Abstract
Objective
To compare the acute effects of 0.9% saline versus a balanced electrolyte solution on acute kidney injury in a rat model of sepsis.
Design
Controlled laboratory experiment.
Setting
University laboratory.
Subjects
Sixty adult, male Sprague-Dawley rats.
Interventions
We induced sepsis by cecal ligation and puncture and randomized animals to receive fluid resuscitation with either 0.9% saline or Plasma-Lyte solution for 4 hours after 18 hours of cecal ligation and puncture (10 mL/kg in the first hour and 5 mL/kg in the next 3 hr). Blood and urine specimens were obtained from baseline, 18 hours after cecal ligation and puncture, immediately after 4 hours fluid resuscitation, and 24 hours later. We measured blood gas, plasma electrolytes, creatinine, interleukin-6, cystatin C, and neutrophil gelatinase-associated lipocalin concentrations. We also analyzed urine for cystatin C and neutrophil gelatinase-associated lipocalin. We used Risk, Injury, Failure, Loss and End-stage criteria for creatinine to assess severity of acute kidney injury. We observed all animals for survival up to 1 day after resuscitation. Surviving animals were killed for kidney histology. Finally, we carried out an identical study in 12 healthy animals.
Measurements and Main Results
Compared with Plasma-Lyte, 0.9% saline resuscitation resulted in significantly greater blood chloride concentrations (p < 0.05) and significantly decreased pH and base excess. Acute kidney injury severity measured by RIFLE criteria was increased with 0.9% saline compared with Plasma-Lyte resuscitation (p < 0.05), and these results were consistent with kidney histology and biomarkers of acute kidney injury. Twenty-four-hour survival favored Plasma-Lyte resuscitation (76.6% vs 53.3%; p = 0.03). Finally, in healthy animals, we found no differences between fluids and no evidence of acute kidney injury.
Conclusion
Volume resuscitation with Plasma-Lyte resulted in less acidosis and less kidney injury and improved short-term survival when compared with 0.9% saline in this experimental animal model of sepsis.
Keywords: acute kidney injury, hyperchloremia, saline, sepsis
Sepsis is the major cause of death in most ICUs and is the leading cause of acute kidney injury (AKI). Fluid resuscitation remains one of the pillars of sepsis therapy. In North America, 0.9% saline is the most commonly used resuscitation fluid especially for patients with sepsis (1). However, infusing large volumes of saline produces metabolic acidosis by increasing the plasma chloride concentration relative to the plasma sodium concentration, decreasing the strong ion difference (2). Hyperchloremia also affects renal function by decreasing glomerular filtration rate (GFR) (3–5). In one double-blind crossover trial, a 2-L 0.9% saline infusion over 1 hour produced hyperchloremia and decreased renal blood flow velocity and renal cortical perfusion in healthy volunteers when compared with a balanced crystalloid (6). In addition, hyperchloremic acidosis may contribute to AKI by increasing nuclear factor-kappa B DNA binding and inflammatory mediator release (7, 8). Although the clinical significance of these effects is still debated, a retrospective study of more than 30,000 abdominal surgery patients recently demonstrated that intraoperative 0.9% saline use resulted in more complications, including need for dialysis, compared with Plasma-Lyte (PL) (9). Similarly, a recent prospective, sequential period study found a chloride-liberal strategy was associated with a significant increase in the prevalence of AKI and use of renal replacement therapy (RRT) compared with a chloride-restrictive strategy (10).
Given the high risk of AKI in septic patients, avoidance of the harmful effects of hyperchloremia would seem prudent. Solutions containing physiologic levels of chloride and buffer— often called “balanced crystalloids” (e.g., Ringer’s acetate, Ringer’s lactate, and other multiple electrolyte solutions)— are widely available. However, they are used less frequently than 0.9% saline partly because they contain calcium, thereby preventing safe administration with blood products (11, 12). Alternative solutions to 0.9% saline that are physiologically balanced and safe to administer with blood products have not been broadly investigated. PL, a balanced crystalloid, has a chloride concentration much closer to that of plasma, does not contain calcium, and may be a suitable alternative to saline (9, 10) (Table 1). Despite widespread use of PL for patients with renal compromise, there is a paucity of data for comparison with 0.9% saline in patients with sepsis.
Table 1.
Compositions of 0.9% Saline, Plasma-Lyte, and Blood Plasma
| Compositions (mEq/L) | 0.9% Saline | Plasma-Lyte | Blood Plasma |
|---|---|---|---|
| Na+ | 154 | 140 | 140 |
| CI− | 154 | 98 | 100 |
| K+ | 5 | 4.2 | |
| Mg2+ | 3 | 2.5 | |
| Ca2+ | 5 | ||
| Bicarbonate | 25 | ||
| Acetate | 27 | ||
| Gluconate | 23 |
Thus, in this study, we compared the effects of a balanced crystalloid solution, PL, with 0.9% saline on the development of AKI in a cecal ligation and puncture (CLP) animal model of sepsis. We hypothesized that PL resuscitation would decrease acidosis, AKI prevalence, and severity and would improve survival when compared with 0.9% saline resuscitation.
MATERIALS AND METHODS
Experimental Protocol
After approval of the protocol by the University Animal Care Committee and the Federal Authorities for Animal Research, experiments were performed in adherence with the National Institutes of Health Guidelines on the Use of Laboratory Animals. Sixty adult (24–28 weeks old, weight 400–600 g), healthy, male, Sprague-Dawley rats were anesthetized with intraperitoneal injection of pentobarbital sodium (50 mg/kg). The sample size based on the primary outcome (occurrence of AKI) was set at 30 animals per group in order to detect an approximate 30–35% change in the rate of AKI. We performed CLP with a predetermined 25% ligated length of cecum and 18-gauge needle: two punctures inferior to the ileocecal valve. This protocol is associated with a mortality of about 50–60% at day 7 (13). The abdomen was closed and animals were randomized to receive either 20 mL/kg of saline or PL subcutaneously for resuscitation. Topical anesthetic was applied to the surgical wound and rats were returned back to their cages and allowed food and water ad libitum.
Eighteen hours after CLP, the animals were reanesthetized with pentobarbital (induced with 30 mg/kg intraperitoneally, maintained with 20 mg/kg every 2–3 hr). The left femoral vein was cannulated with 1.27-mm PE-90 tubing for fluid resuscitation. The right femoral artery was cannulated with 1.27- mm PE-90 tubing for blood samples and for blood pressure measurements (Siemens Sirecust 1281, Malvern, PA). All rats received 10 mL/kg fluid resuscitation in the first hour with either 0.9% saline or PL (Baxter, Deerfield, IL). For the next 3 hours, animals received the same fluid at 5 mL/kg/hr. We chose fluid resuscitation at 18 hours after CLP as in our previous studies (13, 14), and we have determined that 18 hours is the time at which an animal subjected to this severity of CLP becomes symptomatic such that they resemble patients presenting medical attention. This would represent the earliest time point that fluid resuscitation could commence in a clinical situation. The total volume of 25 mL/kg in 4 hours is based on the Fluid Explansion As Supportive Therapy trial protocol in humans (15). After 4-hour treatment was stopped and catheters were removed, the rats were sent back to cages in the animal facility and allowed food and water ad libitum. The animals were observed for survival over an additional 24 hours. Moribund animals defined as severe lethargy, decreased activity, and unresponsiveness to stimulation were euthanized. Surviving animals were killed for histology. To examine the effects of fluid choice of animals without sepsis, the same study was carried out in healthy animals (n = 6 each).
Measurements and Calculations
Blood (1 mL) was drawn from the arterial line, and urine (1–2 mL) was taken from the bladder at 0 hours (baseline), 18 hours (before fluid resuscitation), 22 hours (after resuscitation), and 46 hours after CLP. Similar time points were obtained for healthy controls. Isolated plasma was kept at −80°C for subsequent interleukin (IL)-6, neutrophil gelatinase-associated lipocalin (NGAL), cystatin C, and creatinine (Cr) measurements.
Arterial blood gases, blood urea, and base excess (BE) were analyzed immediately with a blood gas analyzer (I-Stat, Abbott Point of Care, Princeton, NJ). Electrolytes, including sodium (Na+), potassium (K+), chloride (Cl−), as well as urea and hemoglobin (Hb), were also immediately measured with the I-Stat. Plasma IL-6 was measured with an enzyme-linked immunosorbent assay (ELISA) (R & D Systems, Minneapolis, MN). Plasma NGAL and urine NGAL were determined using ELISA (BioPorto Diagnostics, Gentofte, Denmark). Plasma creatinine was detected with a creatinine enzymatic assay kit (BioVision Technologies, Mountain View, CA). Plasma cystatin C and urine cystatin C were measured by ELISA (BioVendor LLC, Candler, NC). The severity of AKI was assessed using the serum creatinine portion of the RIFLE criteria (13), which classified risk (R), injury (I), and failure (F), on the basis of maximum creatinine increase of 150%, 200%, and 300%, respectively, in the 2 days following CLP.
Evaluation of Kidney Histology
Rat kidneys were fixed in 10% neutral buffered formalin, dehydrated in graded anhydrous absolute ethanol, and embedded in paraffin. Histological sections (5 μm) of kidney were stained with hematoxylin and eosin (HE) and periodic acid Schiff (PAS). We considered the morphological changes indicating AKI as the loss of brush border, the vacuolization of tubular epithelial cells, and the presence of intratubular debris. Histological abnormalities were assessed by a pathologist blinded to treatment group.
Statistical Analysis
Data are expressed as medians with interquartile range where appropriate or means ± SE. The analysis of variance and unpaired Student t test were applied to compare normally distributed variables within and among groups. Mann-Whitney U test was used for data not normally distributed. Categorical variables are expressed as proportions and compared using the chi-square test. The association between plasma IL-6 and severity of AKI was examined with multivariable regression analysis. Survival analysis was assessed by Kaplan-Meier statistics and compared using log rank test. We also report differences in proportions of animals alive at 24 hours (chi-square test). A two-sided p value less than 0.05 was considered statistically significant.
RESULTS
Effects on Electrolytes and Status of Acid-Base
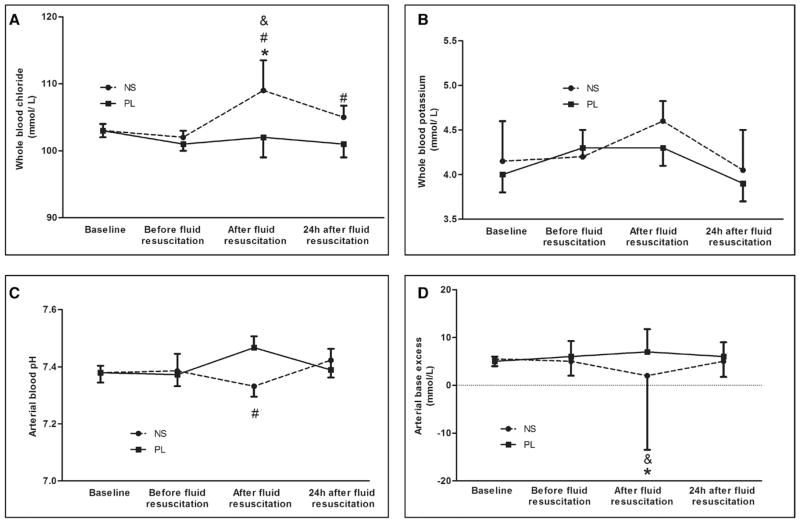
0.9% Saline-treated animals showed significantly higher levels of serum chloride after fluid resuscitation (109 vs 102 mmol/L; p < 0.05), whereas PL infusion did not induce hyperchloremia (102 vs 101 mmol/L; p > 0.05) (Fig. 1A). There were no differences in serum potassium levels (Fig. 1B). Compared with PL, there were significant decreases in pH (7.32 vs 7.45) and BE (2 vs 5 mmol/L) immediately after fluid resuscitation in 0.9% saline-treated animals (p < 0.05) (Fig. 1, C and D).
Figure 1.
Effects of resuscitation fluids on electrolytes and acid-base status in septic rats. Data are expressed as medians and interquartile range. All animals contributed data to the first 3 time points; 16 animals in the saline group and 23 in the Plasma-Lyte (PL) group contributed data to the 24-hr time point. Dashed line: Animals with cecal ligation and puncture (CLP) treated with 0.9% saline. Solid line: Animals with CLP treated with PL. *p < 0.05, versus baseline in the same group; #p < 0.05, comparison between groups at the same time point; &p < 0.05, versus the values before fluid resuscitation in the same group. A, Whole blood chloride concentrations (mmol/L). B, Whole blood potassium concentrations (mmol/L). C, Arterial blood pH. D, Arterial base excess (mmol/L). NS = 0.9% saline.
Effects on Renal Function
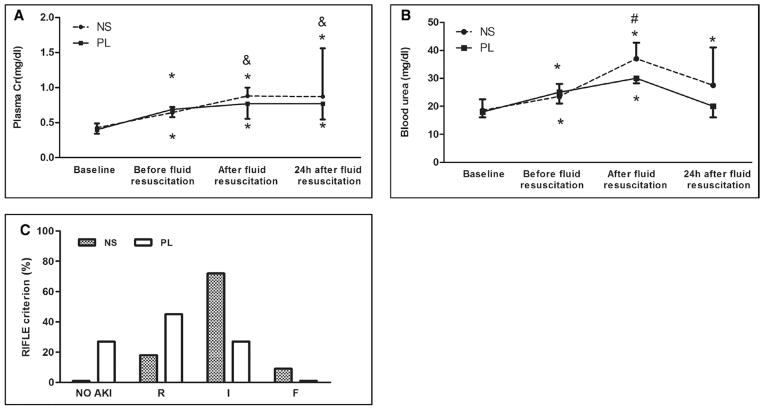
The effects of fluid resuscitation on serum creatinine and urea are shown in Figure 2, A and B. Fluid resuscitation with 0.9% saline resulted in significantly greater rates of AKI (100% vs 76%) and also worsened AKI severity calculated by RIFLE criteria compared to PL resuscitation (RIFLE-I or F: 83% vs 28%; p < 0.001) (Fig. 2C).
Figure 2.
Effects of resuscitation fluids on renal function in septic rats. Data are expressed as medians and interquartile range for A and B; 16 animals in the saline group and 23 in the Plasma-Lyte (PL) group contributed data to the 24-hr time point. Dashed line: Animals with cecal ligation and puncture (CLP) treated with 0.9% saline. Solid line: Animals with CLP treated with PL. *p < 0.05, versus baseline in the same group; #p < 0.05, comparison between groups at the same time point; &p < 0.05, versus the values before fluid resuscitation in the same group. A, Plasma creatinine concentration (mg/dL). B, Blood urea concentration (mg/dL). C, Percentage of different severity of acute kidney injury (AKI) measured by RIFLE categories. R, I, F = AKI RIFLE classes Risk (creatinine change, 150–199%), Injury (creatinine change, 200–299%), and Failure (creatinine change, ≥ 300%). Saline resulted in significantly greater rates of AKI and severe (I or F) AKI (p < 0.001). NS = 0.9% saline.
Effects on Biomarkers of AKI and Kidney Histology
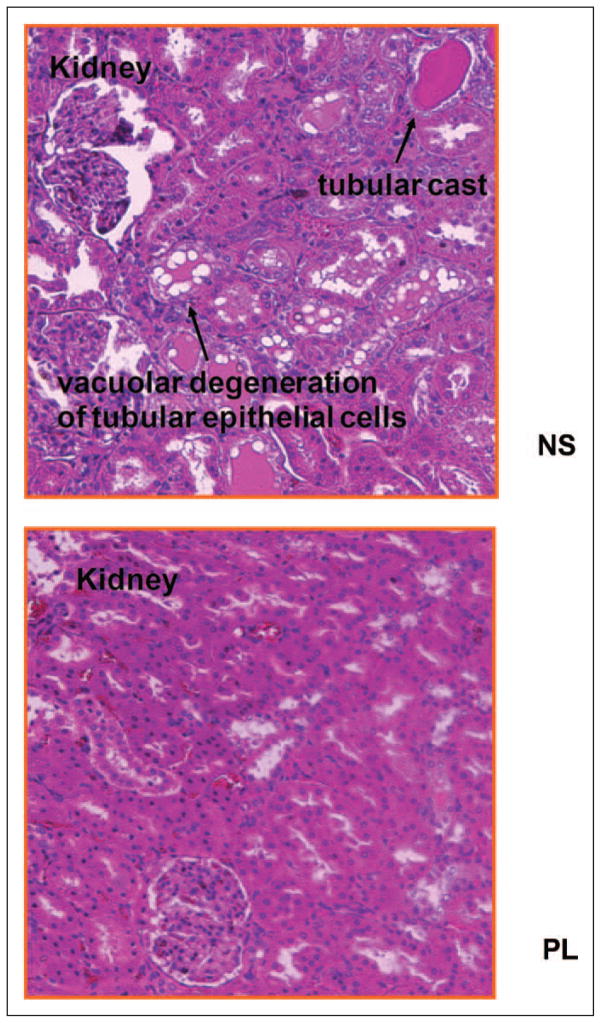
There were no significant increases in plasma cystatin C after sepsis, but plasma cystatin C was higher with saline resuscitation compared to baseline values (Fig. 3A). Plasma NGAL increased significantly with sepsis, but there were no differences between 0.9% saline and PL resuscitation (Fig. 3B). However, urine cystatin C and urine NGAL levels with 0.9% saline resuscitation were significantly greater compared with the values with PL resuscitation (3,013 vs 1,660 IU/mL and 2,408 vs 1,912 IU/mL, respectively; p < 0.05) (Fig. 3, C and D). Figure 4 shows kidney histology using HE and PAS stains under light microscopy with original magnification of ×400. Loss of brush border, vacuolization, and dilation of the tubular lumen were more prominent in 0.9% saline-treated animals.
Figure 3.
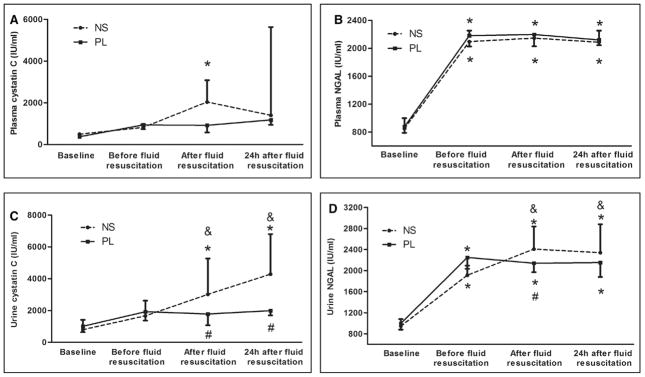
Effects of resuscitation fluids on biomarkers of acute kidney injury in septic rats. Data are expressed as medians and interquartile range. Sixteen animals in the saline group and 23 in the Plasma-Lyte (PL) group were alive at 24 hr. Dashed line: Animals with cecal ligation and puncture (CLP) treated with 0.9% saline. Solid line: Animals with CLP treated with PL. *p < 0.05, versus baseline in the same group; #p < 0.05, comparison between groups at the same time point; &p < 0.05, versus the values before fluid resuscitation in the same group. A, Plasma neutrophil gelatinase-associated lipocalin (NGAL) (IU/mL). B, Plasma cystatin C (IU/mL). C, Urine NGAL (IU/mL). D, Urine cystatin C (IU/mL). NS = 0.9% saline.
Figure 4.
Effects of resuscitation fluids on kidney histopathology in septic rats. Kidney tissues were collected 24 hr after fluid resuscitation (46 hr after cecal ligation and puncture [CLP]). Histological sections (5 μm) of kidney were stained with hematoxylin and eosin and periodic acid Schiff. NS: Animals with CLP treated with 0.9% saline; Plasma-Lyte (PL): Animals with CLP treated with PL. Saline-treated rats showed significant loss of brush border and vacuolization in tubules, these changes in PL-treated rats are milder.
Effects on IL-6
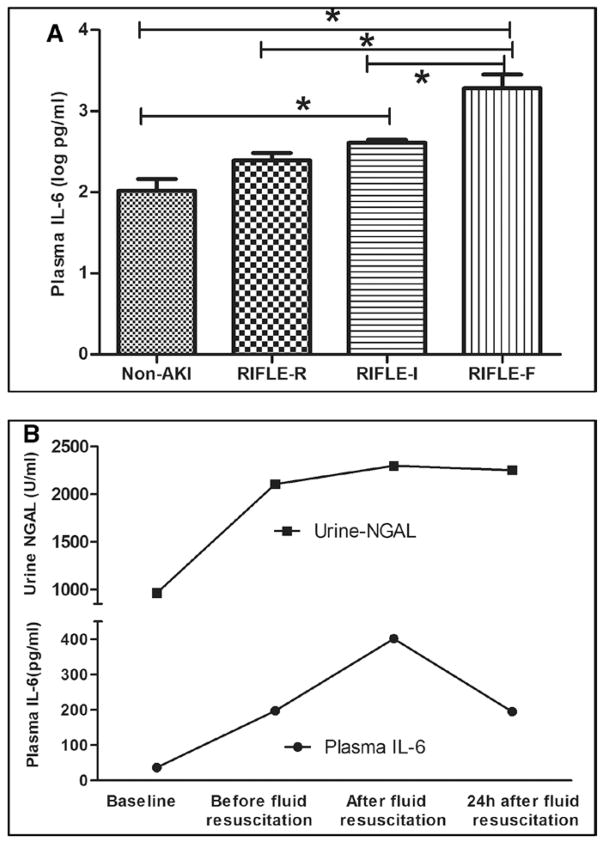
IL-6 was used to evaluate the inflammatory response. Plasma IL-6 levels increased after CLP and continuously increased after fluid resuscitation. However, the IL-6 was significantly greater after 0.9% saline compared with PL resuscitation (2.87 vs 2.50 log pg/mL; p = 0.04) (Fig. 5).
Figure 5.
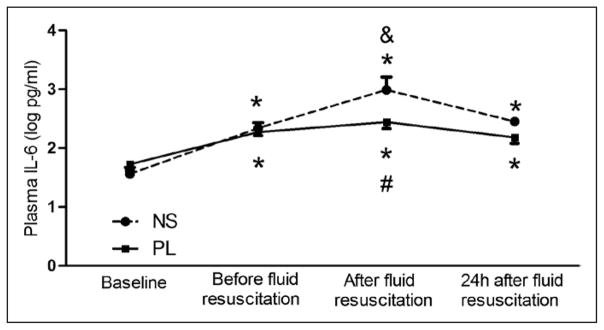
Effects of resuscitation fluids on plasma interleukin (IL)-6 in septic rats. Data are expressed as mean and SE after log transformation (log pg/mL). Sixteen animals in the saline (NS) group and 23 in Plasma-Lyte (PL) group were alive at 24 hr. Dashed line: Animals with CLP treated with 0.9% saline. Solid line: Animals with CLP treated with PL. *p < 0.05, versus baseline in the same group; #p < 0.05, comparison between groups at the same time point; &p < 0.05, versus the values before fluid resuscitation in the same group.
Association Between IL-6 and AKI
In logistic regression analysis, the plasma IL-6 concentration after 4 hours of fluid resuscitation was independently associated with AKI (no AKI vs AKI). The relative risk was 1.20 (95% CI, 1.10–1.34; p = 0.03). The log risk of AKI increases by 0.02 for every one unit change in IL-6 (per 1 pg/mL). Furthermore, the median IL-6 concentrations after 4 hours of fluid resuscitation were related to AKI severity, which were 2.02, 2.40, 2.61, and 3.28 log pg/mL, in non-AKI, RIFLE-R, RIFLE-I, and RIFLE-F, respectively (p < 0.01) (Fig. 6A). Furthermore, changes of urine NGAL were consistent with the changes of IL-6 (Fig. 6B).
Figure 6.
Association between interleukin (IL)-6 and acute kidney injury (AKI). A, IL-6 levels in the different severity of AKI in all animals (data are expressed as mean and SE after log transformed, log pg/mL). *p < 0.05. B, Blood IL-6 and urine neutrophil gelatinase-associated lipocalin (NGAL) levels with time in all animals (data were expressed as median, IL-6: pg/mL; NGAL: IU/mL). RIFLE = Risk, Injury, Failure, Loss and End-stage.
Effects on Vital Signs and Survival
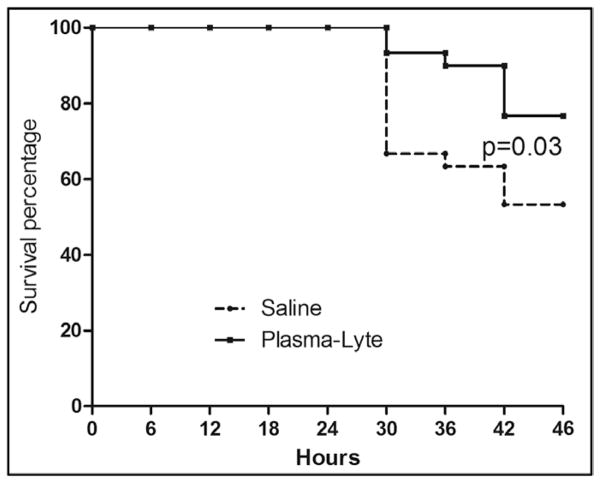
Survival to 24 hours after resuscitation (46 hr after CLP) favored PL resuscitation (23 [76.6%] vs 16 [53.3%], p = 0.01, as did survival time, hazard ratio: 2.53; 95% CI: 1.04–6.13; p = 0.03) (Fig. 7). One of the seven animals dying in PL group was euthanized, as were two of the 14 in saline group as per the euthanization protocol for moribund animals. There were no significant differences in either heart rate or blood pressure between 0.9% saline- and PL-treated animals (data not shown).
Figure 7.
Effects of resuscitation fluids on short-term survival in septic rats. Dashed line: Animals with cecal ligation and puncture (CLP) treated using 0.9% saline. Solid line: Animals with CLP treated using Plasma-Lyte (n = 30 each). Fluid resuscitation began 18 hr after CLP and continued for 4 hr. Animals were followed for survival for an additional 24 hr (46 hr after CLP).
Effect of Different Resuscitation Fluids in Healthy Rats
There was a trend toward hyperchloremia after saline loading. However, healthy animals maintained arterial blood pH within normal limits, and there were no significant differences in creatinine or urea (Table 2).
Table 2.
Effects of 0.9% Saline Infusion and Plasma-Lyte Infusion in Healthy Animals (n = 6, Mean and SE)
| Baseline | Before Fluid Resuscitation | After Fluid Resuscitation | 24 Hr After Fluid Resuscitation | |
|---|---|---|---|---|
| 0.9% Saline infusion | ||||
| Blood CI− (mEq/L) | 100.83 ± 1.60 | 101 ± 2.20 | 103.67 ± 2.58 | 102.17 ± 1.47 |
| Arterial PH | 7.36 ± 0.04 | 7.34 ± 0.09 | 7.32 ± 0.07 | 7.34 ± 0.03 |
| Arterial BE (mmol/L) | 5.87 ± 1.17 | 6.16 ± 3.14 | 6.90 ± 2.64 | 6.83 ± 2.31 |
| Blood Cr (mg/dL) | 0.43 ± 0.10 | 0.46 ± 0.21 | 0.49 ± 0.19 | 0.47 ± 0.20 |
| Blood urea (mg/dL) | 20.83 ± 1.47 | 20.83 ± 4.88 | 25.80 ± 3.77 | 25.00 ± 5.00 |
| Blood IL-6 (log pg/mL) | 1.41 ± 0.01 | 1.44 ± 0.03 | 1.45 ± 0.04 | 1.43 ± 0.05 |
| Urine cystatin C (IU/mL) | 803 ± 28 | 816 ± 64 | 862 ± 102 | 858 ± 73 |
| Urine NGAL (IU/mL) | 1,103 ± 204 | 1,204 ± 251 | 1,262 ± 317 | 1,221 ± 321 |
|
| ||||
| Plasma-Lyte infusion | ||||
| Blood CI− (mEq/L) | 101.50 ± 0.84 | 99.67 ± 2.94 | 99.67 ± 3.00 | 100.05 ± 1.05 |
| Arterial PH | 7.34 ± 0.03 | 7.34 ± 0.04 | 7.35 ± 0.19 | 7.37 ± 0.06 |
| Arterial BE (mmol/L) | 5.33 ± 1.51 | 6.00 ± 3.45 | 6.20 ± 3.49 | 6.13 ± 1.02 |
| Blood Cr (mg/dL) | 0.46 ± 0.10 | 0.42 ± 0.09 | 0.46 ± 0.15 | 0.43 ± 0.08 |
| Blood urea (mg/dL) | 20.50 ± 2.67 | 19.20 ± 2.68 | 23.60 ± 2.51 | 21.67 ± 2.94 |
| Blood IL-6 (log pg/mL) | 1.51 ± 0.17 | 1.55 ± 0.21 | 1.59 ± 0.32 | 1.57 ± 0.43 |
| Urine cystatin C (IU/mL) | 842 ± 102 | 837 ± 273 | 853 ± 283 | 848 ± 279 |
| Urine NGAL (IU/mL) | 1,036 ± 241 | 1,103 ± 198 | 1,153 ± 287 | 1,123 ± 392 |
BE = base excess, IL = interleukin, NGAL = neutrophil gelatinase-associated lipocalin.
DISCUSSION
In this study, we compared the acute effects of saline versus PL on acid-base status and AKI during experimental sepsis in the rat. First, our results confirmed prior studies showing 0.9% saline resuscitation induces hyperchloremic acidosis (2, 3, 9, 10). Interestingly, we found that this effect was seen with relatively small volumes of fluid (25 mL/kg over 4 hr) but only in septic animals—healthy animals were able to maintain blood pH. Second, we found that 0.9% saline worsened sepsis-induced AKI both by RIFLE criteria for creatinine as well as by various biomarkers for AKI and kidney histology. There was significantly less AKI with PL resuscitation as well as prevention of the marked increase in plasma chloride induced by 0.9% saline. Finally, volume resuscitation with PL improved short-term survival.
Although IV infusion of 0.9% saline has been reported to induce hyperchloremic acidosis for many years, it has remained the standard resuscitation fluid in sepsis, especially in North America. One reason may be ongoing debate about the clinical consequences of hyperchloremic acidosis (1, 16). Our study provides further evidence that hyperchloremia occurs in sepsis with 0.9% saline resuscitation volumes comparable to those used in clinical practice and provides evidence that 0.9% saline worsens sepsis-induced AKI. By contrast, volume resuscitation with PL resulted in lower plasma chloride concentrations and was associated with milder acidosis and AKI. Finally, short-term survival was adversely impacted by 0.9% saline resuscitation compared with PL.
Our data shed additional light on recent observations in humans where hyperchloremic solutions were associated with increased risk for severe AKI and use of dialysis. In a recent large-scale analysis of fluid choice across multiple hospitals in the United States, Shaw et al (9) found that 0.9% saline infusion on the day of major surgery was associated with more postoperative morbidity (including renal failure requiring dialysis) compared with PL. Yunos et al (10) recently reported results from a prospective, open-label, sequential period study, in which restricted availability of high chloride fluids resulted in a significant decrease in the prevalence of AKI and use of RRT. However, these observational studies cannot establish a causal relationship between 0.9% saline and AKI. By contrast, our study establishes this link, although cannot distinguish harm from saline versus benefit from PL.
The mechanisms responsible for effects of 0.9% saline on renal function are incompletely understood. One possible explanation is that boluses of 0.9% saline overwhelm the proximal tubule capacity to reabsorb chloride resulting in greater chloride delivery to the thick ascending limb of the distal tubule resulting in activation of tubuloglomerular feedback by the macula densa and a reduction in GFR (3, 17, 18). An already compromised kidney, in the setting of sepsis, might have less capacity to handle the chloride load. Furthermore, chloride infusion may induce thromboxane release with associated vasoconstriction (19) as well as an enhanced responsiveness to vasoconstrictor agents (20).
However, although these effects can explain the change in GFR, actual injury to the kidney, as seen in our study by both biomarkers and histopathology, requires an alternate explanation. One possibility is that acidosis might lead to an alteration in the expression of inflammatory cytokines. Jensen et al (21) exposed macrophages to lactic acid (to a pH of 6.75) and found that tumor necrosis factor secretion was increased secondary to increased gene transcription. We have previously shown that hyperchloremic acidosis increases nuclear factor-κB DNA binding in lipopolysaccharide-stimulated RAW 264.7 cells (22) and significantly increases cytokine expression in a dose-dependent fashion in normotensive septic animals (8). Consistent with previous reports in experimental animals (23–27), our results demonstrated that hyperchloremic acidosis increased cytokine (plasma IL-6) and biomarkers for AKI (urine NGAL, cystatin C, etc.). Here, we also established the association between the increased IL-6 and biomarkers for AKI (independent of fluid type used for resuscitation), which is similar to our previous studies in animals (13) and humans (28). All these results suggest that increased cytokine expression plays an important role in the development of AKI during sepsis. Also of interest is that urine but not plasma NGAL differed by fluid type. The source of plasma NGAL is controversial and may largely reflect neutrophil activation (13) as well as being synthesized in peritoneal mesothelial cells and induced by the peritoneal and gut damage (29). Thus, plasma NGAL is not only a marker of AKI but also a main marker for bacterial infection and systemic inflammation (30, 31). By contrast, urine NGAL may be more specific of renal cell damage.
Interestingly, unlike previous studies (7, 26, 27), we did not observe hypotension as a result of hyperchloremic acidosis. This may reflect the resuscitation volumes used. However, acidemia stimulates vasopressin, adrenocorticotropic hormone, and aldosterone release in animal models (32, 33) and may counteract any effect of acidosis on blood pressure, as well as influencing the inflammatory response. Importantly, normal blood pressure does not exclude hemodynamic compromise as a confounding effect on the inflammatory response (34) and kidney injury in these animals. We can only conclude that the effects of 0.9% saline on inflammation and AKI are independent of overt hypotension.
Our study was designed to answer a clinically important question as to whether 0.9% saline resuscitation can induce AKI, a question not satisfactorily answered by the existing literature. However, our design precludes us from determining whether this effect is due to hyperchloremia per se, some other effect of 0.9% saline, or possibly a beneficial effect of PL. Further study will be required to answer these questions.
We also examined the effect of 0.9% saline and PL on acid-base balance and renal function in healthy animals. We found that healthy animals could maintain blood pH, while sustaining only mild hyperchloremia with 0.9% saline with no evidence of AKI (Table 2). These findings are clinically important because they may indicate that some other exposure (e.g., sepsis) is necessary before 0.9% saline will result in renal injury. Furthermore, in humans it has been shown that renal excretion of a 0.9% saline load is delayed when compared with balanced crystalloids (35); healthy human volunteers can take more than 2 days to excrete a rapid infusion of 2 L of 0.9% saline (36). Thus, the risks associated with 0.9% saline may extend past the initial period of resuscitation.
In conclusion, fluid resuscitation with 0.9% saline in animals with sepsis resulted in hyperchloremic metabolic acidosis, worsened AKI, and increased mortality when compared with resuscitation with a balanced crystalloid (PL) solution. Given the mounting evidence from observational studies and sequential period studies in humans that chloride-rich fluids can increase the risk of AKI, these data provide additional rationale to seek alternatives to 0.9% saline for fluid resuscitation in patients with sepsis.
Acknowledgments
Dr. Peng received support for article research from the National Institutes of Health (NIH) (R01DK070910). Dr. Cove received support for article research from the NIH (T32HL007820). Dr. Singbartl consulted for Baxter and received support for article research from the NIH. His institution received grant support from the NIH and Baxter. Dr. Kellum consulted for Baxter and received support for article research from the NIH (R01DK070910). His institution received grant support from the NIH and Baxter (various grants to University of Pittsburgh). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases, the National Heart Lung and Blood Institute, or the NIH.
Footnotes
See also p. 1009.
The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Awad S, Allison SP, Lobo DN. The history of 0. 9% saline. Clin Nutr. 2008;27:179–188. doi: 10.1016/j.clnu.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 2.Scheingraber S, Rehm M, Sehmisch C, et al. Rapid saline infusion produces hyperchloremic acidosis in patients undergoing gynecologic surgery. Anesthesiology. 1999;90:1265–1270. doi: 10.1097/00000542-199905000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Wilcox CS. Regulation of renal blood flow by plasma chloride. J Clin Invest. 1983;71:726–735. doi: 10.1172/JCI110820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hansen PB, Jensen BL, Skott O. Chloride regulates afferent arteriolar contraction in response to depolarization. Hypertension. 1998;32:1066–1070. doi: 10.1161/01.hyp.32.6.1066. [DOI] [PubMed] [Google Scholar]
- 5.Imig JD, Passmore JC, Anderson GL, et al. Chloride alters renal blood flow autoregulation in deoxycorticosterone-treated rats. J Lab Clin Med. 1993;121:608–613. [PubMed] [Google Scholar]
- 6.Chowdhury AH, Cox EF, Francis ST, et al. A randomized, controlled, double-blind crossover study on the effects of 2-L infusions of 0. 9% saline and plasma-lyte® 148 on renal blood flow velocity and renal cortical tissue perfusion in healthy volunteers. Ann Surg. 2012;256:18–24. doi: 10.1097/SLA.0b013e318256be72. [DOI] [PubMed] [Google Scholar]
- 7.Kellum JA, Song M, Venkataraman R. Effects of hyperchloremic acidosis on arterial pressure and circulating inflammatory molecules in experimental sepsis. Chest. 2004;125:243–248. doi: 10.1378/chest.125.1.243. [DOI] [PubMed] [Google Scholar]
- 8.Kellum JA, Song M, Almasri E. Hyperchloremic acidosis increases circulating inflammatory molecules in experimental sepsis. Chest. 2006;130:962–967. doi: 10.1378/chest.130.4.962. [DOI] [PubMed] [Google Scholar]
- 9.Shaw AD, Bagshaw SM, Goldstein SL, et al. Major complications, mortality, and resource utilization after open abdominal surgery: 0. 9% saline compared to Plasma-Lyte. Ann Surg. 2012;255:821–829. doi: 10.1097/SLA.0b013e31825074f5. [DOI] [PubMed] [Google Scholar]
- 10.Yunos NM, Bellomo R, Hegarty C, et al. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA. 2012;308:1566–1572. doi: 10.1001/jama.2012.13356. [DOI] [PubMed] [Google Scholar]
- 11.Stephens R, Mythen M. Optimizing intraoperative fluid therapy. Curr Opin Anaesthesiol. 2003;16:385–392. doi: 10.1097/01.aco.0000084478.59960.76. [DOI] [PubMed] [Google Scholar]
- 12.Lobo DN. Intravenous 0. 9% saline and general surgical patients: A problem, not a solution. Ann Surg. 2012;255:830–832. doi: 10.1097/SLA.0b013e318250766c. [DOI] [PubMed] [Google Scholar]
- 13.Peng ZY, Wang HZ, Srisawat N, et al. Bactericidal antibiotics temporarily increase inflammation and worsen acute kidney injury in experimental sepsis. Crit Care Med. 2012;40:538–543. doi: 10.1097/CCM.0b013e31822f0d2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peng ZY, Wang HZ, Carter MJ, et al. Acute removal of common sepsis mediators does not explain the effects of extracorporeal blood purification in experimental sepsis. Kidney Int. 2012;81:363–369. doi: 10.1038/ki.2011.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maitland K, Kiguli S, Opoka RO, et al. FEAST Trial Group: Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364:2483–2495. doi: 10.1056/NEJMoa1101549. [DOI] [PubMed] [Google Scholar]
- 16.Guidet B, Soni N, Della Rocca G, et al. A balanced view of balanced solutions. Crit Care. 2010;14:325. doi: 10.1186/cc9230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salomonsson M, Gonzalez E, Kornfeld M, et al. The cytosolic chloride concentration in macula densa and cortical thick ascending limb cells. Acta Physiol Scand. 1993;147:305–313. doi: 10.1111/j.1748-1716.1993.tb09503.x. [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto S, Kawata T, Schnermann J, et al. Chloride channel blockade attenuates the effect of angiotensin II on tubuloglomerular feedback in WKY but not spontaneously hypertensive rats. Kidney Blood Press Res. 2004;27:35–42. doi: 10.1159/000075621. [DOI] [PubMed] [Google Scholar]
- 19.Bullivant EM, Wilcox CS, Welch WJ. Intrarenal vasoconstriction during hyperchloremia: Role of thromboxane. Am J Physiol. 1989;256:F152–F157. doi: 10.1152/ajprenal.1989.256.1.F152. [DOI] [PubMed] [Google Scholar]
- 20.Quilley CP, Lin YS, McGiff JC. Chloride anion concentration as a determinant of renal vascular responsiveness to vasoconstrictor agents. Br J Pharmacol. 1993;108:106–110. doi: 10.1111/j.1476-5381.1993.tb13447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen JC, Buresh C, Norton JA. Lactic acidosis increases tumor necrosis factor secretion and transcription in vitro. J Surg Res. 1990;49:350–353. doi: 10.1016/0022-4804(90)90036-2. [DOI] [PubMed] [Google Scholar]
- 22.Kellum JA, Song M, Li J. Lactic and hydrochloric acids induce different patterns of inflammatory response in LPS-stimulated RAW 264. 7 cells. Am J Physiol Regul Integr Comp Physiol. 2004;286:R686–R692. doi: 10.1152/ajpregu.00564.2003. [DOI] [PubMed] [Google Scholar]
- 23.Kellum JA. Fluid resuscitation and hyperchloremic acidosis in experimental sepsis: Improved short-term survival and acid-base balance with Hextend compared with saline. Crit Care Med. 2002;30:300–305. doi: 10.1097/00003246-200202000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Almac E, Aksu U, Bezemer R, et al. The acute effects of acetate-balanced colloid and crystalloid resuscitation on renal oxygenation in a rat model of hemorrhagic shock. Resuscitation. 2012;83:1166–1172. doi: 10.1016/j.resuscitation.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 25.Todd SR, Malinoski D, Muller PJ, et al. Lactated Ringer’s is superior to normal saline in the resuscitation of uncontrolled hemorrhagic shock. J Trauma. 2007;62:636–639. doi: 10.1097/TA.0b013e31802ee521. [DOI] [PubMed] [Google Scholar]
- 26.Pedoto A, Nandi J, Oler A, et al. Role of nitric oxide in acidosis-induced intestinal injury in anesthetized rats. J Lab Clin Med. 2001;138:270–276. doi: 10.1067/mlc.2001.118176. [DOI] [PubMed] [Google Scholar]
- 27.Pedoto A, Caruso JE, Nandi J, et al. Acidosis stimulates nitric oxide production and lung damage in rats. Am J Respir Crit Care Med. 1999;159:397–402. doi: 10.1164/ajrccm.159.2.9802093. [DOI] [PubMed] [Google Scholar]
- 28.Murugan R, Karajala-Subramanyam V, Lee M, et al. Genetic and Inflammatory Markers of Sepsis (GenIMS) Investigators: Acute kidney injury in non-severe pneumonia is associated with an increased immune response and lower survival. Kidney Int. 2010;77:527–535. doi: 10.1038/ki.2009.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leung JC, Lam MF, Tang SC, et al. Roles of neutrophil gelatinase-associated lipocalin in continuous ambulatory peritoneal dialysis-related peritonitis. J Clin Immunol. 2009;29:365–378. doi: 10.1007/s10875-008-9271-7. [DOI] [PubMed] [Google Scholar]
- 30.Xu SY, Pauksen K, Venge P. Serum measurements of human neutrophil lipocalin (HNL) discriminate between acute bacterial and viral infections. Scand J Clin Lab Invest. 1995;55:125–131. doi: 10.3109/00365519509089604. [DOI] [PubMed] [Google Scholar]
- 31.Mårtensson J, Bell M, Oldner A, et al. Neutrophil gelatinase-associated lipocalin in adult septic patients with and without acute kidney injury. Intensive Care Med. 2010;36:1333–1340. doi: 10.1007/s00134-010-1887-4. [DOI] [PubMed] [Google Scholar]
- 32.Augustinsson O, Forslid A. Aldosterone secretion during acute respiratory acidosis and NH4Cl-induced metabolic acidosis in the goat. Acta Physiol Scand. 1989;136:339–345. doi: 10.1111/j.1748-1716.1989.tb08674.x. [DOI] [PubMed] [Google Scholar]
- 33.Wood CE, Chen HG. Acidemia stimulates ACTH, vasopressin, and heart rate responses in fetal sheep. Am J Physiol. 1989;257:R344–R349. doi: 10.1152/ajpregu.1989.257.2.R344. [DOI] [PubMed] [Google Scholar]
- 34.Ayala A, Perrin MM, Chaudry IH. Defective macrophage antigen presentation following haemorrhage is associated with the loss of MHC class II (Ia) antigens. Immunology. 1990;70:33–39. [PMC free article] [PubMed] [Google Scholar]
- 35.Reid F, Lobo DN, Williams RN, et al. (Ab)normal saline and physiological Hartmann’s solution: A randomized double-blind crossover study. Clin Sci (Lond) 2003;104:17–24. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 36.Lobo DN, Stanga Z, Simpson JA, et al. Dilution and redistribution effects of rapid 2-litre infusions of 0. 9% (w/v) saline and 5% (w/v) dextrose on haematological parameters and serum biochemistry in normal subjects: A double-blind crossover study. Clin Sci (Lond) 2001;101:173–179. [PubMed] [Google Scholar]