Abstract
Numerous efforts have been made to understand fundamental biology of diseases based on gene expressions. However, the relationship between gene expressions and onset of diseases often remains obscure. The great advances in protein microarrays allow us to investigate this unclear question through protein profiles, which are regarded as more reliable than gene expressions to serve as the harbinger of disease onset or as the biomarker of disease treatment monitoring. We review two relatively new platforms of protein arrays, along with an introduction to the common basis of protein array technologies. Immobilization of proteins on the surface of arrays and neutralizing reactive areas after the immobilization are key practical issues in the field of protein array. One of the emerging protein array technologies is the magneto-nanosensor array where giant magnetoresistive (GMR) sensors are used to quantitatively measure analyte of interest which are labeled with magnetic nanoparticles (MNP). Similar to GMR, several different ways of utilizing magnetic properties for biomolecular detection have been developed and are reviewed here. Another emerging protein array technology is Nucleic Acid Programmable Protein Arrays (NAPPA), which have thousands of protein features directly expressed by nucleic acids on array surface. We anticipate these two emerging protein array platforms can be combined to produce synergistic benefits and open new applications in proteomics and clinical diagnostics.
Keywords: Protein array, GMR sensors, Magnetic Immunoassays, Self-assembling assays, Molecular detection, Protein interactions
1. Introduction
The marked advances in genomics have revolutionized biology, including better understanding of gene expression changes or detection of gene mutations associated with diseases [1–4]. Although many efforts have been made to translate the expansion of this information base into a functional knowledge of how protein interactions or functions change during pathology, most of the relationships still remain unexplored and unknown. Importantly, it is recognized that gene expression changes does not necessarily reflect changes in protein expression within the cell. Better tools are needed to accurately probe the protein activity levels. Compared to widely used DNA arrays in genomics and molecular diagnostics, protein arrays are arguably underachieving their potentials as they are perceived not as precise (or as reproducible) as DNA arrays. Thus, the era of functional proteomics is in its infancy relative to genomics, but functional proteomics still holds great promises that it could result in greater understandings of the biology of disease at the individual patient level, and leads to more precise therapies which defines the concept of personalized medicine [5–7].
Efforts to move functional proteomics into more widespread practice have resulted in a number of platforms to assess protein concentration or functional characterization in high throughput assays [8–12]. Protein arrays (used synonymously with protein microarrays or protein biochips) are one of the solutions to the high throughput study of protein interaction networks or immune reactivity [9, 13, 14]. There is a dichotomy in microarray platform development, i.e., the division of forward arrays and reverse arrays. Forward protein arrays either utilize antibodies spotted on array surface to quantitate protein levels in biological samples, or are comprised of individually addressed unique proteins on array surface for assessing protein-protein interactions or anti-protein antibody levels in biological samples [15, 16]. The nucleic acid programmable protein array (NAPPA) [17] is a version of the forward array in which coding regions of genes are subcloned into expression plasmids so that the expressed product would contain a fusion tag. Instead of printing proteins, the plasmids containing the coding region of unique proteins are co-printed along with an anti-tag capture antibody. Proteins are created by in vitro transcription and translation and the nascent proteins are captured in situ. In contrast, reverse protein arrays are printed with extracts from clinically derived samples and then probed with relevant proteins or antibodies. Reverse protein arrays are often used to assess functional state levels in samples, e.g., level of phosphorylation in disease versus healthy controls [18–20]. Each array format (forward or reverse) has its own merits and disadvantages, and its usefulness may vary depending upon the specific technical question under inquiry. Similar to fluorescent detection systems, which is the dominant technology used in DNA arrays and protein arrays, the protein arrays based on magneto-nanosensors [21], an emerging diagnostic platform and the major focus of this review, can be used in both forward and reverse formats. The main difference is that the fluorescent labels are replaced with magnetic nanoparticle tags, and readout is done by giant magnetoresistive (GMR) sensors embedded in a silicon die. The magneto-nanosensors can also monitor any assembly of molecules involved with magnetic tags. Furthermore, the magneto-nanosensors can be combined with NAPPA as discussed later in this article. A major application of the magneto-nanosensors is to measure the abundance of proteins in biological samples like mass spectroscopy (MS) [22], which is widely used as a discovery tool of diagnostic, prognostic, and therapeutic protein biomarkers. Since MS relies mainly on the mass-to-charge ratios to distinguish different proteins, it does not require recognition molecules while the protein arrays are dependent on the availability of specific recognition molecules. MS-based methods can screen numerous proteins simultaneously, but its sensitivity is limited to approximately 1 ng/mL. In contrast, emerging protein arrays such as those based on magneto-nanosensors can detect many proteins simultaneously with sensitivity down to about 1 pg/mL. Importantly, the protein array allows us to readily investigate protein-protein interactions.
2. Biology and Chemistry of Arrays
2.1 Types of Assays
The most common method of detecting proteins in a solution is a sandwich assay, e.g., the Enzyme-linked Immunosorbent Assay (ELISA). The sandwich assay involves the formation of a three-layered structure where capture probes and detection probes form a sandwich around a protein, the analyte of interest as shown in Figure 1. The capture probes are preferably antibodies but could be aptamers [23, 24], diabodies [25], Fab fragments [26, 27], etc. For autoantibody detection, the capture probes can be autoproteins or peptides, and the detection probes are anti-species antibodies so that they form a sandwich around the autoantibody [28, 29]. The capture antibody is directly immobilized on the surface of a solid (Figure 1A). To have a highly specific assay, preferably a monoclonal antibody is used, and it will bind to a certain type of binding site, called an epitope, on a protein. If polyclonal antibodies that bind to multiple epitopes are used as capture probes, other highly abundant proteins that share the same epitope might bind to the antibodies and prevent the target protein from binding, which in turn hampers detection of target protein. Normally, polyclonal antibodies can have a higher probability of non-specific binding if multiplexing measurements are performed, where multiple probes and analytes are involved.
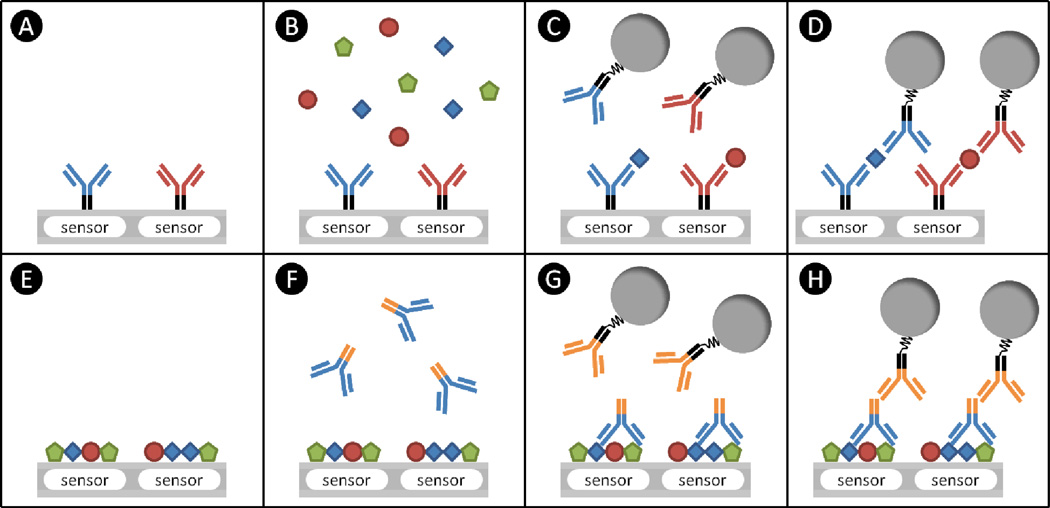
Figure 1. Schematics of sandwich (A-D) and reverse-phase (E-H) assays.
(A) The sensors are immobilized with capture probes. In this case, antibodies are selected as capture probes. (B) After blocking the rest of surface, the sample containing target proteins is introduced and incubated. The complementary pairs of antibody and its antigen are displayed in the same color. Thus, irrelevant proteins shown as green pentagons are not captured by capture probes. (C) After washing, the detection probes are added. They can be pre-conjugated with tags, which are shown as grey circles, or additional steps can be performed to attach the tags to them. (D) If the detection probes do not cross-react with other proteins or capture probes, the final assembly becomes three-layered structure of sandwich assays. (E) In the reverse-phase assays, the samples containing target proteins are spotted on the sensors. (F) After neutralizing the surface, the primary antibodies are added and incubated. These antibodies can recognize their target proteins which are shown as blue squares. (G) After washing unbound primary antibodies, the secondary antibodies with tags are introduced. These secondary antibodies bind to the other parts of the primary antibodies, which are indicated in orange. (H) The final assembly of reverse-phase assays.
The second antibody, known as the detection probe, is delivered in solution after the protein of interest is incubated and captured by the capture probes. It binds to a second epitope on the captured protein, and offers additional specificity in conjunction with the capture probes. If there is another protein that has the first epitope that binds to the capture probes but not the second epitope, this protein will not bind the detection probes. This is a major advantage that the sandwich assays have over other direct assays where only one type of antigen-specific antibodies is used. In addition, the detection probes are pre-modified with a reactive chemistry, enabling easy attachment of the probes to the tag of interest which converts binding events into readable signals. In the ELISA, the tag of interest is enzymatic or fluorescent, while it is magnetic when using magnetic immunoassays. Sometimes, the detection probes are directly conjugated with the tag of interest, but normally the probes are biotinylated and the tag of interest is conjugated with avidin or streptavidin to bind them together using biotin-avidin interaction, which is one of the strongest forms of non-covalent binding.
Another type of assay widely used is a reverse phase assay where many patients' blood samples are analyzed simultaneously [20, 30]. In the reverse phase assay, patient samples containing proteins of interest are immobilized on the surface instead of attaching capture probes to the surface (Figure 1E). Then, a solution containing primary antibodies specific to the protein of interest is introduced (Figure 1F), and they will bind to the immobilized protein of interest on the surface. Subsequently, a secondary antibody, which is conjugated with the tag of interest or biotinylated, is delivered to the surface, and it will bind to the primary antibodies already bound to the protein of interest. Usually, the patient samples are a mixture of numerous kinds of proteins if not purified. When they are immobilized on the surface, all proteins bind to the surface competitively if no specific attachment method is applied. For the protein that has a relatively low concentration, the number of bound proteins is lower than other proteins, and this provides the primary antibodies with fewer binding sites. As a result, a reverse phase assay has relatively low sensitivity compared to sandwich assays.
2.2 Protein Immobilization
The capture probes, or the proteins of interest in a sample in the case of the reverse phase assay, can be immobilized on the surface via a variety of different methods. Ideally, the probes will be attached to the surface without any conformational changes and with their activity intact. One of the methods is nonspecific physical absorption where electrostatic forces between the probes and thin film polymer on the surface dominate [31, 32]. For instance, a cationic polymer can be deposited on the surface to attract negatively charged proteins or antibodies [33, 34]. Poly-L-Lysine has been widely used in protein microarrays [35]. It has also been reported that polymers such as poly-ethyleneimine (PEI) provide both an easy and effective means of physical absorption-based antibody binding [36, 37].
Another method is to employ covalent binding chemistry where new molecular bonds are formed [31, 35]. This method of immobilization is typically more effective and reproducible than physical absorption, because only certain reactive groups on the probes and a layer on the surface are involved to form new bonds. A common reactive group of the proteins is primary amines on lysines and arginines. Essentially, all proteins can be immobilized by amine-reactive chemistry that provides stable amide bonds. N-Hydroxysuccinimide (NHS) and 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) conjugation is the most common amine-reactive chemistry. The EDC reacts with a carboxyl group on a layer of the surface, forming an amine-reactive intermediate. This intermediate can react with an amine group on the proteins to form an amide bond, but it is unstable and short-lived in aqueous solution due to hydrolysis. The addition of NHS stabilizes the intermediate by converting it to an amine-reactive NHS ester, which increases the efficiency of amino-reactive chemistry mediated by the EDC [38]. N-hydroxysulfo-succinimide (Sulfo-NHS) is a negatively charged analog of NHS, and it can be an alternative choice for stabilizing the intermediate activated by EDC [39].
Alternatively, thiol-gold conjugation can be utilized to attach the probes with thiol groups to the gold surface [40,41]. Since all antibodies contain inter-chain disulfide bonds, it is possible to reduce these bonds selectively by using reducing agents such as 2-mercaptoethylamine or dithiothreitol to make thiol groups [42,43]. This method will create a portion of half antibodies because the disulfide bonds can be broken. The half antibodies can be separated from a mixture of antibodies by column chromatography. If this kind of antibody is not desired in an experiment, 2-iminothiolane, also known as Traut's reagent, can be used to create a free sulfhydryl group by reacting with amine groups on antibodies [44,45]. Once sulfhydryl groups are generated, the target surface, an area where you want to attach antibodies, is coated with gold and the antibodies with sulfhydryl groups can be immobilized on the surface.
2.3 Surface Blocking
After immobilizing the capture probes on the surface of arrays, the remaining reactive area must be neutralized or blocked to prevent any future nonspecific binding of proteins, detection probes, or tags [46]. Otherwise, nonspecific binding elevate the background signal such that readout signals can be overestimated. It is difficult to subtract these nonspecific signals from the readout signals, because they are not necessarily constant within an array or between arrays. Another problem we may encounter is that sensitivity at a low concentration of analyte can be impaired because the analyte can be captured outside of sensing area and becomes depleted in the reaction well. To get reproducible and reliable signals, the remaining reactive surface is treated with highly abundant proteins such as albumin or casein. The most common blocking reagent is Bovine Serum Albumin (BSA) at a range of concentration from 1% to 3% [47]. Nonfat milk blocking buffers have been used to block the surface, but BSA is usually more effective than nonfat milk for biotin-avidin systems because it contains a single purified protein [48]. Generally, a blocking solution with fewer kinds of proteins has a lower chance of cross-reactivity. We observed that BSA blocks well in GMR sensors, and used the sensors coated with BSA as negative control sensors.
3. Magnetic Sensor Arrays
3.1 Magnetic Sensors for Biodetection
In 1997, the first demonstration of detecting biological molecules by employing magnetic labels was achieved with Superconducting Quantum Interference Device (SQUID) [49]. SQUID detects a magnetic field by measuring the voltage induced by the current in a superconducting loop, which is generated by the magnetic flux penetrating the loop. The measurement of collagen type III in the system utilized antibody-conjugated magnetic particles to detect the antigens bound to the sample tube. Although later studies successfully improved the performance of devices in terms of the minimum number of particles and concentration of molecules that it can detect [50,51], the sensor is not suitable for integrated arrays due to its large size and requirement of liquid helium or nitrogen for cooling.
One year later, Baselt first reported that the detection of biological molecules is possible by counting the number of magnetic particles using giant magnetoresistive (GMR) sensors [52], which had been introduced in hard disk drive industry as read head sensors around 1997, but later superseded by magnetic tunnel junction (MTJ) sensors [53] after 2004. As we use a hard disk drive without cooling or magnetic shielding, GMR sensors can be operated at ease in microelectronics or biology and have sufficiently high performance at room temperature. GMR sensors consist of multiple layers of magnetic and non-magnetic materials with nanometer scale thicknesses, and the direction of magnetization in one of the magnetic layers, called free layer or sensing layer, can be rotated by magnetic fields generated from magnetic objects. The angle between the magnetizations of the free layer and the reference layer, which is separated from the free layer by a noble metal spacer (typically Cu), modulates spin-dependent electrical resistances of the GMR stacks. The detailed principle of GMR is explained in the next section.
As we can expect, magnetic tunnel junction (MTJ) has also been utilized to develop biosensors [54, 101], which is the current technology widely used for read heads in hard disk drives [55]. While GMR relies on spin-dependent scattering of electrons between magnetic layers, MTJ is involved with spin-dependent tunneling effect. An MTJ stack has a tunnel barrier between two magnetic layers, and a current is applied across the layers to tunnel through the insulating barrier layer. The magnetization of one of the magnetic layers, called free layer or sensing layer just like in GMR sensors, can be switched by an external magnetic field, and the angle between the two magnetic layers adjacent to the tunnel barrier modulates the amplitude of the tunneling current. A preferred barrier material is magnesium oxides, MgO. MgO-based MTJ sensors offer a higher magnetoresistance ratio (MR) and therefore a higher sensitivity is possible. However, there are obstacles that we need to overcome in order to make MTJ sensors the next generation of biosensor arrays. As active sensor areas become larger, there is a higher possibility to have pinholes in the MTJ stack, which significantly reduces the spin-dependent tunneling effect.
Another approach to magnetic biodetection is to use Hall sensors [56,57]. In the Hall effect, charge carriers in a conductor are pushed to one side of the conductor when an external magnetic field is applied perpendicular to the conductor. The built-up charges at the sides of the conductor generate a measurable electric field or Hall voltage in a direction perpendicular to both the applied magnetic field and the current. To measure the concentration of biomolecules by counting the number of magnetic particles that bind to the molecules, the size of Hall sensor is desired to be comparable with that of the magnetic particle because the magnetic field from the particle should interact with most parts of the sensor to maximize the signal. This could be a limitation for Hall sensors in biodetection as a massive Hall sensor array is required to achieve a proper dynamic range of magnetic nanoparicles for just one analyte at different concentrations, and it requires a huge number of electrical contacts between the sensors and signal read out system.
Another method to use magnetic particles for biodetection is based on nuclear magnetic resonance (NMR) technology that measures spin-spin relaxation times (T2 relaxation times, also called transverse relaxation times) of water molecules [58]. The superparamagnetic particles are conjugated with antibodies specific to the target molecule, and they dephase the nuclear spins of surrounding water protons, enhancing T2 relaxation (i.e., shortening T2 time). When superparamagnetic particles bind to the target and form small clusters, mobile clusters of magnetic nanoparticles leads to more efficient dephasing of the nuclear spins of neighboring water molecules, further shortening the T2 time. (Note immobile large clusters of magnetic nanoparticles could lead to an increase in T2 time.) The concentration of analyte, which is proportional to the number of assemblies, is measured by detecting the change in T2 relaxation times with NMR devices. To measure multiple biomarkers, an array of 8 microcoils has been fabricated [59], but each coil is still larger than a few millimeters. Thus, it is not yet easy to integrate a massive array of coils into a small piece of chip.
Among these technologies, the most effective approach to utilize magnetic particles for multiplex biodetection in an array of sensors is arguably the approach based on GMR or MTJ sensors which are highly scalable and well proven in hard disk drive industry. Due to their sizes, NMR and SQUID are not as easily integrated in a single chip device. The noise levels of both GMR and SQUID are compared by a research group, and it is more convenient and cost-effective to implement GMR sensors into the biodetection system [60]. Although Hall sensors can be easily fabricated with Complementary metal-oxide-semiconductor techniques, the sensitivity of the sensors and thus the necessity of developing a massive array of Hall sensors is a bottleneck to fulfilling the requirements in biological applications. The GMR sensor technology has already been developed into an array of sensors ideal for multiplex biodetection, each sensor has a broad dynamic range of magnetic nanoparticles and a sensitivity of protein concentrations down to femto-molar range [21]. Although MTJ sensors are in their developing stage and do not show enough sensitivity yet, they have a high potential to become a very sensitive platform if their high MR ratios can be fully exploited. The results of the studies performed on biological molecule detection by various groups are summarized in Table 1. Although studies about detecting magnetic labels with magnetic sensors are interesting, only studies that demonstrate detection of biological molecules are included in the table. This article will focus on GMR sensors in the next section as they appear to be in the most advanced stage of development.
Table 1.
Biological molecule detection with magnetic sensors.
| Study | Sensor type |
Principal Investigators |
Magnetic labels | The lowest concentration measured |
Ref. |
|---|---|---|---|---|---|
| Kötitz et al (1997) | SQUID | L. Trahms | dextran coated iron oxide particles (13 nm) | 100 pM of collagen type III | [49] |
| Chemla et al (2000) | SQUID | J. Clarke | Magnetite (30–40 nm, multiple cores) | 5×104 of nanoparticles (700 µM of liposome) | [50] |
| Enpuku et al (2006) | SQUID | K. Enpuku | Fe3O4 (25 nm) | 40 fM of IgE | [51] |
| Edelstein et al (2000) | GMR | L. Whitman | Dynal M-280 (2.8 µm) | DNA hybridization | [61] |
| Graham et al (2005) | GMR | P. Freitas | Nanomag-D (250 nm) | 10 pM of DNA hybridization | [62] |
| Dittmer et al (2008) | GMR | M. Prins | Ademtech (500 nm) | 0.8 pM of parathyroid hormone (PTH) | [63] |
| Gaster et al (2009) | GMR | S. X. Wang | Miltenyi (50 nm) | 5 fM of CEA (50 aM of CEA with amplification) | [21] |
| Shen et al (2008) | MTJ | G. Xiao | Fe3O4 (16 nm) | 2.5 µM of DNA hybridization | [54] |
| Aytur et al (2006) | Hall sensor | B. Boser | 15% magnetite (4.1 µm) | 6.7 pM of IgG (1ng/mL) | [64] |
| Perez et al (2002) | NMR | R. Weissleder | CLIO nanoparticle (25–40 nm, multiple 3–5 nm cores) | A nM range of GFP and CA-125 | [58] |
3.2 GMR Sensors
3.2.1 The Principle of GMR
Basically, the GMR sensors transduce the stray field from magnetic labels to an electrical signal because the electrical resistance of the sensor changes as the magnetic field is applied. There are two main types of GMR sensors; one is the multilayer stack, which consists of antiferromagnetically coupled magnetic layers, and the other is the spin-valve. Here, we will focus on the latter as it is more suitable for detecting magnetic nanoparticles. A simple spin-valve structure has an antiferromagnetic layer at the bottom to pin the magnetization of a magnetic layer above, called the pinned layer, by exchange bias. Above the pinned layer are a thin Ru layer and another magnetic layer, called the reference layer. These layers form the so-called synthetic antiferromagnet which is magnetically fixed and does not respond to an applied magnetic field. Above the reference layer are a thin Cu layer and another magnetic layer, called the free layer. The free layer is the one that interacts with an external magnetic field, and its magnetization can be easily rotated along the field, which results in a relative angular change with respect to the pinned layer. If magnetizations of the reference and free layer are aligned in parallel, the resistance of the films becomes the lowest. In anti-parallel alignment, it is the highest due to spin-dependent scattering of electrons at both layers. Therefore, if we measure the resistances in the absence and presence of magnetic particles, the change between the two signals can be used to quantify the number of particles.
3.2.2 Double modulation scheme
The stray field from magnetic labels is extremely small because the magnetic moment of the label is also very small. Although the GMR sensors are preferred to measure this minute field due to its high sensitivity, it is still challenging to detect this tiny stray field. Obviously, a high signal-to-noise ratio (SNR) is required to meet the requirement of biological sensing. There are two ways of increasing the SNR. The first one is to use a bigger magnetic particle with higher magnetic moments to increase the signal itself. However, larger particles limit the dynamic range of measurement, and they are more likely to settle during the measurement so an additional washing step is needed to remove the particles that do not specifically bind to the target molecules. The second way of increasing the SNR is of course to reduce the noise. While the signals from optical detection methods are convoluted with signals from autofluorescent molecules, most of the biological molecules are non-magnetic, which leads to a very low “magnetic” background noise level. Thus, the electrical noise of the sensor and related electronics is the main source for the noise in the GMR sensors and other magnetic sensors. Note we still have to contend with the noise and interference originated from nonspecific binding, cross reactivity, temperature drift, etc., but the rule of thumb in biodetection is to make the electrical noise not a limiting factor in the overall performance. To reduce the electrical noise, a double modulation scheme has been introduced to extract magnetic-related signals from the original signal in spectral domain [65,66]. In this scheme, the current across the sensor and the magnetic field applied to the sensor are modulated at different frequencies, and the read-out signal is a cross-product term of the current and magnetic field in the time domain. The signal related to the nominal resistance (quiescent resistance) can be found at the frequency of the current, while the signals indicating changes in resistances due to the magnetoresistive effect are generated at two different frequencies away from the frequency of the current, added or subtracted by the frequency of the magnetic field in spectral analysis. Since the noise at low frequencies is dominated by the flicker noise, sometimes called 1/f noise, which has an inverse proportionality to frequency dependence, we can move the signal peaks to higher frequencies by choosing the frequencies of the current and the magnetic field appropriately so that the noise is reduced down to the thermal noise floor.
3.2.3 Real-time measurement
The magnetoresistive response is very fast; usually on the time scale of nanoseconds or less. It is a direct function of magnetic fields generated from the particles in close proximity to the sensor. These features allow us to do real-time measurement of binding events of magnetic particles with the GMR sensors. Real-time readout capabilities have been demonstrated with an array of individually accessible 64 GMR sensors [36]. Association rate constants of epithelial cell adhesion molecule (EpCAM), carcinoembryonic antigen (CEA), and vascular endothelial growth factor (VEGF) to their specific antibodies were measured and compared with the results from surface plasmon resonance (SPR) [67], which is the current gold standard for measuring kinetics of molecular interactions. Since an array of sensors is desirable for biological analysis, the total readout time increases linearly with the number of sensors. One way of reading out sensors with real-time measurement capabilities is to minimize the signal acquisition time on each sensor. However, this will increase the noise in the spectral domain. Another method, which requires a different hardware setup but is more effective, is to measure several sensors simultaneously by using the Frequency Division Multiplexing (FDM) technique [68]. In FDM, the currents across the different sensors are modulated at different frequencies, and the outputs of the sensors are combined into one single signal line. This combined signal is decoupled in the spectral domain, and allows us to recover all the original signals from different sensors.
Another requirement for real-time measurement is the stability of magnetic particles. If the gravitational force is more dominant than Brownian motion, or if the particles are not properly functionalized, they may settle to the bottom of the reaction well during the measurement. Once they precipitated on the surface of sensors, we cannot differentiate them from the particles that specifically bind to the analytes (or analyte-detection antibody complexes), so these particles produce non-specific signals. Thus, properly functionalized magnetic nanoparticles are preferred for multiplex magnetic biodetection because they are well suspended in a solution and their Brownian motion dominates gravitation. In addition, if the nanoparticles are ferromagnetic, the inter-particle magnetostatic interaction would result in the clustering of particles. As biomolecular tags, the magnetic nanoparticles are desired to have zero magnetic remanence in the absence of an external field. Superparamagnetic nanoparticles fulfill this requirement. Based on thermodynamics, the mixing of nanoparticles with a solution should have a negative Gibb's free energy of mixing, which can be achieved by coating the surface of nanoparticles with hydrophilic materials [69]. Therefore, superparamagnetic nanoparticles with hydrophilic coatings are an ideal choice for real-time measurement.
Quantitative measurement of the concentration of an analyte of interest is relatively easy compared to real-time measurement because the former only requires one to measure the difference between the signal in the absence of magnetic nanoparticles and the signal at the saturation of binding of nanoparticles. The difference is typically monotonically related to the concentration of the analyte, and often characterized by the so-called standard curve which may vary from analyte to analyte. In several studies [52,61], large-sized particles were used if the settled-down particles are removed by washing the surface of the sensors, which leaves only particles that specifically bind to the analytes on the surface. However, to avoid the additional washing, nanoparticles are more desirable for quantitative measurement. Multiplex protein assays on CEA, eotaxin, graulocyte colony-stimulating factor (GCSF), interleukin-1-alpha (IL-1α), interleukin-10 (IL-10), interferon-gamma (IFN-γ), lactoferrin, and tumor necrosis factor alpha (TNF-α) have been demonstrated with an array of GMR sensors using magnetic nanoparticle labels [21,36]. The comparison between traditional approaches and GMR sensors is summarized in Table 2.
Table 2.
Comparison between existing techniques and GMR-based sensors.
| ELISA | Bead-based optical detection |
Protein Chip | GMR sensors | |
|---|---|---|---|---|
| Multiplex level | 1 | ~ 20 | ~ 100 – 1000 | 4 – 64 |
| Limit of Detection (pM) | ~ 1 | 5 | > 10 | ~ 0.01 |
| Dynamic range (logs) | 3 | 3 | < 2 | 6 |
| Sample volume (µl) | 50 – 200 | 100 | > 100 | 10 – 50 |
3.2.4 Screening protein cross-reactivity
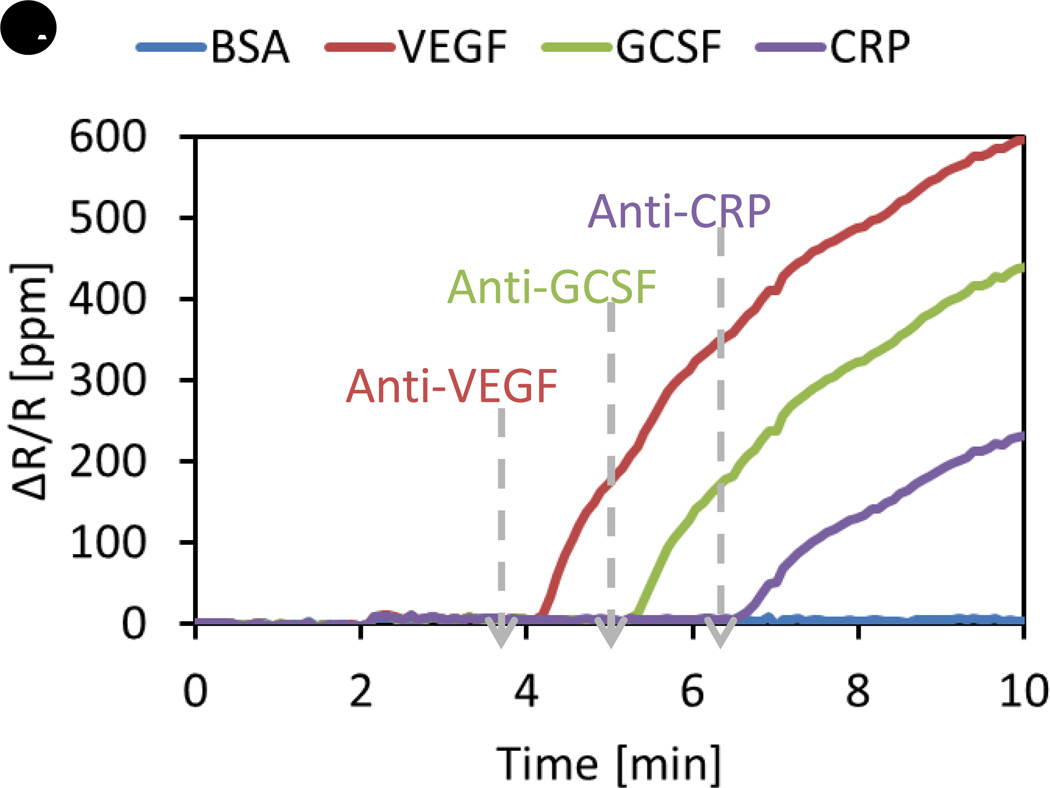
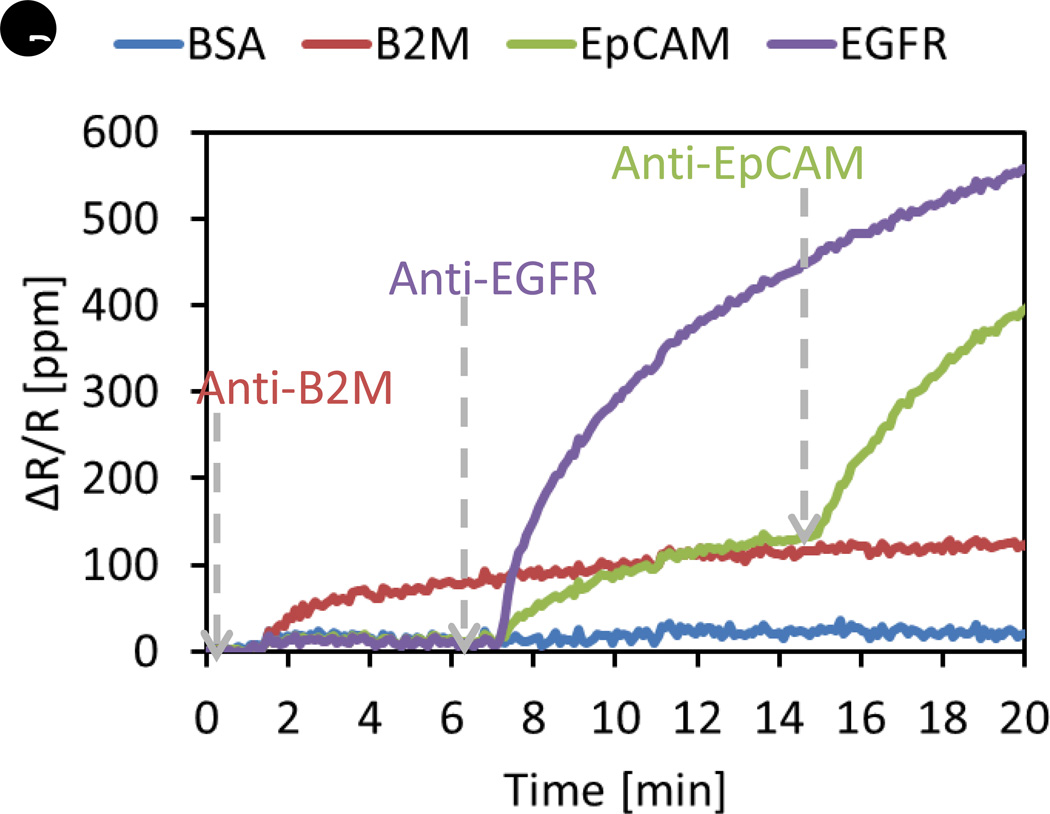
One major problem that we encounter when we develop multiplex assays is the cross-reactivity of reagents [70]. Cross-reactivity means one antibody specific to a certain antigen can bind to another or multiple antigens that are not supposed to bind with the antibody. In a sandwich assay, cross-reaction happens between layers, such as between capture probes and analytes, capture probes and detection probes, or analytes and detection probes. To check whether there is cross-reaction between the reagents by using ELISA, many different possible combinations of assays should be conducted in individual reaction wells. For example, in order to check whether a capture probe reacts with other analytes, a solution that contains all analytes except its target analyte can be assayed in the reaction well coated with the capture probe. Therefore, the number of assays we need to perform to figure out the cross-reactivity is at least the same as the number of proteins we are trying to assay. With the real-time readout capability and the ability of multiplexing GMR sensors, the cross-reactivity test can be done with a single experiment where reagents are sequentially added into the reaction well. Figure 2 shows examples of screening the cross-reactivity between reagents for protein assay development. In Figure 2A, recombinant proteins of VEGF, GCSF, and C-Reactive Protein (CRP) are immobilized on the different sensors. BSA-coated sensors are negative control sensors from which we do not expect any significant signal. The gray dashed arrows indicate the time point at which we introduce one antibody of interest. In this experiment, anti-VEGF antibodies are added to the reaction well at around 4 minutes, and then anti-GCSF and anti-CRP antibodies sequentially. We observe three distinct and separate binding curves, indicating no cross-reactivity between reagents. However, Figure 2B exhibits a very different behavior. In this case, recombinant proteins of beta-2-microglobulin (B2M), epidermal growth factor receptor (EGFR), and EpCAM are immobilized on the different sensors, along with BSA as negative control. When anti-B2M antibodies are added at about 1.5 minutes, we observe only binding curves from the B2M-coated sensors as expected. In contrast, when anti-EGFR antibodies are added at around 7 minutes, signals from both EGFR-coated and EpCAM-coated sensors start to increase simultaneously, which means these particular anti-EGFR antibodies used in this experiment can recognize both EGFR and EpCAM. When anti-EpCAM antibodies are added at about 15 minutes, we see that the slope of the binding curve of the EpCAM-coated sensor increases dramatically due to much better affinity between EpCAM antigen and antibody than that of the cross-reactivity between EpCAM and anti-EGFR. This clearly confirms that the cross-reaction between anti-EGFR antibodies and EpCAM is observed in the real-time measurement. Thus, this GMR-based magneto-nanosensor platform with magnetic nanoparticle labels enables us to screen very specific antibodies much more efficiently than ELISA.
Figure 2. Screening cross-reactivity.
(A) Binding signals of anti-VEGF, anti-GCSF, and anti-CRP antibodies after the antibodies are sequentially added. No cross-reactivity is observed. (B) Binding signals of anti-B2M, anti-EGFR, and anti-EpCAM antibodies after the antibodies are sequentially introduced. Cross-reactivity between anti-EGFR and EpCAM antigen is observed. ∆R/R: Changes in resistance over nominal (quiescent) resistance; B2M: β2-microglobulin; BSA: Bovine serum albumin; CRP: C-reactive protein; EGFR: EGF receptor; EpCAM: Epithelial cell adhesion molecule.
Another application of GMR sensors is apparently biomarker development. Unlike mass spectroscopy, it is difficult to use GMR sensors at the current level of this technique to discover biomarkers from a complex mixture of proteins, but they can be incorporated in validation procedure. Once the biomarkers are found, the developed assays can be easily translated into diagnostic tools. If the system of GMR sensors is miniaturized and becomes end-user friendly, it can also serve as a point-of-care (POC) device [71]. On the other hand, GMR sensors can be used as antigen microarrays where various antigens are spotted on the surface to detect serum antibodies. Autoantibodies are a hallmark of most immune diseases. Using a library of the proteins expressed by human cells, the profiles of autoantibodies can be measured with GMR sensors. Moreover, by spotting vaccine itself or antigens from virus, studies on antiviral antibody response can be carried out to diagnose viral infections, develop effective vaccines, and estimate the efficacy of vaccination.
4. Nucleic Acid Progammable Protein Arrays (NAPPA)
4.1. NAPPA Overview
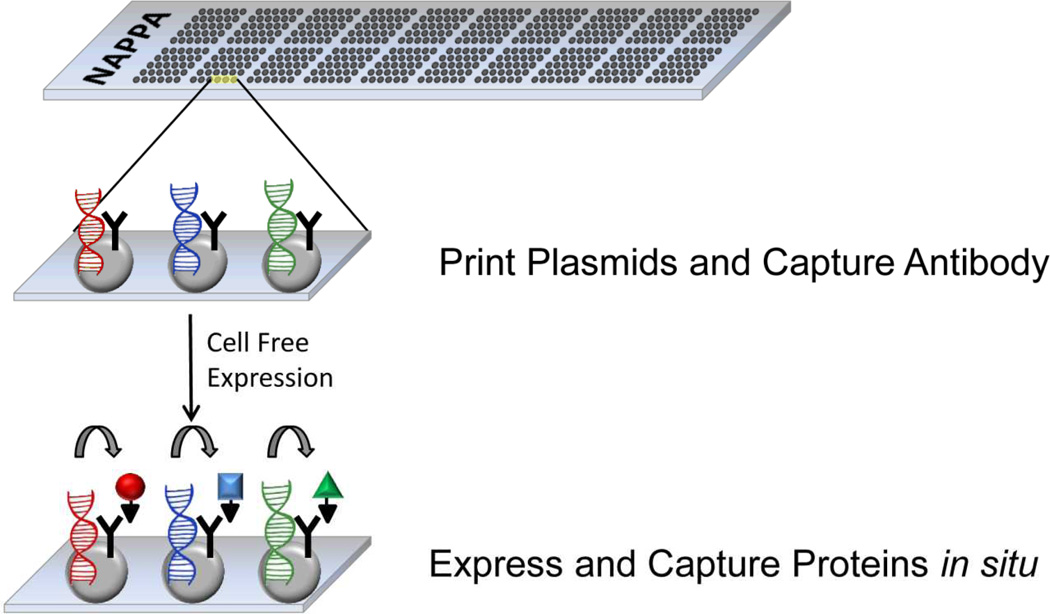
A major departure from the traditional strategy of printing pre-purified proteins arrived with the NAPPA process. The key innovation came with the concept of creating an array by printing discrete spots of individually addressable expression plasmids containing the open reading frame of a tagged target protein as shown in Figure 3. At the time of assay, the proteins are produced by a coupled in vitro transcription and translation (IVTT) system. During IVTT, RNA is transcribed from the plasmid DNA coding region which is subsequently translated to create the nascent protein. Utilization of a constant fusion tag as part of the plasmid construction provides a mechanism to capture the protein by the incorporation of an anti-tag antibody printing mix. There are multiple advantages of printing plasmids as a source of proteins. Chief among these are the stability of the DNA during storage. A cold chain is not needed to maintain reactivity for multiple months when the slides are stored under dry conditions. In addition, the chemistry for producing and purifying plasmids is relatively inexpensive when compared to mass production and purification of proteins. Once the proteins are produced on the array, antibodies against the proteins can be detected in sera from disease cases and healthy controls in an ELISA style immunoassay. The primary incubation of the subject serum allows the antibodies in the serum or plasma to bind to the cognate protein. This interaction is detected by either a fluorescently labeled anti-species secondary antibody. Alternatively, the signals can be amplified by utilizing tyramide signal amplification using HRP-labeled secondary antibodies. Signal acquisition and data processing allow for the detection of significant signal over the background. Alternatively, protein-protein interactions can be tested utilizing differentially tagged proteins with appropriate anti-heterologous tag antibodies. The cumulative result is an array of freshly produced proteins for functional characterization in a high throughput fashion.
Figure 3. Overview of NAPPA slide production.
APTES-coated glass slides are used to print a mixture of open-reading frame containing plasmids admixed with anti-tag capture antibody, BSA, and BS3 cross-linker. To create the protein array, the slide is treated with coupled in vitro transcription/translation lysate which transcribes RNA leading to localized protein production and local capture. Thereafter, the slides can be utilized for protein-interaction analysis or screening for binding with antibodies in clinical samples.
4.2. Expression plasmid libraries
Before a library of proteins can be produced by NAPPA, the library of open reading frames needs to be prepared. Significant effort has gone into the clone production library. Originally started at the Harvard Institute of Proteomics and now migrated to the Virginia G. Piper Center for Personalized Diagnostics (AZ, USA), a large library of open reading frames of human, as well as targeted microbial genomes, is under production, accumulation, storage, and distribution. The prime strategy has been to utilize recombinant cloning strategies to first create sequence-verified coding regions of defined proteins. To date, more than 12,000 human proteins and the complete coding regions of Bacillus anthracis, Vibrio cholera, Francisella tularensis, and soon to be completed Mycobacterium tuberculosis. Utilizing a cloning vector system, master DONR vectors are created through traditional PCR-based cloning. Each clone is sequence verified and placed into a physical (DNASU) and bioinformatics (FlexGene) repository. Once verified, the open reading frames can be transitioned to any number of expression vectors with a simple enzymatic transfer followed by appropriate selection. This process is amenable to high- throughput techniques utilizing robotic handling from PCR, glycerol stocks, to processing of bacteria for DNA minipreps for sequence validation. Once transferred, the clones are organized into sets of glycerol stocks containing the desired gene sets arrayed into 96 well plates. To obtain the plasmid, bacteria are grown under antibiotic selection and the bacteria collected for plate-based DNA minipreps. One of the key expression plasmids in our human clone set creates a c-terminal tag of Glutathione S Transferase (GST) fused to the protein of interest. This tag is a robust fusion partner that has a wealth of experimental data showing that it functions very well for capturing the protein of interest with anti-GST polyclonal antibodies.
4.3. NAPPA Chemistry
The chemistry for creating the NAPPA expression spot has evolved over time. The original version NAPPA utilized biotinylated plasmid DNA that was co-printed in a printing master mix containing Avidin, anti-GST antibody, and the homobifunctional crosslinker sulfosuccinimidyl suberate (BS3). The mix of plasmid and master mix components were contact printed onto 3-aminopropyltriethoxysilane (APTES) –coated glass microscope slides. This chemistry functioned well and the original paper describing the NAPPA process [17] described highly specific interaction of proteins with monoclonal antibodies. Also reported was a network of protein-protein interactions utilizing 29 human DNA replication proteins. The interactions studies found 110 interactions that reflected 47 previously known interactions but 63 newly discovered potential interactions. Further analysis comparing results of studies utilizing purified proteins revealed that 85 % (17 out of 20) were detected also in the NAPPA platform. Comparing the results of co-immunoprecipitation studies, 53% (19 of 36) interactions were also detected on the NAPPA platform. This lower level of sensitivity could be the result of the fact that NAPPA will assess only binary 1:1 protein interaction pairs whereas co-immunoprecipitation can detect multi-protein complexes. This initial publication describing NAPPA provided the foundation that NAPPA was robust and provided a platform suitable for anti-protein antibody characterization as well as protein-protein interaction studies. The next iteration of the NAPPA chemistry was revealed in 2008 with two key changes of forgoing biotinylation of plasmid DNA and incorporating bovine serum albumin into the printing mix [72].
4.4. Characterization Human Antibody Responses Utilizing NAPPA Arrays
Recent experiments have focused on using NAPPA microarrays to study infectious diseases and we performed a comprehensive serological screen of outer membrane proteins of Pseudomonas aeruginosa PAO1 proteins [73]. Serum from 38 patients with documented P. aeruginosa infections identified 12 proteins that commonly trigger an adaptive immune response in acutely infected patients both with and without cystic fibrosis. The results from this study provide valuable information about which bacterial proteins are actually recognized by the immune system in vivo during the natural course of a pulmonary infection.
4.5. GMR Sensors with NAPPA Technique
Since GMR sensor arrays have high sensitivity and NAPPA platform includes a huge variety of protein libraries, adapting NAPPA technique to GMR sensor array platform can bring us many new applications. Autoimmunity is one of the fields where the combined system can play a unique role. The concentration of autoantibody is lower than that of serum antibody generated by humoral response against antigen challenges. Due to high sensitivity of GMR sensors, it could be possible to monitor autoantibody profiles at earlier stages of diseases with the combined system. The proteins from NAPPA libraries serve as capture probes like in antigen microarrays, and complementary autoantibodies from autoimmune patient samples bind to these probes if they exist in the samples. Anti-human immunoglobulin detection probes with magnetic tags are then introduced to read out the signals. Another study on which the combined system can have a significant impact is kinetics of protein interactions. Various proteins from the NAPPA library are expressed on the surface of GMR sensors, and binding of target proteins to the proteins on the surface can be monitored in real-time measurements if the target proteins are properly labeled with magnetic particles. The characterization of protein-protein interactions is critical in the field of pharmacodynamics.
Expert Commentary
Many different types of proteins arrays such as antibody arrays and peptide arrays have been reported. In addition, new techniques are introduced to replace or enhance the state of the art. For example, bead-based assays appear to have surpassed planar arrays for multiplexed protein assays in terms of reliability and commercial success. However, magnetic nanoparticle labeling and GMR sensors in combination have made great strides to create a reliable and sensitive planar protein array platform, which can be further scaled up and improved with the NAPPA technique. The great potential of emerging protein assay platforms for studying fundamental questions of proteomics such as protein-protein interactions is also apparent. Growing attentions have been paid to the role of protein arrays in medicine development. They can be used to detect diseases early, diagnose the stage of diseases, stratify patients, and predict the efficacy of therapy, all of which are becoming more and more important for realizing the vision of personalized medicine.
Five-Year View
Future high impact studies with emerging microarray technologies is post-translational modification of proteins. New technologies can also help us to elucidate protein expressions in complex diseases as well as activities of various enzymes in many biological processes.
From technological point of view, more reliable and higher density protein arrays will be available in the near future. Protein arrays are ideal for immune response monitoring since binding of one or more target molecules to multiple capture molecules can be measured simultaneously and efficiently. If the number of capture molecules is scaled up, more information can be obtained from a single array. For cytokine measurements, protein arrays should be improved in both functional sensitivity and probe density. Multiplexed cytokine measurements require more efforts than antibody response measurements, and there are many practical problems such as cross-reactivity and reagent availability which need to be solved.
Key Issues.
-
-
Antibodies have to be chosen wisely in immunoassay, either forward or reverse phase assays, to precisely detect the analyte of interest.
-
-
Many different immobilization methods can be used to attach the capture molecules on the surface.
-
-
Proper blocking of the reactive surface after the immobilization of proteins is important to reduce non-specific binding.
-
-
Many different approaches to utilize magnetic tags for biomolecular detection have been studied in many research groups.
-
-
GMR sensor array is one of the most promising platforms among emerging protein microarrays.
-
-
Double modulation scheme offers higher signal-to-noise ratios, which enable the GMR sensors to achieve higher sensitivity in biodetection.
-
-
Due to the GMR sensors’ ability of real-time measurement, reagents can be screened easily for specificity and cross-reactivity.
-
-
The NAPPA platform is ideal for monitoring immune response, and it has many benefits including longer storage life of printed arrays and no need for expensive purification step of protein probes.
Acknowledgments
Financial Disclosure/Acknowledgements
This work was supported by Physical Science Oncology Center (U54CA143907), Center for Cancer Nanotechnology Excellence (U54CA151459), Innovative Molecular Analysis Technologies (R33CA138330), and Stanford Medical School Medical Scientist Training Program and National Science Foundation Graduate Fellowship (R.S.G.). R.S.G. and S.X.W. have related patent or patent applications assigned to Stanford University and out-licensed for potential commercialization.
Contributor Information
Jung-Rok Lee, Department of Mechanical Engineering, Stanford University, 476 Lomita Mall, Room 208, Stanford, California 94305, USA, Tel.: +1 650 723 4015, Fax: +1 650 736 1984, jungrok@stanford.edu.
Dewey Mitchell Magee, Biodesign Institute, Arizona State University, 727 East Tyler Street, Tempe, Arizona 85287, USA, Tel.: +1 480 727 0857, Fax: +1 480 965 2747, Mitch.Magee@asu.edu.
Richard Samuel Gaster, Department of Bioengineering, Stanford University, 476 Lomita Mall, Room 208, Stanford, California 94305, USA, Tel.: +1 310 801 3374 , Fax: +1 650 736 1984, RGaster@stanford.edu.
Joshua LaBaer, Biodesign Institute, Arizona State University, 727 East Tyler Street, Tempe, Arizona 85287, USA, Tel.: +1 480 965 2805, Fax: +1 480 965 2747, Joshua.LaBaer@asu.edu.
Shan X. Wang, Department of Materials Science and Engineering, Stanford University, 476 Lomita Mall, Room 351, Stanford, California 94305, USA, Tel.: +1 650 723 8671, Fax: +1 650 736 1984, sxwang@stanford.edu.
References
- 1.Schena M, Shalon D, Davis RW, Brown PO. Quantitative Monitoring of Gene-Expression Patterns with a Complementary-DNA Microarray. Science. 1995;270(5235):467–470. doi: 10.1126/science.270.5235.467. [DOI] [PubMed] [Google Scholar]
- 2.Golub TR, Slonim DK, Tamayo P, et al. Molecular classification of cancer: Class discovery and class prediction by gene expression monitoring. Science. 1999;286(5439):531–537. doi: 10.1126/science.286.5439.531. [DOI] [PubMed] [Google Scholar]
- 3.Alizadeh AA, Eisen MB, Davis RE, et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. 2000;403(6769):503–511. doi: 10.1038/35000501. [DOI] [PubMed] [Google Scholar]
- 4.Van't Veer LJ, Dai HY, Van De Vijver MJ, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415(6871):530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 5.Hamburg MA, Collins FS. The Path to Personalized Medicine. New. Engl. J Med. 2010;363(4):301–304. doi: 10.1056/NEJMp1006304. [DOI] [PubMed] [Google Scholar]
- 6.Kingsmore SF. Multiplexed protein measurement: technologies and applications of protein and antibody arrays. Nat. Rev. Drug. Discov. 2006;5(4):310–320. doi: 10.1038/nrd2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitchell P. A perspective on protein microarrays. Nat. Biotechnol. 2002;20(3):225–229. doi: 10.1038/nbt0302-225. [DOI] [PubMed] [Google Scholar]
- 8.Stoevesandt O, Taussig MJ, He MY. Protein microarrays: high-throughput tools for proteomics. Expert Rev. Proteomic. 2009;6(2):145–157. doi: 10.1586/epr.09.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uetz P, Giot L, Cagney G, et al. A comprehensive analysis of protein-protein interactions in Saccharomyces cerevisiae. Nature. 2000;403(6770):623–627. doi: 10.1038/35001009. [DOI] [PubMed] [Google Scholar]
- 10.Haab BB, Dunham MJ, Brown PO. Protein microarrays for highly parallel detection and quantitation of specific proteins and antibodies in complex solutions. Genome Biol. 2001;2(2) doi: 10.1186/gb-2001-2-2-research0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macbeath G. Protein microarrays and proteomics. Nat. Genet. 2002;32:526–532. doi: 10.1038/ng1037. [DOI] [PubMed] [Google Scholar]
- 12.Zhu H, Snyder M. Protein chip technology. Curr. Opin. Chem. Biol. 2003;7(1):55–63. doi: 10.1016/s1367-5931(02)00005-4. [DOI] [PubMed] [Google Scholar]
- 13.Ito T, Chiba T, Ozawa R, Yoshida M, Hattori M, Sakaki Y. A comprehensive two-hybrid analysis to explore the yeast protein interactome. P. Natl. Acad. Sci. USA. 2001;98(8):4569–4574. doi: 10.1073/pnas.061034498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanash S. Disease proteomics. Nature. 2003;422(6928):226–232. doi: 10.1038/nature01514. [DOI] [PubMed] [Google Scholar]
- 15.Nielsen UB, Geierstanger BH. Multiplexed sandwich assays in microarray format. J. Immunol. Methods. 2004;290(1–2):107–120. doi: 10.1016/j.jim.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 16.Liotta LA, Espina V, Mehta AI, et al. Protein microarrays: Meeting analytical challenges for clinical applications. Cancer Cell. 2003;3(4):317–325. doi: 10.1016/s1535-6108(03)00086-2. [DOI] [PubMed] [Google Scholar]
- 17.Ramachandran N, Hainsworth E, Bhullar B, et al. Self-assembling protein microarrays. Science. 2004;305(5680):86–90. doi: 10.1126/science.1097639. [DOI] [PubMed] [Google Scholar]
- 18.Tibes R, Qiu YH, Hennessy B, Andreeff M, Miiis GB, Kornblau SM. Reverse phase protein array: validation of a novel proteomic technology and utility for analysis of primary leukemia specimens and hematopoietic stem cells. Mol. Cancer Ther. 2006;5(10):2512–2521. doi: 10.1158/1535-7163.MCT-06-0334. [DOI] [PubMed] [Google Scholar]
- 19.Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411(6835):375–379. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- 20.Paweletz CP, Charboneau L, Bichsel VE, et al. Reverse phase protein microarrays which capture disease progression show activation of pro-survival pathways at the cancer invasion front. Oncogene. 2001;20(16):1981–1989. doi: 10.1038/sj.onc.1204265. [DOI] [PubMed] [Google Scholar]
- 21.Gaster RS, Hall DA, Nielsen CH, et al. Matrix-insensitive protein assays push the limits of biosensors in medicine. Nat. Med. 2009;15(11):1327–U1130. doi: 10.1038/nm.2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keshishian H, Addona T, Burgess M, et al. Quantification of Cardiovascular Biomarkers Patient Plasma by Targeted Mass Spectrometry and Stable Isotope Dilution. Mol. Cell Proteomics. 2009;8(10):2339–2349. doi: 10.1074/mcp.M900140-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirby R, Cho EJ, Gehrke B, et al. Aptamer-based sensor arrays for the detection and quantitation of proteins. Anal. Chem. 2004;76(14):4066–4075. doi: 10.1021/ac049858n. [DOI] [PubMed] [Google Scholar]
- 24.Mairal T, Ozalp VC, Sanchez PL, Mir M, Katakis I, O'sullivan CK. Aptamers: molecular tools for analytical applications. Anal. Bioanal. Chem. 2008;390(4):989–1007. doi: 10.1007/s00216-007-1346-4. [DOI] [PubMed] [Google Scholar]
- 25.Nimmagadda SV, Aavula SM, Biradhar N, et al. Recombinant Diabody-Based Immunocapture Enzyme-Linked Immunosorbent Assay for Quantification of Rabies Virus Glycoprotein. Clin. Vaccine Immunol. 2010;17(8):1261–1268. doi: 10.1128/CVI.00204-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eriksson S, Vehniainen M, Jansen T, et al. Dual-label time-resolved immunofluorometric assay of free and total prostate-specific antigen based on recombinant Fab fragments. Clin. Chem. 2000;46(5):658–666. [PubMed] [Google Scholar]
- 27.Peluso P, Wilson DS, Do D, et al. Optimizing antibody immobilization strategies for the construction of protein microarrays. Anal. Biochem. 2003;312(2):113–124. doi: 10.1016/s0003-2697(02)00442-6. [DOI] [PubMed] [Google Scholar]
- 28.Wang XJ, Yu JJ, Sreekumar A, et al. Autoantibody signatures in prostate cancer. New. Engl. J. Med. 2005;353(12):1224–1235. doi: 10.1056/NEJMoa051931. [DOI] [PubMed] [Google Scholar]
- 29.Robinson WH, Digennaro C, Hueber W, et al. Autoantigen microarrays for multiplex characterization of autoantibody responses. Nat. Med. 2002;8(3):295–301. doi: 10.1038/nm0302-295. [DOI] [PubMed] [Google Scholar]
- 30.Sheehan KM, Calvert VS, Kay EW, et al. Use of reverse phase protein microarrays and reference standard development for molecular network analysis of metastatic ovarian carcinoma. Mol. Cell Proteomics. 2005;4(4):346–355. doi: 10.1074/mcp.T500003-MCP200. [DOI] [PubMed] [Google Scholar]
- 31.Rusmini F, Zhong ZY, Feijen J. Protein immobilization strategies for protein biochips. Biomacromolecules. 2007;8(6):1775–1789. doi: 10.1021/bm061197b. [DOI] [PubMed] [Google Scholar]
- 32.Elbert DL, Hubbell JA. Surface treatments of polymers for biocompatibility. Annu. Rev. Mater. Sci. 1996;26:365–394. [Google Scholar]
- 33.Graves HCB. The Effect of Surface-Charge on Non-Specific Binding of Rabbit Immunoglobulin-G in Solid-Phase Immunoassays. J. Immunol. Methods. 1988;111(2):157–166. doi: 10.1016/0022-1759(88)90123-8. [DOI] [PubMed] [Google Scholar]
- 34.Chen H, Yuan L, Song W, Wu ZK, Li D. Biocompatible polymer materials: Role of protein-surface interactions. Prog. Polym. Sci. 2008;33(11):1059–1087. [Google Scholar]
- 35. Kusnezow W, Jacob A, Walijew A, Diehl F, Hoheisel JD. Antibody microarrays: An evaluation of production parameters. Proteomics. 2003;3(3):254–264. doi: 10.1002/pmic.200390038. * Different chemicals for immobilization of proteins are investigated.
- 36. Osterfeld SJ, Yu H, Gaster RS, et al. Multiplex protein assays based on real-time magnetic nanotag sensing. P. Natl. Acad. Sci. USA. 2008;105(52):20637–20640. doi: 10.1073/pnas.0810822105. ** Multiplexing measurements are demonstrated with an array of GMR sensors.
- 37.Yakovleva J, Davidsson R, Lobanova A, et al. Microfluidic enzyme immunoassay using silicon microchip with immobilized antibodies and chemiluminescence detection. Anal. Chem. 2002;74(13):2994–3004. doi: 10.1021/ac015645b. [DOI] [PubMed] [Google Scholar]
- 38.Staros JV, Wright RW, Swingle DM. Enhancement by N-Hydroxysulfosuccinimide of Water-Soluble Carbodiimide-Mediated Coupling Reactions. Anal. Biochem. 1986;156(1):220–222. doi: 10.1016/0003-2697(86)90176-4. [DOI] [PubMed] [Google Scholar]
- 39.Grabarek Z, Gergely J. Zero-Length Crosslinking Procedure with the Use of Active Esters. Anal. Biochem. 1990;185(1):131–135. doi: 10.1016/0003-2697(90)90267-d. [DOI] [PubMed] [Google Scholar]
- 40.Bain CD, Troughton EB, Tao YT, Evall J, Whitesides GM, Nuzzo RG. Formation of Monolayer Films by the Spontaneous Assembly of Organic Thiols from Solution onto Gold. J. Am. Chem. Soc. 1989;111(1):321–335. [Google Scholar]
- 41.Oh SJ, Hong BJ, Choi KY, Park JW. Surface modification for DNA and protein microarrays. Omics. 2006;10(3):327–343. doi: 10.1089/omi.2006.10.327. [DOI] [PubMed] [Google Scholar]
- 42.Carlsson J, Drevin H, Axen R. Protein Thiolation and Reversible Protein-Protein Conjugation - N-Succinimidyl 3-(2-Pyridyldithio)Propionate, a New Heterobifunctional Reagent. Biochem. J. 1978;173(3):723–737. doi: 10.1042/bj1730723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Karyakin AA, Presnova GV, Rubtsova MY, Egorov AM. Oriented immobilization of antibodies onto the gold surfaces via their native thiol groups. Anal. Chem. 2000;72(16):3805–3811. doi: 10.1021/ac9907890. [DOI] [PubMed] [Google Scholar]
- 44.Mcdevitt MR, Chattopadhyay D, Kappel BJ, et al. Tumor targeting with antibody-functionalized, radiolabeled carbon nanotubes. J. Nucl. Med. 2007;48(7):1180–1189. doi: 10.2967/jnumed.106.039131. [DOI] [PubMed] [Google Scholar]
- 45.Liu Z, Tabakman SM, Chen Z, Dai HJ. Preparation of carbon nanotube bioconjugates for biomedical applications. Nat. Protoc. 2009;4(9):1372–1382. doi: 10.1038/nprot.2009.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vogt RF, Phillips DL, Henderson LO, Whitfield W, Spierto FW. Quantitative Differences among Various Proteins as Blocking-Agents for Elisa Microtiter Plates. J. Immunol. Methods. 1987;101(1):43–50. doi: 10.1016/0022-1759(87)90214-6. [DOI] [PubMed] [Google Scholar]
- 47.Pruslin FH, To SE, Winston R, Rodman TC. Caveats and Suggestions for the Elisa. J. Immunol. Methods. 1991;137(1):27–35. doi: 10.1016/0022-1759(91)90390-2. [DOI] [PubMed] [Google Scholar]
- 48.Hoffman WL, Jump AA. Inhibition of the Streptavidin Biotin Interaction by Milk. Anal. Biochem. 1989;181(2):318–320. doi: 10.1016/0003-2697(89)90250-9. [DOI] [PubMed] [Google Scholar]
- 49.Kötitz R, Matz H, Trahms L, et al. SQUID based remanence measurements for immunoassays. IEEE Trans. Appl. Supercon. 1997;7(2):3678–3681. [Google Scholar]
- 50.Chemla YR, Crossman HL, Poon Y, et al. Ultrasensitive magnetic biosensor for homogeneous immunoassay. P. Natl. Acad. Sci. USA. 2000;97(26):14268–14272. doi: 10.1073/pnas.97.26.14268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Enpuku K, Soejima K, Nishimoto T, et al. Quantitative evaluation of magnetic immunoassay with remanence measurement. Supercond. Sci. Tech. 2006;19(5):S257–S260. [Google Scholar]
- 52. Baselt DR, Lee GU, Natesan M, Metzger SW, Sheehan PE, Colton RJ. A biosensor based on magnetoresistance technology. Biosens. Bioelectron. 1998;13(7–8):731–739. doi: 10.1016/s0956-5663(98)00037-2. * The first demonstration of GMR sensors used for detection of biological molecules.
- 53.Moodera JS, Kinder LR, Wong TM, Meservey R. Large Magnetoresistance at Room-Temperature in Ferromagnetic Thin-Film Tunnel-Junctions. Phys. Rev. Lett. 1995;74(16):3273–3276. doi: 10.1103/PhysRevLett.74.3273. [DOI] [PubMed] [Google Scholar]
- 54. Shen W, Schrag BD, Carter MJ, Xiao G. Quantitative detection of DNA labeled with magnetic nanoparticles using arrays of MgO-based magnetic tunnel junction sensors. Appl. Phys. Lett. 2008;93(3):033903. ** Biological measurement by MTJ sensors is demonstrated and the design of sensor array is proposed.
- 55.Mao S, Linville E, Nowak J, et al. Tunneling magnetoresistive heads beyond 150 Gb/in2. IEEE Trans. Magn. 2004;40(1):307–312. [Google Scholar]
- 56.Aytur TS, Beatty PR, Boser B, Anwar M, Ishikawa T. An immunoassay platform based on CMOS hall sensors. Solid-State Sens. Actuator and Microsystems Workshop. 2002:126–129. [Google Scholar]
- 57.Besse PA, Boero G, Demierre M, Pott V, Popovic R. Detection of a single magnetic microbead using a miniaturized silicon Hall sensor. Appl. Phys. Lett. 2002;80(22):4199–4201. [Google Scholar]
- 58.Perez JM, Josephson L, O'loughlin T, Hogemann D, Weissleder R. Magnetic relaxation switches capable of sensing molecular interactions. Nat. Biotechnol. 2002;20(8):816–820. doi: 10.1038/nbt720. [DOI] [PubMed] [Google Scholar]
- 59.Lee H, Sun E, Ham D, Weissleder R. Chip-NMR biosensor for detection and molecular analysis of cells. Nat. Med. 2008;14(8):869–874. doi: 10.1038/nm.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Carr C, Matlachov AN, Sandin H, Espy MA, Kraus RH. Magnetic sensors for bioassay: HTS SQUIDs or GMRs? IEEE Trans. Appl. Supercon. 2007;17(2):808–811. * Comparison between SQUID and GMR is made in terms of noise levels and their capability of integration.
- 61.Edelstein RL, Tamanaha CR, Sheehan PE, et al. The BARC biosensor applied to the detection of biological warfare agents. Biosens. Bioelectron. 2000;14(10–11):805–813. doi: 10.1016/s0956-5663(99)00054-8. [DOI] [PubMed] [Google Scholar]
- 62.Graham DL, Ferreira HA, Feliciano N, Freitas PP, Clarke LA, Amaral MD. Magnetic field-assisted DNA hybridisation and simultaneous detection using micron-sized spin-valve sensors and magnetic nanoparticles. Sensor Actuat. B-Chem. 2005;107(2):936–944. [Google Scholar]
- 63.Dittmer WU, De Kievit P, Prins MWJ, Vissers JLM, Mersch MEC, Martens MFWC. Sensitive and rapid immunoassay for parathyroid hormone using magnetic particle labels and magnetic actuation. J. Immunol. Methods. 2008;338(1–2):40–46. doi: 10.1016/j.jim.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 64.Aytur T, Foley J, Anwar M, Boser B, Harris E, Beatty PR. A novel magnetic bead bioassay platform using a microchip-based sensor for infectious disease diagnosis. J. Immunol. Methods. 2006;314(1–2):21–29. doi: 10.1016/j.jim.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 65. Han SJ, Xu L, Wilson RJ, Wang SX. A novel zero-drift detection method for highly sensitive GMR biochips. IEEE Trans. Magn. 2006;42(10):3560–3562. * A double modulation scheme is introduced to readout the signals in spectral domain and reduce the noise.
- 66.De Boer BM, Kahlman JaHM, Jansen TPGH, Duric H, Veen J. An integrated and sensitive detection platform for magneto-resistive biosensors. Biosens. Bioelectron. 2007;22(9–10):2366–2370. doi: 10.1016/j.bios.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 67. Gaster RS, Xu L, Han SJ, et al. Quantification of protein interactions and solution transport using high-density GMR sensor arrays. Nat. Nanotechnol. 2011;6(5):314–320. doi: 10.1038/nnano.2011.45. ** Monitoring of protein-protein interactions is demonstrated with GMR sensors.
- 68.Hall DA, Gaster RS, Lin T, et al. GMR biosensor arrays: A system perspective. Biosens. Bioelectron. 2010;25(9):2051–2057. doi: 10.1016/j.bios.2010.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Harris LA, Goff JD, Carmichael AY, et al. Magnetite nanoparticle dispersions stabilized with triblock copolymers. Chem. Mater. 2003;15(6):1367–1377. [Google Scholar]
- 70.Gaster RS, Hall DA, Wang SX. Autoassembly Protein Arrays for Analyzing Antibody Cross-Reactivity. Nano Lett. 2011;11(7):2579–2583. doi: 10.1021/nl1026056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gaster RS, Hall DA, Wang SX. nanoLAB: An ultraportable, handheld diagnostic laboratory for global health. Lab Chip. 2011;11(5):950–956. doi: 10.1039/c0lc00534g. [DOI] [PubMed] [Google Scholar]
- 72.Ramachandran N, Raphael JV, Hainsworth E, et al. Next-generation high-density self-assembling functional protein arrays. Nat. Methods. 2008;5(6):535–538. doi: 10.1038/nmeth.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Montor WR, Huang J, Hu YH, et al. Genome-Wide Study of Pseudomonas aeruginosa Outer Membrane Protein Immunogenicity Using Self-Assembling Protein Microarrays. Infect. Immun. 2009;77(11):4877–4886. doi: 10.1128/IAI.00698-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang SX, White RL, Webb CD, Li G. Magnetic nanoparticles, magnetic detector arrays, and methods for their use in detecting biological molecules. No 7,682,838. US Patent. 2010 Mar 23;