Abstract
Due to the limited information of the contribution of various antibiotic resistance mechanisms in clinical Burkholderia cepacia complex isolates, Antibiotic resistance mechanisms, including integron analysis, identification of quinolone resistance-determining region mutations, measurement of efflux pump activity, and sequence analysis of efflux pump regulators, were investigated in 66 clinical B. cepacia complex isolates. Species were identified via recA-RFLP and MALDI-TOF. Four genomovars were identified by recA-RFLP. B. cenocepacia (genomovar III) was the most prevalent genomovar (90.1%). Most isolates (60/66, 90.9%) were correctly identified by MALDI-TOF analysis. Clonal relatedness determined by PFGE analysis revealed 30 pulsotypes, including two major pulsotypes that comprised 22.7% and 18.2% of the isolates, respectively. Seventeen (25.8%) isolates harboured class 1 integron with various combinations of resistance genes. Among six levofloxacin-resistant isolates, five had single-base substitutions in the gyrA gene and three demonstrated efflux pump activities. Among the 42 isolates exhibiting resistance to at least one antimicrobial agent, 94.4% ceftazidime-resistant isolates (17/18) and 72.7% chloramphenicol-resistant isolates (16/22) demonstrated efflux pump activity. Quantitation of efflux pump RNA level and sequence analysis revealed that over-expression of the RND-3 efflux pump was attributable to specific mutations in the RND-3 efflux pump regulator gene. In conclusion, high-level expression of efflux pumps is prevalent in B. cepacia complex isolates. Mutations in the RND-3 efflux pump regulator gene are the major cause of efflux pump activity, resulting in the resistance to antibiotics in clinical B. cepacia complex isolates.
Introduction
The Burkholderia cepacia complex is comprised of Gram-negative, non-fermenting bacilli that are commonly found in natural and hospital environments [1], [2]. The B. cepacia complex includes at least nine species [2], [3]. Among B. cepacia complex infection in cases of cystic fibrosis, B. multivorans (genomovar II) and B. cenocepacia (genomovar III) are common and may cause outbreaks [1], [2]. B. cepacia complex has emerged as important pathogens in patients with cystic fibrosis and chronic granulomatous disease over the past two decades [4]–[6]. The difficulty in treating B. cepacia complex infections is attributed to its intrinsic resistance to multiple antibiotics and disinfectants [2], [7], [8]. The causes of multiple antibiotic resistance in the B. cepacia complex include decreased outer membrane permeability [9], [10], alterations to antibiotic targets [11], integrons [12], [13], and active efflux pumps [14]–[17]. Four RND (resistance-nodulation-division) efflux pumps, including RND-3, RND-4, RND-9 and RND-10, were shown to facilitate multiple antibiotic resistance in laboratory reference strains of the B. cepacia complex, whereas any contribution of these efflux pumps to resistance of clinical isolates has not been reported [14], [15], [17].
Because the contribution of various antibiotic resistance mechanisms in clinical B. cepacia complex isolates is not well understood, we investigated the roles of the class 1 and 2 integrons, the quinolone resistance-determining regions (QRDRs) of topoisomerase II and IV, and active efflux pumps in 66 clinical B. cepacia complex isolates. We also differentiated the B. cepacia complex to the species level by molecular typing and matrix-assisted laser desorption ionisation time-of-flight (MALDI-TOF) analysis in order to investigate the resistance mechanisms in different species.
Materials and Methods
Bacterial isolates
A total of 66 B. cepacia complex isolates were identified with the Vitek 2 system (bioMérieux) from blood (n = 45), sputum (n = 10), lung abscesses (n = 5), urine (n = 3), surgical wounds (n = 2), and pus (n = 1) between 2009 and 2011 at Kaohsiung Medical University Hospital, a 1,600-bed medical centre in Taiwan. Antibiotic resistant mechanisms of bacterial isolates were analysed in this study whereas the human specimens or patient's information were not included.
Ethics statement
This study was approved by the Institutional Review Board of Kaohsiung Medical University Chung-Ho Memorial Hospital, Kaohsiung, Taiwan (KMUH-IRB-20130181). The study subjects were bacterial isolates and the informed consents were waived.
Restriction fragment length polymorphism (RFLP) analyses and sequence of the recA gene
The genomovar typing of B. cepacia complex isolates was described by Mahenthiralingam et al. [18]. Briefly, recA PCR was performed with the primer pair consisting of BCR1 and BCR2 (Table 1), followed by HaeIII digestion of the PCR products. Species were identified based on recA-RFLP types according to previous studies [18], [19]. After identifying five recA-RFLP patterns, the complete recA gene was sequenced in two 500-bp fragments using combinations of the PCR primers BCR1, BCR2, BCR3, and BCR4 (Table 1); nucleotide sequences were compared with sequence database using the BLAST sequence algorithm (National Center for Biotechnology Information).
Table 1. Primers used in this study.
| Target | Primer name | Sequence (5′ to 3′) | Amplicon size (bp) | Reference |
| int1 | Int1F | CGCGGCATAGACTGTAC | 921 | [13] |
| Int1R | TTCGAATGTCGTAACCGC | |||
| int2 | Int2F | GCAAATGAAGTGCAACGC | 465 | [13] |
| Int2R | ACACGCTTGCTAACGATG | |||
| sul1 | sul1F | CTTCGATGAGAGCCGGCGGC | 437 | [12] |
| sul1R | GCAAGGCGGAAACCCGCGCC | |||
| Class I integron cassette | 5′CS | GGCATCCAAGCAGCAAGC | variable | [12] |
| 3′CS | AAGCAGACTTGACCTGAT | |||
| Class II integron cassette | 125′CS | TTTTTGTGCTGCCATATCCGTG | variable | [24] |
| 23′CS | TGGGCTGAGAGAGTGGT | |||
| gyrA | gyrA-F | ATCTCGATTACGCGATGAGC | 449 | [11] |
| gyrA-R | GCCGTTGATCAGCAGGTT | |||
| parC | parC-F | ATTGGTCAGGGTCGTGAAGA | 229 | [11] |
| parC-R | GTAGCGCAGCGAGAAATCCT | |||
| recA | BCR1 | TGACCGCCGAGAAGAGCAA | 1043 | [18] |
| BCR2 | CTCTTCTTCGTCCATCGCCTC | |||
| recA sequencing primers | BCR3 | GTCGCAGGCGCTGCGCAA | [18] | |
| BCR4 | GCGCAGCGCCTGCGACAT | |||
| BCAM0918 (control gene) | 0918F | GAGATGAGCACCGATCACAC | 143 | [26] |
| 0918R | CCTTCGAGGAACGACTTCAG | |||
| BCAL1674 (RND-3) | 1674F | TTGTATCGGCGGCGAATGAT | 129 | This study |
| 1674R | CTTGTCGCCCTTTCCGCATC | |||
| BCAL2822 (RND-4) | 2822F | GCGGTGTTCCCGAACCCGAAT | 168 | [25] |
| 2822R | GCTCGACCTTGTTGCTGGCGT | |||
| BCAM1947 (RND-9) | 1947F | CGACGTCGCAGGAAGAACTC | 118 | This study |
| 1947R | ACTTCGGTGAAGCCGAGATT | |||
| BCAM2551 (RND10) | 2551F | AACACCGACCAGGACAAGAA | 84 | This study |
| 2551R | CTGCATCCCTTGCTGCACTT | |||
| BCAL1672 (RND-3 regulator) | 1672F | AGTCTACCGATGTTCCGCAAA | 930 | This study |
| 1672R | GCCACAGGGTGCGTTTGTTA | |||
| BCAL2823 (RND-4 regulator) | 2823F | GGGGTTGCGGACCCTATATT | 855 | This study |
| 2823R | AATTTCGCGGCGGTGATGTC | |||
| BCAM1948 (RND-9 regulator) | 1948F | CCGTTAAATTCGTCCGCGAG | 944 | This study |
| 1948R | ATACGCGTTTCTTCGGCGAC | |||
| BCAM2554 (RND-10 regulator) | 2554F | TAGAGACGCAGCACGATGTC | 1419 | This study |
| 2554R | GGTTTACTGCGGATTCGGGA |
Matrix-assisted laser desorption ionisation time-of-flight (MALDI-TOF) analysis
B. cepacia complex isolates were grown on blood agar and incubated for 24 h at 37°C. Sample preparation and crystallisation were performed as described by Degand et al. [20]. MALDI-TOF MS was performed using a MALDI Biotyper system (Microflex LT, Bruker Daltonik GmbH, Bremen, Germany) and a 60-Hz nitrogen laser (337 nm wavelength). Spectra were collected in the linear positive mode in the mass range of 2,000 to 20,000 m/z for bacterial identification using MALDI Biotyper software (version 3.1.66; Bruker Daltoniks GmbH, Bremen, Germany).
Antimicrobial susceptibility testing
Antimicrobial susceptibility testing was performed by the standard broth microdilution method according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI) [21]. The MIC was defined as the lowest concentration of antibiotic that prevented bacterial growth after 20 to 24 h of incubation at 37°C. The following antimicrobial agents were tested: ceftazidime, meropenem, minocycline, trimethoprim/sulfamethoxazole, ticarcillin/clavulanic acid, levofloxacin, and chloramphenicol.
Efflux pump activity testing
To evaluate the efflux pump activity of B. cepacia complex isolates, MIC resistance patterns were measured with the efflux pump inhibitor carbonyl cyanide-m-chlorophenyl hydrazone (CCCP; Sigma- Aldrich, St. Louis, MO) by the broth microdilution method [22]. Briefly, 100 µl of the final bacterial inoculation was adjusted to 5×105 CFU/ml in cation-adjusted Mueller-Hinton broth (CAMHB) and incubated with 100 µl of serial two-fold dilutions of antibiotics in a 96-well plate. Subsequently, 1.2 µl CCCP (10 mM in dimethyl sulfoxide [DMSO]) was added to a final concentration of 60 µM. The MIC was defined as the lowest concentration of antibiotic that prevented bacterial growth after 20 to 24 h of incubation at 37°C. Given that 240 µM and 120 µM CCCP inhibited the growth of B. cepacia complex strains, a final concentration of 60 µM CCCP was used in this test. Presence of efflux pump activity was defined as any strain exhibiting at least a 4-fold decrease in MIC with CCCP.
Pulsed-field gel electrophoresis (PFGE)
PFGE typing of DNA digested with XbaI (New England BioLabs, Ipswich, MA) was performed in accordance with previously described methods [23]. Restriction fragments ranging from 50 to 500 kb in size were separated on a CHEF Mapper apparatus (Bio-Rad Laboratories, Hercules, CA) for 20 h at 200 V and 14°C. The gels were then stained with ethidium bromide and photographed under UV light. Dice similarity indices were employed to construct a dendrogram of pulsotype relationships via the unweighted pair group method using arithmetic averages (UPGMA) with BioNumerics software version 6.5 (Applied Maths, Sint-Martens-Latem, Belgium). Pulsotypes were assigned to the same clusters if they exhibited 80% similarity in the dendrogram.
PCR amplification and DNA sequencing
Genomic DNA was extracted with the Pure gene DNA Isolation Kit (Gentra Systems, Inc., Minneapolis, Minn.). PCR assays were performed to amplify the full sequences of the class 1 and 2 integron cassettes (int1 and int2) (Table 1) [12], [13], [24]. To detect quinolone resistance-determining regions (QRDRs) in topoisomerase II and IV, the gyrA and parC genes were sequenced. 4 RND efflux pump regulators (BCAL1672, BCAL2823, BCAM1948, and BCAM2554) were also sequenced. Primers used for amplification of the sequences are listed in Table 1 [11]. Purified PCR amplicons were sequenced via the dideoxy chain-termination method with an automated DNA Sequencer (Perkin-Elmer ABI3700), and nucleotide sequences were compared with sequence database using the BLAST sequence algorithm (National Center for Biotechnology Information).
RNA extraction and synthesis of cDNA
Total RNA was extracted from 5 ml of log-phase cultures using TRIzol reagent (Life Technologies, Carlsbad, CA, USA) according to the manufacturer's recommendations. Contaminating DNA was removed with DNase I (Life Technologies, Carlsbad, CA, USA) digestion for 45 min at 37°C, followed by phenol-chloroform extractions, isopropanol precipitation, and resuspension of total RNA in nuclease-free water. Reverse transcription was performed with 5 µg RNA using random hexamers and M-MLV reverse transcriptase (Life Technologies, Carlsbad, CA, USA).
Quantitative reverse transcription-PCR for efflux pump (efflux pump RNA expression)
PCR reactions were performed in 96-well plates in reaction buffer containing 1× FastStart Universal SYBR Green Master (Roche), 300 nM primers and 2 µl cDNA. Reactions were carried out in a ABI7000 machine following the manufacturer's protocol. Forward and reverse primers were designed using Primer-BLAST and compared to the B. cenocepacia J2315 genome to verify their specificity. The primers are listed in Table 1 [25], [26]. The non-induced efflux strain No. 30 was designated as a reference strain. Relative fold changes in the transcript level of indicated genes were normalised to the BCAM0918 gene and calculated with the 2−ΔΔCT method. The BCAM0918 gene, which was stably expressed under antibiotics, was evaluated as an internal control [26].
Statistical analysis
Categorical variables were analysed with the Chi-square test. The Mann-Whitney U test was used to determine if there were differences between isolates in terms of the expression level of efflux pumps, both for isolates that did and did not exhibit resistance to antimicrobial agents. All tests were two-tailed, and a P-value of<0.05 was considered statistically significant.
Results
Species identification and molecular typing
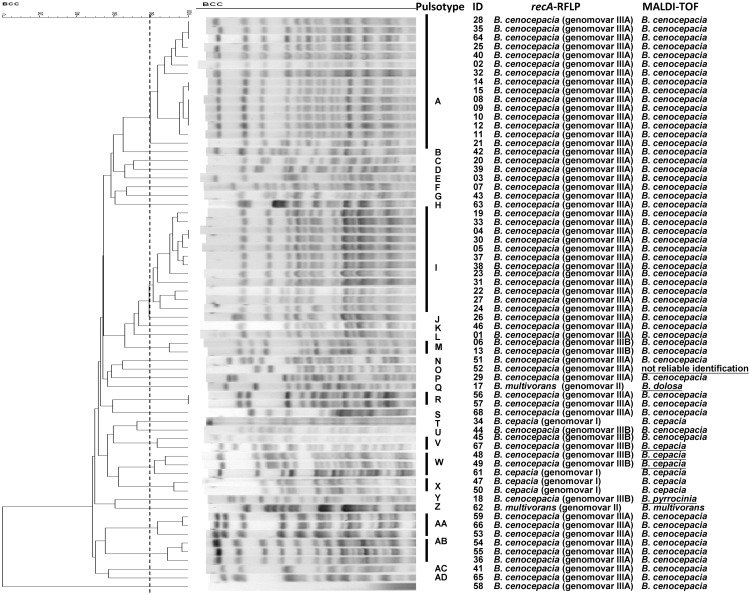
Among 66 B. cepacia complex isolates, five different patterns were identified by recA-RFLP as the previous report [18]. Fifty-two B. cenocepacia (genomovar IIIA) isolates were identified as pattern G (recA sequence to be identical to B. cenocepacia J2315; accession no. AM747720). Eight B. cenocepacia (genomovar IIIB) isolates were identified as pattern H (B. cenocepacia C1394; accession no. AF143783). Four B. cepacia (genomovar I) isolates identified to be one isolate of pattern D (B. cepacia ATCC 25416; accession no. AF143786) and three isolates being pattern E (B. cepacia ATCC 17759; accession no. AF143788). Two B. multivorans (genomovar II) isolates was identified as pattern C (B. multivorans C1576; accession no. AF143774) (Fig. 1). Most isolates (60/66, 90.9%) were correctly identified by MALDI-TOF analysis, whereas 5 isolates (7.6%) were mistakenly identified as different species and one isolate (1.5%) had a score lower than 1.7, which indicates an unreliable identification. An isolate that was unidentified by MALDI-TOF was B. cenocepacia (genomovar IIIA) according to recA-RFLP analysis. In addition, four B. cenocepacia (genomovar IIIB) isolates were misidentified as B. cepacia (n = 3) and B. pyrrocinia (n = 1) by MALDI-TOF analysis. One B. multivorans (genomovar II) isolate was misidentified as B. dolosa by MALDI-TOF analysis (Fig. 1). PFGE analysis revealed 30 pulsotypes, including two major pulsotypes, A (n = 15) and I (n = 12), which were all B. cenocepacia (genomovar IIIA). Except for ceftazidime, no difference in the other antimicrobial resistance rates was observed between isolates of the two major pulsotypes (Table S2).
Figure 1. A dendrogram of pulsotype relationships developed via the unweighted pair group method using arithmetic averages (UPGMA) with BioNumerics software version 6.5 (Applied Maths).
Pulsotypes were assigned to the same clusters if they exhibited 80% similarity in the dendrogram. Species identification was performed by recA-RFLP and MALDI-TOF analysis for 66 B. cepacia complex isolates.
Antimicrobial susceptibility testing
Antimicrobial susceptibility results are presented in Table 2 and the MIC data for each isolate was supplied in file S1. The susceptibility rates for minocycline and trimethoprim/sulfamethoxazole among the 66 B. cepacia complex isolates were highest: 90% and 97%, respectively. The susceptibility rate for meropenem was 86% and that for levofloxacin was 80%. The non-susceptibility rate for chloramphenicol was 55% and for ceftazidime was 35%. All isolates were resistant to ticarcillin/clavulanic acid. The MIC50 for chloramphenicol and ticarcillin/clavulanic acid were 16 (intermediate) and 128/2 mg/L (resistant), respectively. The antimicrobial susceptible rates for the tested antimicrobial agents of three species are revealed in Table S1. No significant differences in those rates between species are found.
Table 2. Antimicrobial susceptibilities of 66 B. cepacia complex isolates.
| Antibiotic | MIC (µg/ml) | No. (%) of total isolates | ||||
| Range | MIC50 | MIC90 | S | I | R | |
| Chloramphenicol | 4–128 | 16 | 64 | 30 (45) | 15 (23) | 21 (32) |
| Ceftazidime | 2–128 | 8 | 64 | 43 (65) | 7 (11) | 16 (24) |
| Meropenem | 1–128 | 4 | 16 | 57 (86) | 0 (0) | 9 (14) |
| Levofloxacin | 0.25–64 | 2 | 4 | 53 (80) | 7 (11) | 6 (9) |
| Minocycline | 0.5–64 | 2 | 4 | 60 (90) | 3 (5) | 3 (5) |
| Ticarcillin/clavulanic acid | ≥128/2 | 128/2 | ≥128/2 | 0 (0) | 0 (0) | 66 (100) |
| Trimethoprim/sulfamethoxazole | ≤0.5/9.5-4/76 | ≤0.5/9.5 | 1/19 | 64 (97) | 0 (0) | 2 (3) |
Integron cassette analysis
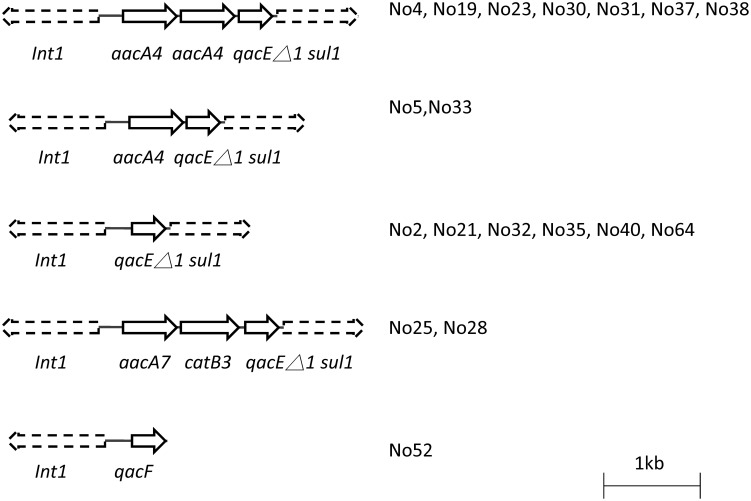
Class 1 integron was identified in 18 B. cenocepacia isolates (Fig. 2). One isolate harboured the class 1 integrase (intI1) and qacF genes (quaternary ammonium compound-resistance protein), which were identical to the sequences for GenBank accession number FN827339. The other 17 isolates demonstrated the typical class 1 backbone structure consisting of a 5′ conserved segment (intI1) and a 3′ conserved segment, which included the qacEΔ1 and sulfamethoxazole resistance gene (sul1). Six isolates exhibited the In0 structure (GenBank accession number: M73819.1), which is an ancestor of more complex integrons [27]. Among 11 isolates containing aminoglycoside resistant genes, 2 isolates harboured one copy of aacA4 gene, which encodes aminoglycoside 6′-N-acetyltransferase, in the class 1 integron cassette, and 7 isolates contained two copies of the aacA4 gene in the class 1 integron cassette (Fig. 2). aacA7, which encodes aminoglycoside 6′-N-acetyltransferase was found in the integrons of two isolates. Two isolates with integron containing catB3 gene, which encodes chloramphenicol acetyltransferase were resistant to chloramphenicol. The result revealed integron's role in the resistance to sulfamethoxazole, chloramphenicol, and aminoglycoside.
Figure 2. Class 1 integron cassette analysis.
Genes are shown as arrows with the direction of transcription indicated by the arrowheads. Int1: class 1 integrase, qacF: quaternary ammonium compound-resistance protein, qacEΔ1: remnants of quaternary ammonium compound resistance protein, sul1: sulphonamides resistance gene, aacA4: aminoglycoside 6′-acetyltransferase, aacA7: aminoglycoside 6′-acetyltransferase, and catB3: chloramphenicol acetyltransferase.
Effect of efflux pumps on antibiotic MICs (efflux pump activity)
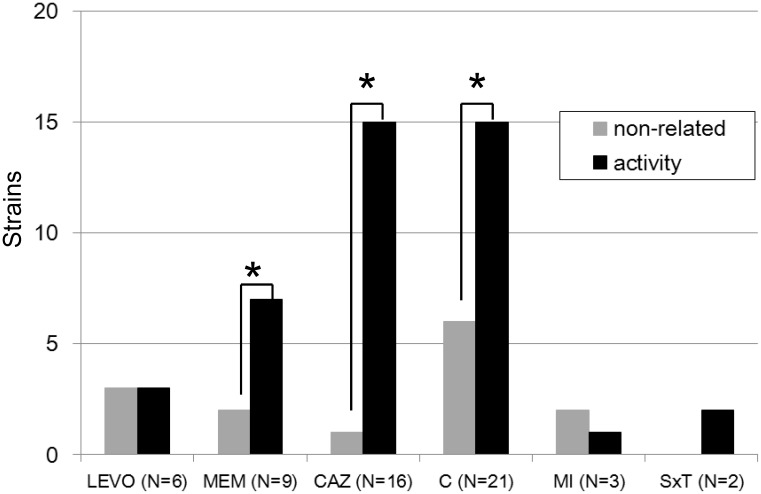
To understand the contribution of efflux pumps to antibiotic resistance, 42 isolates that were resistant to at least one antimicrobial agent, including ceftazidime, meropenem, minocycline, trimethoprim/sulfamethoxazole, levofloxacin, and chloramphenicol, were utilised for further testing. The detailed results of efflux pump activities were revealed in File S1 (CCCP method). 78.6% isolates (33/42) demonstrated presence of efflux pump activity. The proportions of strains exhibiting efflux pump activity for various antimicrobial agents are revealed in Fig. 3. 50% of levofloxacin-resistant isolates (3/6) and 70% meropenem-resistant isolates (7/10) demonstrated efflux pump activity. Most ceftazidime-resistant (17/18, 94.4%) and chloramphenicol-resistant (16/22, 72.7%) isolates also exhibited efflux pump activity. Besides, efflux pump activity was identified in both trimethoprim/sulfamethoxazole-resistant isolates in the study. The presence of efflux pump activity was significantly correlated with resistance to any of the following antimicrobial agents: ceftazidime, meropenem, and chloramphenicol (all p<0.05 according to the Mann-Whitney U test).
Figure 3. Among 42 antibiotic-resistant clinical isolates, the efficacy of efflux pumps was tested via antibiotic susceptibilities: ceftazidime (CAZ), meropenem (MEM), minocycline (MI), trimethoprim/sulfamethoxazole (SxT), levofloxacin (LEVO), and chloramphenicol (C) in the presence or absence of 60 µM CCCP.
Efflux pump activity was defined as present at least 4-fold decrease of MIC in the presence of CCCP. *, p<0.05 (statistical analysis using the Mann-Whitney U test).
Characteristics of levofloxacin-resistant mechanisms
Resistant mechanism of levofloxacin has been known via accumulation of mutations in the QRDR of topoisomerase genes and efflux pump activation [7], [11]. Among the 66 B. cepacia complex isolates, resistance to levofloxacin was identified in 6 clinical isolates. Sequence analysis determined that single-base substitutions in the QRDR regions of the gyrA gene occurred at codons 81 (Gly81Asp), 83 (Thr83Ile), and 87 (Asp87His). No mutation was found in the QRDR region of the parC gene. Besides, higher efflux pump activity was found in 50% levofloxacin- resistant isolates (3/6) and could be related to levofloxacin resistance (Table 3).
Table 3. Characteristics of QRDR mutations and efflux pump activity of six fluoroquinolone-resistant B. cepacia complex isolates.
| Strain | MIC of levofloxacin (µg/ml) | Efflux pump activity | Mutation in | ||
| CCCP (−) CCCP (+) | gyrA gene | parC gene | |||
| No. 1 | 8 | 8 | none | Thr83Ile (ACC→ATC) | no amino acid change |
| No. 21 | 8 | 2 | activity | no amino acid change | no amino acid change |
| No. 26 | 8 | 8 | none | Thr83Ile (ACC→ATC) | no amino acid change |
| No. 27 | 32 | 8 | activity | Asp87His ( GAC→ CAC) | no amino acid change |
| No. 34 | 16 | 16 | none | Thr83Ile (ACC→ATC) | no amino acid change |
| No. 52 | 64 | 8 | activity | Gly81Asp (GGC→GAC) | no amino acid change |
Quantitative RNA expression of RND efflux pumps (efflux pump RNA expression)
According to previous studies, four active RND pumps (RND-3, RND-4, RND-9 and RND-10) were linked to bacterial antibiotic resistance in B. cenocepacia laboratory stains; however, their contributions in clinical isolates had not been elucidated [14], [15], [17]. To understand whether these efflux pumps were expressed in clinical isolates, some representative isolates with efflux pump activity (no. 4, no. 23, and no. 27) and without efflux pump activity (no. 30, and no. 38) were selected from pulsotype (pulsotype I) and the transcript abundance of these four efflux pumps quantified by qRT-PCR (Table 4). Expression of RND-4 (BCAL2822) and RND-10 (BCAM2551) were no significant difference in these clinical isolates. High expression level of RND-3 (BCAL1674) and RND-9 (BCAM1947) were identified in all clinical isolates demonstrating efflux pump activity (Table 4).
Table 4. Quantitative RNA expression of four RND efflux systems in five selected B. cenocepacia (genomovar IIIA) isolates.
| Efflux pump activity | Fold change in expressiona | |||
| BCAL1674 (RND-3) | BCAL2822 (RND-4) | BCAM1947 (RND-9) | BCAM2551 (RND-10) | |
| Activity | ||||
| No. 4 | 7.41±0.77 | 1.44±0.65 | 3.77±0.88 | 2.02±1.25 |
| No. 23 | 6.94±0.92 | 1.47±0.89 | 2.99±0.54 | 1.78±0.56 |
| No. 27 | 8.54±1.31 | 1.00±0.27 | 3.47±0.73 | 0.88±0.53 |
| Non-induced | ||||
| No. 30 | 1b | 1b | 1b | 1b |
| No. 38 | 0.87±0.05 | 1.09±0.22 | 0.74±0.17 | 1.34±0.11 |
. Fold change was determined by quantitative reverse transcription-PCR (quantitative RT-PCR).
. No. 30 was a strain with a non-induced efflux pump that was utilised as a reference strain.
Sequence analysis of regulators in RND efflux pump genes
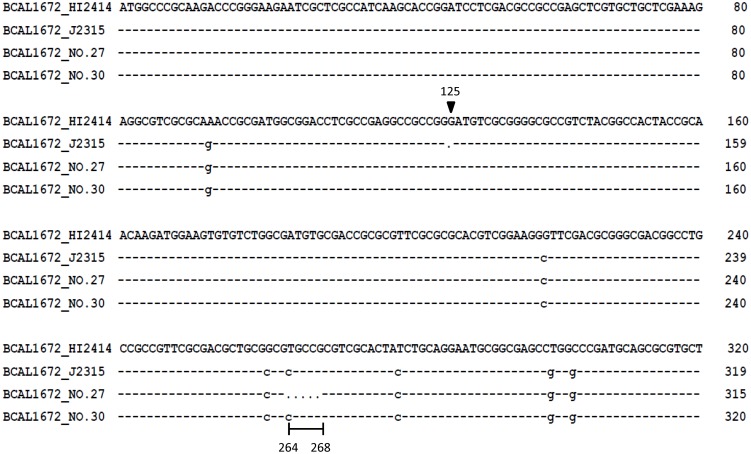
Based on previous studies that determined that the over-expression of multidrug resistance efflux pumps was usually correlated with mutations in regulator genes in clinical Pseudomonas aeruginosa isolates [28], [29], two regulator genes (RND-3, and RND-9) including their promoter regions were sequenced in the five B. cenocepacia (genomovar IIIA) isolates. The regulators of RND-9 demonstrated no amino acid changes when compared to B. cenocepacia J2315 (GenBank accession numbers AM747720.1). Because the regulator of RND-3 (BCAL1672) is a pseudogene in B. cenocepacia J2315, sequence analysis of the BCAL1672 gene was compared with B. cenocepacia HI2424 (GenBank accession numbers CP000458.1) [30]. For three of isolates with efflux pump activity (no. 4, no. 23 and no. 27), five nucleotide deletions (from position 264 to 268) were identified in the BCAL1672 gene (GenBank accession numbers KJ882899). The deletion of a guanine nucleotide at position 125 could result in the translational frameshifting in B. cenocepacia J2315 (Fig. 4). The five nucleotide deletions from position 264 to 268 caused protein frameshifting. These results indicated that mutations in the BCAL1672 repressor could affect RND-3 efflux pump expression in B. cenocepacia clinical isolates.
Figure 4. Comparison of the BCAL1672 gene (the first 320 bp) in B. cenocepacia isolates.
The arrow (at position 125) indicates the deletion of a guanine nucleotide in B. cenocepacia J2315. Deleted nucleotides (between positions 264 and 268) were identified in clinical isolate No. 27.
Correlation of BCAL1672 gene mutations with efflux pump expression in clinical B. cenocepacia (genomovar IIIA) isolates
To investigate the BCAL1672 gene, which is a regulator of the RND-3 efflux pump in clinical B. cenocepacia (genomovar IIIA) isolates, 40 isolates were included for analysis. Ten isolates were susceptible to all tested antimicrobials. Thirty isolates were resistant to at least one antimicrobial agnet (25 isolates with efflux pump activity and five isolates without the activity). The five nucleotide deletions constituted predominant mutations observed in 8 of 25 isolates with efflux pump activity (Table 5). One isolate had 12 nucleotide deletions at the end of BCAL1672 (from position 586 to 598), and six isolates harboured a Ser174Pro (TCG→CCG) variation. When the groups demonstrating efflux pump activity and without activity were compared, these three variations of BCAL1672 were statistically correlated with RND-3 efflux pump activity (Chi-square test, p<0.05). Investigation of these three mutants and antibiotic resistance in these 25 isolates with efflux pump activity found that mutation of BCAL1672 could be related to chloramphenicol (12/14; 86%), meropenem (3/5; 60%), and levofloxacin (2/3; 67%) resistance. However, only three ceftazidime resistant isolates had such mutations (3/10; 30%) (Table 6). These results indicated that mutations of the BCAL1672 gene are associated with efflux pumps activity causing antibiotic resistance whereas ceftazidime resistance could be related to other unidentified mechanisms.
Table 5. Sequence analysis of regulators for the RND-3 efflux systems in B. cenocepacia (genomovar IIIA).
| Mutation in BCAL1672 (RND-3 regulator)a | Efflux activity isolates (N = 25) | Non-induced (N = 5) and all susceptible isolates (N = 10)b |
| 264 GCGTG 268; 5-nt deletion c | 8 | 0 |
| 586 GCTTCGACGCCG 598; 12 nt deletion c | 1 | 0 |
| Ala140Val (GCG→GTG) | 1 | 0 |
| Val153Ala (GTC→GCC) | 16 | 15 |
| Ser174Pro ( TCG→ CCG) c | 6 | 0 |
| Ala199Thr ( GCC→ ACC) | 1 | 6 |
| Arg208Gly ( CGA→ GGA) | 16 | 6 |
| Arg208Gly ( CGA → GGG ) | 0 | 9 |
| Asp215Ser ( GAC→ AGC) | 1 | 0 |
. Sequence compared with B. cenocepacia HI2424 (GenBank accession number: CP000458.1).
. All susceptible isolates were tested for their antimicrobial susceptibilities to CAZ, LVX, C, MEM, MI, and SXT.
. The variations in BCAL1672 that are related to RND-3 efflux pump activity are indicated in bold.
Table 6. Correlation between BCAL1672 mutants and antibiotic resistance in 25 efflux activity B. cenocepacia (genomovar IIIA) isolates.
| Mutation in BCAL1672 (RND-3 regulator)a | Antibiotic resistanceb | |||||
| C (n = 14) | CAZ (n = 10) | MEM (n = 5) | LVX (n = 3) | MI (n = 1) | SXT (n = 2) | |
| 264 GCGTG 268; 5-nt deletion c (n = 8) | 8 | 1 | 0 | 0 | 0 | 2 |
| 586 GCTTCGACGCCG 598; 12 nt deletion c (n = 1) | 1 | 0 | 1 | 1 | 0 | 0 |
| Ser174Pro ( TCG→ CCG) c (n = 6) | 3 | 2 | 2 | 1 | 0 | 0 |
. Sequence compared with B. cenocepacia HI2424 (GenBank accession number: CP000458.1).
. C = chloramphenicol, CAZ = ceftazidime, MEM = meropenem, LVX = levofloxacin, MI = minocycline, and SXT = Trimethoprim/sulfamethoxazole
. The variations in BCAL1672 that are related to RND-3 efflux pump activity are indicated in bold.
Discussion
Most studies of the B. cepacia complex in Taiwan were reported at least ten years ago [31]–[33]. Here, we present the updated data revealing that the non-susceptibility rate for ceftazidime increased from 13.9% (a previous report in 2011) [5] to 35% (this study). All 66 isolates (100%) studies were resistant to ticarcillin/clavulanic acid and the non-susceptibility rates of chloramphenicol and ceftazidime were 55% and 35%, respectively (Table 2). The above indicated antimicrobial resistance of B. cepacia complex to cause a serious clinical concern. Seventeen of 18 ceftazidime-resistant isolates and 16 of 22 chloramphenicol-resistant isolates exhibited efflux pump activity (Fig. 3). Half of the levofloxacin-resistant isolates demonstrated efflux pump activity resulting in at least a 4-fold change in MIC. These data indicate that efflux pump activity plays an important role in causing antibiotic resistance in the B. cepacia complex.
In recent decades, efflux pump activity has emerged and become a significant resistant mechanism in many species of bacteria [7], [34]. In silico analysis of the B. cenocepacia genome sequence revealed 16 putative RND efflux pumps [35], [36]. Genetic analysis indicated that 4 active RND pumps (RND-3, RND-4, RND-9 and RND-10) were correlated with antibiotic resistance in the B. cepacia complex. However, the function of the RND pumps in clinical isolates was not clearly understood [14], [15], [17]. The genes associated with the RND-3 efflux pump include a regulator gene (BCAL1672) and tripartite component genes (BCAL1674 through BCAL1676). Two studies revealed that the RND-3 efflux pump was strongly induced by chloramphenicol and correlated with the extrusion of nalidixic acid [15], [35]. Our results revealed that the RND-3 efflux pump is the most up-regulated among the RND pumps tested in clinical isolates. Quantitative RT-PCR demonstrated that mutations in BCAL1672 result in the activation of the RND-3 efflux pump. Correlation of antibiotic resistance and efflux pump activation revealed that all 14 B. cenocepacia (genomovar IIIA) isolates with efflux pump activity were resistant to chloramphenicol. Among them, 12 had mutations in the regulator gene BCAL1672 (Table 6). The RND-9 efflux operon (comprising BCAM1945 through BCAM1947) belongs to the HAE-1 family, which includes proteins responsible for the extrusion of aminoglycosides, ethidium bromide, fluoroquinolones and β-lactams [14], [37]. In this study, three clinical isolates exhibited increased transcriptional expression of RND-9 (BCAM1947), whereas no mutations were found in the regulator BCAM1948 or the promoter region (Table 4). Up-regulation of RND-9 efflux pump expression could be activated by an unknown transcriptional factor like the situation that the global activator MarA of E.coli that can increase the acrAB efflux pump expression [38]. The efflux pump mechanisms for cefazidime resistance in B. cenocepacia (genomovar IIIA) isolates warrant further investigation (Table 6). Among the 6 fluoroquinolone-resistant B. cepacia complex isolates, amino acid changes were found in the QRDR region of the gyrA gene, including Gly81Asp, Thr83Ile, and Asp87His. No mutation was found in the QRDR region of the parC gene (Table 3). A previous study showed that fluoroquinolone resistance was correlated to a Thr83Ile or Asp87Asn mutation in the gyrA gene [11]. The Gly81Asp and Asp87His mutations in the gyrA gene in B. cepacia complex isolates are newly reported in this study.
Only a few studies have described integrons in the B. cepacia complex. Ramírez et al. described a class 2 integron that harboured a streptothricin acetyltransferase in B. cenocepacia (IIIB) [13]. Crowley et al. reported a class 1 integron containing bla OXA (β-lactamase) and aac(6′)-1a (encoded aminoglycoside 6′-N-acetyltransferase type 1b) in B. cenocepacia (III) [12]. We reported class 1 integron harbouring genes in 18 isolates (18/66, 27.3%). Only one isolate contained a class 1 integrase and the qacF gene (quaternary ammonium compound-resistance protein); the other isolates harboured the typical integron structure (In0) and acquired different copy of the aacA4 gene. No class 2 integron was found. Our results indicate that the class 1 integron cassette is an important vehicle to transmit resistance to aminoglycoside, sulfamethoxazole and chloramphenicol in B. cepacia complex isolates.
The recA-RFLP typing performed that B. cenocepacia (IIIA and IIIB had 52 and 8 isolates, respectively) was prevalent among B. cepacia complex isolates. Because molecular methods consume time and are costly, the more rapid and cost efficient MALDI-TOF analysis has been evaluated for species identification. Species identification accuracy was 90.9% (60/66) for MALDI-TOF analysis. This result indicated that MALDI-TOF has the potential for application in the clinical microbiology laboratory to differentiate B. cepacia complex to species level. Though two major pulsotypes were identified, PFGE analysis revealed high discrimination power and high genotypic diversity of B. cepacia complex.
In summary, antibiotic resistance mechanisms, including mutation of the QRDR region of topoisomerase II, the presence of the class 1 integron, and overexpression of efflux pumps, were common in B. cepacia complex clinical isolates. Mutation of the regulator gene of the RND-3 efflux pump was a major cause of high-level efflux pump expression in clinical B. cepacia complex isolates. Given the contribution of efflux pumps to antibiotic resistance in clinical B. cepacia complex isolates, the development of antimicrobial drugs targeting efflux pumps may counteract antimicrobial-resistant bacteria in the future.
Supporting Information
Antimicrobial susceptibility patterns of species of 66 B. cepacia complex isolates.
(DOCX)
Antimicrobial susceptibility patterns of pulsotype A (n = 15) and I (n = 12) B. cepacia complex isolates.
(DOCX)
Antimicrobial susceptibilities of each B. cepacia complex isolates, efflux pump activity testing and correlation between BCAL1672 mutants and antibiotic resistance in 25 efflux activity B. cenocepacia (genomovar IIIA) isolates.
(XLSX)
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by KMU-Q102005 from Kaohsiung Medical University Research Foundation and NSYSUKMU102-I004 from the NSYSU-KMU Joint Research Project, Taiwan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Drevinek P, Mahenthiralingam E (2010) Burkholderia cenocepacia in cystic fibrosis: epidemiology and molecular mechanisms of virulence. Clin Microbiol Infect 16: 821–830. [DOI] [PubMed] [Google Scholar]
- 2. Mahenthiralingam E, Urban TA, Goldberg JB (2005) The multifarious, multireplicon Burkholderia cepacia complex. Nat Rev Microbiol 3: 144–156. [DOI] [PubMed] [Google Scholar]
- 3. Mahenthiralingam E, Baldwin A, Dowson CG (2008) Burkholderia cepacia complex bacteria: opportunistic pathogens with important natural biology. J Appl Microbiol 104: 1539–1551. [DOI] [PubMed] [Google Scholar]
- 4. Ku NS, Han SH, Kim CO, Baek JH, Jeong SJ, et al. (2011) Risk factors for mortality in patients with Burkholderia cepacia complex bacteraemia. Scand J Infect Dis 43: 792–797. [DOI] [PubMed] [Google Scholar]
- 5. Liao CH, Chang HT, Lai CC, Huang YT, Hsu MS, et al. (2011) Clinical characteristics and outcomes of patients with Burkholderia cepacia bacteremia in an intensive care unit. Diagn Microbiol Infect Dis 70: 260–266. [DOI] [PubMed] [Google Scholar]
- 6. Siddiqui AH, Mulligan ME, Mahenthiralingam E, Hebden J, Brewrink J, et al. (2001) An episodic outbreak of genetically related Burkholderia cepacia among non-cystic fibrosis patients at a university hospital. Infect Control Hosp Epidemiol 22: 419–422. [DOI] [PubMed] [Google Scholar]
- 7. Nikaido H, Pages JM (2012) Broad-specificity efflux pumps and their role in multidrug resistance of Gram-negative bacteria. FEMS Microbiol Rev 36: 340–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rushton L, Sass A, Baldwin A, Dowson CG, Donoghue D, et al. (2013) Key role for efflux in the preservative susceptibility and adaptive resistance of Burkholderia cepacia complex bacteria. Antimicrob Agents Chemother 57: 2972–2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Moore RA, Hancock RE (1986) Involvement of outer membrane of Pseudomonas cepacia in aminoglycoside and polymyxin resistance. Antimicrob Agents Chemother 30: 923–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Aronoff SC (1988) Outer membrane permeability in Pseudomonas cepacia: diminished porin content in a beta-lactam-resistant mutant and in resistant cystic fibrosis isolates. Antimicrob Agents Chemother 32: 1636–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pope CF, Gillespie SH, Pratten JR, McHugh TD (2008) Fluoroquinolone-resistant mutants of Burkholderia cepacia . Antimicrob Agents Chemother 52: 1201–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Crowley D, Daly M, Lucey B, Shine P, Collins JJ, et al. (2002) Molecular epidemiology of cystic fibrosis-linked Burkholderia cepacia complex isolates from three national referral centres in Ireland. J Appl Microbiol 92: 992–1004. [DOI] [PubMed] [Google Scholar]
- 13. Ramirez MS, Vargas LJ, Cagnoni V, Tokumoto M, Centron D (2005) Class 2 integron with a novel cassette array in a Burkholderia cenocepacia isolate. Antimicrob Agents Chemother 49: 4418–4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bazzini S, Udine C, Sass A, Pasca MR, Longo F, et al. (2011) Deciphering the role of RND efflux transporters in Burkholderia cenocepacia . PLoS One 6: e18902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Buroni S, Pasca MR, Flannagan RS, Bazzini S, Milano A, et al. (2009) Assessment of three Resistance-Nodulation-Cell Division drug efflux transporters of Burkholderia cenocepacia in intrinsic antibiotic resistance. BMC Microbiol 9: 200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fehlner-Gardiner CC, Valvano MA (2002) Cloning and characterization of the Burkholderia vietnamiensis norM gene encoding a multi-drug efflux protein. FEMS Microbiol Lett 215: 279–283. [DOI] [PubMed] [Google Scholar]
- 17. Nair BM, Cheung KJ Jr, Griffith A, Burns JL (2004) Salicylate induces an antibiotic efflux pump in Burkholderia cepacia complex genomovar III (B. cenocepacia). J Clin Invest 113: 464–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mahenthiralingam E, Bischof J, Byrne SK, Radomski C, Davies JE, et al. (2000) DNA-Based diagnostic approaches for identification of Burkholderia cepacia complex, Burkholderia vietnamiensis, Burkholderia multivorans, Burkholderia stabilis, and Burkholderia cepacia genomovars I and III. J Clin Microbiol 38: 3165–3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Petrucca A, Cipriani P, Valenti P, Santapaola D, Cimmino C, et al. (2003) Molecular characterization of Burkholderia cepacia isolates from cystic fibrosis (CF) patients in an Italian CF center. Res Microbiol 154: 491–498. [DOI] [PubMed] [Google Scholar]
- 20. Degand N, Carbonnelle E, Dauphin B, Beretti JL, Le Bourgeois M, et al. (2008) Matrix-assisted laser desorption ionization-time of flight mass spectrometry for identification of nonfermenting gram-negative bacilli isolated from cystic fibrosis patients. J Clin Microbiol 46: 3361–3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Clinical and Laboratory Standards Institute Performance standards for antimicrobial susceptibility testing. Twenty-third informational supplement. Document M100-S23 CLSI, Wayne, PA (2013). [Google Scholar]
- 22. Zhang Y, Bao Q, Gagnon LA, Huletsky A, Oliver A, et al. (2010) ampG gene of Pseudomonas aeruginosa and its role in β-lactamase expression. Antimicrob Agents Chemother 54: 4772–4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ma L, Lu PL, Siu LK, Hsieh MH (2013) Molecular typing and resistance mechanisms of imipenem-non-susceptible Klebsiella pneumoniae in Taiwan: results from the Taiwan surveillance of antibiotic resistance (TSAR) study, 2002–2009. J Med Microbiol 62: 101–107. [DOI] [PubMed] [Google Scholar]
- 24. Ramirez MS, Pineiro S, Centron D (2010) Argentinian Integron Study G (2010) Novel insights about class 2 integrons from experimental and genomic epidemiology. Antimicrob Agents Chemother 54: 699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Coenye T, Van Acker H, Peeters E, Sass A, Buroni S, et al. (2011) Molecular mechanisms of chlorhexidine tolerance in Burkholderia cenocepacia biofilms. Antimicrob Agents Chemother 55: 1912–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sass A, Marchbank A, Tullis E, Lipuma JJ, Mahenthiralingam E (2011) Spontaneous and evolutionary changes in the antibiotic resistance of Burkholderia cenocepacia observed by global gene expression analysis. BMC Genomics 12: 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bissonnette L, Roy PH (1992) Characterization of In0 of Pseudomonas aeruginosa plasmid pVS1, an ancestor of integrons of multiresistance plasmids and transposons of gram-negative bacteria. J Bacteriol 174: 1248–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vettoretti L, Plesiat P, Muller C, El Garch F, Phan G, et al. (2009) Efflux unbalance in Pseudomonas aeruginosa isolates from cystic fibrosis patients. Antimicrob Agents Chemother 53: 1987–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Adewoye L, Sutherland A, Srikumar R, Poole K (2002) The mexR repressor of the mexAB-oprM multidrug efflux operon in Pseudomonas aeruginosa: characterization of mutations compromising activity. J Bacteriol 184: 4308–4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. LiPuma JJ, Spilker T, Coenye T, Gonzalez CF (2002) An epidemic Burkholderia cepacia complex strain identified in soil. Lancet 359: 2002–2003. [DOI] [PubMed] [Google Scholar]
- 31. Huang CH, Jang TN, Liu CY, Fung CP, Yu KW, et al. (2001) Characteristics of patients with Burkholderia cepacia bacteremia. J Microbiol Immunol Infect 34: 215–219. [PubMed] [Google Scholar]
- 32. Lu DC, Chang SC, Chen YC, Luh KT, Lee CY, et al. (1997) Burkholderia cepacia bacteremia: a retrospective analysis of 70 episodes. J Formos Med Assoc 96: 972–978. [PubMed] [Google Scholar]
- 33. Yu WL, Wang DY, Lin CW, Tsou MF (1999) Endemic burkholderia cepacia bacteraemia: clinical features and antimicrobial susceptibilities of isolates. Scand J Infect Dis 31: 293–298. [DOI] [PubMed] [Google Scholar]
- 34. Fernandez L, Hancock RE (2012) Adaptive and mutational resistance: role of porins and efflux pumps in drug resistance. Clin Microbiol Rev 25: 661–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Guglierame P, Pasca MR, De Rossi E, Buroni S, Arrigo P, et al. (2006) Efflux pump genes of the resistance-nodulation-division family in Burkholderia cenocepacia genome. BMC Microbiol 6: 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Holden MT, Seth-Smith HM, Crossman LC, Sebaihia M, Bentley SD, et al. (2009) The genome of Burkholderia cenocepacia J2315, an epidemic pathogen of cystic fibrosis patients. J Bacteriol 191: 261–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Perrin E, Fondi M, Papaleo MC, Maida I, Buroni S, et al. (2010) Exploring the HME and HAE1 efflux systems in the genus Burkholderia . BMC Evol Biol 10: 164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Grkovic S, Brown MH, Skurray RA (2002) Regulation of bacterial drug export systems. Microbiol Mol Biol Rev 66: 671–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Antimicrobial susceptibility patterns of species of 66 B. cepacia complex isolates.
(DOCX)
Antimicrobial susceptibility patterns of pulsotype A (n = 15) and I (n = 12) B. cepacia complex isolates.
(DOCX)
Antimicrobial susceptibilities of each B. cepacia complex isolates, efflux pump activity testing and correlation between BCAL1672 mutants and antibiotic resistance in 25 efflux activity B. cenocepacia (genomovar IIIA) isolates.
(XLSX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.