Abstract
We have explored the mechanism(s) underlying 1,25 dihydroxyvitamin D’s (1,25(OH)2D) suppression of agonist-induced vascular smooth muscle cells (VSMC) proliferation. Quiescent cultured adult rat VSMC were treated with 1,25(OH)2D for 48 hours and endothelin (ET) or angiotensin II (AII) for the final 24 hours. We show that VSMC responded to 1,25(OH)2D or its less hypercalcemic analogue RO 25-6760 with ~70% inhibition of ET-dependent 3H-thymidine incorporation. The inhibition was linked to a comparable reduction in ET-stimulated cyclin dependent kinase 2 (Cdk2) activity and suppression of an ET-induced Cdk2 activator, cell division cycle 25 homolog A (Cdc25A). Both 1,25(OH)2D and RO 25-6760 completely inhibited the ET-dependent increase in Cdc25A mRNA and protein levels, phosphatase and promoter activities. 1,25(OH)2D also suppressed AII-induced DNA synthesis, Cdk2 activity and Cdc25A gene transcription. Inhibition of Cdc25A gene expression using a siRNA approach resulted in significant inhibition of ET or AII-dependent Cdk2 activity and 3H-thymidine incorporation. The Cdc25A siRNA-mediated inhibition of ET or AII-induced Cdk2 activity and DNA synthesis was not additive with that produced by 1,25(OH)2D treatment. These data demonstrate that 1,25(OH)2D inhibits VSMC proliferation through a Cdc25A-dependent mechanism and suggest that this hormone may prove useful in the management of disorders characterized by aberrant proliferation of VSMC in the vascular wall.
Keywords: vitamin D, endothelin, Cdk2, Cdc25A, vascular smooth muscle cell
1. Introduction
Cyclin dependent kinase 2 (Cdk2) plays a central role at the G1/S transition and during S phase of the cell cycle [1]. Activation of Cdk2 is responsible for the inactivating phosphorylation of the retinoblastoma gene product, pRB, and the related protein p130 that allows for cell cycle progression [2, 3]. It also phosphorylates other key substrates whose activation is necessary to trigger and organize DNA synthesis [4]. Blockade of Cdk2 expression by Cdk2 antisense oligonucleotide [5] or suppression of Cdk2 activity with a specific kinase inhibitor [6] dramatically reduces aberrant vascular smooth muscle cell (VSMC) proliferation in animal models. Cdk2 activation is initiated by the binding of a cyclin partner, cyclin E or cyclin A, followed by an activating phosphorylation of Thr160 by Cdk-activating kinase (Cdk7/cyclin H), recruitment of the Cdk inhibitor p21 and dephosphorylation of inhibitory phosphates on Thr14 and Tyr15 by Cdc25A, a key cell cycle phosphatase [7, 8].
Numerous studies have shown that Cdc25A plays a rate-limiting role in Cdk2 activation [7, 9]. Microinjection of Cdc25A antibodies arrests cells in G1 before S phase [10]. Ectopic expression of Cdc25A activates Cdk2 in complex with cyclin E or cyclin A and accelerates S phase entry [9]. Rapid degradation of Cdc25A results in sustained inhibitory phosphorylation of Cdk2, leading to suppression of Cdk2 activity and a block in the progression from G1 to S phase [11].
VSMC proliferation is stimulated by a number of vasoactive peptides including endothelin (ET) [12] and angiotensin (AII) [13]. We have shown previously that ET selectively activates Cdk2 activity in neonatal VSMC through an extracellular signal regulated kinase (ERK)-dependent mechanism involving Cdc25A [14], suggesting a possible mechanism for its pro-proliferative activity.
The nuclear receptor ligand 1,25 dihydroxyvitamin D3 (1,25(OH)2D) and its less hypercalcemic analogues have been shown to inhibit proliferation of mitogen-activated VSMC proliferation in vitro [15-18]. Our previous work showed that 1,25(OH)2D inhibits ET-induced activation of cyclin dependent kinase 2 (Cdk2) activity in neonatal rat VSMC [18], a cell line which can be passaged in culture while retaining some features of the adult phenotype [19]. Beyond this there is little information available to clarify the mechanism(s) behind the 1,25(OH)2D-dependent inhibition, particularly in fully differentiated adult VSMC.
In the present study, we show that ET increases Cdc25A gene promoter activity, gene expression and phosphatase activity and that these increases are required for ET’s pro-proliferative activity in primary cultures of mature, adult rat aortic VSMC. Importantly, we find that 1,25(OH)2D exerts its anti-proliferative effects through the suppression of ET-dependent Cdc25A gene transcription, expression and activity. 1,25(OH)2D had a similar inhibitory effect on AII-induced DNA synthesis, Cdk2 activity and Cdc25A gene transcription. These results suggest that 1,25(OH)2D analogues could, through the inhibition of Cdc25A gene expression, prove to be of significant value in the management of vascular diseases characterized by aberrant VSMC proliferation.
2. Materials and Methods
2.1. Materials
Anti-Cdk2, -Cdc25A, -glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and -VDR antibodies were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). 3H-Thymidine and ATP were purchased from Perkin Elmer Life Sciences (Boston, MA), PD098059 from Research Biochemicals International (Natick, MA), ET from American Peptide (Sunnyvale, CA), AII from Phoenix Pharmaceuticals (Burlingame, CA) and 1,25(OH)2D from Calbiochem Inc. (La Jolla, CA). RO 25-6760 was kindly provided by M. Uskokovic of Bioxell Inc (Nutley, NJ). The −450 Cdc25A-luciferase reporter was from R. Prywes [20]. Other reagents were purchased from standard commercial suppliers.
2.2. Cell Culture
Adult rat aortic VSMC were prepared from 250 gram adult male Sprague-Dawley rats using published techniques[14]. The investigations conformed to the Guide for the Care and Use of Laboratory Animals (NIH publication no. 85-23, 1996) and all animal-related experimental protocols were approved by the Institutional Animal Care and Use Committee at University of California San Francisco. Cells were used after reaching 75-80% confluence at passage 4-7.
2.3. 3H-Thymidine Incorporation
Cells cultured in growth media [12] were changed to serum-substitute media containing insulin and transferrin [14] for the ensuing 24-hour period. Cells were then treated with 1,25(OH)2D, RO 25-6760, or vehicle for 48 hours and vehicle, 10−7 mol/L ET or 10−7 mol/L AII (these concentrations were used in all experiments) for the final 24 hours. 3H-thymidine incorporation was measured as previously described [12].
2.4. Total RNA Isolation and Real-time PCR
Serum-deprived cells were treated with 10−8 mol/L 1,25(OH)2D or 10−9 mol/L RO 25-6760 for 48 hours and ET, AII or vehicle for the final 9 hours. Total RNA from cells was isolated using the RNeasy kit (Qiagen, Germany) and reverse transcribed into cDNA. Real-time PCR was carried out with SYBR Green primers (rat-Cdc25A-sense: GACTGTCCCCTGTCACCAAC, rat-Cdc25A-antisense: CAGAGGAGCCCATTCTCTGT; rat-18S rRNA sense: AAACGGCTACCACATCCAAG, rat-18S rRNA antisense: TCTTGGCAAATGCTTTCGC). Cdc25A mRNA levels were normalized to 18S rRNA expression. Real-time PCR were carried out on the ABI Prism 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA).
2.5. Transfection and Luciferase Assay
Serum-deprived cells were transiently co-transfected with 0.5 μg of the −450 Cdc25A luciferase and 0.5 μg Renilla-Luc using lipofectin reagent (Invitrogen Corp, Carlsbad, CA.). Cells were then incubated with 10−8 mol/L 1,25(OH)2D or 10−9 mol/L RO 25-6760 in serum substitute-containing DMEM for 48 hours with or without ET for the final 20 hours. At the end of the treatment, cells were lysed and luciferase activity was measured using the Dual-Luciferase® reporter assay system (Promega). Cdc25A luciferase activity was normalized for Renilla luciferase activity to control for transfection efficiency.
2.6. Cdc25A Phosphatase Assay
Quiescent cells were treated with 10−8 mol/L 1,25(OH)2D or 10−9 mol/L RO 25-6760 for 48 hours and ET for the final 20 hours. 100 μg of cellular lysate was incubated with 1 μg of anti-Cdc25A antibody and 10 μl of protein G-Sepharose for 2 hours at 4°C. Immunoprecipitates were washed and analyzed for phosphatase activity [14] by adding 40 μmol/L O-methylfluorescein monophosphate (OMFP) at 23°C for 1 hour. Fluorescent emissions from the hydrolysis of OMFP to OMF were measured using a Quantum Master Fluorometer. Data were fit to the appropriate equation using nonlinear least-squares analysis in ProFit 5.1.0 (Quantum Soft) [14].
2.7. Immunoprecipitation and Kinase Assay
Quiescent cells were incubated with vehicle, 10−8 mol/L 1,25(OH)2D or 10−9 mol/L RO 25-6760 for 48 hours in presence or absence of ET or AII for the final 24 hours and lysed with buffer [12] containing protease inhibitors (1 Complete™ tablet/50 ml buffer, Roche Applied Science, Indianapolis, IN). 150 μg of supernatant protein was incubated with 1 μg of anti-Cdk2, and 10 μl of protein G-Sepharose for 1-2 hours at 4°C. Immunoprecipitates were collected and kinase reactions were carried out as described previously [12] with appropriate substrate (1 μg histone 1). Reaction products were subjected to electrophoresis on SDS-polyacrylamide gels which were then dried and exposed to X-ray film.
2.8. Immunoblotting
Twenty micrograms of supernatant protein was denatured at 100°C for 4 min, subjected to 10% SDS-polyacrylamide gel electrophoresis, and transferred onto polyvinylidene fluoride (PVDF) membranes. The membranes were blocked with 5% nonfat milk in TBS-T [12] for one hour and probed with anti-VDR, -Cdk2 or -Cdc25A antibodies. Blots were then rinsed in TBS-T and incubated with horseradish peroxidase-conjugated secondary antibodies for 1 hour at room temperature. ECL® reagent (Amersham Life Sciences, Arlington Heights, IL) was used to visualize the presence of targeted proteins. The same membranes were washed and reprobed with anti-GAPDH antibody for signal normalization.
2.9. siRNA silencing of Cdc25A gene
Twenty one-nucleotide annealed duplex siRNAs (5′-CCAAUGGACGUGAGAAAUAtt-3′ sense strand) directed against rat Cdc25A gene sequence were chemically synthesized and purified using a commercial source (Ambion, Austin, TX), as was a negative control siRNA (Ambion) containing a 19-bp scrambled sequence. Cells were transfected with 100 pmol of Cdc25A siRNA or control siRNA using oligofectamine (Invitrogen). After transfection for 48 hours, cells were treated with or without ET or AII for 20-24 hours. Cellular lysates were prepared and analyzed for Cdc25A and GAPDH expression by Western blot analysis. In a separate experiment, cells were treated with Cdc25A siRNA or the negative control, then subjected to 3H-thymidine incorporation or immunoprecipitation and kinase assay.
2.10. Statistical Analysis
Data was analyzed by one-way ANOVA using Student-Newman-Keuls post-hoc test to assess significance.
3. Results
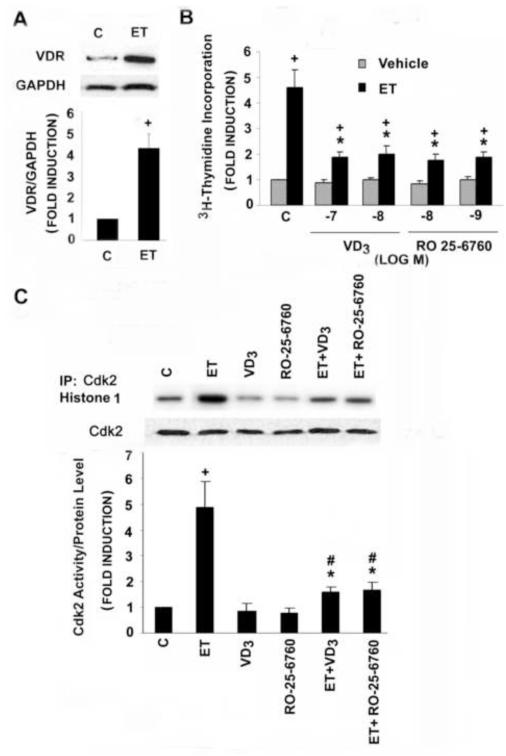
The vitamin D receptor (VDR) is present in our adult rat aortic VSMC population. As shown in Fig. 1A, an immunoreactive band of the appropriate size (~55 kDa) was readily detected in extracts of the VSMC. Of note, treatment with the pro-mitogenic vasoactive peptide ET resulted in a 4-5 fold increase in VDR levels.
Fig. 1.
ET increases VDR expression and VDR ligands suppress ET-stimulated DNA synthesis and Cdk2 activity in adult rat VSMC. A: Quiescent cells were treated with ET for 20 hours; cell extracts were generated and Western blot analysis for VDR was performed as described in Methods. B: Quiescent cells were incubated with different concentrations of 1,25(OH)2D(VD3) or RO 25-6760 (RO), as indicated, for 48 hours in the presence or absence of ET for the final 24 hours. 3H-thymidine incorporation was measured as described. Pooled data were from 3-4 separate experiments. C: Cells were exposed to vehicle, 10−8 mol/L 1,25(OH)2D or 10−9 mol/L RO 25-6760 for 48 hours and ET for the final 20 hours prior to generation of extracts. Cdk2 was immunoprecipated with anti-Cdk2 antibody. Cdk2 kinase activity and protein levels were measured as described. Cdk2 activity was normalized to Cdk2 protein levels. Pooled data from 3-4 separate experiments are shown. *P<0.01 vs. ET alone; +P<0.01, #P<0.05 vs. control.
Treatment with ET increased 3H-thymidine incorporation 4-5 fold in the adult rat VSMC (Fig. 1B). Pretreatment with either 1,25(OH)2D or its less calcemic analogue RO 25-6760 reduced the ET effect by as much as 70% at maximum inhibition. The ET-dependent increase in DNA synthesis was accompanied by an increase in Cdk2 activity (Fig. 1C). While 1,25(OH)2D and RO 25-6760 had relatively modest effects on basal Cdk2 activity, they significantly reduced the ET-dependent induction, findings that are in agreement with those reported previously for neonatal VSMC [18].
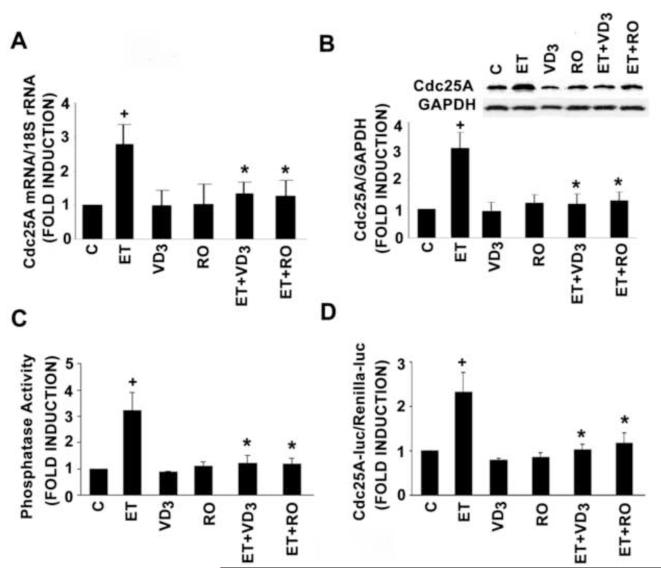
Cdk2 activity is tightly regulated by the Cdk2-activating phosphatase Cdc25A [7, 9]. We asked whether 1,25(OH)2D might act through inhibition of Cdc25A activity. As shown in Fig.2A, ET increased Cdc25A mRNA levels 3-fold. While 1,25(OH)2D and RO-25-6760 had only a modest effect on basal levels of this transcript, each blocked the ET-dependent increase in transcript levels. Similar findings were obtained when levels of Cdc25A protein (Fig. 2B), Cdc25A phosphatase activity (Fig. 2C) or Cdc25A promoter activity (Fig. 2D) were examined. Collectively these data suggest that 1,25(OH)2D and its analogues block agonist-dependent DNA synthesis by reducing expression of the Cdc25A gene, thereby reducing Cdk2 activity and progression of the VSMC through the G1-S transition.
Fig. 2.
1,25(OH)2D (10−8 mol/L) and RO 25-6760 (RO) (10−9 mol/L) treatment (48 hours) inhibit ET-stimulated Cdc25A expression and activity in adult rat VSMC. A: Cdc25A mRNA level was measured by real-time PCR and normalized to 18S rRNA. B: Cdc25A protein level was determined by Western blot analysis and normalized to GAPDH. Representative blot is presented above the bar graph. C: Cdc25A phosphatase activity was assayed by measuring fluorescent emission from the hydrolysis OMFP to OMF. D: −450 Cdc25A-luciferase activity was measured in transfected cells and normalized for Renilla luciferase activity. Pooled data were derived from 3-4 independent experiments. *P<0.01 vs. ET alone, +P<0.01 vs. control.
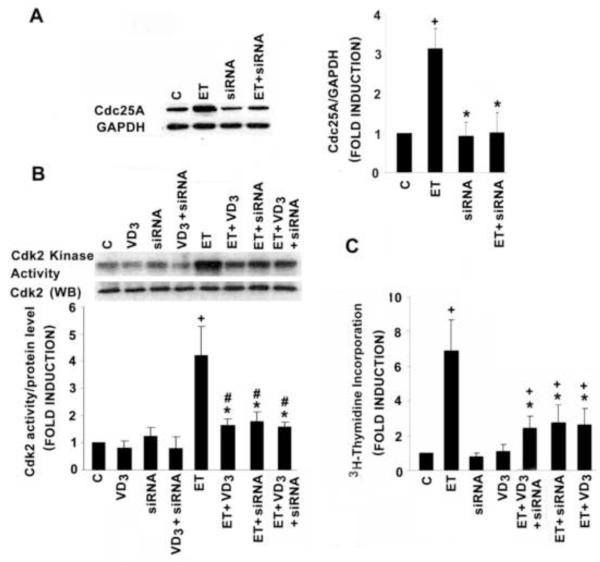
To demonstrate a mechanistic link between Cdc25A, the ET-dependent stimulation and 1,25(OH)2D-dependent inhibition of VSMC proliferative activity, we used an siRNA approach to suppress Cdc25A gene expression. As shown in Fig. 3A, siRNA that specifically targets Cdc25A led to a modest reduction in Cdc25A protein levels at baseline but completely abrogated the ET-dependent induction. This was accompanied by a significant inhibition of the ET-dependent Cdk2 activity (Fig. 3B) and 3H-thymidine incorporation (Fig. 3C). Noteworthy, 1,25(OH)2D and Cdc25A siRNA treatment each effected a similar reduction in ET-induced Cdk2 activity and 3H-thymidine incorporation. However, as shown in Fig. 3B and C, these two agents, when used in maximal inhibitory concentrations, failed to display additive activity suggesting that they operate over a common (i.e. Cdc25A-dependent) signaling pathway to promote the inhibition.
Fig. 3.
Cdc25A siRNA (siRNA) reduces ET-dependent Cdk2 activity and DNA synthesis in the adult VSMC. A: Cdc25A siRNA reduces ET-stimulated Cdc25A protein expression. B: Cdc25A siRNA and 10−8 mol/L 1,25(OH)2D individually reduce Cdk2 activity but the combination failed to display additivity. Cdk2 activity was normalized to Cdk2 protein level. Representative blot is presented above the bar graph. C: Treatment with Cdc25A siRNA results in a significant reduction in DNA synthesis. The reduction was not additive with 10−8 mol/L 1,25(OH)2D, included for the 48 hours of treatment. All control groups were treated with siRNA containing scrambled sequence. Pooled data came from 3-5 separate experiments. *P<0.01 vs. ET alone; +P<0.01, #P<0.05 vs. control.
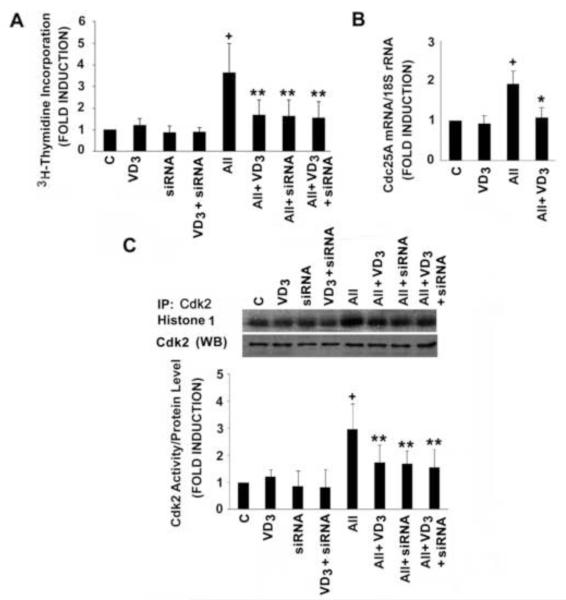
The 1,25(OH)2D -dependent inhibition was not confined to ET-induced VSMC proliferation. As shown in Fig. 4A and C, 1,25(OH)2D significantly inhibited AII-stimulated DNA synthesis and Cdk2 activity, and this was accompanied by 1,25(OH)2D -dependent suppression of AII-induced Cdc25A gene transcription (Fig. 4B). As seen with ET, AII-induced VSMC proliferation and Cdk2 activity are Cdc25A dependent. Treatment with siRNA against Cdc25A led to a dramatic reduction in AII-stimulated DNA synthesis and Cdk2 activity. Addition of 1,25(OH)2D did not further amplify this effect (Fig. 4 A and C). Collectively, these data suggest that 1,25(OH)2D suppresses agonists-induced VSMC proliferation through the inhibition of Cdc25A expression.
Fig. 4.
1,25(OH)2D inhibits AII-induced DNA synthesis, Cdc25A gene expression and Cdk2 activity. A: After transfection with Cdc25A siRNA or scrambled siRNA sequence (C) for 24 hours, cells were treated with 10−8 mol/L 1,25(OH)2D or vehicle for 48 hours. Where indicated, AII (10−7 mol/L) was included for the final 24 hours prior to measurement of 3H-thymidine incorporation. B: Cells were exposed to 10−8 mol/L 1,25(OH)2D for 48 hours and AII for the last 9 hours of the incubation prior to measurement of Cdc25A gene expression. Cdc25A mRNA levels were measured by real-time PCR and normalized to 18S rRNA. C: After treatment, as described in Panel A, cells were lysed, immune complex kinase assays and Western blot (WB) analyses for Cdk2 were carried as described in Methods. Cdk2 activity was normalized to Cdk2 protein levels. Representative results and pooled data are shown. Experiments were repeated 3-4 times. *P<0.01 or **P<0.05 vs. AII alone. +P<0.01 vs. control.
4. Discussion
ET is thought to play a pivotal role in aberrant VSMC proliferation [21-23]; however, the mechanism underlying its activity remains only partially understood. We have shown previously that the ET-dependent increase in Cdk2 activity is associated with increased Cdc25A protein expression and phosphatase activity [14]. Now we provide further support for this association by demonstrating that ET increases Cdc25A promoter activity and gene expression. We have gone on to show that siRNA directed against Cdc25A reduces ET-dependent Cdk2 activity and 3H-thymidine incorporation, solidifying a mechanistic link between ET and the Cdcd25A gene in promoting VSMC proliferation. Interestingly, AII-stimulated DNA synthesis is also accompanied by an increase in Cdk2 activity and Cdc25A gene transcription suggesting that this stimulatory activity may be shared by a number of G protein coupled, vasoconstrictor agents. These data provide strong support for a pivotal role for Cdc25A in promoting VSMC proliferation and suggest that Cdc25A may represent a potential therapeutic target in vascular diseases characterized by aberrant VSMC growth.
1,25(OH)2D regulates gene transcription through binding to the vitamin D receptor (VDR), and its heterodimeric partner, the retinoid X receptor (RXR), in target cells [24]. The antiproliferative effects of 1,25(OH)2D and it less hypercalcemic analogues on cancer cells are most prominent in those tissues with high levels of endogenous VDR expression [25]. This suggests that there may be a threshold level of VDR expression that is critical for manifestation of its antiproliferative activity. Data presented above are compatible with such a model. 1,25(OH)2D had a modest effect on basal 3H-thymidine incorporation in VSMC while promoting a significant reduction of ET-dependent 3H-thymidine incorporation. The latter effect occurs in parallel with an ET-dependent induction in VDR expression. This induction would be predicted to increase sensitivity to 1,25(OH)2D and close the negative feedback loop that regulates the magnitude and duration of the proliferative response.
Extensive studies of the molecular mechanism(s) underlying the 1,25(OH)2D -dependent inhibition of proliferative activity in cancer cells have demonstrated that it arrests cell cycle regulators (most of them in G1 and S phase) in a cell type-specific fashion. G1 cyclins, cyclin-dependent kinases, cyclin-dependent kinase inhibitors and E2F transcription factors have been shown to be regulated by 1,25(OH)2D [26]. There is less data available exploring the mechanism underlying 1,25(OH)2D’s anti-proliferative activity in VSMC, and until now, there have been no reports to show 1,25(OH)2D -dependent anti-proliferative activity involving Cdc25A inhibition in any cell type. The present study establishes a link between and 1,25(OH)2D and Cdc25A and ties this link to a reduction in VSMC proliferative activity, suggesting that 1,25(OH)2D or, more likely, its less hypercalcemic analogues may prove useful in the management of disorders characterized by excessive VSMC proliferative activity.
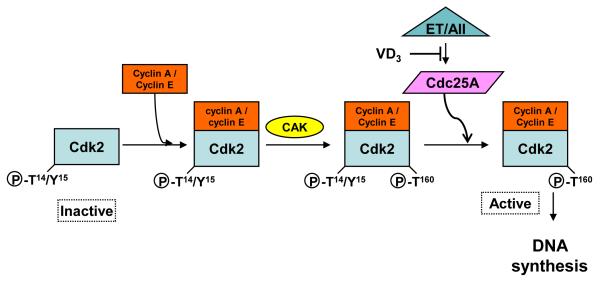
In summary, we have shown that 1,25(OH)2D and RO 25-6760 suppress ET or AII-stimulated DNA synthesis and that the suppression is linked to a reduction in the activity but not the protein level of Cdk2. This reduction appears to be linked to inhibition of ET or AII-induced Cdc25A gene expression (Fig. 5). Collectively, these data suggest a plausible mechanism to account for 1,25(OH)2D -dependent inhibition of VSMC growth and imply a potential role for this agent or its analogues in the treatment of disorders affecting the vascular wall.
Fig. 5.
1,25(OH)2D inhibits ET or AII-activated Cdk2 activity and DNA synthesis through reduction of Cdc25A expression and activity. Following the formation of the Cdk2/Cyclin A or E complex, Cdk-activating kinase (CAK) phosphorylates Thr160. ET increases Cdc25A expression and phosphatase activity which dephosphorylates inhibitory Thr14/Tyr15 phospho-residues and drives cell cycle activity.
Acknowledgements
Funding source: This work was supported by the National Institutes of Health [HL45637 to DGG and HL096047 to SC], the American Heart Association [Grant-in-Aid 0565016Y to SC] and the ExtenD Program of Abbott Laboratories to S.C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Morgan DO. Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu Rev Cell Dev Biol. 1997;13:261–291. doi: 10.1146/annurev.cellbio.13.1.261. [DOI] [PubMed] [Google Scholar]
- [2].Kelly BL, Wolfe KG, Roberts JM. Identification of a substrate-targeting domain in cyclin E necessary for phosphorylation of the retinoblastoma protein. Proc Natl Acad Sci U S A. 1998;95(5):2535–2540. doi: 10.1073/pnas.95.5.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Weinberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995;81(3):323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- [4].Zhao J, Kennedy BK, Lawrence BD, Barbie DA, Matera AG, Fletcher JA, Harlow E. NPAT links cyclin E-Cdk2 to the regulation of replication-dependent histone gene transcription. Genes Dev. 2000;14(18):2283–2297. [PMC free article] [PubMed] [Google Scholar]
- [5].Morishita R, Gibbons GH, Ellison KE, Nakajima M, von der Leyen H, Zhang L, Kaneda Y, Ogihara T, Dzau VJ. Intimal hyperplasia after vascular injury is inhibited by antisense cdk 2 kinase oligonucleotides. J Clin Invest. 1994;93(4):1458–1464. doi: 10.1172/JCI117123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Brooks EE, Gray NS, Joly A, Kerwar SS, Lum R, Mackman RL, Norman TC, Rosete J, Rowe M, Schow SR, Schultz PG, Wang X, Wick MM, Shiffman D. CVT-313, a specific and potent inhibitor of CDK2 that prevents neointimal proliferation. J Biol Chem. 1997;272(46):29207–29211. doi: 10.1074/jbc.272.46.29207. [DOI] [PubMed] [Google Scholar]
- [7].Coulonval K, Bockstaele L, Paternot S, Roger PP. Phosphorylations of cyclin-dependent kinase 2 revisited using two-dimensional gel electrophoresis. J Biol Chem. 2003;278(52):52052–52060. doi: 10.1074/jbc.M307012200. [DOI] [PubMed] [Google Scholar]
- [8].Gu Y, Rosenblatt J, Morgan DO. Cell cycle regulation of CDK2 activity by phosphorylation of Thr160 and Tyr15. EMBO J. 1992;11(11):3995–4005. doi: 10.1002/j.1460-2075.1992.tb05493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Blomberg I, Hoffmann I. Ectopic expression of Cdc25A accelerates the G(1)/S transition and leads to premature activation of cyclin E- and cyclin A-dependent kinases. Mol Cell Biol. 1999;19(9):6183–6194. doi: 10.1128/mcb.19.9.6183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hoffmann I, Draetta G, Karsenti E. Activation of the phosphatase activity of human cdc25A by a cdk2-cyclin E dependent phosphorylation at the G1/S transition. EMBO J. 1994;13(18):4302–4310. doi: 10.1002/j.1460-2075.1994.tb06750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mailand N, Falck J, Lukas C, Syljuasen RG, Welcker M, Bartek J, Lukas J. Rapid destruction of human Cdc25A in response to DNA damage. Science. 2000;288(5470):1425–1429. doi: 10.1126/science.288.5470.1425. [DOI] [PubMed] [Google Scholar]
- [12].Chen S, Gardner DG. Retinoic acid uses divergent mechanisms to activate or suppress mitogenesis in rat aortic smooth muscle cells. J Clin Invest. 1998;102(4):653–662. doi: 10.1172/JCI3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zheng S, Qian Z, Wen N, Xi L. Crocetin suppresses angiotensin II-induced vascular smooth-muscle cell proliferation through inhibition of ERK1/2 activation and cell-cycle progression. J Cardiovasc Pharmacol. 2007;50(5):519–525. doi: 10.1097/FJC.0b013e31813c114e. [DOI] [PubMed] [Google Scholar]
- [14].Chen S, Gardner DG. Suppression of WEE1 and stimulation of CDC25A correlates with endothelin-dependent proliferation of rat aortic smooth muscle cells. J Biol Chem. 2004;279(14):13755–13763. doi: 10.1074/jbc.M310064200. [DOI] [PubMed] [Google Scholar]
- [15].Carthy EP, Yamashita W, Hsu A, Ooi BS. 1,25-Dihydroxyvitamin D3 and rat vascular smooth muscle cell growth. Hypertension. 1989;13(6 Pt 2):954–959. doi: 10.1161/01.hyp.13.6.954. [DOI] [PubMed] [Google Scholar]
- [16].Mitsuhashi T, Morris RC, Jr., Ives HE. 1,25-dihydroxyvitamin D3 modulates growth of vascular smooth muscle cells. J Clin Invest. 1991;87(6):1889–1895. doi: 10.1172/JCI115213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wu-Wong JR, Nakane M, Ma J, Ruan X, Kroeger PE. Effects of Vitamin D analogs on gene expression profiling in human coronary artery smooth muscle cells. Atherosclerosis. 2006;186(1):20–28. doi: 10.1016/j.atherosclerosis.2005.06.046. [DOI] [PubMed] [Google Scholar]
- [18].Chen S, Law CS, Gardner DG. Vitamin D-dependent suppression of endothelin-induced vascular smooth muscle cell proliferation through inhibition of CDK2 activity. J Steroid Biochem Mol Biol. 2010;118(3):135–141. doi: 10.1016/j.jsbmb.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jones PA, Scott-Burden T, Gevers W. Glycoprotein, elastin, and collagen secretion by rat smooth muscle cells. Proc Natl Acad Sci U S A. 1979;76(1):353–357. doi: 10.1073/pnas.76.1.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chen X, Prywes R. Serum-induced expression of the cdc25A gene by relief of E2F-mediated repression. Mol Cell Biol. 1999;19(7):4695–4702. doi: 10.1128/mcb.19.7.4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Dzau VJ, Braun-Dullaeus RC, Sedding DG. Vascular proliferation and atherosclerosis: new perspectives and therapeutic strategies. Nat Med. 2002;8(11):1249–1256. doi: 10.1038/nm1102-1249. [DOI] [PubMed] [Google Scholar]
- [22].Rahmani M, Cruz RP, Granville DJ, McManus BM. Allograft vasculopathy versus atherosclerosis. Circ Res. 2006;99(8):801–815. doi: 10.1161/01.RES.0000246086.93555.f3. [DOI] [PubMed] [Google Scholar]
- [23].Wallitt EJ, Jevon M, Hornick PI. Therapeutics of vein graft intimal hyperplasia: 100 years on. Ann Thorac Surg. 2007;84(1):317–323. doi: 10.1016/j.athoracsur.2007.02.035. [DOI] [PubMed] [Google Scholar]
- [24].Chen S, Costa CH, Nakamura K, Ribeiro RC, Gardner DG. Vitamin D-dependent suppression of human atrial natriuretic peptide gene promoter activity requires heterodimer assembly. J Biol Chem. 1999;274(16):11260–11266. doi: 10.1074/jbc.274.16.11260. [DOI] [PubMed] [Google Scholar]
- [25].Bouillon R, Eelen G, Verlinden L, Mathieu C, Carmeliet G, Verstuyf A. Vitamin D and cancer. J Steroid Biochem Mol Biol. 2006;102(1-5):156–162. doi: 10.1016/j.jsbmb.2006.09.014. [DOI] [PubMed] [Google Scholar]
- [26].Eelen G, Gysemans C, Verlinden L, Vanoirbeek E, De Clercq P, Van Haver D, Mathieu C, Bouillon R, Verstuyf A. Mechanism and potential of the growth-inhibitory actions of vitamin D and ana-logs. Curr Med Chem. 2007;14(17):1893–1910. doi: 10.2174/092986707781058823. [DOI] [PubMed] [Google Scholar]