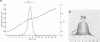Abstract
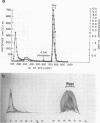
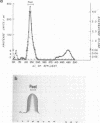
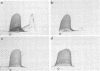
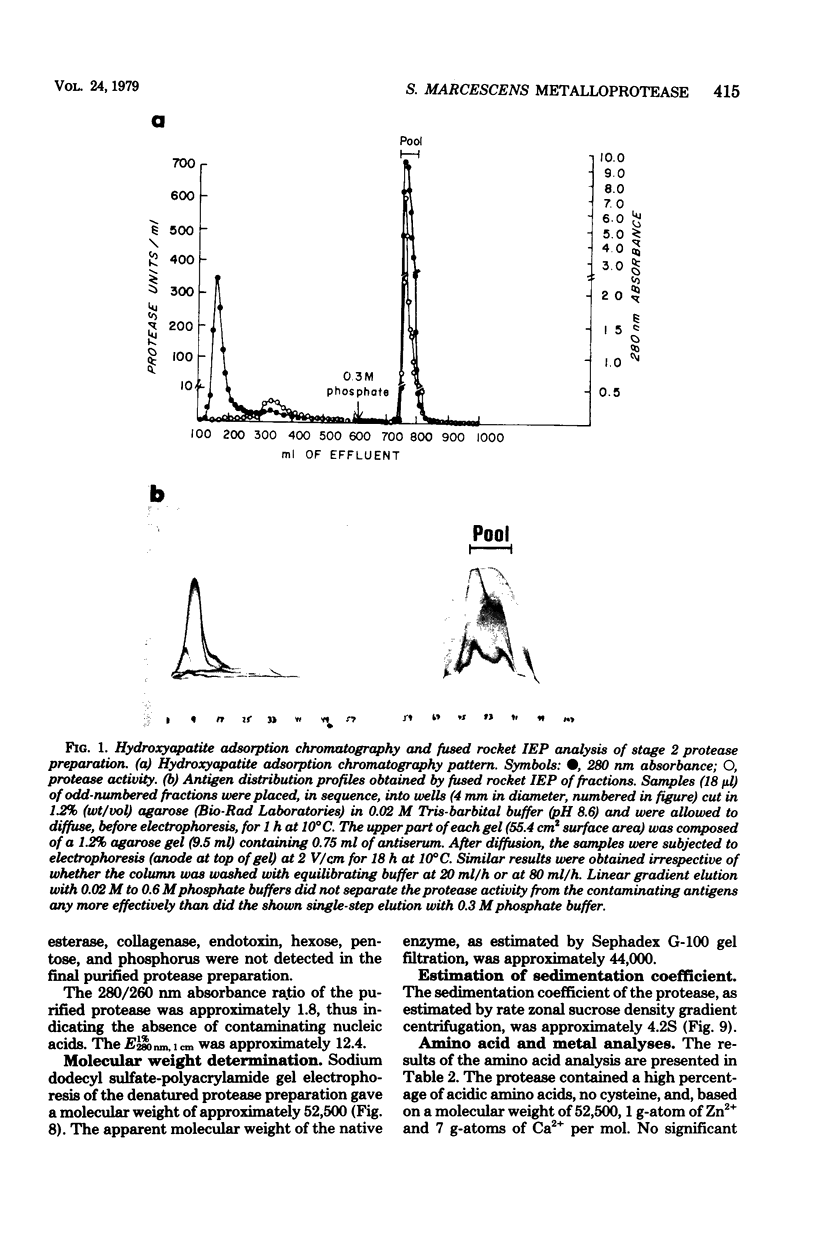
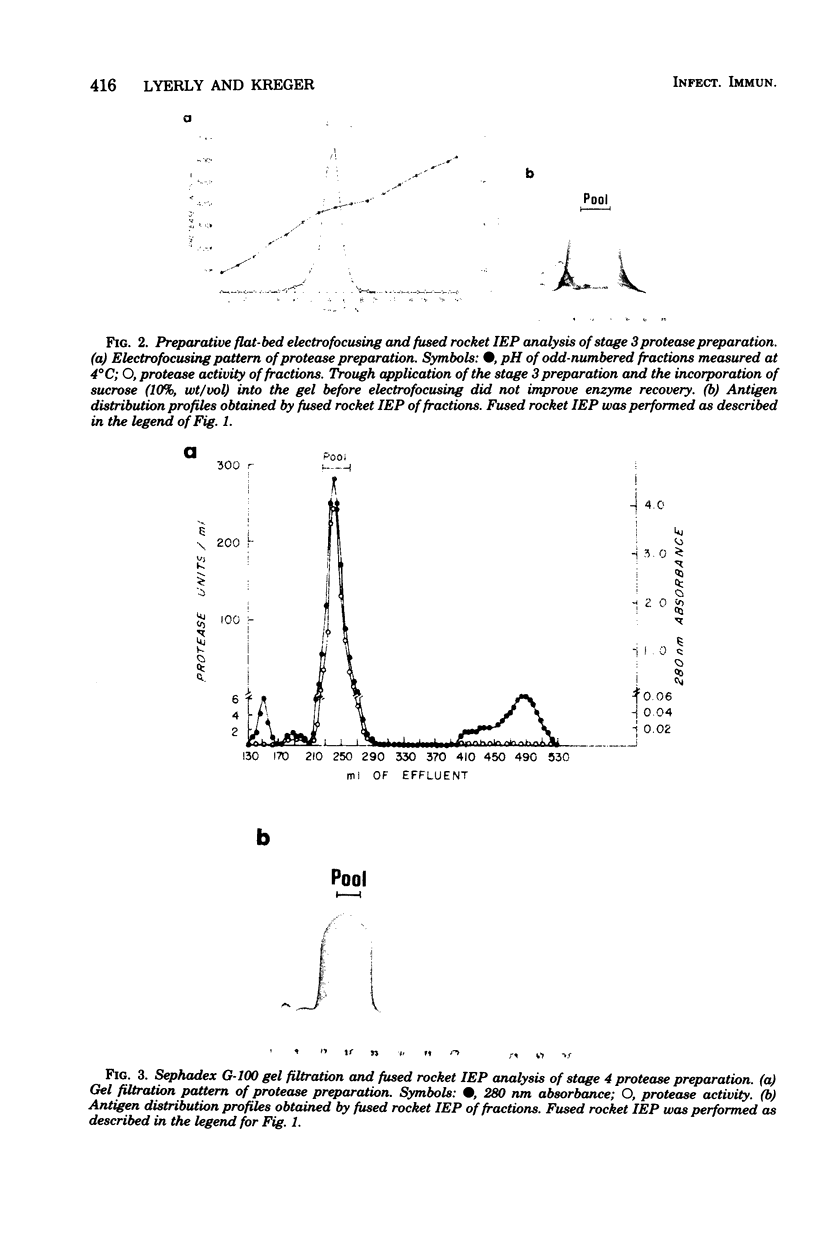
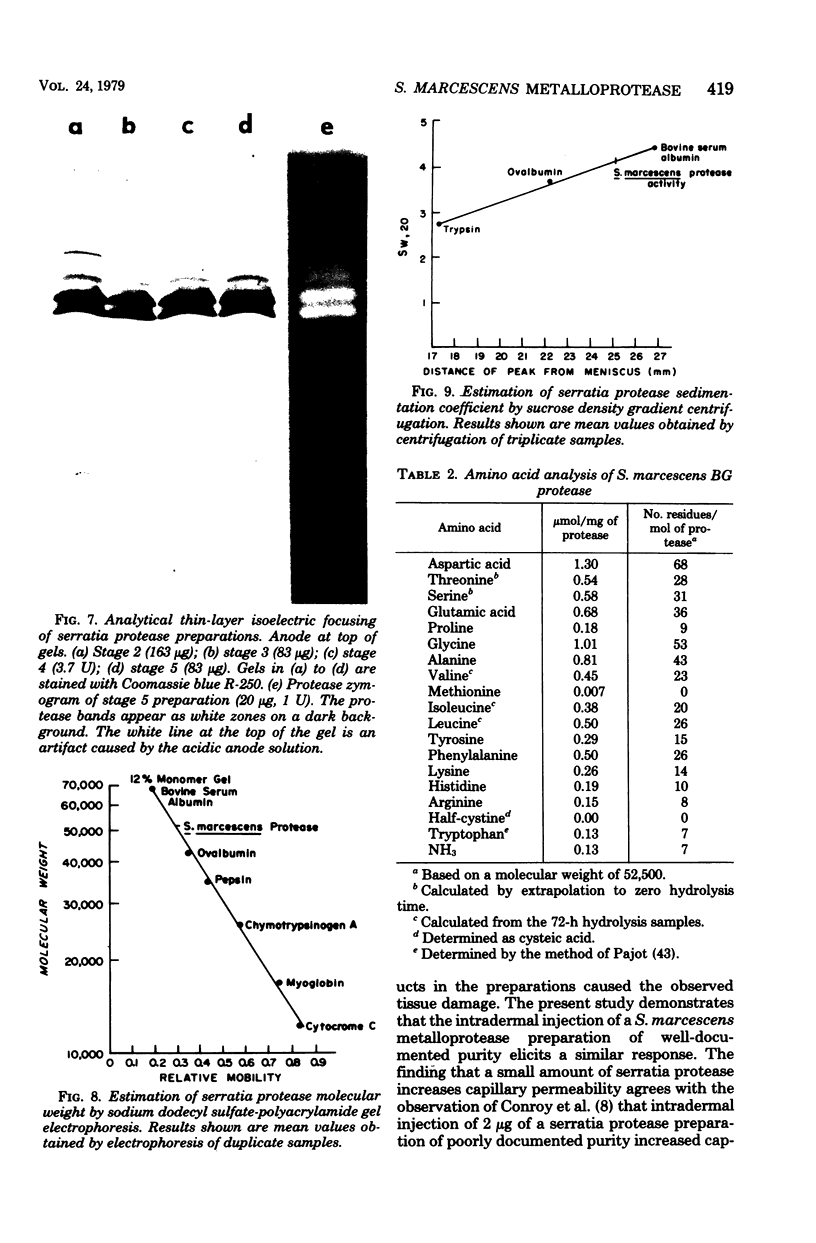
An extracellular, nonelastolytic, neutral metalloprotease of Serratia marcescens was purified by sequential ammonium sulfate precipitation, hydroxyapatite adsorption chromatography, flat-bed isoelectric focusing, and Sephadex G-100 gel filtration. The protease preparation had a 280/260 nm absorbance ratio of 1.8, was free of detectable amounts of endotoxin, carbohydrate, phosphorus, and other known extracellular enzymes of S. marcescens, and was homogeneous by Ouchterlony double immunodiffusion and Grabar-Williams immunoelectrophoresis. Crossed immunoelectrophoresis, thin-layer electrofocusing in polyacrylamide gel, and polyacrylamide disc gel electrophoresis showed three to four closely migrating, Coomassie blue-staining components in the protease preparation. However, zymogram analyses of the patterns showed that protease activity was associated with each component and that the protease was, therefore, microheterogeneous. The isoelectric point and sedimentation coefficient of the protease were approximately 5.3 to 5.4 and 4.2S, respectively, and the molecular weight estimated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and by gel filtration was approximately 52,500 and 44,000, respectively. The pH optimum range, with azocasein as the substrate, was 5.5 to 7.5. The enzyme contained a high percentage of acidic amino acids, no cysteine, and 1 g-atom of Zn2+ and 7 g-atoms of Ca2+ per mol. Various heavy metal ions and chelating agents and heating at 60°C for 15 min inactivated the enzyme. Intracorneal, intratracheal, and intradermal administration of the protease into rabbits elicited rapid and extensive tissue damage. The minimum lethal intravenous dose for mice was approximately 17 mg/kg of body weight.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiyappa P. S., Harris J. O. The extracellular metalloprotease of Serratia marcescens: I. Purification and characterization. Mol Cell Biochem. 1976 Nov 30;13(2):95–100. doi: 10.1007/BF01837059. [DOI] [PubMed] [Google Scholar]
- Altemeier W. A., Culbertson W. R., Fullen W. D., McDonough J. J. Serratia marcescens septicemia. A new threat in surgery. Arch Surg. 1969 Aug;99(2):232–238. doi: 10.1001/archsurg.1969.01340140104015. [DOI] [PubMed] [Google Scholar]
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidson S., Wadström T. Detection of proteolytic activity after isoelectric focusing in polyacrylamide gel. Biochim Biophys Acta. 1973 Jun 15;310(2):418–420. doi: 10.1016/0005-2795(73)90124-4. [DOI] [PubMed] [Google Scholar]
- Conroy M. C., Bander N. H., Lepow I. H. Effects in the rat of intradermal injection of purified proteinases from streptococcus and Serratia marcescens. Proc Soc Exp Biol Med. 1975 Dec;150(3):801–806. doi: 10.3181/00379727-150-39128. [DOI] [PubMed] [Google Scholar]
- DISCHE Z. Spectrophotometric method for the determination of free pentose and pentose in nucleotides. J Biol Chem. 1949 Nov;181(1):379–392. [PubMed] [Google Scholar]
- Davis J. T., Foltz E., Blakemore W. S. Serratia marcescens. A pathogen of increasing clinical importance. JAMA. 1970 Dec 21;214(12):2190–2192. doi: 10.1001/jama.214.12.2190. [DOI] [PubMed] [Google Scholar]
- De Siervo A. J. Alterations in the phospholipid composition of Escherichia coli B during growth at different temperatures. J Bacteriol. 1969 Dec;100(3):1342–1349. doi: 10.1128/jb.100.3.1342-1349.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorwart B. B., Abrutyn E., Schumacher H. R. Serratia arthritis. Medical eradication of infection in a patient with rheumatoid arthritis. JAMA. 1973 Sep 24;225(13):1642–1643. doi: 10.1001/jama.225.13.1642. [DOI] [PubMed] [Google Scholar]
- Epstein E., Carson T. E. Serratia granuloma. JAMA. 1973 Feb 5;223(6):670–671. [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- GAREN A., LEVINTHAL C. A fine-structure genetic and chemical study of the enzyme alkaline phosphatase of E. coli. I. Purification and characterization of alkaline phosphatase. Biochim Biophys Acta. 1960 Mar 11;38:470–483. doi: 10.1016/0006-3002(60)91282-8. [DOI] [PubMed] [Google Scholar]
- GRASSMANN W., NORDWIG A. [Quantitative colorimetric test for collagenase]. Hoppe Seylers Z Physiol Chem. 1960 Dec 31;322:267–272. doi: 10.1515/bchm2.1960.322.1.267. [DOI] [PubMed] [Google Scholar]
- Kaska M., Lysenko O., Chaloupka J. Exocellular proteases of Serratia marcescens and their toxicity to larvae of Galleria mellonella. Folia Microbiol (Praha) 1976;21(6):465–473. doi: 10.1007/BF02876938. [DOI] [PubMed] [Google Scholar]
- Kreger A. S., Gray L. D. Purification of Pseudomonas aeruginosa proteases and microscopic characterization of pseudomonal protease-induced rabbit corneal damage. Infect Immun. 1978 Feb;19(2):630–648. doi: 10.1128/iai.19.2.630-648.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreger A. S., Griffin O. K. Cornea-damaging proteases of Serratia marcescens. Invest Ophthalmol. 1975 Mar;14(3):190–198. [PubMed] [Google Scholar]
- Kreger A. S., Griffin O. K. Physicochemical fractionation of extracellular cornea-damaging proteases of Pseudomonas aeruginosa. Infect Immun. 1974 May;9(5):828–834. doi: 10.1128/iai.9.5.828-834.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurioka S., Liu P. V. Improved assay method for phospholipase C. Appl Microbiol. 1967 May;15(3):551–555. doi: 10.1128/am.15.3.551-555.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU P. V. Observations on the specificities of extracellular antigens of the genera Aeromonas and Serratia. J Gen Microbiol. 1961 Jan;24:145–154. doi: 10.1099/00221287-24-1-145. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lazachek G. W., Boyle G. L., Schwartz A. L., Leopold I. H. Serratia marcescens, an ocular pathogen; new considerations. Arch Ophthalmol. 1971 Nov;86(5):599–603. doi: 10.1001/archopht.1971.01000010601020. [DOI] [PubMed] [Google Scholar]
- MARKS J. Recognition of pathogenic staphylococci: with notes on non-specific staphylococcal haemolysin. J Pathol Bacteriol. 1952 Jan;64(1):175–186. doi: 10.1002/path.1700640118. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- MORIHARA K., TSUZUKI H., OKA T., INOUE H., EBATA M. PSEUDOMONAS AERUGINOSA ELASTASE. ISOLATION, CRYSTALLIZATION, AND PRELIMINARY CHARACTERIZATION. J Biol Chem. 1965 Aug;240:3295–3304. [PubMed] [Google Scholar]
- Maki D. G., Hennekens C. G., Phillips C. W., Shaw W. V., Bennett J. V. Nosocomial urinary tract infection with Serratia marcescens: an epidemiologic study. J Infect Dis. 1973 Nov;128(5):579–587. doi: 10.1093/infdis/128.5.579. [DOI] [PubMed] [Google Scholar]
- McQuade A. B., Crewther W. G. Activity against a synthetic substrate by a preparation of extracellular proteinase from Serratia marcescens. Biochim Biophys Acta. 1969;191(3):762–764. doi: 10.1016/0005-2744(69)90382-9. [DOI] [PubMed] [Google Scholar]
- Meltz D. J., Grieco M. H. Characteristics of Serratia marcescens pneumonia. Arch Intern Med. 1973 Sep;132(3):359–364. [PubMed] [Google Scholar]
- Mills J., Drew D. Serratia marcescens endocarditis: a regional illness associated with intravenous drug abuse. Ann Intern Med. 1976 Jan;84(1):29–35. doi: 10.7326/0003-4819-84-1-29. [DOI] [PubMed] [Google Scholar]
- Nagai Y., Lapiere C. M., Gross J. Tadpole collagenase. Preparation and purification. Biochemistry. 1966 Oct;5(10):3123–3130. doi: 10.1021/bi00874a007. [DOI] [PubMed] [Google Scholar]
- Nestle M., Roberts W. K. An extracellular nuclease from Serratia marcescens. I. Purification and some properties of the enzyme. J Biol Chem. 1969 Oct 10;244(19):5213–5218. [PubMed] [Google Scholar]
- Ovenfors C., Marklund M., Johnsson E., Lundblad G. Investigation of the protease forming ability of Serratia marcescens and one of its mutant strains. Acta Chem Scand. 1973 Oct;27(10):3791–3800. doi: 10.3891/acta.chem.scand.27-3791. [DOI] [PubMed] [Google Scholar]
- Pajot P. Fluroescence of proteins in 6-M guanidine hydrochloride. A method for the quantitative determination of tryptophan. Eur J Biochem. 1976 Mar 16;63(1):263–269. doi: 10.1111/j.1432-1033.1976.tb10228.x. [DOI] [PubMed] [Google Scholar]
- ROLAND C. G. STRADDLING A HOBBY: AVOCATIONAL BOOKS BY CANADIAN PHYSICIANS. JAMA. 1965 Apr 12;192:114–117. doi: 10.1001/jama.1965.03080150044010. [DOI] [PubMed] [Google Scholar]
- Rydén A. C., von Hofsten B. Some properties of the extracellular proteinase and the cell-bound peptidase of Serratia. Acta Chem Scand. 1968;22(9):2803–2808. doi: 10.3891/acta.chem.scand.22-2803. [DOI] [PubMed] [Google Scholar]
- Salceda S. R., Lapuz J., Vizconde R. Serratia marcescens endophthalmitis. Arch Ophthalmol. 1973 Feb;89(2):163–166. doi: 10.1001/archopht.1973.01000040165023. [DOI] [PubMed] [Google Scholar]
- Susor W. A., Kochman M., Rutter W. J. Heterogeneity of presumably homogeneous protein preparations. Science. 1969 Sep 19;165(3899):1260–1262. doi: 10.1126/science.165.3899.1260. [DOI] [PubMed] [Google Scholar]
- TIETZ N. W., BORDEN T., STEPLETON J. D. An improved method for the determination of lipase in serum. Am J Clin Pathol. 1959 Feb;31(2):148–154. doi: 10.1093/ajcp/31.2.148. [DOI] [PubMed] [Google Scholar]
- Traub W. H., Kleber I. Detection of fibrinolytic and elastase activity among clinical isolates of Serratia marcescens. Pathol Microbiol (Basel) 1974;41(6):334–340. doi: 10.1159/000162708. [DOI] [PubMed] [Google Scholar]
- Weber K., Pringle J. R., Osborn M. Measurement of molecular weights by electrophoresis on SDS-acrylamide gel. Methods Enzymol. 1972;26:3–27. doi: 10.1016/s0076-6879(72)26003-7. [DOI] [PubMed] [Google Scholar]