Abstract
Carcinoembryonic antigen (CEA) is a cancer vaccines target. Several feature of recombinant adeno-associated virus (rAAV) are attractive for vaccine applications. Combining other viral vector vaccines with Toll like receptor (TLR) agonists enhances antitumor immunity. Wild-type and CEA transgenic (Tg) mice were immunized with rAAV expressing CEA, the TLR9 agonist, ODN1826, and the TLR7 agonist, imiquimod. Mice were challenged with MC38 colon tumor cells and MC38 cells expressing CEA. rAAV-CEA immunization combined with ODN1826 or imiquimod enhanced CEA-specific T-helper-1 immunity and protected against tumor challenge in wild-type but not in CEA-Tg mice. In contrast, immunization with rAAV-CEA in CEA-Tg mice could abrogate the antitumor effects of ODN1826 and promote tumor growth. Compared to wild-type, CEA-Tg mice were characterized by a greater myeloid suppressor cell and T-helper 2 response to TLR agonists and to syngeneic tumors. Depleting PDCA1+ plasmacytoid dendritic cells and Gr1+ myeloid cells increased anti-CEA immune responses in CEA-Tg mice to rAAV-CEA-ODN1826 immunization; depleting CD25+ T cells did not. There are differences in the response of wild-type and CEA-Tg mice to rAAV-CEA, TLR agonists, and syngeneic tumor. In CEA-Tg mice tumor growth can be promoted with rAAV-CEA and TLR agonists. Dendritic and myeloid cells play a regulatory role.
Keywords: toll like receptors, dendritic cells, myeloid suppressor cells, regulatory T cells, Thelper cells
Introduction
Recombinant adeno-associated virus (rAAV), a nonpathogenic parvovirus, is being tested as a vector for gene therapy because of its safety profile and ability to produce durable transgene expression in a wide variety of cells. In this testing it become apparent that gene transfer is not devoid of immune response and that rAAV can serve as a vaccine vector and elicit biologically significant immune responses to microbial and other antigens, including tumor-associated antigens.1 Several features of rAAV are attractive for vaccine approaches for cancer, including the limited viral vector antigens presented as well as the efficient and persistence of antigen expression. In terms of generating the T helper (Th) 1 cellular immune responses considered essential to effective antitumor immunity, however, rAAV vectors have been relatively weak immunogens.2,3,4
We have demonstrated that rAAV expressing carcinoembryonic antigen (CEA), an antigen over-expressed by many common cancers, administered with bacterial plasmids that did and did not express immunostimulatory cytokines could enhance antitumor immune responses in mouse models.5 Plasmids have been effective in eliciting Th1-associated immunity in part due to their expression of unmethylated CpG motifs found in bacterial but not mammalian DNA.6 Through interactions with Toll like receptor (TLR) 9, CpG oligodinucleotides (ODN) support the maturation/activation of professional antigen presenting cells, and promote the induction of Th1-associated cytokines. We have also found the synthetic TLR9 and TLR7 agonists can improve the antitumor immune activity of rAAV expressing the melanoma associated antigen, Trp2, in mouse B16 melanoma.7
In our previous studies of rAAV-CEA, wild-type C57BL/6 mice were immunized and challenged with syngeneic MC38 tumor cells expressing CEA. Thus, in these models CEA was a non-self/allo-antigen. Mouse models expressing human CEA as a transgene have been developed to evaluate CEA-based cancer vaccines. A number of CEA vaccine approaches have demonstrated antitumor activity in CEA-Tg mice including poxvirus,8 genetically modified cell,9 plasmid DNA,10 and dendritic cell (DC)11 constructs. CpG ODNs have been effectively applied in CEA-Tg mice as an immune adjuvant to adenoviral,12 DC,13 and anti-idiotype antibody-based14 vaccine approaches. We examined the effects of immunizing with rAAV-CEA with the TLR7 agonist, imiquimod, and the TLR9 agonist, ODN1826, synthetic TLR agonists that have been effectively applied as vaccine adjuvants, including with genetic immunization.6 Differential effects were observed in wild-type and CEA-Tg mice. Whereas antitumor immune responses could be effectively elicited in wild-type mice, in CEA-Tg mice tumor growth could be promoted.
Materials and methods
Mice and cell lines
CEA transgenic mice [C57BL/6J-TgN (CEA Ge) 18FJP] (H-2b) were obtained from Dr. F. James Primus, Vanderbilt University Medical Center (Nashville, TN), and bred at the animal care facility of the Cleveland Clinic Foundation Lerner Research Institute. Newborn mice were screened for the CEA transgene by polymerase chain reaction (PCR) analysis of mouse tail DNA.15 Wild-type female C57BL/6 mice were obtained from Taconic (Germantown, NY). Mouse colon carcinoma cell line MC38 and MC38 transfected with human CEA (MC38-CEA) were obtained from Dr. Primus. Six- to 8-week-old mice were used for the experiments. All animal procedures were performed in accordance with recommendations for the proper care and use of laboratory animals.
Immunization and tumor challenge
rAAV serotype 2 encoding CEA was constructed and administered intramuscularly (i.m.) at 1011 particles in 50 µl normal saline as previously described.5 ODN1826 (The Midland Certified Reagent Company, Inc., Midland, TX) and imiquimod (InvivoGen, San Diego, CA) were administered at 50 µg in 0.1 ml phosphate buffered saline (PBS) subcutaneously (s.c.) and pNGVL3 (National Gene Vector Laboratories, Ann Arbor, MI), at 10 µg i.m., at the immunization site. ODN1826 was also administered intratumorally at 50 µg. Mice were challenged with 3 × 105 tumor cells subcutaneously in the flank. Tumor size was measured at least twice every week with a digital caliper for longest axis (L) and shortest axis (W), and tumor volume calculated using the formula: volume in mm3 = (L × W2)/2. When tumor growth exceeded 2000 mm3 or tumors became ulcerated, animals were euthanized. Mice were also sacrificed to obtain tumor, lymph nodes, and spleens for immune response assessment.
Serum antibody
Anti-CEA antibody was measured by enzyme-linked immunosorbent assay (ELISA) as previously described, using plates coated with human CEA protein (Fitzgerald Industries International, Concord MA) and alkaline phosphatase-conjugated goat anti-mouse IgG.5 Absorbance was measured at 405 nm on a VersaMax microplate reader using SoftMax Pro software (Molecular Devices, Sunnyvale, CA). Absorbance on CEA coated plates was corrected for absorbance on parallel plates coated with ovalbumin (Sigma). COL-1 mouse monoclonal γ2a antibody to CEA (Neomarkers, Freemont, CA) was used as a positive control.
Cytokine release assays
Single-cell suspensions of splenocytes were prepared and stimulated with 25 µg/ml human CEA, media alone or 50 µg/ml ovalbumin as negative controls, and 5 µg/ml ConA as a positive control, as previously described.5 After 3 days, culture supernatants were collected and assayed for IFN-γ by ELISA (R&D Systems, Minneapolis, MN) or for IL-2, IL-4, IL-6, IFN-γ, TNF-α, IL-17A, IL-10 using BD Cytometric Bead Array (CBA) Mouse Th1/Th2/Th17 Cytokine Kit (BD Biosciences, San Jose, CA) according to the manufacturer's instructions.
Flow cytometry
Cells were washed twice in PBS - 1% bovine serum albumin plus 0.05% sodium azide and stained for 30 minutes on ice with phycoerythrin or fluorescein isothiocyanate conjugated monoclonal antibodies to B220, CD11b, CD11c, and Gr1 (BD Biosciences, San Jose, CA) and CD4 and FoxP3 (eBioscience Inc., San Diego, CA). Appropriate isotype controls were used. After incubation, the cells were washed and fixed with 2% paraformaldehyde. All samples were analyzed using an EPICS Altra flow cytometer (Beckman Coulter, Fullerton, CA).
Quantitative real-time PCR (qRT-PCR)
Dissected tumors and lymph nodes were placed in RNA Later (Ambion, Austin, TX) and stored at −4°C. RNA was then extracted with RNeasy method (Qiagen, Valencia, CA) according to the manufacturer’s direction and stored at −80°C. An ABI Prism 7500 Sequence Detection System and pre-standardized primers and TaqMan probes for mouse IL-10, IL-12(p40), transforming growth factor (TGF) β, arginase (Arg1), CD206 (Mrc1), CXCL10, and FoxP3 were used (Applied Biosystems, Foster City, CA). Glyceraldehyde-3-phosphate dehydrogenase was used as the endogenous control. Reverse transcription and PCR was accomplished using a one-step protocol and TaqMan Universal Master Mix (Applied Biosystems) according to the recommendations of the manufacturer. Ct values were determined, and the relative number of copies of mRNA (RQ) was calculated using the ΔΔCt method (Relative Quantitation of Gene Expression, User Bulletin #2, ABI Prism 7700 Sequence Detection System, Applied Biosystems).
Immunohistochemistry
Tissue sections were incubated for 1 hour at 37°C with the primary antibody to mouse CD4, CD8, CD11b, and CD11c (BD Biosciences) diluted 1:100 in PBS containing 2% rabbit serum. After washing with PBS, sections were incubated for 15 minutes at 37°C with biotinylated secondary antibody, followed by streptoavidin-biotin peroxidase complex (Vector Laboratories, Burlingame, CA). Slides were washed, developed with 3,3’ diaminobenzidine (Vector Laboratories) and hydrogen peroxide, and counterstained with hematoxylin. The intensity of the immunohistochemical reactions was quantified by a Leica Image Analysis System with Image Pro, version 6.2 (Media Cybernetics, Bethesda, MD).
Cell depletion
For depletion of myeloid derived suppressor cells (MDSC), 200 µg of anti-Gr-1 antibody (RB6-8C5; BioExpress, Lebanon, NH) was injected intraperitoneally (i.p.) every other day for 3 doses.16 This treatment reduced the percentage of Gr-1+ cells to <5%. For depletion of plasmacytoid DCs (pDCs), 500 µg of anti-PDCA-1 antibody (eBio927; eBiosciences) was administrated i.p. every other day for 2 doses.17 This treatment reduced the percentage of PDCA-1+ cells within the CD11c+ cells from to <5%. For depletion of regulatory T (Treg) cells, 0.5 mg of anti-CD25 antibody (PC61; American Type Culture Collection, Manassa, VA) was administrated i.p. every other day for 3 doses18 This treatment reduced the percentage of Foxp3+ cells within lymph node CD4+ T cells to <2%. Purified rat IgG (Sigma-Aldrich, St. Louis, MO) was used as a control.
Statistical analysis
Standard errors of the mean (SEM) or standard deviations (SD) for each set of measurements were calculated and represented as y-axis error bars on each graph. Tumor volume data were analyzed using ANOVA. Differences in other parameters between specific groups of replicates were analyzed by two-sided Student’s t test. Changes were considered significant if P < 0.05.
RESULTS
Tumor growth effects of rAAV-CEA and TLR agonists
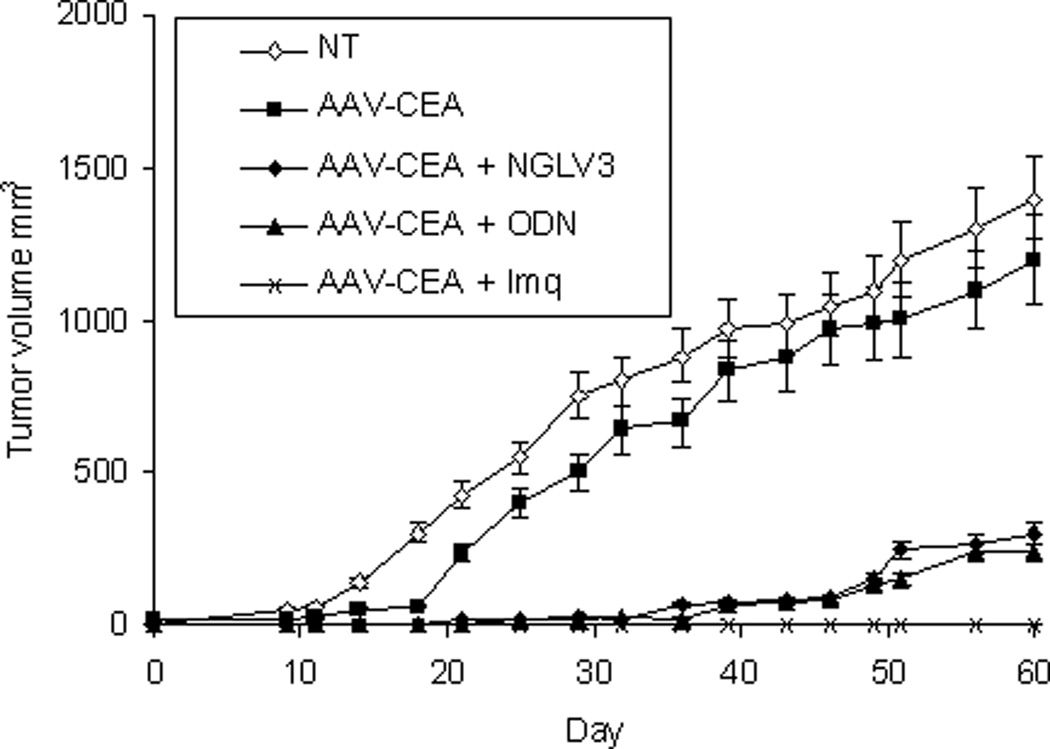
Immunizations were first examined in C57Bl/6 wild type mice (Figure 1). Mice received a single i.m. dose of rAAV-CEA. Two weeks later, when CEA protein is maximally expressed, mice were injected at the same site with ODN1826, imiquimod, or pNGVL3, the plasmid backbone previously shown to be an effective adjuvant.5 Significant tumor protection against MC38-CEA tumor cell challenge was again observed when rAAV-CEA was followed by pNGVL3 (P < 0.003, compared to rAAV-CEA alone) as well as when followed by ODN1826 (P < 0.001) and by imiquimod (P < 0.000001). The same rAAV-CEA-TLR-agonist approach was then tested in CEA-Tg mice (Figure 2). There was no evidence that applying ODN1826 (Figure 2A) or imiquimod (Figure 2B) after AAV-CEA promoted an antitumor effect. All mice developed tumors. Furthermore, a small increase in tumor growth was observed in mice immunized with rAAV-CEA followed by imiquimod (P < 0.05) and by ODN1826 (P < 0.03) compared to un-immunized control mice (Figure 2A). To determine whether an anti-CEA response was operational in this tumor growth, CEA-Tg were immunized with rAAV-CEA and ODN1826 and then challenged with MC38-CEA cells as well as with MC38 cells not expressing CEA. A small increase in tumor growth was again observed in immunized CEA-Tg mice compared to un-immunized control mice challenged with MC38-CEA (P < 0.03, Figure 2C). No differences in tumor growth were induced with immunization in CEA-Tg mice challenged with MC38 cells. CEA-Tg mice with established MC38-CEA tumors were then treated with ODN1826 intratumorally, which has consistently demonstrated antitumor activity in animal tumor models,19 with and without pre-immunization with rAAV-CEA (Figure 2D). The antitumor activity of the intratumoral administration of ODN1826 was confirmed; pre-immunization with rAAV-CEA resulted in less antitumor activity (P < 0.004).
Figure 1. Effects of immunization on tumor growth in wild-type mice.
C57Bl/6 wild-type mice were immunized with rAAV-CEA on day 1 followed by ODN1826 (ODN) on days 15 and 17, imiquimod (Imq) on days 15–18, or pNGVL3 on day 15. Mice were challenged with MC38–CEA tumor cells on day 21 (day 0 on graph). NT = no treatment control. Data are presented as means ± SEM, n = 7 mice per group.
Figure 2. Effects of immunization on tumor growth in CEA-Tg mice.
CEA-Tg mice were immunized with rAAV-CEA on day 1 followed by (A) ODN1826 (ODN) on days 15 and 17 or (B) imiquimod (Imq) on days 15–18. Mice were challenged with MC38-CEA tumor cells on day 21 (day 0 on graph). NT = no treatment control. Data are presented as means ± SEM, n = 10 mice per group. (C) CEA-Tg mice were immunized with rAAV-CEA on day 1 followed by ODN1826 (ODN) on days 15 and 17. Mice were challenged with MC38 or with MC38-CEA tumor cells on day 21 (day 0 on graph). NT = no treatment control. Data are presented as means ± SEM, n = 8 mice per group. (D) CEA-Tg mice were immunized with rAAV-CEA on day 1. Mice were implanted with MC38-CEA tumor cells on day 15 (day 0 on graph). On days 21 and 24, when tumor was palpable, mice were treated with ODN intratumorally (IT). Data are presented as means ± SEM, n = 7 mice per group.
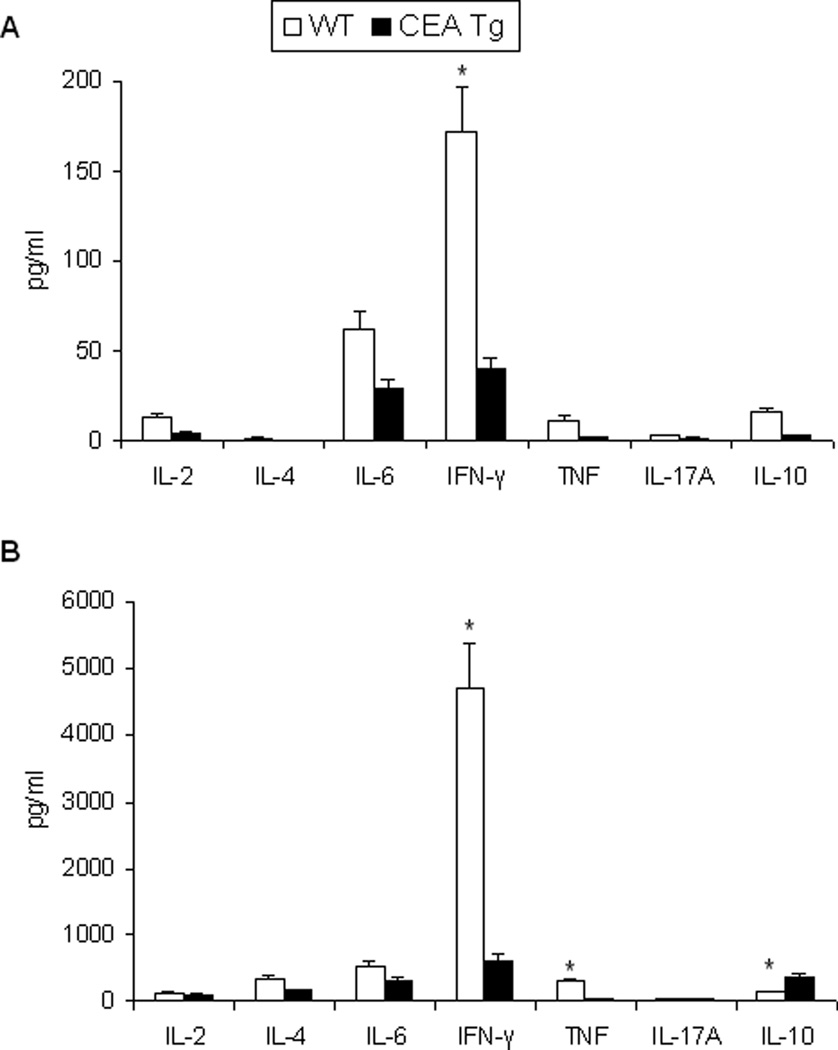
As expected, immunization of wild-type and CEA-Tg mice with rAAV-CEA alone was not effective in eliciting anti-CEA cellular immune responses, as assessed by splenocyte CEA-specific IFN-γ production, nor were the administrations of the ODN1826 or imiquimod alone (Figure 3). In wild-type mice, rAAV-CEA followed by ODN1826 or followed by imiquimod elicited strong cellular as well as humoral immune responses. Although substantially less than that observed in wild-type mice, CEA-Tg mice also responded with CEA-specific IFN-γ production (Figure 3A). CEA-specific humoral responses, which were elicited with rAAV-CEA alone, were also less in CEA-Tg mice (Figure 3B). The CEA-specific cellular response, in terms of Th1/Th2/Th17 bias, was compared in a follow-up study (Figure 4). Although again substantially less than that elicited in wild-type mice, CEA-specific Th1-associated IFN-γ production was observed in CEA-Tg mice (Figure 4A). The production of Th2-associated cytokines in response to CEA did not vary between wild-type and CEA-Tg mice. As a control for these studies, splenocytes were also simulated with ConA. The cytokine release profile differed significantly (Figure 4B). Less Th1-associated IFN-γ and TNF-α and more Th2-associated IL-10 were observed in CEA-Tg mice to this nonspecific stimulation.
Figure 3. Effect of immunization on CEA-specific cellular and humoral immune response.
C57Bl/6 wild-type (WT) and CEA-Tg mice were immunized with rAAV-CEA on day 1 followed by ODN1826 (ODN) on days 15 and 17. Splenocytes and sera were collected on day 21. (A) CEA specific IFN-γ production was assessed by ELISA. Data represent means ± SD, n = 4 mice per group. (B) Total serum IgG antibody levels against CEA were determined by ELISA. Data represent means ± SD, n = 4 mice per group. * = P < 0.05, versus NT; ** P < 0.05, WT versus CEA-Tg.
Figure 4. Effect of immunization on CEA-specific Th1/Th2/Th17 response.
C57Bl/6 wild-type (WT) and CEA-Tg mice were immunized with rAAV-CEA on day 1 followed by ODN1826 on days 15 and 17. (A) Splenocytes were collected on day 21. CEA-specific Th1/Th2/Th17 cytokine production was assessed by CBA. Data represent means ± SD, n = 4 mice per group. (B) Splenocytes from mice immunized with rAAV-CEA on day 1 followed by ODN on days 15 and 17 were stimulated with ConA. Th1/Th2/Th17 cytokine production was assessed by CBA. Data represent means ± SD, n = 4 mice per group. * P < 0.05, WT versus CEA-Tg.
Response to TLR agonists and syngeneic tumor
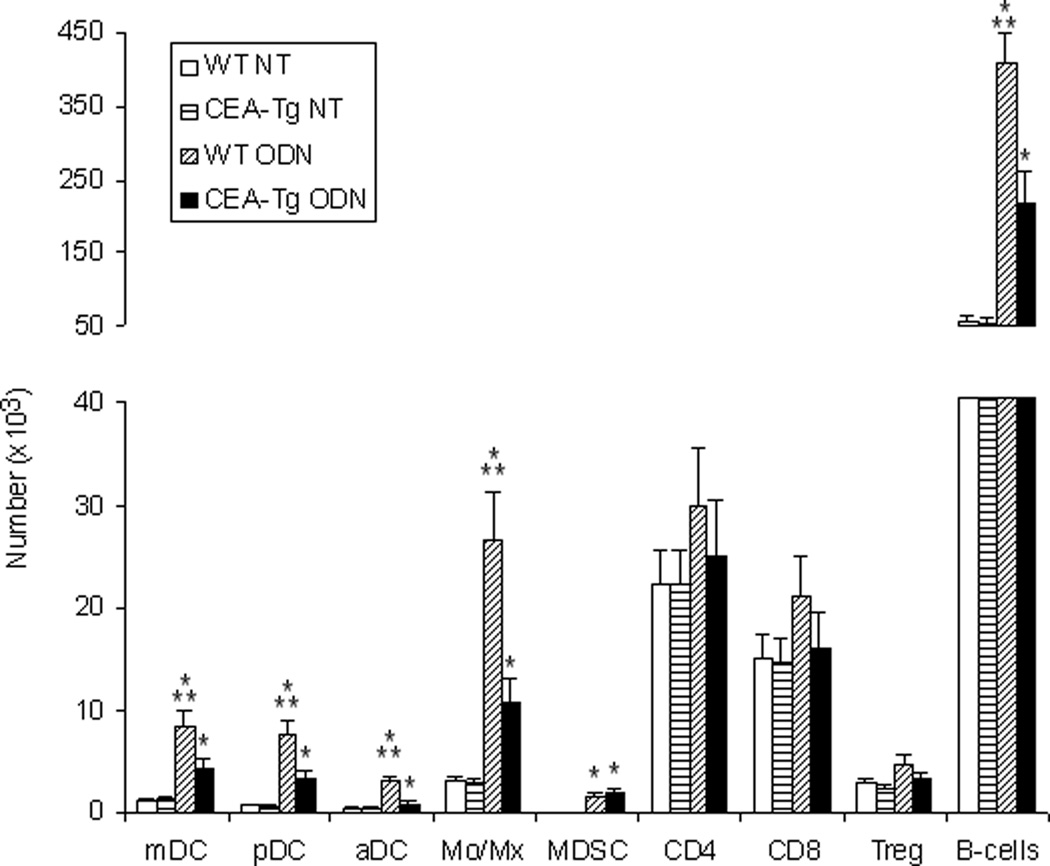
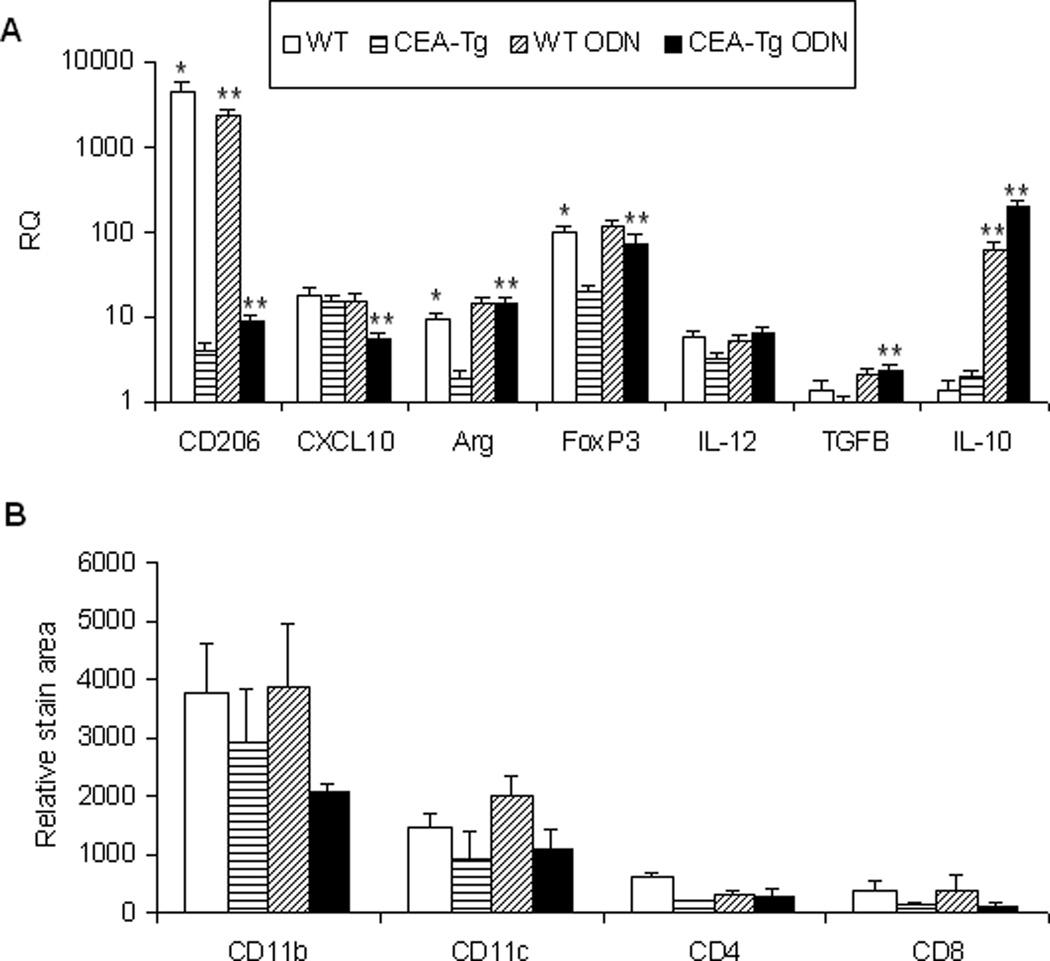
TRL agonists interact either directly or indirectly with a variety of immune cells. Significant differences in the number of lymph node mDC, pDC, activated DC (aDC), Treg cells, MDSC, macrophage, B cell, CD4 and CD8 populations of wild-type and CEA-Tg mice at baseline were not observed. With the exception of CD4 and CD8 cells, wild-type mice manifested greater increases in these populations in response to ODN1826 (Figure 5). To examine responses to self/auto-, tumor-associated antigens, a PCR-based technique was used to examine these populations intratumorally in syngeneic MC38 tumors that did not express CEA (Figure 6A). Compared to when establish in wild-type mice, MC38 tumors established in CEA-Tg mice expressed less CD206, a marker of M2-polarization,20 less arginase, a product of MDSC and M2-polarized macrophage;21 and less FoxP3, expression of which in mice is highly restricted to Treg cells.22 Intratumoral IL-12(p40), a product of M1-polarized macrophages, and CXCL10, a marker of M1-polarization,23 did not vary significantly; nor did IL-10 or TGF-β, cytokines implicated in tumor progression. Paralleling the lymph node cellular infiltration data, the response of these markers to TLR9 stimulation also varied. Relative increases in intratumoral arginase, FoxP3, TGF-β, and IL-10 were greater in CEA-Tg compared to wild type mice. Whereas CD206 decreased and CXCL10 was unchanged in wild type, CD206 increased and CXCL10 decreased in CEA-Tg mice. Tumor DC, macrophage, CD4 and CD8 cellular infiltration was also examined by immunohistochemistry (Figure 6B). Although there was a trend toward less macrophage and DC infiltration in CEA-Tg mice compared to wild type, differences at baseline and in response to ODN1826 did not reach the level of significance.
Figure 5.
Effects of ODN1826 (ODN) on lymph node cells in wild-type (WT) and CEA-Tg mice. MC38 tumor cells were implanted s.c. on day 1 into C57Bl/6 wild-type and CEA-Tg mice. Groups of mice were treated with ODN on days 8 and 10. NT = no treatment control. Draining lymph nodes were harvested on day 15. The number of CD11b+ monocyte/macrophages, B220+ B cells, CD4+FoxP3+ Treg cells, CD11b+Gr1+ MDSC, CD11c+CD11b+ mDC, CD11c+B220+ pDC, and CD11c+CD86+ aDC were assessed by flow cytometry. Data represent means ± SD, n = 4 mice per group. * = P < 0.05, ODN versus NT; ** P < 0.05, WT versus CEA-Tg.
Figure 6. Effect of ODN1826 (ODN) on the tumor microenvironment in wild-type (WT) and CEA-Tg mice.
MC38 tumor cells were implanted s.c. on day 1 into C57Bl/6 WT and CEA-Tg mice. Groups of mice were treated with ODN on days 15 and 17 and spleens harvested on day 17. (A) The relative levels of immunoregulatory cytokines/factors were determined by QRT-PCR at baseline. Data represent means ± SD, n = 4 mice per group. (b) Phenotypes of infiltrating cells were assessed by immunohistochemistry. Data represent means ± SD, n = 4 mice per group. * = P < 0.05, WT versus CEA-Tg; ** P < 0.05, ODN versus no treatment.
Depletion of pDC, MDSC, and Treg cells
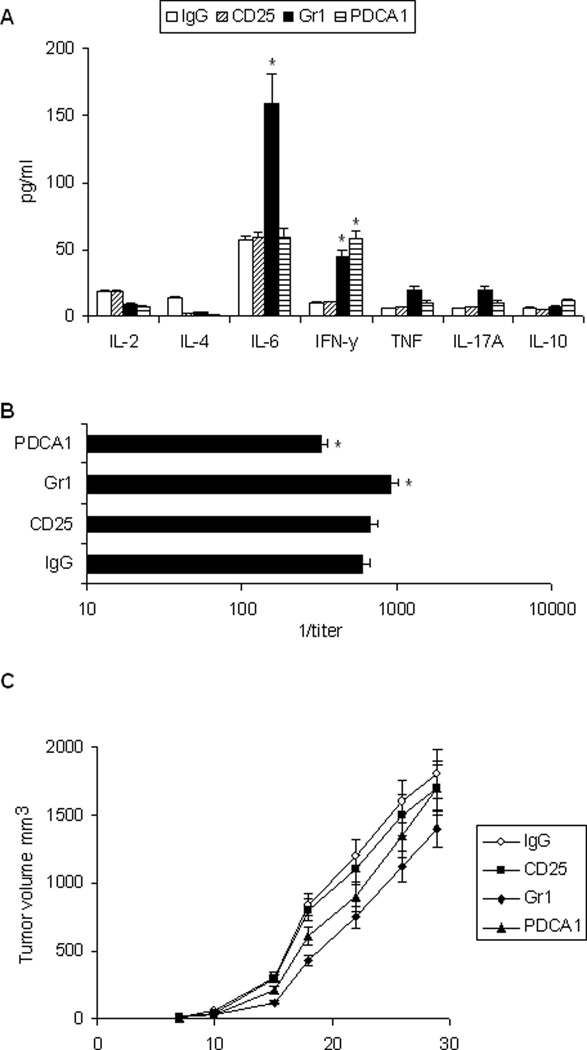
Whether MDSC, pDC, and Treg cells in CEA-Tg mice regulated the response to rAAV-CEA-ODN1826 immunization was assessed by depleting these cell populations using antibody-based methods (Figure 7). Effects of cellular and humoral immune responses were examined initially. There was a small increase in CEA-specific IFN-γ response in CEA-Tg mice depleted of pDC with PDCA1 antibody and depleted of MDSC with Gr1 antibody. IL-6 also increased with MDSC depletion (Figure 7A). There was also a small increase in CEA-specific antibody response after MDSC depletion and a small decrease with pDC depletion (Figure 7B). Depletion of Treg cells with CD25 antibody did not significantly modulate CEA specific cellular or humoral immune responses in CEA-Tg mice. The effects on antitumor activity was then examined (Figure 7C). Depletion of MDSC improved antitumor activity of rAAV-CEA-ODN1826 immunization (P < 0.02). There was a trend toward improved antitumor activity with pDC depletion but this did not reach the level of significance. Depletion of Treg cells did not have an impact. All mice in these experiments developed tumor, and the antitumor activity was manifest only in terms of a decrease in tumor growth rate.
Figure 7. Effects of pDC, Treg, and MDSC depletion on immunization in CEA-Tg mice.
CEA-Tg mice were immunized with rAAV-CEA on day 1 and ODN1826 (ODN) on days 15 and 17. Anti-CD25, anti-Gr1, or control IgG antibody was given i.p. on days 13, 15, and 17; anti-PDCA-1 i.p. on days 13 and 15. Sera and spleen cells were harvested on day 22. (A) CEA-specific Th1/Th2/Th17 cytokine release was assessed by CBA. Data represent means ± SD, n = 3 mice per group. * = P < 0.05 versus IgG control. (B) CEA-specific IgG antibody was measured by ELISA. Data represent means ± SD, n = 3 mice per group. * = P < 0.05 versus IgG control. (C) CEA-Tg mice were immunized with rAAV-CEA, anti-PDCA-1, CD25, Gr1, or control (IgG) antibody, and ODN as above and challenged with MC38-CEA tumor cells on day 21 (day 0 on graph). Data are presented as means ± SEM, n = 6 mice per group.
Discussion
Synthetic TLR9 and TLR7 agonists combined with rAAV-CEA can effectively promote antitumor immune response against CEA-expressing tumors in wild-type mice. Humoral responses to AAV-encoded transgenes have been much stronger and more consistently generated than cellular responses.2,3,4 With TLR7 and TLR9 agonist adjuvants strong cellular immune responses to CEA were produced in wild-type mice, in which CEA is a non-self antigen. Almost complete protection against challenge with MC38-CEA tumors resulted. rAAV vectors can produce durable expression of neo-antigens. Expression of antigens in tissues must be relatively high to facilitate DC priming of naïve CD8+ T cells by cross presentation.24 Furthermore, the duration of the activity of cytolytic T lymphocytes (CTL) has been shown to parallel the kinetics of antigen presentation.25
rAAV-CEA combined with synthetic TLR agonists also elicited a CEA-specific Th1-associated cellular immune response in CEA-Tg mice in which CEA is a self antigen. As expected, this response was substantially less than that in wild-type mice; it was also not protective. Somewhat unexpected were observations that immunization with rAAV-CEA and TLR agonists could promote the growth of MC38-CEA tumors in CEA-Tg mice despite eliciting a measurable Th1-associated response to CEA. Not all attempts of enhancing antitumor activity in CEA-Tg mice with TLR agonists have been effective. Although CD4+-mediated IFN-γ production was induced in CEA-Tg mice only when immunization with adenoviral vectors was performed in the presence of TLR adjuvants, the combination did not significantly impact the growth of MC38-CEA tumors.12 Therapeutic synergy was also not observed with a synthetic TLR7 agonist, SM360320, and immunization with a CEA plasmid vaccine (with electroporation), despite inducing a switch in anti-CEA antibody from IgG1 to IgG2a consistent with a Th1 response.26 That tumor growth can be promoted in CEA-Tg mice with an immunization strategy, however, has not been previously reported. We also found that humoral immune response was attenuated in CEA-Tg mice. Cellular immune responses are considered to be central to antitumor immunity, but passive transfer studies in CEA-Tg mice and clinical observations have demonstrated that antibodies as well as T cells contributed to the antitumor effects of CEA vaccines.18,27
That rAAV vectors can promote immune tolerance has been reported. Several factors appeared to influence immunogenicity of rAAV expressed transgenes, including the dose and route of administration, the subcellular localization and levels of the expressed protein, and the presence of inflammation at the administration site.28,29 The tolerance induced does not appear to be driven by Treg cells.29 That TLR stimulation can promote immune tolerance is also well recognized.30 Specific DC and B-cell subsets appears to be involved in the tolerance promoted by TLR9 stimulation.31,32 ODN1826 has also been shown to induced MDSC that efficiently suppress T cell-mediated immunoreactivity and graft-versus-host disease in a murine model of allogeneic cell therapy.33 Relative increases in response to ODN186 in intratumoral arginase and TGF-β were greater in CEA-Tg compared to wild type mice in our studies. Both are associated with the suppression of antitumor immunity mediated by tumor-associated, M2-polarized macrophages.20 Signaling through TLR7 has been shown to enhance the immunosuppressive activity of murine Treg cells.34
The mechanism of tolerance in CEA-Tg mice is not established. T-cell response against CEA appears to be blunted by both thymic and peripheral tolerance.35 We could not implicate a central role for Treg cells in the observations made. At baseline there was evidence that the intratumoral Treg response was actually less in CEA-Tg than in wild-type mice. Depletion of Treg cells in CEA-Tg mice resulted in an increased immune response and antitumor effect with adenovirus immunization.36 In this study, tumor challenge was intrasplenic, which may contribute immunologic activity and also has been reported to increased CD25+ cells.37 Our studies do implicate MDSC and pDC. CEA-specific cellular and/or humoral response did increase in CEA-Tg mice depleted of MDSC and pDC with antibody. The tumor progression that was observed with the combination of rAAV-CEA and CpG was lessened in mice depleted of MDSC. All mice, however, did develop tumor. Inhibiting MDSC has been shown to improve cancer vaccine response.38 In some tumor models depletion of Gr1+ cells has been shown to promote tumor regression;16,39 in others, however, tumor growth is promoted.40,41 Although pDC play a central role in regulating immune responses to microbial antigens, there are little data regarding the role of pDC, which express TLR7 and TLR9, in regulating cancer vaccine responses, viral vectored or other. pDCs that infiltrate solid tumor have been shown to present tumor antigens and induce Treg cells that inhibit antitumor immunity, including that promoted by CpG ODN.42 It should be noted that C57Bl/6 mice have been characterized by less Treg cell expansion than other mouse strains.43
We observed qualitative and quantitative differences in the immune response to TLR agonists and syngeneic, non-CEA expressing tumors, in wild-type and CEA-Tg mice. CEA is a carcinoembryonic antigen-related cell adhesion molecule (CEACAM), a group of mammalian, immunoglobulin-related glycoproteins (reviewed in ref. 44). CEA, designated CEACAM5, was first characterized in gastrointestinal cancers, and measuring circulating CEA is used in the monitoring of patients with colorectal and other types of carcinomas. Although a murine CEA gene family has been identified, no direct counterpart to human CEA has been described. CEACAMS have been implicated in a variety of functions including cell-cell adhesion, host-pathogen interactions, and immune modulation. This includes inhibition of natural killer, Th cells, and CTL.45,46 CEA has also been implicated in suppressing TLR signaling of human DC and increasing their production of immunosuppressive cytokines such as IL-6 and IL-10.47 The effects of CEA of MDSC have not been reported. In pancreatic carcinoma patients the immune response to CEA is Th2-deviated.48 We did observe a Th2 bias in CEA-Tg mice compared to wild-type to nonspecific immune stimulation. CEA is an attractive target for cancer vaccine approaches. Nonetheless, antitumor activity in the clinical trials reported to date has been limited.49 Antitumor activity of TLR9 agonists in patients with carcinoma has also been limited.50 Our results underscore the complexity of the immune response to self, tumor-associated antigens and a potential limitation of genetic immunization with rAAV-vectored vaccines and TLR agonists.
Acknowledgements
This work was supported by National Institutes of Health grants R01CA118660 and R01CA132077.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Ponnazhagan S. Adenoassociated virus vectors for genetic immunization. Immunol Res. 2002;26:247–253. doi: 10.1385/IR:26:1-3:247. [DOI] [PubMed] [Google Scholar]
- 2.Chirmule N, Xiao W, Truneh A, Schnell MA, Hughes JV, Zoltick P, et al. Humoral immunity to adeno-associated virus type 2 vectors following administration to murine and nonhuman primate muscle. J Virol. 2000;74:2420–2425. doi: 10.1128/jvi.74.5.2420-2425.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang L, Cao O, Swalm B, Dobrzynski E, Mingozzi F, Herzog RW. Major role of local immune responses in antibody formation to factor IX in AAV gene transfer. Gene Ther. 2005;12:1453–1464. doi: 10.1038/sj.gt.3302539. [DOI] [PubMed] [Google Scholar]
- 4.Zhang TP, Jin DY, Wardrop RM, 3rd, Gui T, Maile R, Frelinger JA, et al. Transgene expression levels and kinetics determine risk of humoral immune response modeled in factor IX knockout and missense mutant mice. Gene Ther. 2007;14:429–440. doi: 10.1038/sj.gt.3302881. [DOI] [PubMed] [Google Scholar]
- 5.Ponnazhagan S, Mahendra G, Lima J, Aldrich WA, Jenkins CB, Ren C, et al. Augmentation of antitumor activity of a recombinant adeno-associated virus carcinoembryonic antigen vaccine with plasmid adjuvant. Hum Gene Ther. 2004;15:856–864. doi: 10.1089/hum.2004.15.856. [DOI] [PubMed] [Google Scholar]
- 6.Scheerlinck JY. Genetic adjuvants for DNA vaccines. Vaccine. 2001;19:2647–2656. doi: 10.1016/s0264-410x(00)00495-3. [DOI] [PubMed] [Google Scholar]
- 7.Triozzi PL, Aldrich W, Ponnazhagan S. Regulation of the activity of an adeno-associated virus vector cancer vaccine administered with synthetic Toll-like receptor agonists. Vaccine. 2010;28:7837–7843. doi: 10.1016/j.vaccine.2010.09.086. [DOI] [PubMed] [Google Scholar]
- 8.Hodge JW, Grosenbach DW, Aarts WM, Poole DJ, Schlom J. Vaccine therapy of established tumors in the absence of autoimmunity. Clin Cancer Res. 2003;9:1837–1849. [PubMed] [Google Scholar]
- 9.Mizobata S, Tompkins K, Simpson JF, Shyr Y, Primus FJ. Induction of cytotoxic T cells and their antitumor activity in mice transgenic for carcinoembryonic antigen. Cancer Immunol Immunother. 2000;49:285–295. doi: 10.1007/s002620000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou H, Luo Y, Mizutani M, Mizutani N, Becker JC, Primus FJ, et al. A novel transgenic mouse model for immunological evaluation of carcinoembryonic antigen-based DNA minigene vaccines. J Clin Invest. 2004;113:1792–1798. doi: 10.1172/JCI21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ojima T, Iwahashi M, Nakamura M, Matsuda K, Nakamori M, Ueda K, et al. Successful cancer vaccine therapy for carcinoembryonic antigen (CEA)-expressing colon cancer using genetically modified dendritic cells that express CEA and T helper-type 1 cytokines in CEA transgenic mice. Int J Cancer. 2007;120:585–593. doi: 10.1002/ijc.22298. [DOI] [PubMed] [Google Scholar]
- 12.Salucci V, Mennuni C, Calvaruso F, Cerino R, Neuner P, Ciliberto G, et al. CD8+ T-cell tolerance can be broken by an adenoviral vaccine while CD4+ T-cell tolerance is broken by additional co-administration of a Toll-like receptor ligand. Scand J Immunol. 2006;63:35–41. doi: 10.1111/j.1365-3083.2006.01706.x. [DOI] [PubMed] [Google Scholar]
- 13.Saha A, Bhattacharya-Chatterjee M, Foon KA, Celis E, Chatterjee SK. Stimulatory effects of CpG oligodeoxynucleotide on dendritic cell-based immunotherapy of colon cancer in CEA/HLA-A2 transgenic mice. Int J Cancer. 2009;124:877–888. doi: 10.1002/ijc.24009. [DOI] [PubMed] [Google Scholar]
- 14.Saha A, Baral RN, Chatterjee SK, Mohanty K, Pal S, Foon KA, et al. CpG oligonucleotides enhance the tumor antigen-specific immune response of an anti-idiotype antibody-based vaccine strategy in CEA transgenic mice. Cancer Immunol Immunother. 2006;55:515–527. doi: 10.1007/s00262-005-0009-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clarke P, Mann J, Simpson JF, Rickard-Dickson K, Primus FJ. Mice transgenic for human carcinoembryonic antigen as a model for immunotherapy. Cancer Res. 1998;58:1469–1477. [PubMed] [Google Scholar]
- 16.Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203:2691–2702. doi: 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blasius AL, Giurisato E, Cella M, Schreiber RD, Shaw AS, Colonna M. Bone marrow stromal cell antigen 2 is a specific marker of type I IFN-producing cells in the naive mouse, but a promiscuous cell surface antigen following IFN stimulation. J Immunol. 2006;177:3260–3265. doi: 10.4049/jimmunol.177.5.3260. [DOI] [PubMed] [Google Scholar]
- 18.Facciabene A, Aurisicchio L, Elia L, Palombo F, Mennuni C, Ciliberto G, et al. DNA and adenoviral vectors encoding carcinoembryonic antigen fused to immunoenhancing sequences augment antigen-specific immune response and confer tumor protection. Hum Gene Ther. 2006;17:81–92. doi: 10.1089/hum.2006.17.81. [DOI] [PubMed] [Google Scholar]
- 19.Sharma S, Karakousis CP, Takita H, Shin K, Brooks SP. Cytokines and chemokines are expressed at different levels in small and large murine colon-26 tumors following intratumoral injections of CpG ODN. Neoplasia. 2004;6:523–528. doi: 10.1593/neo.04166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Umemura N, Saio M, Suwa T, Kitoh Y, Bai J, Nonaka K, et al. Tumor-infiltrating myeloid-derived suppressor cells are pleiotropic-inflamed monocytes/macrophages that bear M1- and M2-type characteristics. J Leukoc Biol. 2008;83:1136–1144. doi: 10.1189/jlb.0907611. [DOI] [PubMed] [Google Scholar]
- 21.Bronte V, Zanovello P. Regulation of immune responses by L-arginine metabolism. Nat Rev Immunol. 2005;5:641–654. doi: 10.1038/nri1668. [DOI] [PubMed] [Google Scholar]
- 22.Fontenot JD, Rudensky AY. A well adapted regulatory contrivance: regulatory T cell development and the forkhead family transcription factor Foxp3. Nat Immunol. 2005;6:331–337. doi: 10.1038/ni1179. [DOI] [PubMed] [Google Scholar]
- 23.Kline J, Brown IE, Zha YY, Blank C, Strickler J, Wouters H, et al. Homeostatic proliferation plus regulatory T-cell depletion promotes potent rejection of B16 melanoma. Clin Cancer Res. 2008;14:3156–3167. doi: 10.1158/1078-0432.CCR-07-4696. [DOI] [PubMed] [Google Scholar]
- 24.Kurts CJ, Miller FAP, Subramaniam RM, Carbone FR, Heath WR. Major histocompatibility complex class I-restricted cross presentation is biased toward high-dose antigens and those released during cellular destruction. J Exp Med. 1998;188:409–414. doi: 10.1084/jem.188.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nelson D, Bundell C, Robinson B. In vivo cross-presentation of a soluble protein antigen: kinetics, distribution, and generation of effector CTL recognizing dominant and subdominant epitopes. J Immunol. 2000;165:6123–6132. doi: 10.4049/jimmunol.165.11.6123. [DOI] [PubMed] [Google Scholar]
- 26.Dharmapuri S, Aurisicchio L, Neuner P, Verdirame M, Ciliberto G, La Monica N. An oral TLR7 agonist is a potent adjuvant of DNA vaccination in transgenic mouse tumor models. Cancer Gene Ther. 2009;16:462–472. doi: 10.1038/cgt.2008.91. [DOI] [PubMed] [Google Scholar]
- 27.Ullenhag GJ, Frödin JE, Jeddi-Tehrani M, Strigård K, Eriksson E, Samanci A, et al. Durable carcinoembryonic antigen (CEA)-specific humoral and cellular immune responses in colorectal carcinoma patients vaccinated with recombinant CEA and granulocyte/macrophage colony-stimulating factor. Clin Cancer Res. 2004;10:3273–3281. doi: 10.1158/1078-0432.CCR-03-0706. [DOI] [PubMed] [Google Scholar]
- 28.Ge Y, Powell S, Van Roey M, McArthur JG. Factors influencing the development of an anti-factor IX (FIX) immune response following administration of adeno-associated virus-FIX. Blood. 2001;97:3733–3737. doi: 10.1182/blood.v97.12.3733. [DOI] [PubMed] [Google Scholar]
- 29.Kelly ME, Zhuo J, Bharadwaj AS, Chao H. Induction of immune tolerance to FIX following muscular AAV gene transfer is AAV-dose/FIX-level dependent. Mol Ther. 2009;17:857–863. doi: 10.1038/mt.2009.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ehlers M, Ravetch JV. Opposing effects of Toll-like receptor stimulation induce autoimmunity or tolerance. Trends Immunol. 2007;28:74–79. doi: 10.1016/j.it.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 31.Lenert P, Brummel R, Field EH, Ashman RF. TLR-9 activation of marginal zone B cells in lupus mice regulates immunity through increased IL-10 production. J Clin Immunol. 2005;25:29–40. doi: 10.1007/s10875-005-0355-6. [DOI] [PubMed] [Google Scholar]
- 32.Mellor AL, Baban B, Chandler PR, Manlapat A, Kahler DJ, Munn DH. Cutting edge: CpG oligonucleotides induce splenic CD19+ dendritic cells to acquire potent indoleamine 2,3-dioxygenase-dependent T cell regulatory functions via IFN Type 1 signaling. J Immunol. 2005;175:5601–5605. doi: 10.4049/jimmunol.175.9.5601. [DOI] [PubMed] [Google Scholar]
- 33.Morecki S, Gelfand Y, Yacovlev E, Eizik O, Shabat Y, Slavin S. CpG-induced myeloid CD11b+Gr-1+ cells efficiently suppress T cell-mediated immunoreactivity and graft-versus-host disease in a murine model of allogeneic cell therapy. Biol Blood Marrow Transplant. 2008;14:973–984. doi: 10.1016/j.bbmt.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 34.Forward NA, Furlong SJ, Yang Y, Lin TJ, Hoskin DW. Signaling through TLR7 enhances the immunosuppressive activity of murine CD4+CD25+ T regulatory cells. J Leukoc Biol. 2010;87:117–125. doi: 10.1189/jlb.0908559. [DOI] [PubMed] [Google Scholar]
- 35.Bos R, van Duikeren S, Morreau H, Franken K, Schumacher TN, Haanen JB, et al. Balancing between antitumor efficacy and autoimmune pathology in T-cell-mediated targeting of carcinoembryonic antigen. Cancer Res. 2008;68:8446–8455. doi: 10.1158/0008-5472.CAN-08-1864. [DOI] [PubMed] [Google Scholar]
- 36.Elia L, Aurisicchio L, Facciabene A, Giannetti P, Ciliberto G, La Monica N, et al. CD4+CD25+ regulatory T-cell-inactivation in combination with adenovirus vaccines enhances T-cell responses and protects mice from tumor challenge. Cancer Gene Ther. 2007;14:201–210. doi: 10.1038/sj.cgt.7701004. [DOI] [PubMed] [Google Scholar]
- 37.Dietrich A, Stockmar C, Aust G, Endesfelder S, Guetz A, Sack U, et al. Intraoperative subcutaneous or intrasplenic vaccination with modified autologous tumor cells leads to enhanced survival in a mouse tumor model. J Cancer Res Clin Oncol. 2006;132:379–388. doi: 10.1007/s00432-005-0073-5. [DOI] [PubMed] [Google Scholar]
- 38.Nagaraj S, Youn JI, Weber H, Iclozan C, Lu L, Cotter MJ, et al. Anti-inflammatory triterpenoid blocks immune suppressive function of MDSCs and improves immune response in cancer. Clin Cancer Res. 2010;16:1812–1823. doi: 10.1158/1078-0432.CCR-09-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Terabe M, Matsui S, Park JM, Mamura M, Noben-Trauth N, Donaldson DD, et al. Transforming growth factor-beta production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J Exp Med. 2003;198:1741–1752. doi: 10.1084/jem.20022227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nausch N, Galani IE, Schlecker E, Cerwenka A. Mononuclear myeloid-derived "suppressor" cells express RAE-1 and activate natural killer cells. Blood. 2008;112:4080–4089. doi: 10.1182/blood-2008-03-143776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bergami-Santos PC, Mariano M, Barbuto JA. Dual role of polymorphonuclear neutrophils on the growth of Ehrlich ascites tumor (EAT) in mice. Life Sci. 2004;75:245–255. doi: 10.1016/j.lfs.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 42.Vicari AP, Chiodoni C, Vaure C, Aït-Yahia S, Dercamp C, Matsos F, et al. Reversal of tumor-induced dendritic cell paralysis by CpG immunostimulatory oligonucleotide and anti-interleukin-10 receptor antibody. J Exp Med. 2002;196:541–549. doi: 10.1084/jem.20020732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Melencio L, McKallip RJ, Guan H, Ramakrishnan R, Jain R, Nagarkatti PS, et al. Role of CD4(+)CD25(+) T regulatory cells in IL-2-induced vascular leak. Int Immunol. 2006;18:1461–1471. doi: 10.1093/intimm/dxl079. [DOI] [PubMed] [Google Scholar]
- 44.Thompson JA, Grunert F, Zimmermann W. Carcinoembryonic antigen gene family: molecular biology and clinical perspectives. J Clin Lab Anal. 1991;5:344–366. doi: 10.1002/jcla.1860050510. [DOI] [PubMed] [Google Scholar]
- 45.Stern N, Markel G, Arnon TI, Gruda R, Wong H, Gray-Owen SD, et al. Carcinoembryonic antigen (CEA) inhibits NK killing via interaction with CEA-related cell adhesion molecule 1. J Immunol. 2005;174:6692–6701. doi: 10.4049/jimmunol.174.11.6692. [DOI] [PubMed] [Google Scholar]
- 46.Chen CJ, Shively JE. The cell-cell adhesion molecule carcinoembryonic antigen-related cellular adhesion molecule 1 inhibits IL-2 production and proliferation in human T cells by association with Src homology protein-1 and down-regulates IL-2 receptor. J Immunol. 2004;172:3544–3552. doi: 10.4049/jimmunol.172.6.3544. [DOI] [PubMed] [Google Scholar]
- 47.Nonaka M, Ma BY, Murai R, Nakamura N, Baba M, Kawasaki N, et al. Glycosylation-dependent interactions of C-type lectin DC-SIGN with colorectal tumor-associated Lewis glycans impair the function and differentiation of monocyte-derived dendritic cells. J Immunol. 2008;180:3347–3356. doi: 10.4049/jimmunol.180.5.3347. [DOI] [PubMed] [Google Scholar]
- 48.Tassi E, Gavazzi F, Albarello L, Senyukov V, Longhi R, Dellabona P, et al. Carcinoembryonic antigen-specific but not antiviral CD4+ T cell immunity is impaired in pancreatic carcinoma patients. J Immunol. 2008;181:6595–6603. doi: 10.4049/jimmunol.181.9.6595. [DOI] [PubMed] [Google Scholar]
- 49.Berinstein NL. Carcinoembryonic antigen as a target for therapeutic anticancer vaccines: a review. J Clin Oncol. 2002;20:2197–2207. doi: 10.1200/JCO.2002.08.017. [DOI] [PubMed] [Google Scholar]
- 50.Murad YM, Clay TM. CpG oligodeoxynucleotides as TLR9 agonists: therapeutic applications in cancer. BioDrugs. 2009;23:361–375. doi: 10.2165/11316930-000000000-00000. [DOI] [PubMed] [Google Scholar]