1. Introduction
The electroretinogram (ERG) is an electrical signal produced by the retina induced on a corneal electrode. Under photopic conditions, ERG components elicited by light increments and decrements can be separated using a long-flash paradigm in which relatively long periods of light are alternated. The retinal responses to increments and decrements can be examined separately with this technique. The ON–OFF ERG waveform contains components that reflect the function of photoreceptors and postreceptoral neurons in the retina; the different neural types that contribute vary in response latency and kinetics and some are sign conserving while others are sign preserving. These features make the ON–OFF ERG a noninvasive method that can be useful for drawing inferences about the function of retinal circuitry in humans and animal models and in healthy and diseased eyes.
In species that have multiple cone types and particularly in primates that normally have three cone types, cone isolated ERGs have proven valuable both in basic science and clinical applications [1–3]. ERGs that are generated by modulation of a single class of cone and provide information about the temporal characteristics of the underlying cellular components and the function of retinal circuitry are of particular interest with regard to the S cones since they are affected differentially from the long (L) and middle (M) wavelength sensitive cones in many vision disorders. Also, particularly in primates, the response properties of the photoreceptors and the circuitry in the outer retina (where most of the ERG is generated) differs between S and L/M cones. However, several differences and similarities remain controversial [4,5], and results from the ERG may have important implications for understanding the properties of S-cones and their circuitry.
In the past, the approach for isolating responses to S cones has been to use intense adapting lights to suppress L- and M-cone ERG responses; either alone [6–8], or in combination with a two color silent-substitution alternating short and middle wavelength lights adjusted in intensity to silence M-cone contributions [9,10]. S-cones only make up ∼5% of cone photoreceptors in the retina [11] and they are outnumbered by rods by more than 300 to 1. Short wavelength lights (e.g., ca. 440 nm) usually chosen to elicit responses from S-cones produce responses in the L and M cones that are about 20% of those produced by wavelengths presented near the L/M peak absorptions [12]. Thus, the challenge in recording S-cone ERGs is that methods that reduce contributions of rods and L- and M-cones to just a few percent can still produce ERGs designed to be S-cone isolating that have larger contributions from other photoreceptor types than S-cones. Moreover, intense long wavelength adaptation may affect responses of circuitry under study in analyzing S-cone ERGs. Finally, with previous techniques it has been difficult to assess the degree to which the responses reflect S-cones relative to contamination by other photoreceptor types.
Presented here is a chromatic long-flash ON–OFF protocol that allows ON and OFF responses to be studied independently allowing further separation and analysis of contributions from different neural subtypes. The silent-substitution technique [13] has been a powerful technique in vision research. Here, the ON–OFF ERG is combined with a silent substitution to elicit responses from individual cone photoreceptor classes by modulating the intensities of three color lights between the two periods. For the first time in an ERG protocol, an empirical technique was used to refine and confirm S-cone isolation. Scone ON–OFF ERGs can be verified to be free of contamination from rods and M- and L-cones and silent substitution has the benefit of leaving the retina in a more neutrally adapted state.
2. Methods
A. Human Preparation
The pupil of the right eye of each subject was dilated by administration of Tropicamide (0.5%), and a Dawson, Trick, and Litzkow (DTL) electrode was placed on the sclera just inferior to the cornea resting on the bottom eyelid. For reference and earth ground, the skin was first cleaned with alcohol pads, and gold disc electrodes (F-E5GH, Grass Technologies) were adhered to the upper cheek and temple. A small amount of conductive paste was placed on the gold discs to increase conductivity to the skin, and then the electrodes were held in place with Tegaderm-Film. Subjects were positioned inside the Ganzfeld using a chin cup and forehead rest so that the entire visual field of the test eye received the light stimulus.
B. Baboon Preparation
ERGs were performed while the animal was under sevofluorane anesthesia (1.25%–2.5%). Vital signs including blood pressure, heart rate, blood oxygenation, body temperature, and CO2 in expired air were monitored and maintained in normal physiological limits. The pupil of the right eye of the baboon was dilated by administration of Tropicamide (1%) and a DTL fiber electrode was placed on the sclera inferior to the cornea resting on the bottom eyelid. For reference, a gold disc electrode was placed underneath the top eyelid, held in place by a combination of electrically conductive paste, and the pressure exerted by a speculum used to hold the lid open during the procedure. The cornea was kept moist by administration of artificial tears. A gold-plated earth ground was placed inside the cheek.
C. Apparatus
A color Ganzfeld (model Q450, Roland Consult, Friedrich-Franz-Str. 19, 14770 Brandenburg, Germany) was used along with accompanying Retiport software. The Ganzfeld has five LED primaries with peaks centered at 450, 470, 525, 594, and 641 nm. To better facilitate ERGs on multiple species, the 470 nm was replaced with a 375 nm for work with rodents (the Q450 Ganzfeld was painted by the manufacturer with UV reflective paint), and the 450 nm LED was replaced with a 420 nm for work with primates. The wavelengths of the two replacement LEDs were chosen to yield maximal S-cone quantal contrast in humans and nonhuman primates.
D. Stimulus
An ON–OFF ERG paradigm was constructed by alternating two different spectral stimuli. In this way, both ON and OFF retinal components can be examined separately. By allowing values of greater than 200 ms for both ON and OFF phases, the retina can respond and return to baseline for both light onset and light offset. However, extra time was allowed to ensure the retina could come to equilibrium after the onset of each phase. A value of 1 Hz was chosen somewhat arbitrarily with a 50% duty cycle. The ON phase was 500 ms and the OFF phase is displayed for 450 ms. All ERGs were recorded using a band-pass filter (20–100 Hz, −3 dB cutoff) that removed a significant portion of low frequency noise while retaining biological signals of interest.
Although differences in ERG preparation were apparent between subjects (such as raw amplitude, 60 Hz noise, etc.), final L/M- and S-ERG traces are the result of averaging the ERG examinations for each subject. Here, an “ERG examination” is defined as the trace resulting from final averaged presentation of a particular stimulus paradigm (L/M- or S-isolating). ERGs are noisy measurements prone to contamination from AC, muscle movements, and other random signals; therefore some examinations were excluded on a trial-by-trial basis (exclusion criteria included blink artifacts, issues related to electrode placement, low frequency artifacts, and 60 Hz noise). The process described here for analyzing the data places emphasis on the population L/M- and S-cone responses while de-emphasizing contributions from the individuals who had higher signal and/or more ERG examinations.
E. Silent Substitution Calculation
LED intensities were measured using a SpectroCAL spectroradiometer (Cambridge Research Systems Ltd., 80 Riverside Estate, Sir Thomas Longley Road, Rochester, Kent ME2 4BH England). The spectroradiometer was positioned where the eye of the subject is normally, just inside the outer plane of the integrating sphere. Measured intensity values, ILED(λ), were then converted to quanta, QLED(λ), by the equation
| (1) |
where λ is the wavelength, h is Planck's constant, and c is the speed of light. Estimates of the lens yellowing with age are available for human [14], but an empirical refinement technique developed for Scone isolation and used for every subject individually replaced the need to used and age adjusted lens correction (see next paragraph). A macular pigment correction factor was not included in the calculation as the majority of the signal comes from cone photoreceptors outside of the macular region. For color normal humans, peak sensitivities for S-, M-, and L-cones were chosen at 418, 530, and 559 nm, respectively [15–21], and those peaks were fit with the Neitz photopigment template curve [12]. Estimates of S, M, and L activity, Sact, Mact, and Lact, is given by
| (2) |
| (3) |
| (4) |
where gxxx variables are linear gains between 0 and 1 for each LED, Qxxx(λ) are the spectrum of each LED in quanta, and S(λ), M(λ), and L(λ) are the photopigment sensitivities with an optical density of 0.25. For each cone-isolating condition, quantal contrast was chosen to be equal between S- and L/M-isolating conditions. The final step was to minimize a sum of squared differences for the other two cone activities, using the LED gains as process variables.
All chromatic test lights were presented above the ambient photopic fluorescent lighting conditions (∼300 lux), and therefore rods were not included in the silent substitution calculation. In the ERG traces, no rod contamination can be seen in the signal.
F. Comparison of S-Cone ERGs using Adaptation Methods
The first experiment was set up to compare the findings to the Gouras technique. Five increasing intensities of an adapting white light was set to 0, 950, 4000, and 16,000 td. A bright flash of 420 nm and then 641 nm light was presented for 1 ms at 7 Hz concurrently with the steady white light at each adaptation level. Each trace is the average of 1000 individual presentations, and filter settings were set to 5–10,000 Hz bandpass. The darkest stimulus was presented first, and then the adaptation light was increased. In a second S-cone ERG comparison, an amber light (594 nm) was turned on at 30,000 td and bright 420 nm wavelength light was turned on in the above described ON–OFF ERG paradigm and presented simultaneously. Both of these comparisons were performed on anesthetized old world nonhuman primate in an effort to minimize artifacts.
G. Empirical Refinement Technique
S-cones make up only 5% of the total cone-photoreceptors, and therefore S-cone mediated signal is expected to be small compared to signals from L- and M-cones. Because of this, any error in the S-cone isolating calculation has potential to produce large contamination by the L- and M-cones. To arrive at the true S-cone isolating light conditions, an empirical refinement technique was developed using the L- and M-cone contamination as the measure. The first step in the procedure was to obtain an L/M isolated ERG as shown in Fig. 2(a). Long wavelength lights produce ERGs with no S-cone contamination. The distinctive temporal characteristics of the L/M ERG and the relative amplitudes of different components provide a distinctive “signature.”
Fig. 2.
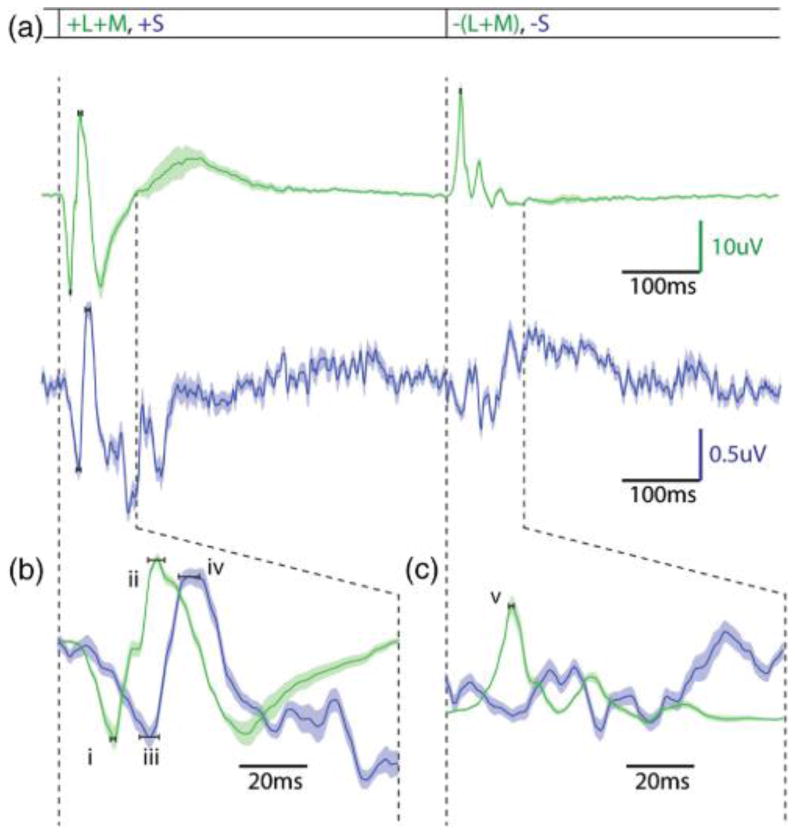
This graph contains the main L/M- and S-ERGs generated by the ON–OFF ERG paradigm. (a) Contains both the L/M-cone mediated trace (green) and the S-cone mediated trace (violet). The L/M-cone trace has got many of the features indicative of ON–OFF ERGs in response to white-light stimuli, with the exception that the tight filtering characteristics has removed much of the lower frequency noise not representative of photoreceptor or bipolar mediated responses. (b) Expands the first 100 ms after light onset. +(L+M) a and b waves occur at 17.3 and 30.8 ms, respectively (i, ii), and S-cone a and b waves occur at 26.2 and 40.3 ms, respectively (iii, iv). (c) Expands the first 100 ms after light offset. A −(L+M) d-wave happens at 20.1 ms after light offset (v), and S-cones have no characteristic depolarization indicative of a d wave. In fact, the waveform seen at the light offset for S-cone responses is opposite in polarity to the d wave.
Figure 1 shows the technique: traces in Fig. 1(a) show ERG responses to different fractions of the theoretically calculated silencing light intensities for S-cone isolation. If the quantal catch for the L- and M-cones is greater in the “OFF” phase than the “ON” phase, then L/M-cone driven a and b waves with temporal properties characteristic of those cones are recorded at light offset. The 0.5 and 0.4 fractions [Fig. 1(a)] show such characteristic L/M-cone driven ERGs at light offset for this reason. The L/M b-wave amplitudes are plotted as negative on the graph in Fig. 1(b) when they occur in the OFF phase. The 0.3 and 0.2 values produce very small L/M ERGs since those values are very close to nulling the L/M-cone response. Since it is not possible to accurately determine the L/M b-wave amplitude when it is close to the S-cone isolation value, those values were excluded in Fig. 1(b). The 0.1 value has an L/M-cone driven response at light onset, indicating that the quantal catch was greater for the L/M-cones in the ON phase. That b-wave amplitude is, thus, plotted as a positive in Fig. 1(b). The data points are fit, and the x intercept is taken as the value that perfectly silences both L- and M-cone responses and isolates the Scone ERG responses.
Fig. 1.
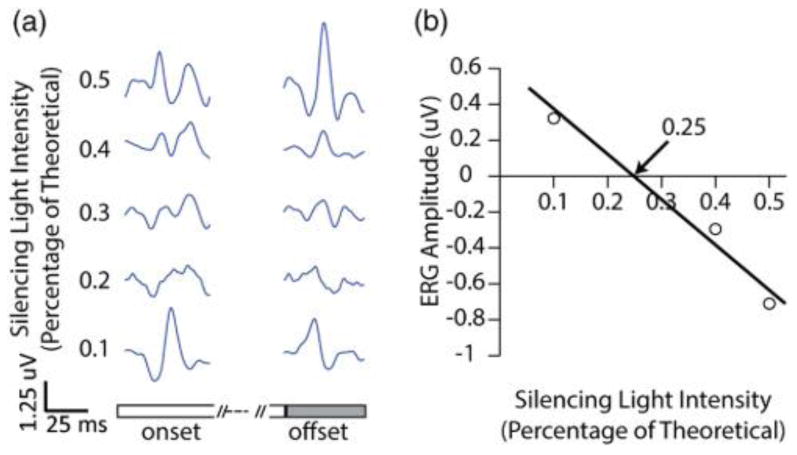
This figure shows how the S-cone ERG settings were systematically adjusted from the theoretically calculated S-isolation point by using the +(L+M) signal contamination. (a) Shows ERG traces with different percentages of long wavelength silencing lights. A value of 1 corresponds to perfect isolation with no estimate for the yellowing of the lens. The top two traces (0.5 and 0.4) contain L/M-contamination at light offset, which indicates the silencing lights are still too bright. The next two traces (0.3 and 0.2) are noisy for the relatively small amount of averages, and the true S-cone ERG is in one or both of those traces; therefore they are not used in the fit. The bottom trace (0.1) contains obvious +(L+M)-contamination at light onset, which indicates the long wavelength silencing lights are too dim. (b) Shows the plots from the +(L+M) b-wave amplitudes. When obvious L/M-contamination showed up at light offset (0.5 and 0.4), b-wave amplitudes were negated and plotted. When obvious L/M contamination showed up at light onset (0.1), b-wave amplitudes were plotted. The data points were fit, and the x intercept was taken as the fine-tuned S-isolated condition. This example would predict turning the long wavelength silencing lights down to 0.25% of their calculated isolation without a lens estimate.
3. Results
A. L/M- and S-Cone ERGs
Figure 2 shows the ERG traces obtained under conditions that isolate S-cones compared ones that isolate the summed response of L/M-cones. In Fig. 2(a), the L/M- and S-cone conditions are plotted above and below each other, and each trace represents average results for five normal subjects. Based on raw amplitude of b waves, the S-cone ERGs are approximately 4% of the L/M-ERG. This is in good agreement with the published values for the percentage of S-cones in the human retina [11,22]; however, it differs from what has been observed for L:M ERGs in which cone responses at low flicker rates are near 1:1 regardless of cone ratio [1].
Figures 2(b) and 2(c) are the same data of Fig. 2(a) but plotted on an expanded time scale. This makes it easier to compare the temporal characteristics of the ON and OFF components of the ERG. The a and b waves of the +(L+M) ERG [Fig. 2(b), i andii] occur at 17.3 and 30.8 ms, respectively. The a and b waves of the +S ERG [Fig. 2(b), iii and iv] occur at 26.2 and 40.2 ms, respectively. These findings are consistent with some of the previously published latencies for S-cone mediated a and b waves [6,8] and inconsistent with others [9].
At light offset [Fig. 2(c)] the S-cone ERG shows no d-wave depolarization. This indicates that compared to L/M-cones that produce a robust d wave, there is no evidence of S-cone specific OFF bipolar cells in humans. This is consistent with both anatomical and electrophysiological results from other primates [23– 25], which have not found evidence for S-cone specific OFF bipolar cells. The only exception is an anatomical study in which OFF midget bipolar cells are reported to contact S-cones in macaque retina [4]. The −(L+M) d wave [Fig. 2(c), v] occurs at 20.1 ms after light offset.
Figure 3(a) shows a table of results from 5 volunteers for which S-cone isolation was determined using the empirical refinement technique. Initially, S-cone silent substitution calculations were performed using the lens estimate of a 22-year-old and then adjustments were made empirically to determine S-cone isolation. The first four subjects had a range of ages between 22 and 46. For the 22-year-old the “22-year-old” theoretical calculation was correct. However, as subjects got older, as expected, relatively increasing amounts of short wavelength light were required to produce S-cone isolation. Additionally, the fifth subject reported in Fig. 3(a) (denoted with a red circle), although the oldest, had undergone cataract surgery in which he received an intraocular lens (IOL) implant. Hence, the “22-year-old” S-isolation values were close to correct. In fact, in Fig. 3(b), using the projected fit from the other four subjects, the lens replacement subject was projected to have the lens of approximately a 19-year-old. This finding is consistent with the use of an age factor for lens yellowing [14], and helps serve to validate the empirical technique. It also suggests that the technique could be used to measure variability of lens yellowing among normal subjects.
Fig. 3.
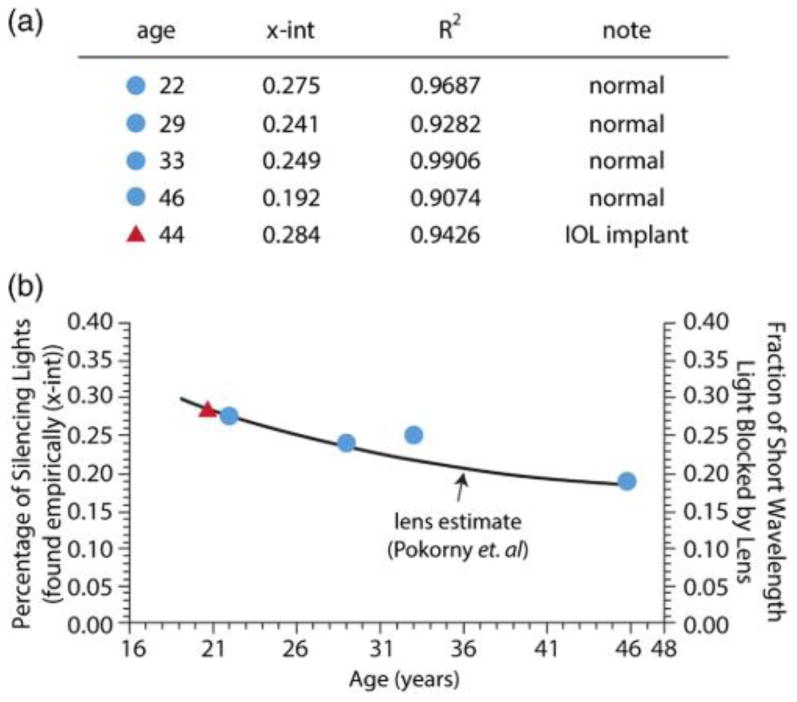
This figure shows the lens is the major factor in S-isolation calculations. (a) Is a table that contains four normal subjects (blue circles) and one subject with an IOL implant (red triangle). The empirical technique described in this paper was used on all of the subjects above, and the amount the silencing lights had to be decreased from a theoretical calculation that did not include a lens estimate is shown as the x intercept. The black line in (b) shows the Pokorny et al. age dependent lens transmission estimate multiplied by the 420 nm LED used in this experiment. The blue dots are the x intercept values found in the empirical technique. There is a close match between the intensity required of the longer wavelength silencing lights and the amount that the lens would decrease the 420 nm light. Although it is important to note that the 33-year-old would have had +(L+M) contamination in the +S phase if the pure theoretical calculation had been used. Additionally, the subject with the IOL implant required brighter long wavelength silencing lights then the theoretical calculation predicts.
B. S-Cone ERG Comparisons
Figure 4 shows the S-cone ERG responses in a baboon using white light to preferentially prime the retina to elicit S-cone responses. The recreation of the original experiment did not produce the characteristic first and second peaks shown by Gouras and MacKay [6]. The timing of the peak that Gouras and MacKay assign to be the S-cone b wave (∼31.5 ms) is early compared to the responses seen in the technique presented in this paper. In fact, the timing of the b wave of the L- and M-cones is 30.8 ms, and most likely what has occurred is, by suppressing the L-, M-, and S-cones, Gouras and MacKay have emphasized normally smaller inner retinal responses and the “second peak” of the putative S-response is simply the L/M-cone response. It is still unexplained how Blue Cone Monochromats tested with the technique by Gouras and MacKay retain the second peak, while tritanopes only have the first. However, it appears that white-light adaptation used in this technique serves to further reduce the amplitude of the Scone mediated responses, not isolate them. Figure 5 shows the response of the baboon in the presence of a bright yellow adapting light. The characteristic two-peaks indicative of this technique are seen [Fig. 5(a)], overlaid is a scaled L/M-cone ERG [Fig. 5(b)], and finally the pure S-cone ERG in the same animal [Fig. 5(c)]. It is possible with the use of brighter adapting lights to further suppress the +(L+M) response; yet it is impossible to fully remove it using chromatic adaptation alone.
Fig. 4.
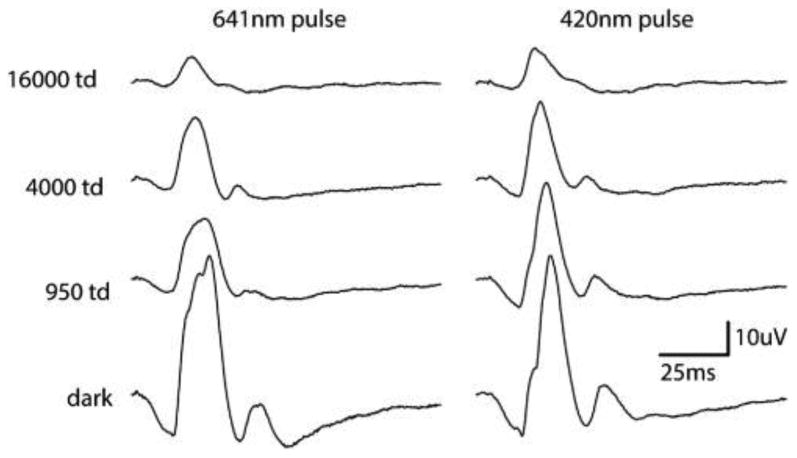
This figure shows the results from both the white-light adaptation studies. The four traces, bottom to top, increase the amount of white light adaptation that is present in the ERGs. The column on the left shows baboon ERG responses to 641 nm LED pulses, and the column on the right shows responses to 420 nm LED pulses. These wavelengths were chosen to best approximate white light placed through Wratten filter 29 and Wratten filter 98 in Fig. 4 of the Gouras and MacKay paper describing the technique. This study was not able to reproduce the results that used white light to preferentially elicit ERG responses from S-cones.
Fig. 5.
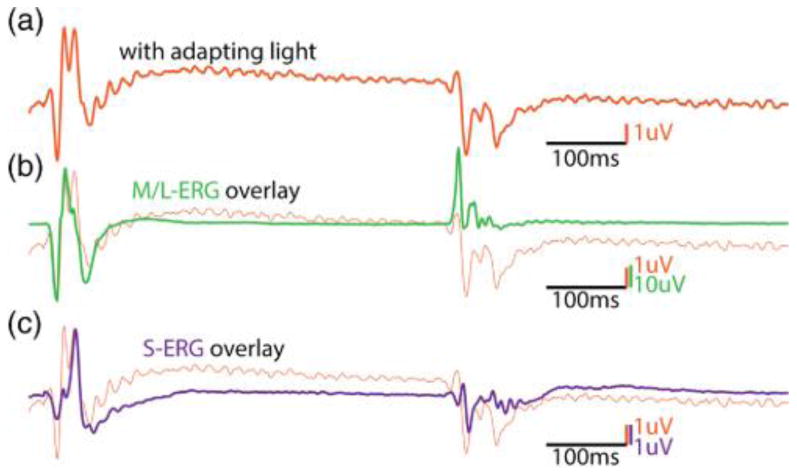
(a) Contains an ERG that was elicited in response to a 420 nm wavelength LED in the presence of a ∼30,000 td594 nm amber suppressing light. At light onset, two distinct peaks can be observed. (b) Overlays the L/M ERG arbitrarily scaled from the same animal. The timing of the peak lines up with the first peak, indicating this is +(L+M) contamination. (c) Overlays the pure S-cone ERG elicited from the same animal using the technique described in this paper. The S-cone peak is aligned with the second peak, indicating this is a pure S-cone mediated signal.
4. Discussion
The ERG waveform is the mass retinal response to a light stimulus; nonetheless, there are approaches that can be used to gain information about the function of individual cell types and their circuits. Since the ERG measures potentials at the cornea in reference to the head surrounding the eye, potential changes in cells with processes aligned in a radial direction such as photoreceptors and bipolar cells produce the greatest contributions to the response.
The primate/human photopic ON–OFF ERGs reported here show tri-phasic “ON-response.” There is an initial hyperpolarizing a wave. Since there is no rod-specific OFF-bipolar cell, scotopic a waves are dominated by contributions from rod photoreceptors. In contrast, L and M cones contact both ON- and OFF-bipolar cells. The photopic ERG a wave reflects a combination of activity of cone photoreceptors and OFF-bipolar cells [26,27]. The hyperpolarization of cones is the first response to light by the retina. The rapid closing of ionotropic glutamate-receptor-gated channels in OFF-bipolar cells cause them to hyperpolarize very quickly in response to the shutoff of glutamate at the cone terminal in response to light onset [26,28]. Thus, in the photopic ERG, the OFF-bipolar response overlaps in time with the cone response, and their combination makes up the a wave [26,27]. The initiation of the ON- and OFF-bipolar cell responses are separated in time because the metabotropic glutamate receptors (mGluR6) of ON bipolar cells use a second-messenger system to invert voltage polarity, which has slower kinetics than the ionotropic glutamate receptors used by OFF bipolar cells [28,29]. This timing difference leads to a depolarization that, in the ERG waveform, is called the b wave. This reflects activity resulting from feedforward connectivity from cones to ON-bipolar cells.
In addition to their intrinsic light response, cones receive feedback from their neighbors via horizontal cells. OFF bipolar cells and horizontal cells each produce hyperpolarizing responses that oppose those produced by ON-bipolar cells [26]. Responses from horizontal cell feedback to the cone pedicle and, in turn, to the ON-bipolar cells is expected to be time delayed both by virtue of traversing extra synapses and because of the slower mGluR receptor and, thus, could be partially responsible for the hyperpolarizing response that comes after the b wave representing the final part of the tri-phasic ON response.
Recently, the possibility has been raised that horizontal cells may employ a signaling pathway using GABA to send feed-forward signals directly to bipolar cells [30–32]. In macaque retina, the molecular components of this putative feed-forward pathway were highly enriched beneath S-cones as compared to M- and L-cones and colocalized with a marker for HII horizontal cells. The enrichment at S-cones was not observed in either mouse or ground squirrel in which labeling was uniform for S and L/M cone-types, so this is something that may have evolved in the primate lineage. The proposed feed-forward signals from horizontal cells are sign inverted and time delayed compared to signals directly from cones having traversed two additional synapses. If a feed-forward pathway exists, it would serve to depolarize the OFF-bipolar cells with a similar time course as the depolarization of the ON-bipolar cells that results from feed forward directly from cones and possibly contribute to the b wave under some conditions such as the presentation of S-cone stimuli in primates (including humans).
In primates, the OFF component of the ON–OFF ERG is primarily monophasic characterized by a massive depolarization often of greater amplitude than the ERG b wave. For test lights of modest intensity, light offset produces a sudden release of large amounts of glutamate from cone pedicle that depolarizes the OFF-bipolar cells to produce the ERG d wave.
Here, methods are described that allow contributions of postreceptoral circuitry, described above, associated with different cone classes to light increments and decrements to be studied both in normal eyes and those affected by clinical disorders. The cone isolating ON–OFF ERG may be a particularly powerful tool for studying chromatic circuits in the retina when used in animal models in conjunction with pharmacological agents administered intraocularly to silence neurotransmitter mechanisms or in humans with mutations that silence specific neural components. With the methods described here, temporal properties of the different cone types can be characterized and compared.
Acknowledgments
This study was supported by NEI grants R01EY09303, P30EY01730, and T32EY007030, and by unrestricted funds from the Research to Prevent Blindness, the Bishop Foundation, and the Ray H. Hill foundation.
References
- 1.Kremers J, Usui T, Scholl HPN, Sharpe LT. Cone signal contributions to electrograms in dichromats and trichromats. Investig Ophthalmol Vis Sci. 1999;40:920–930. [PubMed] [Google Scholar]
- 2.Brainard DH, Calderone JB, Nugent AK, Jacobs GH. Flicker ERG responses to stimuli parametrically modulated in color space. Investig Ophthalmol Vis Sci. 1999;40:2840–2847. [PubMed] [Google Scholar]
- 3.Kremers J. The assessment of L- and M-cone specific electroretinographical signals in the normal and abnormal human retina. Prog Retinal Eye Res. 2003;22:579–605. doi: 10.1016/s1350-9462(03)00049-1. [DOI] [PubMed] [Google Scholar]
- 4.Klug K, Herr S, Ngo I, Sterling P, Schein SJ. Macaque retina contains an S-cone OFF midget pathway. J Neurosci. 2003;39:9881–9887. doi: 10.1523/JNEUROSCI.23-30-09881.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee SCS, Telkes I, Grunert U. S-cones do not contribute to the OFF-midget pathway in the retina of the marmoset, Callithrix jacchus. Eur J Neurosci. 2005;22:437–447. doi: 10.1111/j.1460-9568.2005.04231.x. [DOI] [PubMed] [Google Scholar]
- 6.Gouras P, Mackay CJ. Electroretinographic responses of the short-wavelength-sensitive cones. Investig Ophthalmol Vis Sci. 1990;31:1203–1209. [PubMed] [Google Scholar]
- 7.Crognale M, Jacobs GH, Neitz J. Flicker photometric measurements of short wavelength sensitive cones. In: Drum B, Moreland JD, Serra A, editors. Colour Vision Deficiencies X. Kluwer Academic; 1991. pp. 341–346. [Google Scholar]
- 8.Arden G, Wolf J, Berninger T, Hogg CR, Tzekov R, Holder GE. S-cone ERGs elicited by a simple technique in normals and in tritanopes. Vis Res. 1999;39:641–650. doi: 10.1016/s0042-6989(98)00182-5. [DOI] [PubMed] [Google Scholar]
- 9.Sawusch M, Pokorny J, Smith VC. Clinical electroretinography for short wavelength sensitive cones. Investig Ophthalmol Vis Sci. 1987;28:966–974. [PubMed] [Google Scholar]
- 10.Drasdo N, Aldebasi YH, Chiti Z, Mortlock KE, Morgan JE, North RV. The S-cone PhNR and pattern ERG in primary open angle glaucoma. Investig Ophthalmol Vis Sci. 2001;42:1266–1272. [PubMed] [Google Scholar]
- 11.Hofer H, Carroll J, Neitz J, Neitz M, Williams DR. Organization of the human trichromatic cone mosaic. J Neurosci. 2005;25:9669–9679. doi: 10.1523/JNEUROSCI.2414-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carroll J, McMahon C, Neitz M, Neitz J. Flicker-photometric electroretinogram estimates of L: M cone photoreceptor ratio in men with photopigment spectra derived from genetics. J Opt Soc Am A. 2000;17:499–509. doi: 10.1364/josaa.17.000499. [DOI] [PubMed] [Google Scholar]
- 13.Estévez O, Spekreijse H. The ‘silent substitution’ method in visual research. Vis Res. 1982;22:681–691. doi: 10.1016/0042-6989(82)90104-3. [DOI] [PubMed] [Google Scholar]
- 14.Pokorny J, Smith VC, Lutze M. Aging of the human lens. Appl Opt. 1987;26:1437–1440. doi: 10.1364/AO.26.001437. [DOI] [PubMed] [Google Scholar]
- 15.Stockman A, MacLeod DIA, Johnson NE. Spectral sensitivities of the human cones. J Opt Soc Am A. 1993;10:2491–2520. doi: 10.1364/josaa.10.002491. [DOI] [PubMed] [Google Scholar]
- 16.Kraft TW, Neitz J, Neitz M. Spectra of human L cones. Vis Res. 1998;38:3663–3670. doi: 10.1016/s0042-6989(97)00371-4. [DOI] [PubMed] [Google Scholar]
- 17.Stockman A, Sharpe L, Fach C. The spectral sensitivity of the human short-wavelength cones. Vis Res. 1999;39:2901–2927. doi: 10.1016/s0042-6989(98)00225-9. [DOI] [PubMed] [Google Scholar]
- 18.Stockman A, Sharpe L. Spectral sensitivities of the middle- and long-wavelength sensitive cones derived from measurements in observers of known genotype. Vis Res. 2000;40:1711–1737. doi: 10.1016/s0042-6989(00)00021-3. [DOI] [PubMed] [Google Scholar]
- 19.Stockman A, Sharpe LT, Merbs S, Nathans J. Spectral sensitivities of human cone visual pigments determined in vivo and in vitro. Methods Enzymol. 2000;316:626–650. doi: 10.1016/s0076-6879(00)16754-0. [DOI] [PubMed] [Google Scholar]
- 20.Schnapf JL, Kraft TW, Baylor DA. Spectral sensitivity of human cone photoreceptors. Nature. 1987;325:439–441. doi: 10.1038/325439a0. [DOI] [PubMed] [Google Scholar]
- 21.Sharpe LT, Stockman A, Jägle H, Knau H, Klausen G, Reitner A, Nathans J. Red, green, and red–green hybrid pigments in the human retina: correlations between deduced protein sequences and psychophysically measured spectral sensitivities. J Neurosci. 1998;18:10053–10069. doi: 10.1523/JNEUROSCI.18-23-10053.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roorda A, Williams DR. The arrangement of the three cone classes in the living human eye. Nature. 1999;397:520–522. doi: 10.1038/17383. [DOI] [PubMed] [Google Scholar]
- 23.Calkins DJ, Tsukamoto Y, Sterling P. Microcircuitry and mosaic of a blue–yellow ganglion cell in the primate retina. J Neurosci. 1998;18:3373–3385. doi: 10.1523/JNEUROSCI.18-09-03373.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herr S, Klug K, Sterling P, Schein SJ. Inner S-cone bipolar cells provide all of the central elements for s cones in macaque retina. J Comp Neurol. 2003;457:185–201. doi: 10.1002/cne.10553. [DOI] [PubMed] [Google Scholar]
- 25.Haverkamp S, Wassle H, Duebel J, Kuner T, Augustine GJ, Feng G, Euler T. The primordial, blue-cone color system of the mouse retina. J Neurosci. 2005;25:5438–5445. doi: 10.1523/JNEUROSCI.1117-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sieving PA, Murayama K, Naarendorp F. Push-pull model of the primate photopic electroretinogram: a role for hyperpolarizing neurons in shaping the b-wave. Vis Neurosci. 1994;11:519–532. doi: 10.1017/s0952523800002431. [DOI] [PubMed] [Google Scholar]
- 27.Robson JG, Saszik SM, Ahmed J, Frishman LJ. Rod and cone contributions to the a-wave of the electroretinogram of the macaque. J Physiol. 2003;547:509–530. doi: 10.1113/jphysiol.2002.030304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Copenhagen DR, Ashmore JF, Schnapf JK. Kinetics of synaptic transmission from photoreceptors to horizontal and bipolar cells in turtle retina. Vis Res. 1983;23:363–369. doi: 10.1016/0042-6989(83)90083-4. [DOI] [PubMed] [Google Scholar]
- 29.Burkhardt DA. Contrast processing by ON and OFF bipolar cells. Vis Neurosci. 2011;28:69–75. doi: 10.1017/S0952523810000313. [DOI] [PubMed] [Google Scholar]
- 30.Puller C, Manookin MB, Neitz M, Neitz J. Syntaxin-4 is highly enriched beneath S-cone pedicles in the primate retina. Investig Ophthalmol Vis Sci. 2012;53:6323. ARVO abstract. [Google Scholar]
- 31.Puller C, Manookin MB, Neitz M, Neitz J. Specialized synaptic pathway for chromatic signals beneath S-cone photoreceptors is common to human, Old and New World primates. J Opt Soc Am A. 2014;31:A189–A194. doi: 10.1364/JOSAA.31.00A189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neitz J, Neitz M, Puller C. Department of Ophthalmology, University of Washington; Seattle, Washington 98109, USA: Synaptic elements for GABAergic feed-forward signaling between HII horizontal cells and blue cone bipolar cells are enriched beneath primate S-cones. are preparing a manuscript to be called. [DOI] [PMC free article] [PubMed] [Google Scholar]


