Abstract
Background
The immune response has been implicated in the control of uveal melanoma progression. Epigenetic mechanisms mediated by specific microRNAs (miRs) regulate immune responses.
Methods
Blood was drawn from six patients with uveal melanoma followed from diagnosis, at which time there was no clinical or radiographic evidence of metastasis, until metastasis manifested. Circulating T cell, natural killer (NK), natural killer T (NKT), and myeloid suppressor cell populations were assessed by flow cytometry. CD3+, CD15+, and CD56+ cells were isolated using immunomagnetic beads. Plasma and cellular levels of immune regulatory miRs were determined by quantitative polymerase chain reaction assays.
Results
The development of metastasis was associated with decreases in circulating CD3−CD56dim NK cells and CD8+ and double-negative CD3+CD56+ NKT cells. ICOS+CD4+FoxP3+ T regulatory cells and CD11b+CD14−CD15+ myeloid suppressor cells increased. Plasma levels of miR-20a, 125b, 146a, 155, 181a, and 223 were higher in the study patients at diagnosis compared to controls. Plasma levels of miR-20a, 125b, 146a, 155, and 223 increased, and miR-181a decreased when metastasis manifested. Alterations in immune regulatory miRs were also observed in CD3+, CD15+, and CD56+ cell populations.
Conclusions
The development of metastasis in uveal melanoma is associated with changes in immune effector and regulatory cells consistent with lessening tumor immune surveillance. These changes are associated with changes in plasma and cellular levels of immune regulatory miRs. The results may help guide uveal melanoma immunotherapy and biomarker development.
Keywords: MicroRNA, Uveal melanoma, Biomarkers, Immune response
1. Introduction
Melanoma of the eye’s uveal tract is a rare, aggressive cancer with a high mortality rate because of the development of metastatic disease, primarily in the liver, that almost invariably is refractory to therapy (Singh et al., 2011). Immune mechanisms have been implicated in uveal melanoma progression. Mouse models have implicated cytostatic CD8+ T cells (Eyles et al., 2010), natural killer (NK) cells (Dithmar et al., 2000; Yang et al., 2011), and pro-tumoral macrophages (Ly et al., 2010). Several clinical observations suggest that immune responses are operational. In many solid tumors, including cutaneous melanoma, the presence of tumor infiltrating lymphocytes (TIL) confers a good prognosis. In uveal melanoma, TIL are associated with the development of metastatic disease. Tumor expression of MHC class I antigen, which is necessary for T-cell recognition but renders cells resistant to NK cells, is also associated with a poor prognosis (Blom et al., 1997). We have shown that elevated blood levels of beta2 microglobulin, the soluble MHC class I heavy chain, are associated with tumor monosomy-3, which confers a poor prognosis (Triozzi et al., 2013). Specific HLA-C alleles encoding ligands for inhibitory NK receptors have been associated with disease-free survival (Maat et al., 2009). T regulatory (Treg), natural killer T (NKT), and myeloid derived suppressor cells (MDSC) have also been identified in the tumors or blood of patients (Niederkorn, 2009; McKenna et al., 2009; Mougiakakos et al., 2010; Bricard et al., 2009).
Although immune responses are predominantly controlled at the transcriptional level, epigenetic mechanisms are increasingly being recognized. microRNAs (miRs) are small non-coding RNAs that regulate gene expression at the post-transcriptional level by either degrading or blocking translation of mRNA targets. They play important roles in oncogenesis, and the ability of miR expression profiling to distinguish different cancer types and classify their subtypes has been well-described. miRs also play important regulatory roles in a variety of cellular functions, including immune response, and several miRs with immune regulatory activities have been identified. Predominant among these are miR-125b, 146a, 155, 181a, 223, and miRs of the 17–92 complex (Tsitsiou and Lindsay, 2009). Highly stable in the circulation, miRs hold great promise as a new class of blood biomarkers (Ferracin et al., 2010).
Studies of circulating immune cells in patients with uveal melanoma have been primarily conducted in patients at diagnosis (McKenna et al., 2009; Manyś-Kubacka et al., 2005; Haynie et al., 1997). There is little information regarding circulating levels of immune effector and regulatory cells as patients progress to metastatic disease. How circulating miRs, immune regulatory or other, modulate with the progression of human cancer is also not known. In order to examine the immune mechanisms involved as well as to develop potential biomarkers of disease progression, we compare here immune cell and immune regulatory miRs levels of patients followed prospectively from primary diagnosis to metastatic disease.
2. Patients and methods
2.1. Patients
Six consenting patients with uveal melanoma enrolled on a study approved by the Cleveland Clinic Institutional Review Board were evaluated. Plasma was also obtained from healthy donors without ocular disease, also on an approved study. At the time of diagnosis, each patient underwent comprehensive ophthalmic examination with supporting diagnostic studies. This included computed tomography scans of the chest, abdomen, and pelvis to rule out metastatic disease. Fine needle aspiration biopsy was performed in patients undergoing brachytherapy (plaque radiotherapy) at the time of plaque insertion. Chromosome 3 status was assessed by a fluorescence in situ hybridization technique (Singh et al., 2012). Patients were followed clinically and radiographically using standard-of-care guidelines, which included liver imaging and laboratory tests at least every six months. Metastasis was confirmed cytohistologically in all patients. Blood for the immune studies was drawn prior to primary therapy and at the time of clinical follow-up.
2.2. Flow cytometry
An aliquot of whole peripheral blood was evaluated by multicolor flow cytometry using a FACSCalibur flow cytometer (BD Biosciences, Mountain View, CA). Immune cell populations were identified using phycoerythrin labeled CD11b, FoxP3, and NKG2D; fluorescein isothiocyanate labeled CD3zeta, CD4, and CD14; allophycocyanin labeled CD8 and CD56; and peridinin–chlorophyll–protein complex labeled CD3epsilon and CD15. All labeled antibodies were purchased from BD Biosciences (Mountain View, CA) with the exception of FoxP3, which was purchased from eBiosciences (San Diego, CA). The percentage of populations of interest was determined by using gate statistics.
2.3. Cell isolation
CD3, CD15, and CD56 cells were isolated from peripheral blood mononuclear cells obtained using magnetic cell separation and MicroBeads from Miltenyi Biotec (Auburn, CA) according to the manufacturer’s instruction.
2.4. miRs
Total RNA was isolated from plasma and from cells isolated using the miRNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. Reverse transcription reactions were performed using a TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA) according to the manufacturer’s instructions. Quantitative real-time polymerase chain reaction (qRT-PCR) was performed using the reverse transcription reaction product, TaqMan MicroRNA Assay kit, and TaqMan Universal PCR Master Mix (Applied Biosystems) according to the manufacturer’s instructions. TaqMan MicroRNA Assay kits for human miRs were used. Reactions were loaded onto a 96-well plate and run in duplicate on an ABI 7500 Fast Real-Time PCR System (Applied Biosystems). The reactions were incubated at 50 °C for 20 s and 95 °C for 10 min, followed by 40 cycles of denaturation at 95 °C for 15 s, then 1 min of annealing/extension at 60 °C. The CT method was used to determine relative number of copies (RQ) of miR. Data were normalized to a Caenorhabditis elegans synthetic miR sequence, cel-miR-39 (Qiagen), which was spiked in as a control during RNA isolation.
2.5. Statistical analysis
Data are presented as means ± SD. All statistical analyses were performed using t tests. Differences between primary and metastatic samples were analyzed using two-tailed, paired t tests. P < 0.05 was considered significant.
3. Results
3.1. Immune cells
Blood was drawn from six patients at the time of primary diagnosis and when metastasis manifested (Table 1). All patients had normal laboratory evaluations, including absolute neutrophil, lymphocyte, and monocyte counts and liver function tests, at diagnosis and when metastasis manifested. Levels of T, NK, and NKT phenotypes were evaluated (Figs. 1 and 2). Although CD8+ cells tended to decrease and CD4+ cells tended to increase, significant changes in these cells and their ratios were not observed at metastasis compared to primary diagnosis (Fig. 1). Significant changes were also not observed in CD3+, CD4+, or CD8+ T-cell activation, as assessed by expression of inducible costimulator (ICOS), or suppression, as assessed by expression of T cell receptor (TCR) ζ. When compared to primary diagnosis, metastasis was associated with decreases in circulating CD3−CD56dim NK cells (Fig. 2A) and in CD8+ and doublenegative (DN) CD3+CD56+ NKT cells (Fig. 2B). Although not reaching the level of statistical significance, CD3−CD56bright NK cells tended to increase, and NKG2D+ NK cells and CD8+ NKT tended to decrease. Changes in the frequency of CD4+ NKT were not observed. Treg cell and myeloid suppressor phenotypes were also evaluated (Fig. 3). Although changes in the frequency of CD4+ and CD8+ FoxP3+ Treg cells were not observed (Fig. 3A), CD4+FoxP3+ cells expressing ICOS increased significantly (Fig. 3B). CD11b+CD14−CD15+ cells also increased significantly (Fig. 3C).
Table 1.
Study patients.
| No. | Age/sex | Primary tumor |
Treatment | Time to metastasis (months) | Metastatic involvement | ||
|---|---|---|---|---|---|---|---|
| Diameter × height (mm) | Monosomy 3 | TILs | |||||
| 1 | 58/M | 14 × 11 | Yes | Yes | Enucleation | 24 | Liver |
| 2 | 66/F | 17 × 14 | Yes | Yes | Enucleation | 9 | Liver |
| 3 | 74/F | 20 × 14 | Yes | Yes | Enucleation | 6 | Liver, bone, lung, and subcutaneous tissues |
| 4 | 67/M | 14 × 4 | Yes | Yes | Enucleation | 24 | Liver |
| 5 | 65/M | 13 × 3 | Yes | NDa | Brachytherapy | 15 | Liver |
| 6 | 52/M | 8 × 6 | Yes | Yes | Enucleation | 24 | Liver, bone, and lung |
ND, not determined.
Fig. 1.
(A) T cell populations and (B) T cell receptor ζ chain expression at diagnosis (P) and at metastasis (M) (n = 6).
Fig. 2.
(A) NK and (B) NKT populations at diagnosis (P) and at metastasis (M) (n = 6).
Fig. 3.
(A) Treg cell, (B) ICOS+ Treg cell, and MDSC at diagnosis (P) and at metastasis (M) (n = 6).
3.2. Immune miRs
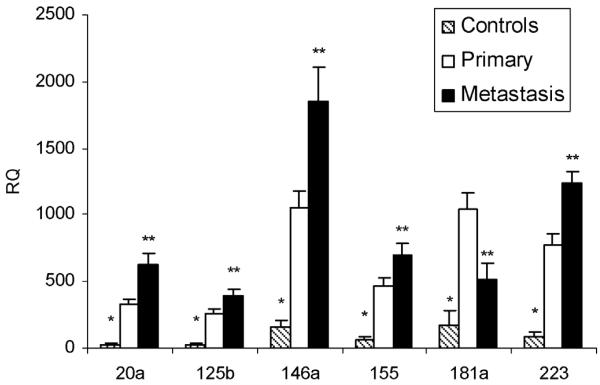
Plasma levels of miR-20a, a miR of the 17–92 complex, and miR-125b, 146a, 155, 181a, and 223 were assessed in patients with uveal melanoma at diagnosis and at the time metastasis manifested. Levels of these immune regulatory miRs were also compared to 26 healthy donor controls (Fig. 4). Levels of all the miRs tested were higher in the study patients when compared to the controls. With the exception of miR-181a, levels of all the immune regulatory miRs tested were higher at metastasis compared to primary diagnosis. miR-181a levels were lower at metastasis compared to primary diagnosis, but still higher than that of the controls. Immune regulatory miR levels were also assessed in blood CD3+, CD15+, and CD56+ cells isolated using immunomagnetic separation from five patients that had sufficient cells (Fig. 5). miR-146a increased in all three populations. miR-181a decreased in CD3+ cells. miR-155 decreased in CD56+ and CD15+ cells. The increases in miR-155 observed in CD3+ cells were not significant. Although miR-223 tended to increase in all three populations, only in the CD56+ population was the increased statistically significant. Increases in miR-20a were also only significant in CD56+ cells.
Fig. 4.
Plasma levels of healthy donor controls (n = 26) and of uveal melanoma patients (n = 6) at primary diagnosis and at metastasis. *P < 0.05 compared to primary and metastasis; **P < 0.05 compared to primary.
Fig. 5.
Immune miR levels of CD3, CD15, and CD56 peripheral blood cells at diagnosis (P) and at metastasis (M) (n = 5).
4. Discussion
The high rate of metastatic disease despite a low rate of local recurrence and the presence of circulating melanoma cells indicate the presence of subclinical micro-metastases at presentation in patients with uveal melanoma (Kujala et al., 2003; Torres et al., 2011). What regulates the progression to macro-metastasis is not known. We compared immune cells in patients at diagnosis, at which time there was no clinical or radiographic evidence of metastasis, to when metastasis manifested. Changes consistent with lessening tumor immune surveillance were observed. Metastasis was associated with decreases in CD3−CD56dim NK cells, which mediate tumor cell cytotoxicity, and CD8+ and DN NKT phenotypes, which may promote antitumor immunity by producing Th1-associated cytokines (Bricard et al., 2009). Increases were observed in the frequency of ICOS+CD4+FoxP3+ Treg cells, a subset that secrete interleukin-10 and transforming growth factor β and suppresses dendritic cells and CD4+ Th cells (Ito et al., 2008). Increases were also observed in CD11b+CD14+CD15+ cells, a neutrophilic MDSC phenotype identified in the blood of patients with uveal (and cutaneous) melanoma (McKenna et al., 2009; Gros et al., 2012). We did not observe changes in expression of ICOS, an activation molecule in the CD28/CTLA-4/B7 family, on T cells. We also did not observe changes in the expression of the TCRζ, an indicator of T cell suppression, which has also been reported among uveal melanoma TIL (Staibano et al., 2006).
The role of miRs in immune regulation is increasingly being recognized. Certain miRs serve in important negative feedback loops in the immune system, whereas others serve to amplify the response of the immune system by repressing inhibitors of the response. miRs are attractive cancer biomarkers. Because of incorporation into microparticles and exosomes, miRs are very stable and can be measured in the circulation (Ferracin et al., 2010). To develop blood biomarkers of uveal melanoma progression, we examined levels of circulating immune regulatory miRs in patients with uveal melanoma. We observed increases in plasma levels of several miRs implicated in immune regulation as metastasis manifested, including miRs-125b, 146a, 155, 20a, and 223. In contrast, miR-181a decreased. Furthermore, we found that all the immune miRs tested were high in study patients compared to normal control plasma. miR-125b, 155, 181a, and miRs of the 17–92 complex have been shown to play central roles in T-cell development and function (Dooley et al., 2013). Of note, reduction of miR-181a has been shown to decrease the strength and increases the threshold required for TCR signaling (Li et al., 2007). miR 146a and miR-155 play roles in NK cells development and function (Leong et al., 2012). Overexpression of miR-146a in NK cell lines inhibited NF-κB signals, suppressed proliferation, induced apoptosis (Ng et al., 2011). miR-181a and miR-223 have been implicated in NKT regulation (Li et al., 2011; Henao-Mejia et al., 2013); miR-125b, 146a, and 155, in Treg cell development (Kohlhaas et al., 2009; Hezova et al., 2010); and miR-223, in MDSC regulation (Liu et al., 2011).
miRs measured in plasma are derived primarily from circulating leukocytes (Mitchell et al., 2008). We examined the possibility that the changes in plasma miR levels could be ascribed to specific immune cell populations. Cell availability limited these analyses. Nonetheless, guided by the flow cytometry data, we examined changes in the immune cell expression of CD3+, CD56+ and CD15+ populations. Whereas miR-146 increased in the plasma and in all three immune populations, changes in the plasma and the cellular levels of miR-155 and miR-181a varied. Significant changes were most frequent in CD56+ cells, which includes NK and NKT cells. Further study of the cellular source of the plasma miRs will be necessary. It would be of interest to examine miR expression in more specific T, NK, NKT, and MDSC populations modulate with disease progression. miR levels of CD4+, CD8+, and CD4+CD25highCD127−Treg cells have been associated with the activity of autoimmune diseases (Hezova et al., 2010; Lorenzi et al., 2012; Pan et al., 2010; Tsitsiou et al., 2012).
Uveal melanoma is a rare cancer, and samples size in this study was small. Further studies of immune cells and immune miRs in cancer patients should help improve the understanding of immune response in tumor surveillance. Cellular phenotypes and miR levels may help guide immunotherapy recommendations. The ICOS pathway is required for optimal antitumor responses medicated by anti-CTLA-4 therapy (Fu et al., 2011). Circulating miRs are being examined as predictive markers. They may lead to novel immunotherapeutic targets. Molecular characterization of immune miRs as potential biomarkers may also lead to future therapies, both indirectly and directly. That supplementing/replenishing miRs in vivo can boost protective immunity against lethal tumors has been demonstrated in mouse models (Cubillos-Ruiz et al., 2012). Methods of directly modifying miR expression are under investigation (Jackson and Linsley, 2010). miRs can also be indirectly modified by currently available drugs (Dubovsky et al., 2010).
Acknowledgments
This work was supported in part by RO1CA136776 from the National Cancer Institute, National Institutes of Health, Bethesda, MD and a gift from the Ratner family, Cleveland, OH.
References
- Blom DJ, Luyten GP, Mooy C, Kerkvliet S, Zwinderman AH, Jager MJ. Human leukocyte antigen class I expression: marker of poor prognosis in uveal melanoma. Invest. Ophthalmol. Vis. Sci. 1997;38:1865–1872. [PubMed] [Google Scholar]
- Bricard G, Cesson V, Devevre E, Bouzourene H, Barbey C, Rufer N, Im JS, Alves PM, Martinet O, Halkic N, Cerottini JC, Romero P, Porcelli SA, Macdonald HR, Speiser DE. Enrichment of human CD4+ V(alpha)24/Vbeta11 invariant NKT cells in intrahepatic malignant tumors. J. Immunol. 2009;182:5140–5151. doi: 10.4049/jimmunol.0711086. [DOI] [PubMed] [Google Scholar]
- Cubillos-Ruiz JR, Baird JR, Tesone AJ, Rutkowski MR, Scarlett UK, Camposeco-Jacobs AL, Anadon-Arnillas J, Harwood NM, Korc M, Fiering SN, Sempere LF, Conejo-Garcia JR. Reprogramming tumor-associated dendritic cells in vivo using miRNA mimetics triggers protective immunity against ovarian cancer. Cancer Res. 2012;72:1683–1693. doi: 10.1158/0008-5472.CAN-11-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dithmar S, Rusciano D, Lynn MJ, Lawson DH, Armstrong CA, Grossniklaus HE. Neoadjuvant interferon alfa-2b treatment in a murine model for metastatic ocular melanoma: a preliminary study. Arch. Ophthalmol. 2000;118:1085–1089. doi: 10.1001/archopht.118.8.1085. [DOI] [PubMed] [Google Scholar]
- Dooley J, Linterman MA, Liston A. MicroRNA regulation of T-cell development. Immunol. Rev. 2013;253:53–64. doi: 10.1111/imr.12049. [DOI] [PubMed] [Google Scholar]
- Dubovsky JA, Villagra A, Powers JJ, Wang HW, Pinilla-Ibarz J, Sotomayor EM. Circumventing immune tolerance through epigenetic modification. Curr. Pharm. Des. 2010;16:268–276. doi: 10.2174/138161210790170120. [DOI] [PubMed] [Google Scholar]
- Eyles J, Puaux AL, Wang X, Toh B, Prakash C, Hong M, Tan TG, Zheng L, Ong LC, Jin Y, Kato M, Prévost-Blondel A, Chow P, Yang H, Abastado JP. Tumor cells disseminate early, but immunosurveillance limits metastatic outgrowth, in a mouse model of melanoma. J. Clin. Invest. 2010;120:2030–2039. doi: 10.1172/JCI42002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferracin M, Veronese A, Negrini M. Micromarkers: miRNAs in cancer diagnosis and prognosis. Expert Rev. Mol. Diagn. 2010;10:297–308. doi: 10.1586/erm.10.11. [DOI] [PubMed] [Google Scholar]
- Fu T, He Q, Sharma P. The ICOS/ICOSL pathway is required for optimal anti-tumor responses mediated by anti-CTLA-4 therapy. Cancer Res. 2011;71:5445–5454. doi: 10.1158/0008-5472.CAN-11-1138. [DOI] [PubMed] [Google Scholar]
- Gros A, Turcotte S, Wunderlich JR, Ahmadzadeh M, Dudley ME, Rosenberg SA. Myeloid cells obtained from the blood but not from the tumor can suppress T-cell proliferation in patients with melanoma. Clin. Cancer Res. 2012;18:5212–5223. doi: 10.1158/1078-0432.CCR-12-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynie GD, Shen TT, Gragoudas ES, Young LH. Flow cytometry analysis of peripheral blood lymphocytes in patients with choroidal melanoma. Am. J. Ophthalmol. 1997;124:357–361. doi: 10.1016/s0002-9394(14)70827-x. [DOI] [PubMed] [Google Scholar]
- Henao-Mejia J, Williams A, Goff LA, Staron M, Licona-Limón P, Kaech SM, Nakayama M, Rinn JL, Flavell RA. The microRNA miR-181 is a critical cellular metabolic rheostat essential for NKT cell ontogenesis and lymphocyte development and homeostasis. Immunity. 2013;38:984–997. doi: 10.1016/j.immuni.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hezova R, Slaby O, Faltejskova P, Mikulkova Z, Buresova I, Raja KR, Hodek J, Ovesna J, Michalek J. microRNA-342, microRNA-191 and microRNA-510 are differentially expressed in T regulatory cells of type 1 diabetic patients. Cell. Immunol. 2010;260:70–74. doi: 10.1016/j.cellimm.2009.10.012. [DOI] [PubMed] [Google Scholar]
- Ito T, Hanabuchi S, Wang YH, Park WR, Arima K, Bover L, Qin FX, Gilliet M, Liu YJ. Two functional subsets of FOXP3+ regulatory T cells in human thymus and periphery. Immunity. 2008;28:870–880. doi: 10.1016/j.immuni.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A, Linsley PS. The therapeutic potential of microRNA modulation. Discov. Med. 2010;9:311–318. [PubMed] [Google Scholar]
- Kohlhaas S, Garden OA, Scudamore C, Turner M, Okkenhaug K, Vigorito E. Cutting edge: the Foxp3 target miR-155 contributes to the development of regulatory T cells. J. Immunol. 2009;182:2578–2582. doi: 10.4049/jimmunol.0803162. [DOI] [PubMed] [Google Scholar]
- Kujala E, Makitie T, Kivela T. Very long-term prognosis of patients with malignant uveal melanoma. Invest. Ophthalmol. Vis. Sci. 2003;44:4651–4659. doi: 10.1167/iovs.03-0538. [DOI] [PubMed] [Google Scholar]
- Leong JW, Sullivan RP, Fehniger TA. Natural killer cell regulation by microRNAs in health and disease. J. Biomed. Biotechnol. 2012:632329. doi: 10.1155/2012/632329. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li QJ, Chau J, Ebert PJ, Sylvester G, Min H, Liu G, Braich R, Manoharan M, Soutschek J, Skare P, Klein LO, Davis MM, Chen CZ. miR-181a is an intrinsic modulator of T cell sensitivity and selection. Cell. 2007;129:147–161. doi: 10.1016/j.cell.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Li K, Seo KH, Gao T, Zheng Q, Qi RQ, Wang H, Weiland M, Dong Z, Mi QS, Zhou L. Invariant NKT cell development and function in microRNA-223 knockout mice. Int. Immunopharmacol. 2011;11:561–568. doi: 10.1016/j.intimp.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Liu Q, Zhang M, Jiang X, Zhang Z, Dai L, Min S, Wu X, He Q, Liu J, Zhang Y, Zhang Z, Yang R. miR-223 suppresses differentiation of tumor-induced CD11b+ Gr1+ myeloid-derived suppressor cells from bone marrow cells. Int. J. Cancer. 2011;129:2662–2673. doi: 10.1002/ijc.25921. [DOI] [PubMed] [Google Scholar]
- Lorenzi JC, Brum DG, Zanette DL, de Paula Alves Souza A, Barbuzano FG, Dos Santos AC, Barreira AA, da Silva WA. miR-15a and 16-1 are downregulated in CD4+ T cells of multiple sclerosis relapsing patients. Int. J. Neurosci. 2012;122:466–471. doi: 10.3109/00207454.2012.678444. [DOI] [PubMed] [Google Scholar]
- Ly LV, Baghat A, Versluis M, Jordanova ES, Luyten GP, van Rooijen N, van Hall T, van der Velden PA, Jager MJ. In aged mice, outgrowth of intraocular melanoma depends on proangiogenic M2-type macrophages. J. Immunol. 2010;185:3481–3488. doi: 10.4049/jimmunol.0903479. [DOI] [PubMed] [Google Scholar]
- Maat W, van der Slik AR, Verhoeven DH, Alizadeh BZ, Ly LV, Verduijn W, Luyten GP, Mulder A, van Hall T, Koning F, Jager MJ, van Bergen J. Evidence for natural killer cell-mediated protection from metastasis formation in uveal melanoma patients. Invest. Ophthalmol. Vis. Sci. 2009;50:2888–2895. doi: 10.1167/iovs.08-2733. [DOI] [PubMed] [Google Scholar]
- Manyś-Kubacka K, Kostrzewa A, Sobieska M, Samborski W. Alterations of lymphocyte subpopulations in choroidal melanoma patients undergoing surgery. Rocz. Akad. Med. Bialymst. 2005;50:289–292. [PubMed] [Google Scholar]
- McKenna KC, Beatty KM, Bilonick RA, Schoenfield L, Lathrop KL, Singh AD. Activated CD11b+ CD15+ granulocytes increase in the blood of patients with uveal melanoma. Invest. Ophthalmol. Vis. Sci. 2009;50:4295–4303. doi: 10.1167/iovs.08-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O’Briant KC, Allen A, Lin DW, Urban N, Drescher CW, Knudsen BS, Stirewalt DL, Gentleman R, Vessella RL, Nelson PS, Martin DB, Tewari M. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. U.S.A. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mougiakakos D, Johansson CC, Trocme E, All-Ericsson C, Economou MA, Larsson O, Seregard S, Kiessling R. Intratumoral forkhead box P3-positive regulatory T cells predict poor survival in cyclooxygenase-2-positive uveal melanoma. Cancer. 2010;116:2224–2233. doi: 10.1002/cncr.24999. [DOI] [PubMed] [Google Scholar]
- Ng SB, Yan J, Huang G, Selvarajan V, Tay JL, Lin B, Bi C, Tan J, Kwong YL, Shimizu N, Aozasa K, Chng WJ. Dysregulated microRNAs affect pathways and targets of biologic relevance in nasal-type natural killer/T-cell lymphoma. Blood. 2011;118:4919–4929. doi: 10.1182/blood-2011-07-364224. [DOI] [PubMed] [Google Scholar]
- Niederkorn JY. Immune escape mechanisms of intraocular tumors. Prog. Retin. Eye Res. 2009;28:329–347. doi: 10.1016/j.preteyeres.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan W, Zhu S, Yuan M, Cui H, Wang L, Luo X, Li J, Zhou H, Tang Y, Shen N. MicroRNA-21 and microRNA-148a contribute to DNA hypomethylation in lupus CD4 T cells by directly and indirectly targeting DNA methyltransferase 1. J. Immunol. 2010;184:6773–6781. doi: 10.4049/jimmunol.0904060. [DOI] [PubMed] [Google Scholar]
- Singh AD, Turell ME, Topham AK. Uveal melanoma: trends in incidence, treatment, and survival. Ophthalmology. 2011;11:1881–1885. doi: 10.1016/j.ophtha.2011.01.040. [DOI] [PubMed] [Google Scholar]
- Singh AD, Aronow ME, Sun Y, Bebek G, Saunthararajah Y, Schoenfield LR, Biscotti CV, Tubbs RR, Triozzi PL, Eng C. Chromosome 3 status in uveal melanoma: a comparison of fluorescence in situ hybridization and single-nucleotide polymorphism array. Invest. Ophthalmol. Vis. Sci. 2012;53:3331–3339. doi: 10.1167/iovs.11-9027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staibano S, Mascolo M, Tranfa F, Salvatore G, Mignogna C, Bufo P, Nugnes L, Bonavolontà G, De Rosa G. Tumor infiltrating lymphocytes in uveal melanoma: a link with clinical behavior. Int. J. Immunopathol. Pharmacol. 2006;19:171–179. [PubMed] [Google Scholar]
- Torres V, Triozzi P, Eng C, Tubbs R, Schoenfiled L, Crabb JW, Saunthararajah Y, Singh AD. Circulating tumor cells in uveal melanoma. Future Oncol. 2011;7:101–109. doi: 10.2217/fon.10.143. [DOI] [PubMed] [Google Scholar]
- Triozzi PL, Elson P, Aldrich W, Achberger A, Tubbs R, Biscotti CV, Singh AD. Elevated blood beta-2 microglobulin is associated with tumor monosomy-3 in patients with primary uveal melanoma. Melanoma Res. 2013;23:1–7. doi: 10.1097/CMR.0b013e32835b7154. [DOI] [PubMed] [Google Scholar]
- Tsitsiou E, Lindsay MA. microRNAs and the immune response. Curr. Opin. Pharmacol. 2009;9:514–520. doi: 10.1016/j.coph.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsitsiou E, Williams AE, Moschos SA, Patel K, Rossios C, Jiang X, Adams OD, Macedo P, Booton R, Gibeon D, Chung KF, Lindsay MA. Transcriptome analysis shows activation of circulating CD8+ T cells in patients with severe asthma. J. Allergy Clin. Immunol. 2012;129:95–103. doi: 10.1016/j.jaci.2011.08.011. [DOI] [PubMed] [Google Scholar]
- Yang W, Li H, Mayhew E, Mellon J, Chen PW, Niederkorn JY. NKT cell exacerbation of liver metastases arising from melanomas transplanted into either the eyes or spleens of mice. Invest. Ophthalmol. Vis. Sci. 2011;52:3094–3102. doi: 10.1167/iovs.10-7067. [DOI] [PMC free article] [PubMed] [Google Scholar]