.4.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAUER D. C., MATHIES M. J., STAVITSKY A. B. Sequences of synthesis of gamma-1 macroglobulin and gamma-2 globulin antibodies during primary and secondary responses to proteins, salmonella antigens, and phage. J Exp Med. 1963 Jun 1;117:889–907. doi: 10.1084/jem.117.6.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger F. M., Fukui G. M., Ludwig B. J., Rosselet J. P. Increased host resistance to infection elicited by lipopolysaccharides from Brucella abortus. Proc Soc Exp Biol Med. 1969 Sep;131(4):1376–1381. doi: 10.3181/00379727-131-34111. [DOI] [PubMed] [Google Scholar]
- Chiller J. M., Skidmore B. J., Morrison D. C., Weigle W. O. Relationship of the structure of bacterial lipopolysaccharides to its function in mitogenesis and adjuvanticity. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2129–2133. doi: 10.1073/pnas.70.7.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho A., Möller G. Editorial: Immune activation of B cells: evidence for 'one nonspecific triggering signal' not delivered by the Ig receptors. Scand J Immunol. 1974;3(2):133–146. [PubMed] [Google Scholar]
- Crewther P., Warner N. L. Serum immunoglobulins and antibodies in congenitally athymic (nude) mice. Aust J Exp Biol Med Sci. 1972 Oct;50(5):625–635. doi: 10.1038/icb.1972.55. [DOI] [PubMed] [Google Scholar]
- Golub E. S., Weigle W. O. Studies on the induction of immunologic unresponsiveness. I. Effects of endotoxin and phytochemagglutinin. J Immunol. 1967 Jun;98(6):1241–1247. [PubMed] [Google Scholar]
- Jacobson E. B., Caporale L. H., Thorbecke G. J. Effect of thymus cell injections on germinal center formation in lymphoid tissues of nude (thymusless) mice. Cell Immunol. 1974 Sep;13(3):416–430. doi: 10.1016/0008-8749(74)90261-5. [DOI] [PubMed] [Google Scholar]
- Janossy G., Greaves M. F. Lymphocyte activation. I. Response of T and B lymphocytes to phytomitogens. Clin Exp Immunol. 1971 Oct;9(4):483–498. [PMC free article] [PubMed] [Google Scholar]
- Kessel R. W., Braun W., Plescia O. J. Endotoxin cytotoxicity: role of cell-associated antibody. Proc Soc Exp Biol Med. 1966 Feb;121(2):449–452. doi: 10.3181/00379727-121-30801. [DOI] [PubMed] [Google Scholar]
- Kreutzer D. L., Scheffel J. W., Draper L. R., Robertson D. C. Mitogenic activity of cell wall components from smooth and rough strains of Brucella abortus. Infect Immun. 1977 Mar;15(3):842–845. doi: 10.1128/iai.15.3.842-845.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüger J., Gershon R. K. DNA synthetic response of thymocytes to a variety of antigens. J Immunol. 1972 Mar;108(3):581–585. [PubMed] [Google Scholar]
- Landy M., Baker P. J. Cytodynamics of the distinctive immune response produced in regional lymph nodes by Salmonella somatic polysaccharide. J Immunol. 1966 Nov;97(5):670–679. [PubMed] [Google Scholar]
- Leong D., Diaz R., Milner K., Rudbach J., Wilson J. B. Some structural and biological properties of Brucella endotoxin. Infect Immun. 1970 Feb;1(2):174–182. doi: 10.1128/iai.1.2.174-182.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mond J. J., Caporale L. H., Thorbecke G. J. Kinetics of B cell memory development during a thymus "independent" immune response. Cell Immunol. 1974 Jan;10(1):105–116. doi: 10.1016/0008-8749(74)90155-5. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Betz S. J., Jacobs D. M. Isolation of a lipid A bound polypeptide responsible for "LPS-initiated" mitogenesis of C3H/HeJ spleen cells. J Exp Med. 1976 Sep 1;144(3):840–846. doi: 10.1084/jem.144.3.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller G. 19S antibody production against soluble lipopolysaccharide antigens by individual lymphoid cells in vitro. Nature. 1965 Sep 11;207(5002):1166–1168. doi: 10.1038/2071166a0. [DOI] [PubMed] [Google Scholar]
- Peavy D. L., Shands J. W., Jr, Adler W. H., Smith R. T. Mitogenicity of bacterial endotoxins: characterization of the mitogenic principle. J Immunol. 1973 Aug;111(2):352–357. [PubMed] [Google Scholar]
- Rank W. R., DiPauli R., Flügge-Rank U. The lipid A immunity system. I. Induction of heterophile antibodies by enterobacterial lipopolysaccharides and their lipid A component. Eur J Immunol. 1972 Dec;2(6):517–522. doi: 10.1002/eji.1830020610. [DOI] [PubMed] [Google Scholar]
- Renoux G., Renoux M., Tinelli R. Phenol-water fractions from smooth Brucella abortus and Brucella melitensis: immunochemical analysis and biologic behavior. J Infect Dis. 1973 Feb;127(2):139–148. doi: 10.1093/infdis/127.2.139. [DOI] [PubMed] [Google Scholar]
- Renoux M., Renoux G., Palat A., Néhémie C. Activité mitogénique des fractions de Brucella sur les lymphocytes en culture. Dev Biol Stand. 1976;31:230–234. [PubMed] [Google Scholar]
- Rudbach J. A. Molecular immunogenicity of bacterial lipopolysaccharide antigens: establishing a quantitative system. J Immunol. 1971 Apr;106(4):993–1001. [PubMed] [Google Scholar]
- Rudbach J. A., Reed N. D. Immunological responses of mice to lipopolysaccharide: lack of secondary responsiveness by C3H/HeJ mice. Infect Immun. 1977 May;16(2):513–517. doi: 10.1128/iai.16.2.513-517.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPINK W. W., McCULLOUGH N. B., HUTCHINGS L. M., MINGLE C. K. A standardized antigen and agglutination technic for human brucellosis. Am J Clin Pathol. 1954 Apr;24(4):496–498. doi: 10.1093/ajcp/24.4_ts.496. [DOI] [PubMed] [Google Scholar]
- Scheffel J. W., Kreutzer D. L., Robertson D. C., Draper L. R. Responsiveness of rabbit spleen and appendix cells to bacterial mitogens. Infect Immun. 1977 May;16(2):493–499. doi: 10.1128/iai.16.2.493-499.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skidmore B. J., Chiller J. M., Morrison D. C., Weigle W. O. Immunologic properties of bacterial lipopolysaccharide (LPS): correlation between the mitogenic, adjuvant, and immunogenic activities. J Immunol. 1975 Feb;114(2 Pt 2):770–775. [PubMed] [Google Scholar]
- Skidmore B. J., Morrison D. C., Chiller J. M., Weigle W. O. Immunologic properties of bacterial lipopolysaccharide (LPS). II. The unresponsiveness of C3H/HeJ Mouse spleen cells to LPS-induced mitogenesis is dependent on the method used to extract LPS. J Exp Med. 1975 Dec 1;142(6):1488–1508. doi: 10.1084/jem.142.6.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultzer B. M. Genetic control of leucocyte responses to endotoxin. Nature. 1968 Sep 21;219(5160):1253–1254. doi: 10.1038/2191253a0. [DOI] [PubMed] [Google Scholar]
- Sultzer B. M., Goodman G. W. Endotoxin protein: a B-cell mitogen and polyclonal activator of C3H/HeJ lymphocytes. J Exp Med. 1976 Sep 1;144(3):821–827. doi: 10.1084/jem.144.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultzer B. M., Nilsson B. S. PPD tuberculin--a B-cell mitogen. Nat New Biol. 1972 Dec 13;240(102):198–200. doi: 10.1038/newbio240198a0. [DOI] [PubMed] [Google Scholar]
- Tingle A. J., Shuster J. The role of T lymphocytes in the primary humoral antibody response to brucellin. J Immunol. 1974 Feb;112(2):716–721. [PubMed] [Google Scholar]
- Watson J., Epstein R., Nakoinz I., Ralph P. The role of humoral factors in the initiation of in vitro primary immune responses. II. Effects of lymphocyte mitogens. J Immunol. 1973 Jan;110(1):43–52. [PubMed] [Google Scholar]
- Watson J., Riblet R. Genetic control of responses to bacterial lipopolysaccharides in mice. I. Evidence for a single gene that influences mitogenic and immunogenic respones to lipopolysaccharides. J Exp Med. 1974 Nov 1;140(5):1147–1161. doi: 10.1084/jem.140.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]


