Abstract
Eutrophication is a water quality issue in lakes worldwide, and there is a critical need to identify and control nutrient sources. Internal phosphorus (P) loading from lake sediments can account for a substantial portion of the total P load in eutrophic, and some mesotrophic, lakes. Laboratory determination of P release rates from sediment cores is one approach for determining the role of internal P loading and guiding management decisions. Two principal alternatives to experimental determination of sediment P release exist for estimating internal load: in situ measurements of changes in hypolimnetic P over time and P mass balance. The experimental approach using laboratory-based sediment incubations to quantify internal P load is a direct method, making it a valuable tool for lake management and restoration.
Laboratory incubations of sediment cores can help determine the relative importance of internal vs. external P loads, as well as be used to answer a variety of lake management and research questions. We illustrate the use of sediment core incubations to assess the effectiveness of an aluminum sulfate (alum) treatment for reducing sediment P release. Other research questions that can be investigated using this approach include the effects of sediment resuspension and bioturbation on P release.
The approach also has limitations. Assumptions must be made with respect to: extrapolating results from sediment cores to the entire lake; deciding over what time periods to measure nutrient release; and addressing possible core tube artifacts. A comprehensive dissolved oxygen monitoring strategy to assess temporal and spatial redox status in the lake provides greater confidence in annual P loads estimated from sediment core incubations.
Keywords: Environmental Sciences, Issue 85, Limnology, internal loading, eutrophication, nutrient flux, sediment coring, phosphorus, lakes
Introduction
As a growing number of lakes worldwide suffer from cultural eutrophication, determination of the causes of water quality degradation is becoming increasingly important for lake management and restoration. Phosphorus (P) loading to lakes is generally implicated in eutrophication, as it is most often the nutrient limiting algal growth1. Historically, quantification of P loading to lakes focused on external sources, or P originating in the watershed via point and nonpoint sources. However, internal loading from lake sediments can account for a large portion, if not the majority, of the total P load in eutrophic lakes2-5. Thus, even substantial reductions in external loading to lakes can fail to result in water quality improvement due to the overriding effect of P release from sediments5-8. Because of the ecological and societal implications of P loading, including the cost and difficulty of P control, it is critical that P loads be accurately identified prior to enacting a management strategy.
At least two different mechanisms are responsible for phosphorus release from sediments. 1) During periods of anoxia or hypoxia, reducing conditions can result in the desorption of phosphate from iron oxyhydroxides at the sediment-water interface, causing diffusion of dissolved phosphate from the sediments into the water column9-11. 2) Disturbance of the sediment surface, through wind-induced resuspension and bioturbation, can result in the release of P into the water column by either desorption of P from resuspended sediment particles or release of dissolved P from the sediment pore water to the water column, respectively11-13.
Three principal approaches are available for quantifying internal P loading to lakes14,15. (1) In situ measurements of changes in hypolimnetic total phosphorus (TP) over time can be used when monitoring data are available. Internal load estimates based on in situ measurements suffer from high variability associated with the inherent spatial and temporal variability of environmental data and can be affected by inadequate monitoring frequency14. (2) Mass balance can be used to estimate internal loading, when complete P budgets can be constructed. However, it is rare that sufficient data are available on P inputs and exports to construct a complete P budget16. (3) Experimentally-determined sediment P release rates can be used, in combination with information on areal extent and duration of P release (i.e. anoxic period), to calculate internal P load. This is a direct method of internal P load quantification, although it too has limitations (see below).
Because management decisions often must be made on compressed time scales due to funding restrictions or societal pressures, experimental determination of internal P load can have greater utility for lake management and restoration since it requires less time and data than the in situ and mass balance approaches. Laboratory incubations of sediment cores, combined with monitoring of external loads, have been used to determine the relative contributions of internal and external P loads, with the goal of guiding management decisions to optimize nutrient source control2,4,17. In two Michigan lakes with extensive shoreline development and high percentages of impervious surface (>25%) in the sub-basins directly adjacent to the lake, internal P load was estimated to account for up to 80% of the total P load, prompting recommendations to focus management efforts on reducing sediment P release2,4. In contrast, experimental studies of sediment from a less developed lake in the same region showed that internal loading composed only 7% of the total P load, prompting a recommendation to focus P management strategies in the watershed17. Sediment core experiments also have been used in a Michigan lake to determine the potential effectiveness of aluminum sulfate (alum) treatment to reduce sediment P release rates2, the most efficient alum dosing concentration and the effects of sediment resuspension13, and the efficacy of an in situ alum treatment 1 year18 and 5 years19 following treatment. Experimental determination of internal P load is an effective approach to providing answers to key management questions in eutrophic lakes.
Protocol
1. Field Sampling
Conduct sampling once during each ice-free season (where applicable) for 1-2 years, if possible (i.e. 3 times/year in a north temperate climate). If time and/or funds prohibit seasonal sampling, conduct sampling once per year during mid-to-late summer.
Select sediment collection sites to cover different geographic regions within the lake. Choosing locations close to historical water quality and/or sediment sampling sites, when available, is often desirable to take advantage of historical data. Otherwise, attempt to select sites that represent different sediment types in the lake.
- Conduct water quality sampling prior to sediment core collection.
- At a minimum, measure water depth and vertical profiles of water temperature and dissolved oxygen. Near-bottom measurements should be taken as close to sediment surface as possible, without disturbing the sediment.
- Collect any other water quality data and samples that are desired to fulfill the specific goals of the study. Examples include vertical profiles of pH, conductivity, and turbidity; Secchi depth; photosynthetically active radiation (PAR) profiles; chlorophyll a; soluble reactive phosphorus (SRP); total phosphorus (TP); and nitrogen species.
At each sampling location, fill a 10 L carboy with water collected 1 m above the sediment surface using a Van Dorn or Niskin bottle. This will be used in the initial set up of sediment cores in the lab and for refilling cores after sampling during incubation. Place the carboy in a cooler with ice.
- Collect 6 sediment cores per site using a piston corer2,20.
- Refer to Fisher et al.20 for specific instructions regarding construction of the coring device. Briefly, the coring device consists of a graduated 0.6 m long polycarbonate core tube (7 cm i.d.), a polyvinyl chloride (PVC) attachment assembly for coupling to aluminum drive rods, a piston constructed of two rubber stoppers and an eye bolt, a plastic-coated piston cable with a swivel clip, and aluminum drive rods. Assemble the coring device according to the following steps:
- Thread the swivel clip end of the piston cable through the top of the PVC attachment assembly. Orient a core tube with bolt holes facing upward and extend the cable through the length of the core tube. Clip the piston cable to the eye bolt of the piston stopper.
- Attach the core tube to the PVC attachment assembly using a wire lock hitch pin. Pull the piston cable to advance the piston 20 cm from the bottom of the core tube to maintain a water layer on top of the sediment surface during core collection.
- Attach an aluminum drive rod to the other end of the PVC attachment assembly using a wire lock hitch pin. Lower coring device vertically into the water, adding additional sections of aluminum drive rod as needed.
- Position the corer vertically at the sediment-water interface and push downward, with the piston cable remaining stationary. To accomplish this, pull the piston cable taut once the corer is in place at the sediment-water interface, attach vice grips to cable, step on the cable to the inside of the vice grips, and then push downward.
- Bring core to the surface and seal with a rubber stopper prior to breaking the water surface. Secure bottom stopper with duct tape.
- Bolt the piston to the top of the core tube to keep it stationary during transit. Place the core tube in a vertical rack and maintain at ambient near-bottom lake temperature, using ice if necessary.
2. Laboratory Incubation
Upon return from the field, adjust cores to contain the desired depth of sediment and overlying water column. Excess sediment can be carefully let out the bottom of the core tube by removing the bottom stopper; add water from the carboy collected at the corresponding site, if needed. Commonly-used sediment and water column depths are 20 cm of sediment with a 25 cm overlying water column2,4,13,17-19, but these amounts can be modified as desired.
Place sediment core tubes into a darkened environmental growth chamber, with the temperature maintained to match ambient bottom water temperatures measured in the field.
Expose the cores to redox treatments. For the oxic treatment, bubble the water column of 3 cores/site with air. Bubble the water column of the remaining three cores per site with N2 (with ~350 ppm CO2 to buffer pH) for the anoxic treatment. Ensure a slow and consistent bubble rate that is nondisruptive to the sediment surface.
On day 1 of core incubation, filter each 10 L carboy containing near-bottom water collected from each site in the field. Using a peristaltic pump and filter cartridge housing, filter water first through a 1 μm filter, followed by a 0.2 μm filter. Store filtered water at 4 °C for the duration of the core incubation.
- Sample cores for P release rate over the duration of the incubation period2,3. Because this is a redox-sensitive experiment, take precautions to maintain redox treatment conditions whenever possible.
- With a syringe, remove a 40 ml water sample through the sampling port of each sediment core on days 0 (i.e. at the time cores are placed in the growth chamber), 1, 2, 4, 6, 8, 12, 20, 24, and 28 of core incubation. (Note: if changes over very short time frames are desired, the sampling regime can be modified to sample at hr 1, 2, 4, 8, etc. However, the system is often still equilibrating through the first 12 hr, so P release dynamics can be quite variable at the start of incubations.)
- Immediately after removal, dispense a 20 ml subsample into a scintillation vial and refrigerate for analysis of TP. Filter the other 20 ml subsample through a 0.45 μm membrane filter and into a scintillation vial and freeze for analysis of SRP.
- Replace the 40 ml subsample with an equal volume of filtered water (see step 2.4) from the corresponding site.
3. P Release Rate Calculation
Calculate flux (release rate) based on the change in water column TP or SRP using the following equation2: Prr = (Ct - C0) × V / A where Prr is the net P release (positive values) or retention (negative values) rate per unit surface area of sediment (mg P/m2/d), Ct is the TP or SRP concentration in the water column at time t, C0 is the TP or SRP concentration at time 0, V is the volume of water in the water column of the core tube, and A is the planar surface area of the sediment cores. Calculate P release rate using the linear portion of the concentration vs. time curve to give the maximum apparent release rate4,13,18,19. To avoid potential short-term bias, choose nonconsecutive sampling dates for Ct and C018,19.
4. Internal P Load Calculation
- Calculate annual P flux.
- For each season during which sampling occurred, multiply anoxic and oxic flux individually by the number of days in that season. Sum the seasonal values to yield annual anoxic and oxic flux (mg/m2/year). If multiple sites in the same lake were sampled, this calculation can be performed either separately for each site or using the mean flux values for all sites (see Section 4.2.2).
- P release from sediments is generally very low during winter due to low water temperatures. If sampling was not conducted during winter, assume that P flux was 0 for that season14,15.
- Because the majority of internal P release occurs during summer, annual internal P flux can be coarsely estimated from summer measurements alone in the absence of seasonal data2,15,17. For this approach, calculate the P flux according to Section 4.1.1 and assume 0 flux for all seasons except summer. Recognize that this will be a conservative estimate of annual P release.
- If available, dissolved oxygen data may be used to refine the annual P flux calculation2,4. Such data may reveal that a lake experiences hypoxia or anoxia for a certain percentage of the year, or during specific seasons. In those cases, use anoxic and oxic flux according to the appropriate proportion or season and sum the values to calculate annual internal P flux.
- For example, if hypoxia or anoxia was measured only during summer, calculate Section 4.1.1 using anoxic flux for summer and oxic flux for the remaining seasons. Sum the values to obtain annual internal P flux. Similarly, if routine dissolved oxygen monitoring data indicate that the lake experiences hypoxia or anoxia 35% of the year, multiply annual anoxic flux from Section 4.1.1 by 0.35 and annual oxic flux from Section 4.1.1 by 0.65 and sum the values to calculate annual internal P flux.
- Polymictic lakes pose a particular challenge to internal P load calculation, due to their frequent mixing and spatial and temporal variability in redox condition14. Nürnberg et al.16 developed a model to calculate the number of anoxic days a polymictic lake may experience during a season or year. The active sediment release area and time (AA), which represents the length of time (days/season) that an area similar to the lake surface area is actively releasing P, can be calculated as follows: AA = -36.2 + 50.2 log (Pseason) + 0.762 z/A0.5 where P is the average water column TP concentration during a given season, z is mean depth, and A is lake surface area. To calculate annual internal P flux, multiply AA by anoxic flux and the number of oxic days by oxic flux for each season, and then sum all values.
- Scale up internal P flux to the entire lake area.
- Multiply the annual P flux from step 4.1 by the entire lake surface area to calculate annual internal P load. Unless annual P flux was calculated according to Sections 4.1.4 or 4.1.5, use the anoxic annual flux to calculate annual internal P load. Otherwise, use the flux calculated in Sections 4.1.4 or 4.1.5.
- If multiple sites in the same lake were sampled, the lake can be divided into geographic zones associated with each site. Multiply the annual anoxic P flux (or annual flux from Sections 4.1.4 or 4.1.5) for each site by the surface area of the zone, then sum the values to obtain annual internal P load for the entire lake4,17. Alternatively, the mean annual P flux of all sites can be used in Section 4.2.1.
- Detailed dissolved oxygen data may indicate that specific areas of the lake experience hypoxic or anoxic conditions (e.g. deep areas), while other areas remain oxic year round. If available, use this information to refine the flux × area calculation (Steinman et al., in prep). Multiply the anoxic surface area by the annual anoxic flux and multiply the oxic surface area by the annual oxic flux, and sum the two values to calculate annual internal P load.
Representative Results
Internal P release was measured from sediment cores collected in Mona Lake, Michigan, to identify the relative contribution of internal versus external P loads4. Four sites were sampled over three seasons to estimate annual internal P load, accounting for spatial variation in P flux. Sediment cores were incubated for 20-28 days under anoxic and oxic conditions, and the overlying water column was sampled for SRP and TP concentrations at regular intervals during the incubation period. The anoxic treatment triggered SRP and TP release from the sediments; however, we are presenting only the TP flux results for illustrative purposes. TP concentrations were highest during summer in anoxic treatments, and spatial variability in TP release was evident during all seasons (Figure 1). Mean internal TP flux was less than 1.4 mg P/m2/day in all oxic cores; negative flux values at 3 of the 4 sites during fall indicated that oxic sediments were acting as a sink rather than a source of P during that season4 (Table 1). TP release rates were considerably higher in anoxic cores, with flux as high as 15.56 mg P/m2/day in the summer and as low as 0.80 mg P/m2/day in the spring4 (Table 1). These flux values were used to calculate seasonal internal P flux based on dissolved oxygen conditions measured at the time of sediment core collection4. Seasonal internal P load was calculated by scaling up the flux at each site to the surface area of the corresponding geographic zone4; seasonal values were summed to estimate annual internal P load, assuming 0 flux during winter. Annual internal P load was estimated to be 3.4 metric tons, with the majority of the load occurring during summer (Table 2). Comparing these results with concurrent external P load estimates, it was estimated that the sediments in Mona Lake contribute between 9-82% of the total annual P load4 (Table 2).
A series of experiments was conducted in Spring Lake, Michigan, to determine 1) the potential effectiveness of aluminum sulfate (alum) treatment in reducing internal P loading2 and 2) the efficacy of an in situ alum treatment18,19. Laboratory experiments simulating a lake-wide application of alum demonstrated a dramatic decline in internal P release with treatment2 (Figure 2). Similar to the example above, we are presenting only TP release from these experiments as representative results. In anoxic cores without alum treatment (simulating natural summer conditions in Spring Lake sediments), mean TP concentrations in the overlying water column reached more than 1.2 mg/L (Figure 2). In contrast, anoxic cores dosed with alum had virtually no P release and concentrations were not different from either of the oxic treatments2 (Figure 2). A sediment core incubation conducted 1 year following lake-wide application of alum in Spring Lake revealed that the treatment was highly effective at reducing sediment P release, with release rates similar between anoxic and oxic treatments18 (Figure 3A). When the experiment was repeated 5 years following alum treatment, TP release remained substantially lower than pretreatment but was greater than that measured 1 year following treatment, suggesting a slight decline in alum efficacy19 (Figure 3B).
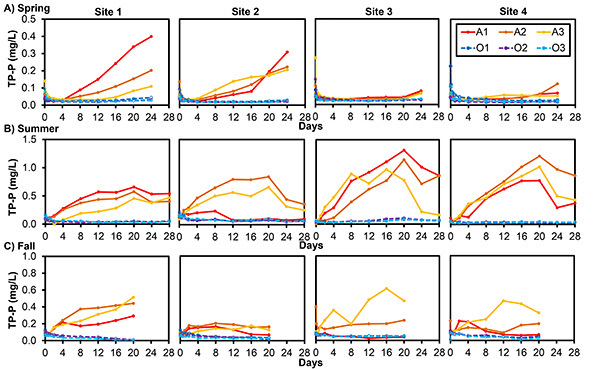 Figure 1. Total phosphorus (TP) concentrations (mg/L) measured during laboratory incubations of sediment cores collected from Mona Lake, Michigan, during spring (A), summer (B), and fall (C)4. TP was measured in the water overlying sediment cores from 4 lake sites over a 20- to 28-day incubation. The letter in the legend refers to redox state (A = anoxic treatment; O = oxic treatment); the number refers to replicate number (1-3). Note the different scales on the y-axes among seasons. Click here to view larger image.
Figure 1. Total phosphorus (TP) concentrations (mg/L) measured during laboratory incubations of sediment cores collected from Mona Lake, Michigan, during spring (A), summer (B), and fall (C)4. TP was measured in the water overlying sediment cores from 4 lake sites over a 20- to 28-day incubation. The letter in the legend refers to redox state (A = anoxic treatment; O = oxic treatment); the number refers to replicate number (1-3). Note the different scales on the y-axes among seasons. Click here to view larger image.
| Season | Site | Anoxic flux, mg P/m2/day | Oxic flux, mg P/m2/day |
| Spring | 1 | 2.77 ± 1.53 | 0.25 ± 0.01 |
| 2 | 2.82 ± 0.83 | 0.26 ± 0.23 | |
| 3 | 0.80 ± 0.07 | 0.17 ± 0.07 | |
| 4 | 1.15 ± 0.71 | 0.12 ± 0.04 | |
| Summer | 1 | 7.06 ± 2.57 | 0.46 ± 0.24 |
| 2 | 9.27 ± 5.99 | 1.36 ± 0.73 | |
| 3 | 15.56 ± 1.00 | 0.90 ± 0.29 | |
| 4 | 13.63 ± 1.82 | 0.59 ± 0.41 | |
| Fall | 1 | 4.48 ± 1.56 | -0.66 ± 0.22 |
| 2 | 2.87 ± 0.97 | -1.14 ± 0.93 | |
| 3 | 3.10 ± 4.08 | 0.51 ± 0.13 | |
| 4 | 6.46 ± 4.66 | -0.79 ± 0.23 |
Table 1.Mean (±SD) maximum apparent TP flux (mg P/m2/day) in sediment cores collected from Mona Lake, Michigan, and incubated under anoxic and oxic conditions4. Flux was calculated from the change in TP concentrations over time, shown in Figure 1.
| Season | Internal P Load, t | External P Load, t | Internal Load Contribution, % |
| Spring | 0.055 | 0.557 | 9.0% |
| Summer | 2.272 | 0.862 | 72.5% |
| Fall | 1.127 | 0.242 | 82.3% |
| Winter | 0.000 | ||
| Annual | 3.454 |
Table 2. Annual and seasonal internal P load estimates (metric tons, t) for Mona Lake, Michigan, calculated based on maximum apparent TP flux4 (shown in Table 1). Seasonal internal P load estimates are compared to external P load estimates to determine the contribution of internal load to total P load.
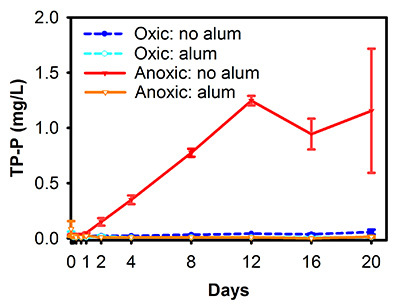 Figure 2. Mean (±SD) TP concentrations (mg/L) measured during laboratory incubations of sediment cores collected from Spring Lake, Michigan, and experimentally treated with aluminum sulfate (alum) under oxic and anoxic conditions2. TP was measured in the water column overlying sediment cores over a 20-day incubation period. This figure has been modified from Steinman et al.2 Reprinted by Permission, ASA, CSSA, SSSA. Click here to view larger image.
Figure 2. Mean (±SD) TP concentrations (mg/L) measured during laboratory incubations of sediment cores collected from Spring Lake, Michigan, and experimentally treated with aluminum sulfate (alum) under oxic and anoxic conditions2. TP was measured in the water column overlying sediment cores over a 20-day incubation period. This figure has been modified from Steinman et al.2 Reprinted by Permission, ASA, CSSA, SSSA. Click here to view larger image.
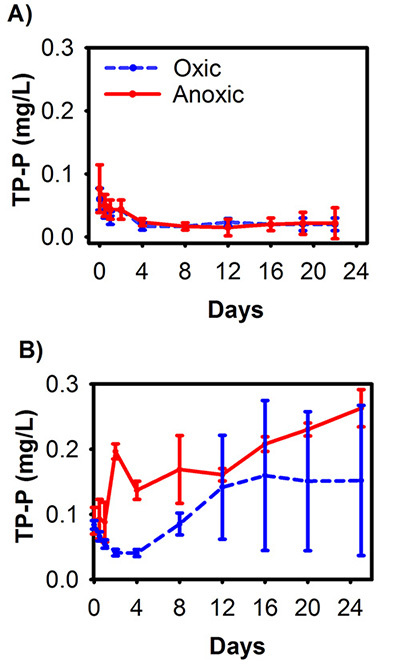 Figure 3. Mean (±SD) TP concentrations (mg/L) measured during laboratory incubations of sediment cores collected from Spring Lake, Michigan 1 year after18 (A) and 5 years after19 (B) a lake-wide application of alum. Sediment cores were subjected to oxic and anoxic treatments and the overlying water column was sampled for TP concentration over a 22-day (A) to 25-day (B) incubation. This figure has been modified from Steinman et al.18; panel A and Steinman et al.19; panel B. Reprinted by Permission, ASA, CSSA, SSSA. Click here to view larger image.
Figure 3. Mean (±SD) TP concentrations (mg/L) measured during laboratory incubations of sediment cores collected from Spring Lake, Michigan 1 year after18 (A) and 5 years after19 (B) a lake-wide application of alum. Sediment cores were subjected to oxic and anoxic treatments and the overlying water column was sampled for TP concentration over a 22-day (A) to 25-day (B) incubation. This figure has been modified from Steinman et al.18; panel A and Steinman et al.19; panel B. Reprinted by Permission, ASA, CSSA, SSSA. Click here to view larger image.
Discussion
Nutrient loading to lakes can result in both environmental and economic impairments21-23; therefore, it is crucial that society understands the nature of the nutrient sources and how to manage them. Costly attempts to reduce nutrient loading may not improve water quality if the appropriate contributing source (i.e. lake sediments or watershed inputs) is not targeted for management action, thereby resulting in setbacks in lake restoration and frustration on the part of stakeholders. Particularly in shallow eutrophic lakes, quantification of internal phosphorus load is a critical step in identifying a management strategy to improve water quality conditions. Even when sediments are implicated as a major source of nutrients, reductions in external P load must be included in any lake management strategy for alleviating eutrophication, since external inputs of P ultimately accumulate in the sediments and fuel future internal loading24,25.
Although other approaches exist to estimate internal P load, experimental determination of P release rates is a direct method that can be adjusted to answer a variety of management and research questions. Laboratory incubations of sediment cores collected from Spring Lake, Michigan, were used to determine the potential effectiveness of an alum treatment2 and the most efficient application concentration13. As a result of the findings from these laboratory-based studies, the stakeholders developed confidence that the alum treatment could control P release in Spring Lake sediments. Consequently, they approved a 10-year assessment to fund an alum treatment; subsequent sediment core incubations revealed that the treatment was effective at reducing sediment P flux 1 year18 and 5 years19 following treatment. Sediment core incubations have also been used to evaluate the effects of sediment resuspension13 and bioturbation (G. Nogaro and A. Harris, unpublished data) on P release.
Several additional sediment analyses can be performed in conjunction with core incubations to provide information that is useful in interpreting sediment P release results. The top 5 or 10 cm of sediment can be extruded from cores for analysis of sediment TP, porewater SRP, sequential P fractionation, and metals4,18,19. An example of sequential P fractionation26 that can be useful in internal loading studies involves determining the amount of P bound to 1) aluminum (Al-P) or iron (Fe-P), which represents a redox insensitive (Al-P) and a redox-sensitive (Fe-P) mineral association that can become soluble under anoxic conditions, and 2) calcium (Ca-P) or magnesium (Mg-P), which are both stable mineral associations. Further, sediment Fe:P ratios can be calculated to provide insight on the potential P-binding capacity of sediments. Iron-rich sediments that remain oxidized have been shown to release very little P when Fe:P ratios are above 15 (by weight)27. These additional sediment analyses can be performed on cores following internal load incubation4,18,19, or on replicate cores taken at the time of internal load core collection but not used for release rate measurements.
Despite the benefits of experimental determination of sediment P flux, the approach is not without limitations. A number of assumptions must often be made that can add uncertainty to the results:
One assumption is that release rates from the sediment cores are representative of conditions in the study lake. To minimize the impact of this assumption, sampling strategies should be designed to represent as much of the spatial and temporal variability as possible in sediment P release. Sampling sites should cover as much geographic range as possible within a lake to capture spatial variation in sediment characteristics2. If available, bathymetric maps can be used to select sites that are representative of the range of bottom depths in the lake. Other considerations for capturing spatial variation include the location of major tributary inputs and the presence of distinct lake basins. When possible, laboratory incubations should be conducted during each ice-free season and over multiple years to capture temporal variation in release rates.
A second assumption is that the incubation conditions are representative of natural conditions. A constant anoxic condition creates an optimal situation for the release of P, which may not naturally occur in the study lake. Thus, anoxic treatments may overestimate sediment P release; therefore, it may be best to think of release rates measured in anoxic treatments as maximum potential rates.
In order to calculate annual internal P load, assumptions about the timing, duration, and spatial extent of hypolimnetic anoxia must be made. For example, in strongly stratified lakes with relatively consistent water depth and confirmed hypolimnetic anoxia, some studies have assumed that the entire lake area is anoxic during stratified periods for the purpose of annual internal P load estimation2,4. However, this may result in an overestimation of load due to oxic sediments in shallow littoral areas4. Thus, a comprehensive dissolved oxygen monitoring strategy that captures diel, seasonal, and spatial variability in redox condition is highly recommended for accurate annual internal load estimation.
Finally, laboratory incubations may introduce experimental artifacts due to the inability to completely simulate natural conditions. For example, because the sediments are enclosed in core tubes, water exchange through permeable sediments is precluded; however, it is possible to design flow-through core tubes that mitigate this issue28. Other artifacts include the inability to mimic major mixing events or wind-wave action, which could disrupt sediment integrity in natural systems.
Given that the sediment core incubation approach can be used to generate reasonable internal P load estimates in as little as one year (although multiple years of data provide more robust information), it is a valuable tool for informing lake management decisions. When used to develop lake management or restoration plans, it can help ensure wise use of financial resources. In lakes where internal P load management has already occurred, sediment core incubations can verify the efficacy of treatment and be used to modify the trajectory of management, if warranted.
Disclosures
The authors have nothing to disclose.
Acknowledgments
The authors gratefully acknowledge the field and lab assistance provided by James Smit and Kurt Thompson. Funding for original studies for which this protocol was developed was provided by the Spring Lake-Lake Board2,13,18,19; the Michigan Department of Environmental Quality4; and Jim Duncan, Dave Farhat, and the President’s Office at Grand Valley State University17.
References
- Schindler DW. The dilemma of controlling cultural eutrophication of lakes. Proc. Royal Soc. B. 2012;279:4322–4333. doi: 10.1098/rspb.2012.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman AD, Rediske R, Reddy KR. The reduction of internal phosphorus loading using alum in Spring Lake. Michigan. J. Env. Qual. 2004;33:2040–2048. doi: 10.2134/jeq2004.2040. [DOI] [PubMed] [Google Scholar]
- Moore PA, Reddy KR, Fisher MM. Phosphorus flux between sediment and overlying water in Lake Okeechobee, Florida: spatial and temporal variations. J. Env. Qual. 1998;27:1428–1439. [Google Scholar]
- Steinman AD, Chu X, Ogdahl M. Spatial and temporal variability of internal and external phosphorus loads in an urbanizing watershed. Aquatic Ecol. 2009;43:1–18. [Google Scholar]
- Søndergaard M, Bjerring R, Jeppesen E. Persistent internal phosphorus loading during summer in shallow eutrophic lakes. Hydrobiologia. 2013;710:95–110. [Google Scholar]
- Björk S. International Congress of European Water Pollution Control Association. Rome, Italy: 1985. Lake restoration techniques. In: Lake pollution and recovery; pp. 293–301. [Google Scholar]
- Graneli W. Internal phosphorus loading in Lake Ringsjon. Hydrobiologia. 1999;404:19–26. [Google Scholar]
- Steinman AD, et al. In: Phosphorus in Lake Okeechobee: sources, sinks, and strategies. In: Phosphorus Biogeochemistry of Subtropical Ecosystems: Florida as a case example. Reddy KR, O’Connor GA, Schelske CL, editors. New York: CRC/Lewis Publ; 1999. pp. 527–544. [Google Scholar]
- Mortimer CH. The exchange of dissolved substances between mud and water in lakes. J. Ecol. 1941;29:280–329. [Google Scholar]
- Marsden MW. Lake restoration by reducing external phosphorus loading: the influence of sediment phosphorus release. Freshwater Biol. 1989;21:139–162. [Google Scholar]
- Søndergaard M, Jensen JP, Jeppesen E. Role of sediment and internal loading of phosphorus in shallow lakes. Hydrobiologia. 2003;506-509:135–145. [Google Scholar]
- Selig U. Particle size-related phosphate binding and P-release at the sediment-water interface in a shallow German lake. Hydrobiologia. 2003;492:107–118. [Google Scholar]
- Steinman AD, Nemeth L, Nemeth E, Rediske R. Factors influencing internal P loading in a western Michigan, drowned river-mouth lake. J. N. Am. Benthol. Soc. 2006;25:304–312. [Google Scholar]
- Nürnberg GK. Assessing internal phosphorus load—problems to be solved. Lake Reservoir Manag. 2009;25:419–432. [Google Scholar]
- Nürnberg GK, LaZerte BD, Loh PS, Molot LA. Quantification of internal phosphorus load in a large, partially polymictic and mesotrophic Lake Simcoe, Ontario. J. Great Lakes Res. 2013;39:271–279. [Google Scholar]
- Nürnberg GK, Tarvainen M, Ventellä A-M, Sarvala J. Internal phosphorus load estimation during biomanipulation in a large polymictic and mesotrophic lake. Inland Waters. 2012;2:147–132. [Google Scholar]
- Steinman AD, Ogdahl M, Luttenton M. In: An analysis of internal phosphorus loading in White Lake Michigan. In: Lake Pollution Research Progress. Miranda FR, Bernard LM, editors. New York: Nova Science Publishers; 2008. pp. 311–325. [Google Scholar]
- Steinman AD, Ogdahl M. Ecological effects after an alum treatment in Spring Lake Michigan. J. Env. Qual. 2008;37:22–29. doi: 10.2134/jeq2007.0142. [DOI] [PubMed] [Google Scholar]
- Steinman AD, Ogdahl ME. Macroinvertebrate response and internal phosphorus loading in a Michigan Lake after alum treatment. J. Env. Qual. 2012;41:1540–1548. doi: 10.2134/jeq2011.0476. [DOI] [PubMed] [Google Scholar]
- Fisher MM, Brenner M, Reddy KR. A simple, inexpensive piston corer for collecting undisturbed sediment/water interface profiles. J. Paleolimnol. 1992;7:157–161. [Google Scholar]
- Carpenter SR, Bolgrien D, Lathrop RC, Stow CA, Reed T, Wilson MA. Ecological and economic analysis of lake eutrophication by nonpoint pollution. Aus. J. Ecol. 1998;23:68–79. [Google Scholar]
- Smith VH. In: Cultural eutrophication of inland, estuarine, and coastal waters. In: Successes, limitations, and frontiers in ecosystem science. Pace ML, Groffman PM, editors. New York: Springer; 1998. pp. 7–49. [Google Scholar]
- Pretty JN, Mason CF, Nedwell DB, Hine RE, Leaf S, Dils R. Environmental costs of freshwater eutrophication in England and Wales. Env. Sci. Technol. 2003;37:201–208. doi: 10.1021/es020793k. [DOI] [PubMed] [Google Scholar]
- Carpenter SR. Eutrophication of aquatic ecosystems: Bistability and soil phosphorus. Proc. Natl. Acad. Sci. U.S.A. 2005;102:10002–10005. doi: 10.1073/pnas.0503959102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson L-A, et al. Biomanipulation as an application of food chain theory: constraints, synthesis and recommendations for temperate lakes. Ecosystems. 1998;1:558–574. [Google Scholar]
- Moore PA, Reddy KR. Role of Eh and pH on phosphorus geochemistry in sediments of Lake Okeechobee, Florida. J. Env. Qual. 1994;23:955–964. doi: 10.2134/jeq1994.00472425002300050016x. [DOI] [PubMed] [Google Scholar]
- Jensen HS, Kristensen P, Jeppesen E, Skytthe A. Iron:phosphorus ratio in surface sediment as an indicator of phosphate release from aerobic sediments in shallow lakes. Hydrobiologia. 1992;235-236:731–743. [Google Scholar]
- Roychoudhury AN, Viollier E, Van Cappellen P. A plug flow-through reactor for studying biogeochemical reactions in undisturbed aquatic sediments. App. Geochem. 1998;13:269–280. [Google Scholar]


