Abstract
Multiple sclerosis (MS) affects myelin sheaths within the central nervous system, concurring to cause brain atrophy and neurodegeneration as well as gradual functional disconnections. To explore early signs of altered connectivity in MS from a structural and functional perspective, the morphology of corpus callosum (CC) was correlated with a dynamic inter-hemispheric connectivity index.
Twenty mildly disabled patients affected by a relapsing-remitting (RR) form of MS (EDSS ≤ 3.5) and 15 healthy subjects underwent structural MRI to measure CC thickness over 100 sections and electroencephalography to assess a spectral coherence index between primary regions devoted to hand control, at rest and during an isometric handgrip.
In patients, an overall CC atrophy was associated with increased lesion load. A less efficacious inter-hemispheric coherence during movement was associated with CC atrophy in sections interconnecting homologous primary motor areas (anterior mid-body). In healthy controls, less efficacious inter-hemispheric coherence at rest was associated with a thinner CC splenium. Our data suggest that in mildly disabled RR-MS patients a covert impairment may be detected in the correlation between the structural (CC thickness) and functional (inter-hemispheric coherence) measures of homologous networks, whereas these two counterparts do not yet differ individually from controls.
Keywords: multiple sclerosis (MS), relapsing-remitting, corpus callosum, Electroencephalography/Event-Related Potentials (EEG/ERPs), sensorimotor control, structural magnetic resonance imaging, inter-hemispheric coherence
Introduction
The disruption of brain and corpus callosum parenchyma affects firing properties and the ability of neuronal assemblies to cooperate via cortico-cortical connectivity. This specific feature can nowadays be investigated by evaluating the coherence of rhythmic oscillations from discrete brain areas (Schnitzler and Gross, 2005; Siegel et al., 2012).
The corpus callosum (CC) is the largest neural fiber bundle connecting the two cerebral hemispheres (Hellige, 1993). It changes in size and shape over time during brain maturation (Luders et al., 2010) and is influenced by individual experiences and specific skills that contribute to finely reshape its subregions (Westerhausen et al., 2004; Luders et al., 2010). Callosal macrostructure is accounted for by variable degrees of axonal myelination, redirection and shortening in normal (Luders et al., 2003) and pathologic conditions (Di Paola et al., 2010). Multiple sclerosis (MS) profoundly affects CC architecture by virtue of its ability to target white and gray matter, determining a “multiple disconnection syndrome” in large-scale cortical networks (Calabrese and Penner, 2007; Bester et al., 2013). Concurrently, demyelination and axonal degeneration, in the short- and long-run, alter the coherence and synchronization patterns of neuronal assemblies at cortico-spinal and cortico-cortical levels (Cader et al., 2006; Tecchio et al., 2008).
From a neurophysiological perspective, the particular arrangement of large myelinated (Pandya et al., 1971) and thin non-myelinated (Aboitiz et al., 1992) fibers along the CC facilitates the transmission of periodic/aperiodic impulses between the two primary sensori-motor areas (S1/M1). The peak density in the posterior mid-body and the splenium was observed associated with the synchronous oscillations that take place during uni- or bi-manual voluntary movements in healthy people (Stancak et al., 2002). In particular, the coherence amplitude between left and right S1/M1 in the lower alpha band (7.8–9.8 Hz) during hand and shoulder movements correlated with the size of a region that includes the rostral body and the posterior mid-body (Stancak et al., 2002).
Abnormal functional connectivity patterns among sensori-motor control regions have been extensively shown as early features of MS in resting state (Lowe et al., 2002), during the execution of simple movements (Casadio et al., 2008; Tomasevic et al., 2013), during the assessment of motor-evoked potentials and intra-cortical inhibition (Wahl et al., 2011), as well as during somato-sensory stimulation (Tecchio et al., 2008). Little is yet known about the relationships between the morphometric variability of CC and the functional connectivity of hand sensori-motor homologous areas.
Our aim was to study the relationship between callosal morphology and an electrophysiological index of S1/M1 inter-hemispheric coupling, while executing a simple isometric handgrip requiring high sensori-motor integration from somesthetic, proprioceptive and visual channels, when the disease severity had few clinical or functional impacts.
Experimental Procedures
Patients
The present study was conducted according to the standards established by Fatebenefratelli Hospital's Institutional Review Board as well as the regulations in biomedical research (Declaration of Helsinki, www.wma.net/en/30publications/10policies/b3).
Twenty patients with defined diagnoses of MS (McDonalds criteria, 2001) were enrolled (see Table 1 for demographic and clinical details). The following inclusion criteria were considered: relapsing-remitting (RR) course of disease (Lublin and Reingold, 1996); low Extended Disability Scale Score (EDSS < 3.5)(Kurtzke, 1983); absence of clinical relapse or radiological evidence of disease activity (i.e., contrast enhancement in T1W MR images) for at least three months preceding the study; no treatment with corticosteroids or psychotropic drugs within the three months preceding the study. In addition, MS patients underwent the following assessments: neurophysiological estimates of inter-hemispheric coherence indices (IHCoh) through electroencephalographic recordings at rest and during an isometric grip performed by left and right hands; magnetic resonance imaging (MRI) examination.
Table 1. Clinical, demographic and morphometric characteristics of the investigated subjects.
| Patient nr | Gender | Age | Dis Durat | EDSS | Les Load | BPF | LrF | LtThal vol | RtThal vol |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 44 | 4 | 3 | 5.9 | 0.76 | 0.027 | 3.40 | 3.83 |
| 2 | M | 26 | 3 | 2.5 | 3.4 | 0.66 | 0.173 | 3.72 | 3.56 |
| 3 | F | 40 | 12 | 1 | 11.8 | 0.69 | 0.017 | 2.94 | 2.60 |
| 4 | F | 43 | 15 | 3.5 | 0.4 | 0.75 | 0.027 | 5.59 | 4.35 |
| 5 | F | 40 | 10 | 1.5 | 8.1 | 0.73 | 0.208 | 2.25 | 2.58 |
| 6 | F | 50 | 16 | 2 | 6.3 | 0.76 | 0.005 | 2.25 | 2.58 |
| 7 | F | 26 | 7 | 1.5 | 93.7 | 0.79 | 0.014 | 3.43 | 3.03 |
| 8 | F | 43 | 10 | 2 | 37.1 | 0.77 | 0.071 | 3.60 | 3.67 |
| 9 | F | 39 | 9 | 1 | 11.4 | 0.75 | 0.02 | 4.37 | 3.96 |
| 10 | F | 33 | 11 | 1.5 | 31.5 | 0.77 | 0.001 | 4.47 | 3.93 |
| 11 | F | 42 | 15 | 1.5 | 116.1 | 0.75 | 0.097 | 3.00 | 2.95 |
| 12 | M | 40 | 3 | 0 | 29.6 | 0.72 | 0.001 | 4.77 | 4.11 |
| 13 | M | 39 | 17 | 0 | 10.6 | 0.79 | 0.001 | 4.24 | 3.80 |
| 14 | F | 44 | 14 | 1.5 | 2.1 | 0.75 | 0.050 | 3.89 | 3.58 |
| 15 | F | 31 | 3 | 1 | 0.9 | 0.75 | 0.009 | 4.16 | 3.44 |
| 16 | M | 28 | 8 | 2 | 75.7 | 0.75 | 0.004 | 4.45 | 3.58 |
| 17 | F | 60 | 28 | 1 | 1.5 | 0.74 | 0.003 | 4.74 | 4.54 |
| 18 | F | 31 | 9 | 2 | 0.7 | 0.77 | 0.035 | 3.35 | 3.67 |
| 19 | F | 43 | 3 | 2.5 | 0.4 | 0.7 | 0.251 | 3.74 | 3.34 |
| 20 | M | 44 | 5 | 3.5 | 1.8 | 0.74 | 0.004 | 4.43 | 3.91 |
| mean (SD) | 15 F 5 M | 39.3 (8.33) | 10.10 (6.12) | range 0-3.5 | 22.4 (33.8) | 0.74 (0.03) | 0.051 (0.074) | 3.84 (0.87) | 3.55 (0.56) |
| HC | Gender | Age | BPF | LtThal vol | RtThal vol | ||||
| mean (SD) | 11 F 4 M | 37.2 (9.1) | 0.82 (0.19) | 4.28 (0.37) | 3.81 (0.44) | ||||
Legend: F=female; M=male; SD=standard deviation; Dis Durat= disease duration, expressed in years; EDSS=Expanded Disability Status Scale; Les Load=lesion load expressed in mm3; BPF=Brain Parenchyma fraction; LrF=Lesion relative Fraction, given by the ratio between the total lesion load and the white matter volume; Lt/RtThal vol=left/right thalamus volume normalized by BPF; HC=Healthy controls.
All twenty RR-MS patients were right-handed (Edinburgh Inventory > 85), had mild clinical disease severity (EDSS range: 0–3.5, median: 1) and variable disease duration (range 3–28 years), lesion load and atrophy impact (Table 1).
Healthy controls
Fifteen right-handed healthy subjects (11 females, age range 25–44) served as controls for the morpho-structural part of the study (corpus callosum thickness/thalamus volumetry) and the coherence estimates gathered from the EEG recordings. They had no clinical neurologic history nor underwent psychoactive drug treatment during the three months preceding the study (See Table 1).
MRI exam and parameter estimates
MRI data acquisition
Imaging was performed with a 1.5 T scanner (Achieva, Philips Medical Systems, Best, The Netherlands), provided with a 33 mT/m gradient amplitude, online 2D/3D geometric distortion correction and a standard quadrature head coil. The acquisition protocol consisted of a high-resolution anatomical 3D sequence optimized for morphometric measurements and routine 2D sequences for lesion characterization. The former was empirically adjusted to allow reliable segmentation of subcortical structures (T1-weighted Turbo Field Echo TR/TE/FA = 7.0 ms/3.16 ms/8°; 256x256 matrix, 160 sagittal contiguous slices, in-plane resolution 1x1 mm). The 2D sequences included Dual Turbo Spin Echo, Fluid Attenuated Inversion Recovery (FLAIR) and two T1-Spin Echo sequences before and after intravenous injection of the contrast agent gadolinium (0.2 mmol/kg), according to the same acquisition parameters as detailed in Tecchio et al. (2008).
Lesion characterization
For aspects regarding lesion characterization, optimized segmentation and volumes calculation for normalization, see Tecchio et al. (2008). Briefly, in the present study, the lesion load was estimated as the lesion relative fraction (LrF), defined as the total lesion volume (TLV) of white matter hyperintensities (WMHy) upon T2-weighted/FLAIR images, normalized for the overall white matter volume. Moreover, as an estimate of whole brain atrophy, the brain parenchymal fraction (BPF) was considered, defined as the ratio between brain parenchymal tissue volume (normal-appearing white matter, TLV and gray matter) and the total intracranial volume (Rudick et al., 1999).
Assessment of thalamic volumes
Volumes of both thalami as anatomical regions of interest (ROI) were calculated upon the segmented T1-weighted images that underwent the automatic algorithms of FMRIB Integrated Registration and Segmentation Tool (FIRST) 1.2, as part of the FSL software package (version 4.1, www.fmrib.ox.ac.uk/fsl/). Each volume was expressed as total number of voxels in the ROI, normalized for brain parenchymal tissue volume and then converted into milliliters for statistics.
Estimate of corpus callosum thickness
First, intensity drifts due to magnetic field inhomogeneity were eliminated from T1-weighted images applying radiofrequency bias-field corrections (Sled et al., 1998). Then, for each subject, the anatomical image volume was manually positioned along the AC–PC line. The most accurate representation of the CC along the mid-sagittal surface was thus obtained, exposing the septum pellucidum anteriorly and a large part of the falx posteriorly. Regional callosal thickness was measured as detailed elsewhere (Luders et al., 2006). Briefly, the upper (Top) and lower (Bottom) callosal boundaries were manually outlined in the mid-sagittal section of each brain. Callosal Top and Bottom sections were re-digitized, resulting in 100 equidistant points. Subsequently, the spatial average of the 100 equidistant surface points representing the Top and Bottom was calculated resulting in a new midline segment (Medial Curve), also consisting of 100 equidistant points. Finally, the distances between 100 corresponding surface points between Medial Curve and Top/Bottom were quantified in millimeters using a mesh-based geometrical modeling algorithm (Thompson et al., 1996). Table 2 shows the correspondence of the 100 points with the classical Witelson's parcellation scheme. The consistency of the IHCoh was further tested within five main anatomofunctional sections (mean thickness across the CC sub-regions listed in Table 2 left column).
Table 2. Corpus callosum sections according to the topography of cerebral connected areas.
| Corpus callosum sections | Hemispheric connected areas |
|
|---|---|---|
Current Study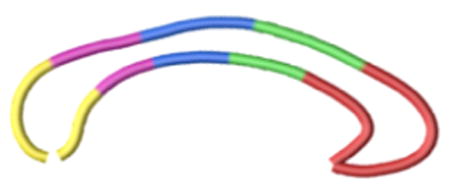
|
Witelson's subdivision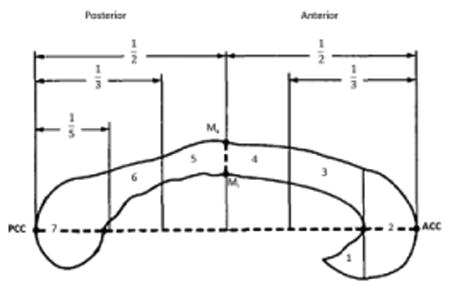
|
|
| 1-33 (red) Section A | Rostrum, Genu, Rostral body (regions 1, 2, 3) | Prefrontal, premotor, supplementary motor |
| 34-50 (green) Section B | Anterior mid-body (region 4) | Primary motor |
| 51-67 (blue) Section C | Posterior mid-body (region 5) | Somesthetic, posterior parietal |
| 68-81 (purple) Section D | Isthmus (region 6) | Superior temporal, posterior parietal |
| 82-100 (yellow) Section E | Splenium (region 7) | Inferior temporal and occipital |
Correspondence between the 100 callosal points clustered in five sections (from A to E), as were used in the present work, compared to the “classical” Witelson's subdivions, according to interhemispheric connected cortical areas.
EEG data and functional inter-hemispheric connectivity measures
EEG recordings and electrophysiological experimental setup
EEG recordings were performed to assess inter-hemispheric connectivity between homologous sensori-motor areas crucial for the fine control of hand movements. Bipolar signals were collected from left and right rolandic areas (F3-C3, F4-C4 respectively) in fronto-central reference, via Ag-AgCl cup electrodes, during the execution of a weak isometric handgrip task. Resting state with eyes open data were also collected. Two additional electrode pairs were placed next to one eye for 7 recording electro-oculogram (to check for eye blinking) and on the chest for recording electrocardiogram. Data were digitally sampled at 1000 Hz (pre-sampling analogical filter 0.1–256 Hz) and stored for off-line processing.
Motor task
All subjects laid comfortably with their arms supported, flexed at the elbow, and with their forearms semi-pronated. They were instructed to maintain a steady isometric opposition of the thumb to the other fingers with resistance against a compliant object, visually monitoring the exerted pressure (pressure sensor 40PC100G1A, Honeywell Sensing and Control, Golden Valley, Minneapolis, USA, Figure 1). Subjects were first asked to perform short (300– 400 ms) grips of a bulb, three times with each hand, at maximum voluntary contraction (MVC). After determining individual MVC as the average of the three repetitions, patients were required to alternate 20 s periods of steady isometric grip with 20 s periods of rest (Figure 1, left), three times with one hand followed by three times with the other hand. To avoid fatigue influences in the test task, the target level was set to 20% MVC. A total of 120 s of contraction were recorded for each hand to ensure at least 100 s of artifact-free data. A separate 120 s period of complete rest was also recorded. The grip accuracy was monitored and analyzed for differences between the left and right hands, in order to exclude behavioral differences in the execution of the task due to the dominant/non-dominant hand used.
Figure 1. Experimental setup for the inter-hemispheric coherence index (IHCoh) estimate.
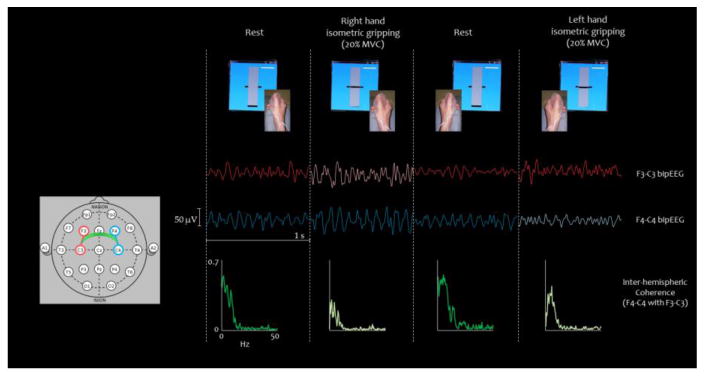
Top panel: visual feedback while performing the task with each hand separately: periods of isometric contractions were intermingled to rest (recovery). Middle panel: representative signal (1 second) of left and right bipolar derivations for each condition (F3-C3 and F4-C4 bipEEG) is shown with color code as sketched on the left (lighter color indicates contra-lateral movement). Bottom Panel: inter-hemispheric coherence between homologous regions in each condition (lighter color indicates the motor tasks).
Inter-hemispheric functional connectivity estimate
The inter-hemispheric sensori-motor functional communication was evaluated by the coherence of rhythmic sinusoids at various frequencies between homologous primary sensori-motor cortices via standard FFT approach. For homologous bipolar derivations (x=F3-C3, y=F4-C4), the squared modulus of coherence was calculated as:
being f the frequency bin, Sxy the cross spectrum between x and y estimated by Welch procedure (average of about 100, 50% overlapped windows of 2 s durations, Hanning windowing), and Sx and Sy the power spectral density of x and y respectively. Frequency bins were integrated in five standard EEG bands: delta (1.5-4 Hz); theta (4.5-8 Hz); alpha (8-12 Hz); beta (12-33 Hz); gamma (33.5-90 Hz, excluding five frequency bins around 50 Hz).
Statistical analysis
Inter-hemispheric coherence (IHCoh) between left and right SM1 in terms of reactivity in specific bands during isometric handgrip with respect to rest and possible differences between the two hands in MS patients and controls were investigated by Analysis of Variance (ANOVA), with Band (delta, theta, alpha, beta, gamma), Condition (rest, movement) and Hand (left, right) as within-subject factors and Group (healthy controls, MS patients) as between-subject factors. The same design with adjunctive Hemisphere (left, right) was preliminarily performed to test EEG SM1 characteristics at rest and during handgrip in the two groups. Post-hoc comparisons were considered whenever a factor showed a significant effect.
The thalamic volumes were analyzed by ANOVA Hemisphere (left, right) as within-subject factors and Group (healthy controls, MS patients) as between-subject factors.
The relationship between callosal thickness and inter-hemispheric coherence (IHCoh) was estimated as the Pearson's correlation at each of the 100 CC surface points, at rest and during left and right hand grip. Pearson's correlations were also calculated between the 100 CC thickness measurements and clinical variables (EDSS score) as well as MRI assessment scores (LrF, thalamic volumes). All correlative estimates were corrected for multiple comparisons using a false discovery rate (FDR) thresholded at 0.05 (Benjamini and Hochberg, 1995). Correlation coefficients (r) corresponding to below-threshold significance were color-coded and projected onto an ad-hoc generated mean callosal surface model. Finally, the CC 5-level thickness measure was correlated with IHCoh.
Results
Corpus callosum and thalamus characteristics
No differences of the corpus callosum morphology were seen between the two groups. In MS patients, callosal thickness did not correlate with EDSS, while a negative correlation was found with LrF across almost the entire callosal surface (FDR corrected p < 0.050). This relationship was confirmed at each of the five CC sections (FDR corrected p < 0.025).
The ANOVA design on thalamic volumes displayed a clear Hemisphere effect [F(1, 30)=31.276, p<.001], corresponding to smaller volumes in the right than in the left dominant hemisphere in both groups (Table 1). A trend of smaller thalami (left and right) in patients was indicated by the Group factor p=0.109.
No relationship appeared between thalamic volume and CC thickness in any region, either for the left or right thalami (p>.200 consistently).
Inter-hemispheric coherence characteristics
EEG spectral characteristics did not differ between patients and healthy controls. IHCoh decreased during handgrip from that of the relaxing periods in frequency bands typically reactive in the Rolandic areas when executing a motor task in both controls and MS patients (full ANOVA model Condition effect [F(1, 21)=8.646; p=.008], Figure 2). In fact, IHCoh showed reactivity in delta, theta and alpha bands by the reduced ANOVA models applied to single bands (Condition factor p<.015 consistently). Absence of interaction with Group either in the full or in reduced models indicated that the IHCoh decrease was present in both patients and healthy controls without differences between the two groups.
Figure 2. Inter-hemispheric coherence properties.
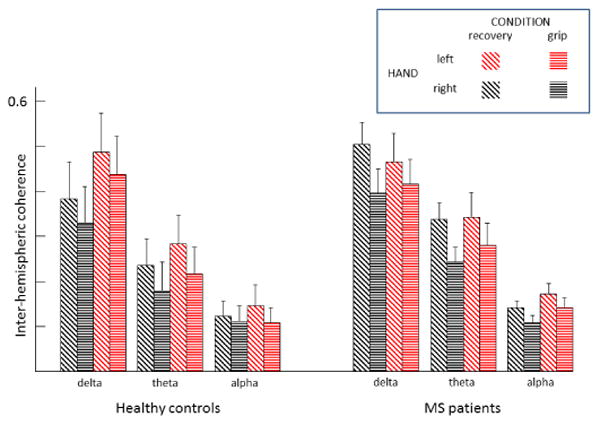
Inter-hemispheric coherence (IHCoh) among homologous Rolandic areas during an isometric grip with the right and left hands (grip) and resting intermingled periods (recovery) investigated in the standard frequency ranges in healthy controls and MS patients.
In alpha band the Hand effect [F(1,21)=6.300, p=.020] indicated lower motor and recovery IHCoh in the right (dominant) hand than in the left hand (Figure 2). Absence of interaction factors with Group (p>.300) indicated that these characteristics did not differentiate MS patients from healthy controls. The absence of main effect Group (p= .491) indicated that there were no differences of inter-hemispheric coherence at any band or condition between controls and patients.
IHCoh did not show any association with lesion load (LrF) or EDSS.
Relationships between corpus callosum thickness and inter-hemispheric coherence
When looking at the relationship between corpus callosum thickness and interhemispheric coherence we observed well-defined dissociations between MS patients and healthy controls. In fact, while both groups featured only negative correlations in the right hand, associations emerged selectively during handgrip in patients and during recovery in controls, in alpha band.
In fact MS patients displayed negative correlations of IHCoh during isometric handgrip within callosal sections connecting homologous sensori-motor cortices (anterior and posterior mid-body, Figure 3). The analysis with the CC thickness estimated in the five anatomofunctional sections confirmed the relationship in section B (anterior mid-body) connecting primary motor areas, r= -.652, q-value (FDR-corrected analog of the p-value q-value ≤ 0.05.
Figure 3. Structural-functional correlation between corpus callosum thickness and interhemispheric functional connectivity in alpha band.
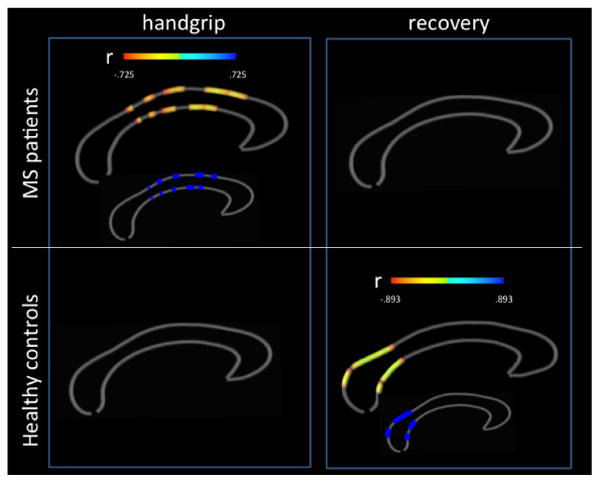
Callosal thickness (100 sections) and inter-hemispheric coherence in alpha band of rolandic areas during an isometric handgrip performed with the right hand by patients and healthy controls and during the recovery period. The color bars encode Pearson's correlation coefficients r at uncorrected significance (p ≤ 0.05) while the smaller maps represent in dark blue the callosal portions surviving FDR-corrections for multiple comparisons (q ≤ 0.05). No significant correlations were found for the left (non dominant) hand in both groups (not shown).
These associations did not emerge during movement in healthy controls. In contrast, healthy controls showed negative correlations between alpha band IHCho during recovery periods and callosal thickness of section E (splenium), connecting inferior-temporal and occipital areas, r= -.899, q-value ≤ 0.05 (Figure 3).
Relationships between thalamic volume and inter-hemispheric coherence
IHCoh did not show associations with left and right thalamic volumes either during isometric handgrip with the right or the left hand or during the recovery period in either controls or patients (FDR corrected p>.200).
Discussion
The current study revealed four main findings: 1. Overall thinner CC was associated with lesion load in a group of mild RR-MS patients, while it was unrelated to clinical severity; 2. CC was thinner in anterior mid-body section in patients displaying a less efficacious interhemispheric coherence during an isometric handgrip task; 3. In healthy controls, CC thickness at the splenium was correlated with sensorimotor inter-hemispheric coherence at rest; 4. Higher sensorimotor inter-hemispheric coherences were found at rest vs. task execution and during the non-dominant vs. dominant handgrip, in both patients and controls.
We detail the discussion of our findings, being aware of the necessity to extend the investigation in wider patient populations and considering cognitive-behavioral measures to evaluate the functional impact of our approach.
Less efficient inter-hemispheric functional connectivity associates with loco-regional corpus callosum atrophy in MS patients
A region-specific impairment of callosal architecture impacting the functional organization of the regions devoted to the execution of an isometric handgrip task was found in a population of 20 RR-MS patients. Inter-hemispheric functional connectivity as expressed by an index of synchronization (IHCoh) between tonically firing corticospinal cells in homologous sensorimotor rolandic cortices was associated with a reduction of callosal thickness at the level of the anterior mid-body. These regions convey few connections between homologous primary sensorimotor areas (Hofer and Frahm, 2006; Zarei et al., 2006), as well as fibers from secondary motor and somatosensory hand regions (Jung et al., 2012). Their relevance in sensorimotor control is confirmed by a selective dependence on handedness in healthy people (Witelson, 1989; Stancak et al., 2000, 2002). Moreover, they are engaged during bimanual object manipulation and exploration (Jung et al., 2012). This supports the concept that CC structure variability reflects inter-individual differences in skilled performance (Johansen-Berg et al., 2007).
The absence of a correlation between corpus callosum thickness and thalamic volume indicates that callosal atrophy affects cortico-cortical connectivity between homologous areas before involving subcortical structures.
Moreover, movement-related inter-hemispheric connectivity did not show any relationship with thalamic atrophy, suggesting a minor role of indirect fibers originating from thalamic nuclei (Dell'Acqua et al., 2010).
Important to note is that the inter-hemispheric coherence index (IHCoh) expressed a sharp association with CC thickness, in particular in the region of primary motor area connection, which was related to a normalized measure of lesion load (LrF). At the same time IHCoh did not associate with LrF, suggesting a stronger compensatory capacity at the neuronal level than at the structural level.
Nature of higher inter-hemispheric functional connectivity with impaired trans-callosal fibers
The finding that inter-hemispheric synchronization is higher at rest than during handgrip led us to hypothesize that higher inter-hemispheric synchrony expresses weaker task-related recruitment specificity. The task-related activity yields a reduced temporo-spatial summation of trans-callosal impulses between homologous sensorimotor cortices. The effects of such summation might be relevant in a slowly conducting and probably di-synaptic or polysynaptic trans-callosal system of excitatory neurons, impinging on inhibitory interneurons (Manson et al., 2008). In particular, local excitatory and inhibitory network interplay mediates pyramidal cell activities of homologous areas. In those patients an impairment of S1 excitatory intra-cortical connectivity was found as a ‘local’ effect of the widespread damage influencing the multi-nodal networks involved in complex cerebral functions (Tecchio et al., 2008). In parallel, the dysfunction of the local inhibitory network as a direct target of trans-callosal fibers might induce a deficit in the crucial local excitation-surrounding inhibition mechanisms to focus activity in neuronal pools required for an efficient behavioral control (Lenzi et al., 2007).
In healthy controls loco-regional corpus callosum thickness associates with interhemispheric functional connectivity in resting state
In healthy controls a thinner splenium - the most posterior part of the corpus callosum connecting reciprocally the parietal lobes and visual portions of the occipital lobes - was associated with a reduced sensorimotor inter-hemispheric functional connectivity in resting state. We speculate that this structural/functional behavior mirrors the necessity of a balanced interplay between homologous hemispheric areas, even at rest (Deco and Corbetta, 2011; Pellegrino et al., 2012). The interesting association found at rest in healthy controls, and not in patients also supports this view.
Dominance-dependent network susceptibility to corpus callosum thinning
MS-related thinning of the corpus callosum topographically related to inter-hemispheric connectivity differed depending upon whether the handgrip was executed with the dominant or the non-dominant hand. Specifically, executing the movement with the left non-dominant hand did not yield significant effects. Furthermore, in healthy controls the association with CC thickness emerged with inter-hemispheric connectivity during recovery periods after executing the right handgrip task. This points towards a greater susceptibility of damage to the nodes of highly specialized and frequently recruited networks controlling the dominant vs. the non-dominant hand (Lenzi et al., 2007; Dell'Acqua et al., 2010). A second, not mutually exclusive explanation may be found in a lower level of the overall functionality of the network devoted to the left hand. The finding that the relationships between corpus callosum thickness and interhemispheric coherence emerged in the alpha band in healthy controls supports such a hypothesis, since inter-hemispheric connectivity displayed a dependence on hand-dominance only in this frequency band.
Conclusions
This study investigated the relationship between inter-hemispheric coherence and corpus callosum (CC) thickness in MS. Correlation analysis revealed that regional CC atrophy increased with lesion load and with a less efficacious inter-hemispheric interplay during the execution of a simple motor task (isometric handgrip). These findings extend the knowledge that sensorimotor network derangement may be among the initial factors impacting the clinical state, as found in secondary and primary progressive MS patients (Rocca et al., 2010).
Highlights.
corpus callosum (CC) and inter-hemispheric connectivity are compromised early in the course of MS
structural MRI and EEG can be used to assess such an early imbalance
Higher coherence in α band at rest involved sensorimotor sections of corpus callosum
inter-hemispheric connectivity displayed a hand-dominance dependence only in α band
Acknowledgments
The authors thank neurologists Drs. Doriana Landi, Anna Ghazarian, Marialuisa Dell'Acqua for their clinical collaboration.
Funding: This study has received funding from: a. FISM - Fondazione Italiana Sclerosi Multipla; Contract grant number: Cod. 2011/R/32; b. MIUR prot. 2010SH7H3F Functional connectivity and neuroplasticity in physiological and pathological aging [ConnAge]; c. PNR-CNR Aging Program 2012-2014.
This work was additionally funded by the National Institutes of Health, Grant P41 RR013642 and P41 EB015922.
Footnotes
Authors Contribution: G.Z. and F.T. designed the research; G.Z., L.T., D.L., E.L. and M.M.F. performed the research; G.Z., L.T., F.T. and E.L. analyzed the data; G.Z. and F.T. wrote the paper; M.F., P.M.R., A.W.T. and P.M.T. supervised the workflow.
Conflict Of Interest: The authors have no disclosures to report.
References
- Aboitiz F, Scheibel AB, Fisher RS, Zaidel E. Fiber composition of the human corpus callosum. Brain Res. 1992;598:143–153. doi: 10.1016/0006-8993(92)90178-c. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a pratical and powerful approach to multiple testing. J R Statist Soc. 1995;57:289–300. [Google Scholar]
- Bester M, Lazar M, Petracca M, Babb JS, Herbert J, Grossman RI, Inglese M. Tract-specific white matter correlates of fatigue and cognitive impairment in benign multiple sclerosis. J Neurol Sci. 2013;330:61–66. doi: 10.1016/j.jns.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cader S, Cifelli A, Abu-Omar Y, Palace J, Matthews PM. Reduced brain functional reserve and altered functional connectivity in patients with multiple sclerosis. Brain. 2006;129:527–537. doi: 10.1093/brain/awh670. [DOI] [PubMed] [Google Scholar]
- Calabrese P, Penner IK. Cognitive dysfunctions in multiple sclerosis--a “multiple disconnection syndrome”? J Neurol. 2007;254(Suppl 2):II18–21. doi: 10.1007/s00415-007-2006-5. [DOI] [PubMed] [Google Scholar]
- Casadio M, Sanguineti V, Morasso P, Solaro C. Abnormal sensorimotor control, but intact force field adaptation, in multiple sclerosis subjects with no clinical disability. Mult Scler. 2008;14:330–342. doi: 10.1177/1352458507085068. [DOI] [PubMed] [Google Scholar]
- Deco G, Corbetta M. The dynamical balance of the brain at rest. Neuroscientist. 2011;17:07–123. doi: 10.1177/1073858409354384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell'Acqua ML, Landi D, Zito G, Zappasodi F, Lupoi D, Rossini PM, Filippi MM, Tecchio F. Thalamocortical sensorimotor circuit in multiple sclerosis: an integrated structural and electrophysiological assessment. Hum Brain Mapp. 2010;31:1588–1600. doi: 10.1002/hbm.20961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paola M, Spalletta G, Caltagirone C. In vivo structural neuroanatomy of corpus callosum in Alzheimer's disease and mild cognitive impairment using different MRI techniques: a review. J Alz Dis. 2010;20:67–95. doi: 10.3233/JAD-2010-1370. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Horwitz B, Ungerleider LG, Maisog JM, Pietrini P, Grady CL. The functional organization of human extrastriate cortex: a PET-rCBF study of selective attention to faces and locations. J Neurosci. 1994;14:6336–6353. doi: 10.1523/JNEUROSCI.14-11-06336.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellige JB. Hemispheric asymmetry: What's right and what's left. Cambridge: Harvard University Press; 1993. [Google Scholar]
- Hofer S, Frahm J. Topography of the human corpus callosum revisited—comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. NeuroImage. 2006;32:989–994. doi: 10.1016/j.neuroimage.2006.05.044. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Della-Maggiore V, Behrens TE, Smith SM, Paus T. Integrity of white matter in the corpus callosum correlates with bimanual co-ordination skills. Neuroimage. 2007;36(Suppl 2):T16–21. doi: 10.1016/j.neuroimage.2007.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung P, Klein JC, Wibral M, Hoechstetter K, Bliem B, Lu MK, Wahl M, Ziemann U. Spatiotemporal dynamics of bimanual integration in human somatosensory cortex and their relevance to bimanual object manipulation. J Neurosci. 2012;32:5667–5677. doi: 10.1523/JNEUROSCI.5957-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- Lenzi D, Conte A, Mainero C, Frasca V, Fubelli F, Totaro P, Caramia F, Inghilleri M, Pozzilli C, Pantano P. Effect of corpus callosum damage on ipsilateral motor activation in patients with multiple sclerosis: a functional and anatomical study. Hum Brain Mapp. 2007;28:636–644. doi: 10.1002/hbm.20305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe MJ, Phillips MD, Lurito JT, Mattson D, Dzemidzic M, Mathews VP. Multiple sclerosis: low-frequency temporal blood oxygen level-dependent fluctuations indicate reduced functional connectivity initial results. Radiology. 2002;224:184–192. doi: 10.1148/radiol.2241011005. [DOI] [PubMed] [Google Scholar]
- Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology. 1996;46:907–911. doi: 10.1212/wnl.46.4.907. [DOI] [PubMed] [Google Scholar]
- Luders E, Narr KL, Zaidel E, Thompson PM, Jancke L, Toga AW. Parasagittal asymmetries of the corpus callosum. Cereb Cortex. 2006;16:346–354. doi: 10.1093/cercor/bhi112. [DOI] [PubMed] [Google Scholar]
- Luders E, Rex DE, Narr KL, Woods RP, Jancke L, Thompson PM, Mazziotta JC, Toga AW. Relationships between sulcal asymmetries and corpus callosum size: gender and handedness effects. Cereb Cortex. 2003;13:1084–1093. doi: 10.1093/cercor/13.10.1084. [DOI] [PubMed] [Google Scholar]
- Luders E, Thompson PM, Toga AW. The development of the corpus callosum in the healthy human brain. J Neurosci. 2010;30:10985–10990. doi: 10.1523/JNEUROSCI.5122-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manson SC, Wegner C, Filippi M, Barkhof F, Beckmann C, Ciccarelli O, De Stefano N, Enzinger C, Fazekas F, Agosta F, Gass A, Hirsch J, Johansen-Berg H, Kappos L, Korteweg T, Polman C, Mancini L, Manfredonia F, Marino S, Miller DH, Montalban X, Palace J, Rocca M, Ropele S, Rovira A, Smith S, Thompson A, Thornton J, Yousry T, Frank JA, Matthews PM. Impairment of movement-associated brain deactivation in multiple sclerosis: further evidence for a functional pathology of interhemispheric neuronal inhibition. Exp Brain Res. 2008;187:25–31. doi: 10.1007/s00221-008-1276-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandya DN, Karol EA, Heilbronn D. The topographical distribution of interhemispheric projections in the corpus callosum of the rhesus monkey. Brain Res. 1971;32:31–43. doi: 10.1016/0006-8993(71)90153-3. [DOI] [PubMed] [Google Scholar]
- Pellegrino G, Tomasevic L, Tombini M, Assenza G, Bravi M, Sterzi S, Giacobbe V, Zollo L, Guglielmelli E, Cavallo G, Vernieri F, Tecchio F. Inter-hemispheric coupling changes associate with motor improvements after robotic stroke rehabilitation. Restor Neurol Neurosci. 2012;30:497–510. doi: 10.3233/RNN-2012-120227. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca MA, Valsasina P, Absinta M, Riccitelli G, Rodegher ME, Misci P, Rossi P, Falini A, Comi G, Filippi M. Default-mode network dysfunction and cognitive impairment in progressive MS. Neurology. 2010;74:1252–1259. doi: 10.1212/WNL.0b013e3181d9ed91. [DOI] [PubMed] [Google Scholar]
- Rudick RA, Fisher E, Lee JC, Simon J, Jacobs L. Use of the brain parenchymal fraction to measure whole brain atrophy in relapsing-remitting MS. Multiple Sclerosis Collaborative Research Group. Neurology. 1999;53:1698–1704. doi: 10.1212/wnl.53.8.1698. [DOI] [PubMed] [Google Scholar]
- Schnitzler A, Gross J. Normal and pathological oscillatory communication in the brain. Nat Rev Neurosci. 2005;6:285–296. doi: 10.1038/nrn1650. [DOI] [PubMed] [Google Scholar]
- Siegel M, Donner TH, Engel AK. Spectral fingerprints of large-scale neuronal interactions. Nat Rev Neurosci. 2012;13:121–134. doi: 10.1038/nrn3137. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE transactions on medical imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Stancak A, Jr, Lucking CH, Kristeva-Feige R. Lateralization of movement-related potentials and the size of corpus callosum. Neuroreport. 2000;11:329–332. doi: 10.1097/00001756-200002070-00021. [DOI] [PubMed] [Google Scholar]
- Stancak A, Lucking CH, Kristeva-Feige R. The size of corpus callosum and functional connectivities of cortical regions in finger and shoulder movements. Brain Res Cogn Brain Res. 2002;13:61–74. doi: 10.1016/s0926-6410(01)00091-x. [DOI] [PubMed] [Google Scholar]
- Tecchio F, Zito G, Zappasodi F, Dell' Acqua ML, Landi D, Nardo D, Lupoi D, Rossini PM, Filippi MM. Intra-cortical connectivity in multiple sclerosis: a neurophysiological approach. Brain. 2008;131:1783–1792. doi: 10.1093/brain/awn087. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Schwartz C, Toga AW. High-resolution random mesh algorithms for creating a probabilistic 3D surface atlas of the human brain. NeuroImage. 1996;3:19–34. doi: 10.1006/nimg.1996.0003. [DOI] [PubMed] [Google Scholar]
- Tomasevic L, Zito G, Pasqualetti P, Filippi M, Landi D, Ghazaryan A, Lupoi D, Porcaro C, Bagnato F, Rossini P, Tecchio F. Cortico-muscular coherence as an index of fatigue in multiple sclerosis. Mult Scler. 2013;19:334–343. doi: 10.1177/1352458512452921. [DOI] [PubMed] [Google Scholar]
- Wahl M, Hubers A, Lauterbach-Soon B, Hattingen E, Jung P, Cohen LG, Ziemann U. Motor callosal disconnection in early relapsing-remitting multiple sclerosis. Hum Brain Mapp. 2011;32:846–855. doi: 10.1002/hbm.21071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerhausen R, Kreuder F, Dos Santos Sequeira S, Walter C, Woerner W, Wittling RA, Schweiger E, Wittling W. Effects of handedness and gender on macro- and microstructure of the corpus callosum and its subregions: a combined high-resolution and diffusion-tensor MRI study. Brain Res Cogn Brain Res. 2004;21:418–426. doi: 10.1016/j.cogbrainres.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Witelson SF. Hand and sex differences in the isthmus and genu of the human corpus callosum. A postmortem morphological study. Brain. 1989;112:799–835. doi: 10.1093/brain/112.3.799. [DOI] [PubMed] [Google Scholar]
- Zarei M, Johansen-Berg H, Smith S, Ciccarelli O, Thompson AJ, Matthews PM. Functional anatomy of interhemispheric cortical connections in the human brain. J Anat. 2006;209:311–320. doi: 10.1111/j.1469-7580.2006.00615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


