Abstract
Purpose
Digital subtraction angiography (DSA) remains the gold standard to diagnose intracranial arteriovenous malformations (AVMs) but is invasive. Existing magnetic resonance angiography (MRA) is suboptimal for assessing the hemodynamics of AVMs. The objective of this study was to evaluate the clinical utility of a novel noncontrast four-dimensional (4D) dynamic MRA (dMRA) in the evaluation of intracranial AVMs through comparison with DSA and time-of-flight (TOF) MRA.
Materials and methods
Nineteen patients (12 women, mean age 26.2±10.7 years) with intracranial AVMs were examined with 4D dMRA, TOF and DSA. Spetzler–Martin grading scale was evaluated using each of the above three methods independently by two raters. Diagnostic confidence scores for three components of AVMs (feeding artery, nidus and draining vein) were also rated. Kendall's coefficient of concordance was calculated to evaluate the reliability between two raters within each modality (dMRA, TOF, TOF plus dMRA). The Wilcoxon signed-rank test was applied to compare the diagnostic confidence scores between each pair of the three modalities
Results
dMRA was able to detect 16 out of 19 AVMs, and the ratings of AVM size and location matched those of DSA. The diagnostic confidence scores by dMRA were adequate for nidus (3.5/5), moderate for feeding arteries (2.5/5) and poor for draining veins (1.5/5). The hemodynamic information provided by dMRA improved diagnostic confidence scores by TOF MRA.
Conclusion
As a completely noninvasive method, 4D dMRA offers hemodynamic information with a temporal resolution of 50–100 ms for the evaluation of AVMs and can complement existing methods such as DSA and TOF MRA.
1. Introduction
Intracranial arteriovenous malformations (AVMs) are congenital vascular abnormalities characterized by direct arteriovenous shunts through a nidus of coiled and tortuous vascular connections without a normal intervening capillary bed. The principal presentation of AVM patients is hemorrhage which accounts for 2–4% of overall hemorrhagic strokes1, conferring significant morbidity and mortality. Detailed information about the architecture and hemodynamics of AVM is of great value with respect to adequate diagnosis and ensuing therapeutic approaches2.
Digital subtraction angiography (DSA) remains the gold standard for the characterization and delineation of intracranial AVMs with both high temporal and spatial resolutions. However, as an invasive technique, it exposes both doctors and patients to the radiation of X-rays and carries risks related to puncture of femoral artery, catheter placement, contrast agents and experience of doctors3. Time-resolved contrast enhanced MRA (CE-MRA) is a promising technique in the assessment of both anatomic and hemodynamic information with extensive coverage, but its temporal resolution is relatively low (generally on the order of seconds) which might not be sufficient to capture the fast-flow hemodynamic information. Also, complications may occur with the application of gadolinium-based contrast agent such as nephrogenic systemic fibrosis (NSF) when the glomerular filtration rate (GFR) is lower than 30 ml/min4. Standard time-of-flight (TOF) MRA provides static vascular images with high spatial resolution, however it cannot provide hemodynamic information. Recently, an unenhanced 4-D time-resolved dynamic MRA technique was introduced by combining arterial spin tagging with multiphase true fast imaging with steady-state precession (TrueFISP) – also termed TrueSTAR5, 6. In preliminary studies, this 4D dynamic MRA technique was able to delineate the dynamic course of blood flow through an AVM with a temporal resolution of a few tens of milliseconds and a spatial resolution of a few cubic millimeters.
In this paper, we attempted to explore the clinical value of the 4D dynamic MRA technique in a cohort of AVM patients, using Spetzler-Martin grading scale (size, eloquence of adjacent brain and pattern of venous drainage)7 (Table 1) as the reference which has been traditionally used to estimate the risk of surgical intervention for patients with intracranial AVMs. In addition, other factors that are important for the evaluation of AVMs or deemed as significant determinants of risks and outcomes such as the pattern of supplying artery and draining vein, AVM related aneurysms, presence of arteriovenous fistula (AVF) and detailed hemodynamic information were evaluated8–10. These results were then compared with those of DSA and TOF MRA respectively to test whether the information derived from dynamic MRA is consistent with the gold standard of DSA, and whether dynamic MRA will improve the existing diagnosis based on TOF MRA.
Table 1.
Spetzler-Martin grading scale
| size of nidus | eloquence | venous drainage | |||
|---|---|---|---|---|---|
| <3cm | 1 | non-eloquent | 0 | superficial | 0 |
| 3cm~6cm | 2 | eloquent | 1 | deep | 1 |
| >6cm | 3 | ||||
2. Materials and methods
2.1. Patients
This study was approved by the institutional review boards of Tian Tan Hospital and the Institute of Biophysics at the Chinese Academy of Sciences. Written informed consent was obtained from all patients. The general contraindications for MR examination were applied and defined as exclusion criteria.
Between July 2009 and November 2010, 19 patients (12 women, 7 men, mean age 26.2±10.7 years) with intracranial arteriovenous malformations scheduled for DSA examination were included in this study. Among them, 4 patients experienced spontaneous intracranial hemorrhage (all occurred within 4 months before they were hospitalized in Tian Tan Hospital) and were treated conservatively in their local hospitals. One patient had gamma-knife treatment and another underwent endovascular interventional treatment. The time interval between DSA and dynamic MRA was 1~68 days (15.4±20.6 days).
2.2. Conventional DSA
DSA was performed according to a standard protocol during routine clinical examination on a biplane angiography system (Advantx LCV+, GE Healthcare, UK) in TianTan Hospital in 16 of the 19 patients because 3 DSAs were performed in the patients’ regional hospitals. A 5-F diagnostic catheter was navigated into internal carotid and vertebral arteries respectively via the right femoral artery to acquire standard anteroposterior and lateral projections, each by manual delivery of 5–7 ml iodinated contrast agent Iodixanol Injection per run (Visipaque, GE Healthcare, IRELAND).
2.3. MR angiography
All imaging was performed on a 3-T MR imager (Tim Trio; Siemens, Erlangen, Germany) with a body coil as the transmitter and a 12-channel head array coil as the receiver.
Conventional MR sequences included axial T1-weighted 3D magnetization prepared rapid acquisition gradient-echo [repetition time (TR)/echo time (TE)=1760/3.1 msec, inversion time=950 msec, spatial resolution= 1mm×1mm×1mm], T2-weighted fast spin-echo (TR/TE=4000/87 msec, spatial resolution=0.43mm×0.43mm×4.8mm), and time-of-flight MR angiography (TR/TE=33/3.86 msec, spatial resolution=0.57mm×0.57mm×0.65 mm).
Dynamic MRA was implemented using flow-sensitive alternating inversion recovery (FAIR)11 for spin tagging. Slice-selective (label) or nonselective (control) inversion recovery signals were continuously sampled by a 3D segmented multiphase TrueFISP sequence, and the difference of the two acquisitions provided 4D dMRA signals. To minimize the effects of cardiac pulsation on dynamic MRA images, cardiac-gated dMRA was performed in 19 AVM patients. A slab of 64 slices was acquired (TR=2.94 ms, TE=TR/2, spatial resolution=1mm×1mm×1mm, flip angle=25°). Depending on the cardiac cycle, 10–17 phases of dMRA images with a temporal resolution of 83 ms were acquired within approximately 6 min. After dynamic MR angiograms were obtained, maximum intensity projection (MIP) images were then generated for each phase along three directions which were displayed as a movie to visualize the dynamic flow patterns of AVM lesions.
2.4. Statistical Analysis
One neuroradiologist (with 14 years' experience) and one neurosurgeon (with 8 years' experience) reviewed the cases independently. They were blinded to the information regarding the patients' name and clinical history. The order of the 19 cases was randomized. First they graded AVMs according to Spetzler–Martin grading scale and evaluated other detailed information (the pattern of supplying artery and draining vein, AVM-related aneurysms and AVF).
This step was carried out with dMRA only. Then they were asked to give diagnostic confidence scores according to the information gathered above for each component of an AVM (feeding artery, nidus and draining vein). One week later, they repeated the evaluation procedures with TOF MRA first and then with combined TOF and dMRA together. Another week later, they repeated the evaluation procedures again but with DSA only. During all evaluation procedures, conflicting readings were resolved by consensus of the two raters. The diagnostic confidence score was defined as follows: score 5, excellent imaging quality with clear detailed vascular architecture and hemodynamic information, with no artifacts and can be diagnosed definitely; score 4, good imaging quality with fine vascular architecture and hemodynamic information, with mild artifacts and relatively clear diagnosis can be drawn; score 3, ordinary imaging quality and detailed vascular architecture and hemodynamic information need to be distinguished with some efforts, with moderate artifacts and possible diagnosis can be obtained; score 2, poor imaging quality and bad vascular architecture and hemodynamic information, with sever artifacts but can be helpful to the diagnosis; score 1, cannot afford any help to diagnosis.
Kendall's coefficient of concordance was calculated to evaluate the reliability between two readers within each modality (dMRA, TOF, TOF plus dMRA). A Kendall's coefficient of concordance higher than 0.75 was considered to indicate excellent agreement. The Wilcoxon signed-rank test was applied to compare the diagnostic confidence scores between each pair of the three modalities for each component of an AVM, respectively. A two-tailed P value of .05 or less indicated significant difference. All statistical analyses were performed with STATA 10.0 software (StataCorp, College Station, TX, USA).
3. Results
All the 19 AVMs demonstrated a typical appearance with one or more feeding arteries, an appreciable nidus and at least one readily identifiable draining vein entering venous sinuses, and no AVM-related aneurysm or AVF was found on DSA. Dynamic MRA was able to depict the entire dynamic blood circulation from arterial feeders to draining veins of cerebral AVM in accordance with DSA (Fig. 1, Supplemental Video 1). It failed to detect AVM lesions in three patients (patient nos. 1, 7, 12). In patient no. 1, severe artifact due to patient's movement during examination led to poor image quality. In patient nos. 7 and 12, both dMRA and TOF MRA showed inabilities to detect intracranial AVMs with low blood flow which was manifested as lightly stained on DSA (Fig. 2).
Figure 1.

Patient No. 4, female, 27 years old. She was admitted to hospital due to deterioration of vision of right eye for 5 years and headache for one month. (a) Axial, (b) sagittal, and (c) coronal dynamic MR angiography MIP images acquired with 3D TrueSTAR show an AVM in right occipital lobe. We can clearly observe the anatomic details of dynamic courses for labeled blood from feeding arteries (MCA and PCA), through abnormal vascular nidus and then into the draining veins sequentially. Furthermore, we can observe the heterogeneity within the nidus. The information was consistent with (d) TOF and (f) DSA. The heterogeneity within the nidus was confirmed to be venous aneurysm but was unclear on TOF. ms = milliseconds. MCA= middle carotid artery. PCA= posterior carotid artery. Arrowheads = feeding arteries. Arrows = nidus. Curved arrows = draining veins.
Figure 2.
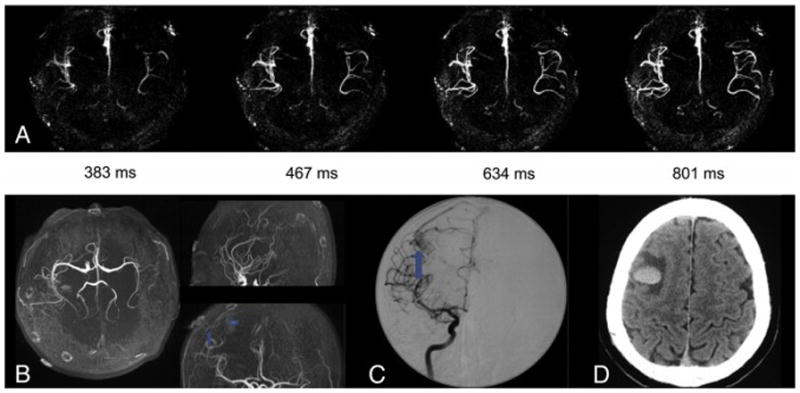
Patient No. 11, male, 27 years old. He had a sudden spontaneous intracranial hemorrhage confirmed by CT (f) and was treated conservatively in his regional hospital. One month after the incident, he was transferred to Tiantan Hospital. (a) Axial, (b) sagittal, and (c) coronal dynamic MR angiography MIP images acquired with 4D dMRA show a small AVM with a maximum diameter of 8mm in left parieto-occipital lobe with left MCA as its feeding artery. The draining vein was invisible due to the limit of vertical slab thickness. This was confirmed by DSA. However, TOF failed to detect the AVM lesion due to the disturbance of high signal intensity cause by methemoglobin and the size of the lesion was enlarged (7cm). Arrowheads = feeding arteries. Arrows = nidus. Curved arrows = draining veins.
3.1. Spetzler-Martin grading scale
Dynamic MRA provided afine delineation with respect to the size and location but a poor depiction as to the detection of draining veins of AVM. TOF encountered similar problems as dMRA. Spetzler–Martin grading scale could be completed only in four patients (patient nos. 4, 9, 13, 14) on dMRA imaging and in six patients on TOF MRA (patient nos. 4, 8, 9, 14, 16, 18).
Dynamic MRA provided correct evaluation of the sizes and locations of all the 16 AVMs detected, and the scores based on Spetzler–Martin classification were in complete accordance with those evaluated by DSA (Table 2). In contrast, TOF provided correct evaluation of AVM sizes in 15 of the 18 AVM lesions detected and was inaccurate in 3 AVMs (patient nos. 7, 11, 18). All of the three patients had a history of spontaneous intracerebral hemorrhage, and the high signal intensity caused by methemoglobin completely obscured (no. 11) or disturbed (no. 18) AVM anatomy. As a result, the apparent size of AVM was enlarged (Fig. 3, Supplemental Video 2), while in another patient (no. 7), the signal due to methemoglobin was mistaken for AVM lesion (Fig. 2). Moreover, dMRA can visualize dynamic bloodflow and thus can provide a better view of AVM size in late stages of the arterial filling phase (Fig. 4). Improved diagnostic accuracy was achieved when dMRA and TOF MRA were combined. In 17 of the 19 patients (excluding no. 7), Spetzler–Martin grading scale of AVM size and location based on dMRA plus TOF completely matched that of DSA (Table 2).
Table 2.
Spetzler-Martin grading score from 4 modalities
| Patient No. | Spetzler-Martin grading score
|
|||
|---|---|---|---|---|
| dMRA | TOF | DMRA plus TOF | DSA | |
| 1 | × | S2E1 | S2E1 | S2E1V0 |
| 2 | S2E0 | S2E0 | S2E0 | S2E0V0 |
| 3 | S1E1 | S1E1 | S1E1 | S1E1V0 |
| 4 | S2E1V0 | S2E1V0 | S2E1V0 | S2E1V0 |
| 5 | S1E1 | S1E1 | S1E1 | S1E1V0 |
| 6 | S2E1 | S2E1 | S2E1 | S2E1V0 |
| 7 | × | S1E1* | S1E1 | S1E1V0 |
| 8 | S2E1 | S2E1V0 | S2E1V0 | S2E1V0 |
| 9 | S2E1V0 | S2E1V0 | S2E1V0 | S2E1V0 |
| 10 | S2E0 | S2E0 | S2E0 | S2E0V0 |
| 11 | S1E1 | S3E1 | S1E1 | S1E1V0 |
| 12 | × | × | × | S1E0V0 |
| 13 | S2E1V0 | S2E1 | S2E1V0 | S2E1V0 |
| 14 | S2E1V0 | S2E1V0 | S2E1V0 | S2E1V0 |
| 15 | S2E0 | S2E0 | S2E0 | S2E0V0 |
| 16 | S3E1 | S3E1V0 | S3E1V0 | S3E1V0 |
| 17 | S2E1 | S2E1 | S2E1 | S2E1V1 |
| 18 | S1E1V0 | S2E1V0 | S1E1V0 | S1E1V0 |
| 19 | S1E1 | S1E1 | S1E1 | S1E1V0 |
Note —S=size of cerebral AVM nidus; E= eloquence of adjacent brain; V=venous drainage pattern. Absence of V (in the form of SE) means this method fails to detect venous drainage pattern. ×means failure to detect intracranial AVM lesion.
although TOF grading score was consistent with DSA, high intensity on TOF was caused by methemoglobin instead of AVM.
Figure 3.
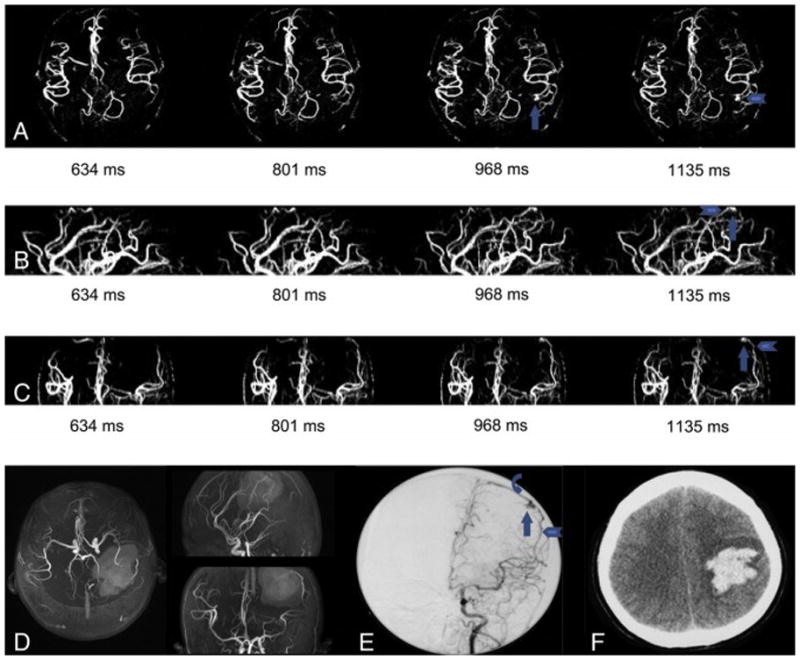
Patient No. 7 male, 40 years old. He experienced spontaneous intracranial hemorrhage (d) and received conservative treatment in his regional hospital 3 month before he was admitted into Tiantan Hospital. dMRA didn’t detect any lesion (a) and TOF seemed to detect a frontal lesion (b, arrowhead). DSA demonstrated a frontal AVM with slow blood flow (c, arrow) and the lesion was lightly stained and should be identified with efforts. We compared DSA and TOF carefully and found that the lesion showed on TOF was actually caused by high signal intensity caused by methemoglobin and the real AVM entity located laterally to the hematoma. This was verified by operation.
Figure 4.
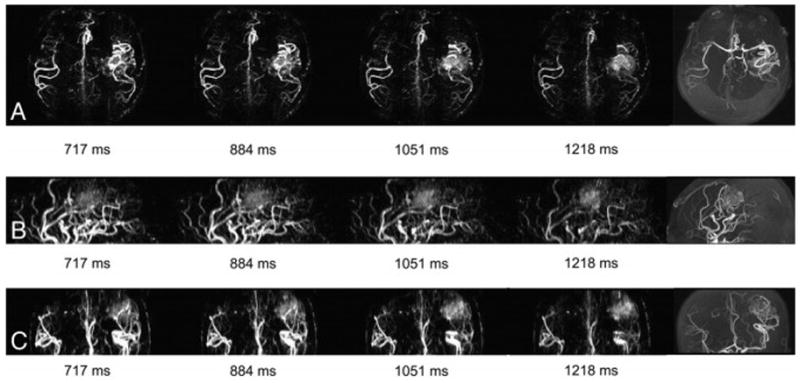
Patient No. 5, female, 30 years old. She had severe headache accompanying vomiting for half a month and then was admitted into Tiantan Hospital. (a) Axial, (b) sagittal, and (c) coronal dMRA show an occipital AVM with right PCA as its feeding artery. Also, we can observe the filling of superior sagittal sinus (arrow) in artery filling phase before the filling of vascular nidus and the occlusion of right transverse sinus (curved arrow). The latter can be confirmed by DSA (e). However, the early filling of superior sagittal sinus wasn’t observed on DSA (d).
3.2. Diagnostic confidence
Compared with DSA, dMRA detected 22 of all the 29 feeding arteries (75.8%) and 5 of all the 25 draining veins (20%) correctly in 19 patients. TOF MRA detected 26 of all the 29 feeding arteries (89.7%) and 7 of all the 25 (28%) draining veins. The two modalities combined together were able to detect 26 of all the 29 feeding arteries (89.7%) and 8 of all the 25 (32%) draining veins. Compared with TOF, dMRA has advantages in the observation of dynamic blood flow with a high temporal resolution (83 ms per frame). In three patients (nos. 1, 5, 17), occlusion of unilateral transverse sinus can be observed via dMRA (Fig. 5). The average scores of diagnostic confidence of the 19 patients for feeding artery, nidus and draining vein by means of dMRA, TOF and dMRA plus TOF, respectively, are displayed in Table 3. The diagnostic confidence scores were relatively high and adequate for diagnosis with respect to the depiction of nidus, moderate when describing feeding arteries and insufficient to make a correct diagnosis for draining veins in all the three modalities. Compared with dMRA, TOF provided a better result in the scoring of feeding arteries with statistical significance (P=.002). TOF and dMRA were not statistically different in the scoring for nidus and draining veins (PN.05). When dMRA was combined with TOF, the obtained diagnostic confidence scores of feeding arteries (P=.0002), nidus (P=.0004) and draining veins (P=.005) were significantly improved compared to those from dMRA alone. The confidence scores obtained from dMRA plus TOF of the nidus (P=.009) and draining veins (P=.046) were also significantly improved compared to those based on TOF MRA alone. The confidence score from dMRA plus TOF of feeding arteries did not change significantly compared to those based on TOF MRA (P > .05).
Figure 5.
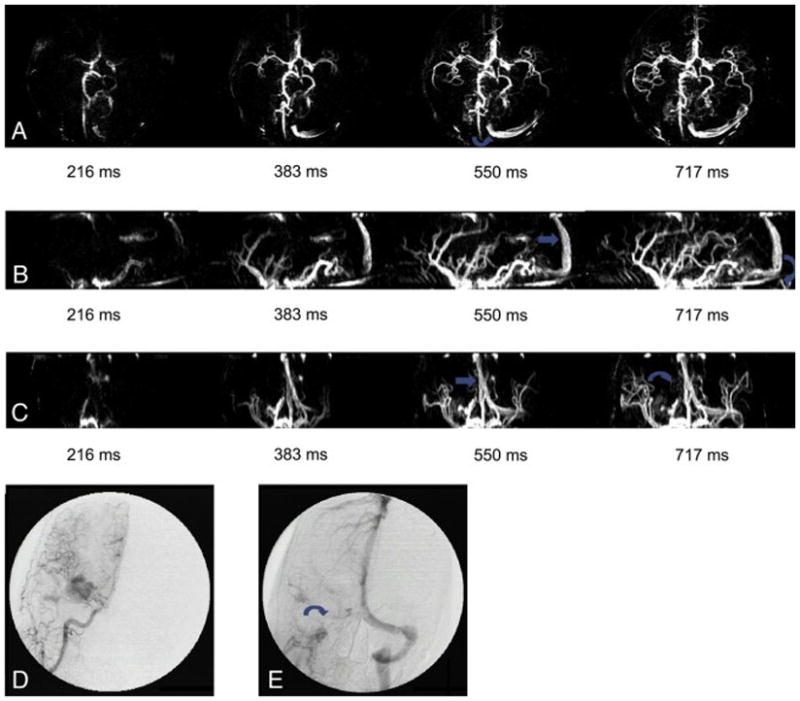
Patient No. 6, female, 43 years old. She had accidental blurred vision of right eye four times in a month. (a) TOF showed AVM located on left fronto-parietal lobes. But the overlapping of vessels disturbed observation of AVM size. (b) Axial, (c) sagittal and (d) coronal dMRA showed the contour of AVM lesion better at late stage of arterial filling phase.
Table 3.
Average scores of diagnostic confidence for components of AVM by means of dMRA, TOF and dMRA plus TOF. P values indicate statistical significance of Kendall's coefficient.
| dMRA | TOF | dMRA plus TOF | |
|---|---|---|---|
| feeder artery | 2.45±0.91 | 3.61±1.13 | 3.76±0.93 |
| nidus | 3.45±1.23 | 3.87±1.14 | 4.24±1.05 |
| draining vein | 1.53±0.84 | 1.97±1.39 | 2.24±1.35 |
| Kendall's coefficient between 2 observers | 0.889 | 0.826 | 0.834 |
| p value | <0.001 | 0.002 | 0.001 |
4. Discussion
Spetzler-Martin grading scale has been traditionally used to estimate the risk of surgical intervention for intracranial AVM and has been validated by its correlation with outcomes for surgically treated patients. All AVM falls into one of six grades and the higher grade, the poorer outcome for operative treatment. Many risk factors have been studied to predict hemorrhage of intracranial AVM. Some research showed that patients with small (=3 cm) AVMs presented more frequently with hemorrhage than did patients with larger AVMs (≤ 3cm)12–14 . Previous rupture of intracranial AVM has been confirmed to be an independent predictor of subsequent hemorrhage1, 15. The yearly risk of death at the first bleeding is about 10% and will increase with each bleeding16. So it is of great importance to correctly evaluate AVM in patients with hemorrhage history.
In this pilot study, we have successfully applied unenhanced 4-D dynamic MR angiography for the clinical evaluation and diagnosis of AVM patients before operations. As a newly emerging technology, TrueSTAR possesses several advantages for dynamic flow imaging such as inherent flow compensation 17, and relatively high contrast-to-noise ratio (CNR) and signal-to-noise ratio (SNR) 6. Compared with DSA and CE-MRA, the 4D dMRA technique is entirely non-invasive and can provide detailed dynamic information of blood flow which is unavailable using TOF MRA. The temporal resolution (83msec per frame) is much higher than that generally acquired by CE-MRA 18, 19. The relatively short acquisition time (6~7 min) makes it feasible to be implemented in routine clinical care. This modality has recently been tested clinically in the evaluation of intracranial collateral flow in patients with steno-occlusive diseases of brain-supplying arteries 20. During the preparation of this manuscript, a similar study was conducted in 15 AVM patients, and the results showed that the consistency between non-contrast enhanced dMRA and DSA was excellent for the arterial feeders, good for the nidus size and moderate for the venous drainage21. Our data are consistent with the reported findings. Furthermore, we performed comparisons between dMRA and TOF MRA.
4.1. dMRA vs. DSA
Compared with DSA, dMRA is a totally non-invasive examination with a faster acquisition time which avoids sophisticated surgical procedures, the injection of iodinated contrast agent and the exposure to ionizing radiation. Also, this technique does not require hospitalization and costs less. Furthermore, it does not increase the emotional stress of patients.
In the 16 positive results detected by dMRA, the demarcation of AVMs was well defined which allowed satisfactory results for both location and size judgments. All the scoring for size measurement and whether it occurs on eloquent areas according to Spetzler-Martin grading scale were completely in accordance with those from DSA. In this respect, dMRA is equal or even better than DSA because dMRA can provide anatomic information and clinical neurosurgeons are more familiar with this presentation form (axial, sagittal, and coronary presentation). Dynamic MRA showed poor ability to detect draining veins mainly due to the short coverage of time window which was insufficient to capture entire venous filling phrase. In the current implementation of dMRA, a tradeoff had to be made to balance the coverage of time window and spatial resolution in the slice direction. In the 16 positive patients, dMRA detected 22 of 26 feeding arteries (84.6%) correctly and did not miss any dominating feeding arteries which are more valuable to surgical strategy. However, it was demonstrated insensitive to tenuous or tiny feeding arteries.
The use of flow-sensitive alternating inversion recovery (FAIR) for spin labeling can cause contamination of labeled venous signals. In this study, 6 AVMs demonstrated the abnormal filling of superior sagittal sinus within artery filling phase before the filling of vascular nidus which indicated a fast blood velocity through AVMs and the existence of arteriovenous fistula were suspected. But this phenomenon was denied by DSA and no early fillings of superior sagittal sinus were detected.
4.2. dMRA vs. TOF
Compared with TOF, dMRA is a dynamic technique with a high temporal resolution allowing the observation of the passage of labeled blood from feeding arteries, through abnormal vascular nidus to draining veins and then into venous sinuses. Thus it is more vivid and has the potential to separate feeding arteries from the draining veins and present more detailed information such as the estimation of blood flow velocity, degree of shunt, detection of arteriovenous fistula and so on. In this study, we observed the occlusion of unilateral transverse sinus in 3 patients via the dynamic images which were invisible on TOF MRA.
As for Spetzler-Martin grading score, dMRA was superior for size measurement in patients who had a history of intracranial hemorrhage and showed the ability to detect small AVMs with a minimum size of 8mm in this study. Dynamic MRA images were obtained by complex subtractions of selective and nonselective inversion-recovery TrueFISP images which offered a better suppression of background signal intensity arising from methemoglobin. TOF did poorly in this aspect and was susceptible to the high signal intensity of methemoglobin which might obscure or disturb AVM anatomy, or might be mistaken for components of AVM22. In all the 4 patients who had previous intracranial hemorrhage, TOF overestimated the size of AVM in 2 patients (figure 2) and mistaken the hemorrhage for AVM in 1 patient (figure 3). On the other hand, TOF provides static vascular imaging and the overlapping of vessels may disturb the observation of an AVM. In contrast, we can achieve a good view of AVM entity via dMRA in the capillary or venous filling phase. Both dMRA and TOF provided a fine and accurate depiction of the location but demonstrated poor ability with regard to the draining veins.
As for diagnostic confidence scoring, TOF had relatively higher average scores than dMRA for each component of AVM. The reason may be that the higher spatial resolution of TOF facilitates the observation of static anatomical structures. However, in some patients, dMRA can provide additional temporal information compared to TOF due to its dynamic features and are more reliable in patients experiencing cerebral hemorrhage. More importantly, improved diagnostic confidence was achieved with combination of dMRA and TOF MRA, which provided high temporal and spatial resolution for 4D vascular imaging respectively. Since both dMRA and TOF are noninvasive procedures, the only cost for their combined use in clinical routine care is an additional scan time of several minutes plus the time for radiologists to view the 4D images.
4.3. Disadvantage of dMRA
Despite its advantages, dMRA encounters challenges for the evaluation of intracranial AVMs as demonstrated by this pilot study. First, it is insensitive to intracranial AVMs with low blood flow. This caused the failure of detection of AVMs in 2 patients and low diagnostic confidence in another 2 patients. Secondly, the time coverage is relatively short (1 s or so in cardiac-gated dMRA) and is not long enough to capture the entire dynamic course of blood flow through AVM lesions, which leads to a poor detection of draining veins that is of great importance for risk evaluation. Finally, contamination of venous blood would mislead observers to give a wrong judgment. Some of these technical limitations are currently being addressed through implementation of fast image acquisition schemes such as undersampled radial acquisition, parallel imaging and compressed sensing etc.23, which allow prolonged time coverage without sacrificing spatial resolution in 4D dMRA. Another potential improvement of TrueSTAR is to apply more efficient spin labeling schemes including the newly introduced pseudo-continuous ASL 24 as well as vessel selective labeling 25, 26 for observing the dynamic inflowing pattern of a particular artery of interest. The present study was conducted at 3 T, which should provide advantages for spin-labeling-based methods. However, a recent study reported that the performance of noncontrast 4D dMRA was superior at 1.5 T compared to 3 T, probably due to reduced susceptibility and saturation effects of SSFP at 1.5 T27. In future clinical studies, the optimal field strength and imaging parameters for noncontrast dMRA need to be evaluated.
5. Conclusion
As a newly emerging and promising technique, noncontrast unenhanced dynamic magnetic resonance angiography can be easily implemented in clinical routines due to its non-invasive feature, fast acquisition time. In this pilot study performed on 19 AVM patients, dMRA was capable in depiction of the size and location of AVM lesions, moderate in description of feeding arteries and poor in detection of draining veins. Most information it provided was in accordance with DSA. Although it can’t replace either DSA or TOF for clinical diagnosis of AVMs according to the results of this study, it can provide complementary temporal information to TOF and thus enhance our diagnostic confidence. In the future, further development should be done to address its shortcomings and challenges.
Supplementary Material
References
- 1.Hernesniemi JA, Dashti R, Juvela S, Vaart K, Niemela M, Laakso A. Natural history of brain arteriovenous malformations: a long-term follow-up study of risk of hemorrhage in 238 patients. Neurosurgery. 2008 Nov;63(5):823–9. doi: 10.1227/01.NEU.0000330401.82582.5E. discussion 9–31. [DOI] [PubMed] [Google Scholar]
- 2.Soderman M, Andersson T, Karlsson B, Wallace MC, Edner G. Management of patients with brain arteriovenous malformations. Eur J Radiol. 2003 Jun;46(3):195–205. doi: 10.1016/s0720-048x(03)00091-3. [DOI] [PubMed] [Google Scholar]
- 3.Willinsky RA, Taylor SM, TerBrugge K, Farb RI, Tomlinson G, Montanera W. Neurologic complications of cerebral angiography: prospective analysis of 2,899 procedures and review of the literature. Radiology. 2003 May;227(2):522–8. doi: 10.1148/radiol.2272012071. [DOI] [PubMed] [Google Scholar]
- 4.Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006 Sep;17(9):2359–62. doi: 10.1681/ASN.2006060601. [DOI] [PubMed] [Google Scholar]
- 5.Bi X, Weale P, Schmitt P, Zuehlsdorff S, Jerecic R. Non-contrast-enhanced four-dimensional (4D) intracranial MR angiography: a feasibility study. Magn Reson Med. 2010 Mar;63(3):835–41. doi: 10.1002/mrm.22220. [DOI] [PubMed] [Google Scholar]
- 6.Yan L, Wang S, Zhuo Y, et al. Unenhanced dynamic MR angiography: high spatial and temporal resolution by using true FISP-based spin tagging with alternating radiofrequency. Radiology. 2010 Jul;256(1):270–9. doi: 10.1148/radiol.10091543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. J Neurosurg. 1986 Oct;65(4):476–83. doi: 10.3171/jns.1986.65.4.0476. [DOI] [PubMed] [Google Scholar]
- 8.Kubalek R, Moghtaderi A, Klisch J, Berlis A, Quiske A, Schumacher M. Cerebral arteriovenous malformations: influence of angioarchitecture on bleeding risk. Acta Neurochir (Wien) 2003 Dec;145(12):1045–52. doi: 10.1007/s00701-003-0143-x. discussion 52. [DOI] [PubMed] [Google Scholar]
- 9.Lawton MT, Kim H, McCulloch CE, Mikhak B, Young WL. A supplementary grading scale for selecting patients with brain arteriovenous malformations for surgery. Neurosurgery. 2010 Apr;66(4):702–13. doi: 10.1227/01.NEU.0000367555.16733.E1. discussion 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Redekop G, TerBrugge K, Montanera W, Willinsky R. Arterial aneurysms associated with cerebral arteriovenous malformations: classification, incidence, and risk of hemorrhage. J Neurosurg. 1998 Oct;89(4):539–46. doi: 10.3171/jns.1998.89.4.0539. [DOI] [PubMed] [Google Scholar]
- 11.Kim SG, Tsekos NV. Perfusion imaging by a flow-sensitive alternating inversion recovery (FAIR) technique: application to functional brain imaging. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 1997 Mar;37(3):425–35. doi: 10.1002/mrm.1910370321. [DOI] [PubMed] [Google Scholar]
- 12.Kader A, Young WL, Pile-Spellman J, et al. The influence of hemodynamic and anatomic factors on hemorrhage from cerebral arteriovenous malformations. Neurosurgery. 1994 May;34(5):801–7. doi: 10.1227/00006123-199405000-00003. discussion 7–8. [DOI] [PubMed] [Google Scholar]
- 13.Langer DJ, Lasner TM, Hurst RW, Flamm ES, Zager EL, King JT., Jr Hypertension, small size, and deep venous drainage are associated with risk of hemorrhagic presentation of cerebral arteriovenous malformations. Neurosurgery. 1998 Mar;42(3):481–6. doi: 10.1097/00006123-199803000-00008. discussion 7–9. [DOI] [PubMed] [Google Scholar]
- 14.Spetzler RF, Hargraves RW, McCormick PW, Zabramski JM, Flom RA, Zimmerman RS. Relationship of perfusion pressure and size to risk of hemorrhage from arteriovenous malformations. J Neurosurg. 1992 Jun;76(6):918–23. doi: 10.3171/jns.1992.76.6.0918. [DOI] [PubMed] [Google Scholar]
- 15.Stapf C, Mast H, Sciacca RR, et al. Predictors of hemorrhage in patients with untreated brain arteriovenous malformation. Neurology. 2006 May 9;66(9):1350–5. doi: 10.1212/01.wnl.0000210524.68507.87. [DOI] [PubMed] [Google Scholar]
- 16.Han PP, Ponce FA, Spetzler RF. Intention-to-treat analysis of Spetzler-Martin grades IV and V arteriovenous malformations: natural history and treatment paradigm. J Neurosurg. 2003 Jan;98(1):3–7. doi: 10.3171/jns.2003.98.1.0003. [DOI] [PubMed] [Google Scholar]
- 17.Miyazaki M, Lee VS. Nonenhanced MR angiography. Radiology. 2008 Jul;248(1):20–43. doi: 10.1148/radiol.2481071497. [DOI] [PubMed] [Google Scholar]
- 18.Hadizadeh DR, von Falkenhausen M, Gieseke J, et al. Cerebral arteriovenous malformation: Spetzler-Martin classification at subsecond-temporal-resolution four-dimensional MR angiography compared with that at DSA. Radiology. 2008 Jan;246(1):205–13. doi: 10.1148/radiol.2453061684. [DOI] [PubMed] [Google Scholar]
- 19.Lim RP, Shapiro M, Wang EY, et al. 3D time-resolved MR angiography (MRA) of the carotid arteries with time-resolved imaging with stochastic trajectories: comparison with 3D contrast-enhanced Bolus-Chase MRA and 3D time-of-flight MRA. AJNR Am J Neuroradiol. 2008 Nov;29(10):1847–54. doi: 10.3174/ajnr.A1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lanzman RS, Kropil P, Schmitt P, et al. Nonenhanced ECG-gated time-resolved 4D Steady-state free precession (SSFP) MR angiography (MRA) for assessment of cerebral collateral flow: comparison with digital subtraction angiography (DSA) Eur Radiol. 2011 Jan 12; doi: 10.1007/s00330-010-2051-9. [DOI] [PubMed] [Google Scholar]
- 21.Xu J, Shi D, Chen C, et al. Noncontrast-enhanced four-dimensional MR angiography for the evaluation of cerebral arteriovenous malformation: a preliminary trial. Journal of magnetic resonance imaging : JMRI. 2011 Nov;34(5):1199–205. doi: 10.1002/jmri.22699. [DOI] [PubMed] [Google Scholar]
- 22.Farb RI, McGregor C, Kim JK, et al. Intracranial arteriovenous malformations: real-time auto-triggered elliptic centric-ordered 3D gadolinium-enhanced MR angiography--initial assessment. Radiology. 2001 Jul;220(1):244–51. doi: 10.1148/radiology.220.1.r01jn15244. [DOI] [PubMed] [Google Scholar]
- 23.Mistretta CA. Undersampled radial MR acquisition and highly constrained back projection (HYPR) reconstruction: potential medical imaging applications in the post-Nyquist era. J Magn Reson Imaging. 2009 Mar;29(3):501–16. doi: 10.1002/jmri.21683. [DOI] [PubMed] [Google Scholar]
- 24.Robson PM, Dai W, Shankaranarayanan A, Rofsky NM, Alsop DC. Time-resolved vessel-selective digital subtraction MR angiography of the cerebral vasculature with arterial spin labeling. Radiology. 2010 Nov;257(2):507–15. doi: 10.1148/radiol.10092333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hendrikse J, van der Grond J, Lu H, van Zijl PC, Golay X. Flow territory mapping of the cerebral arteries with regional perfusion MRI. Stroke; a journal of cerebral circulation. 2004 Apr;35(4):882–7. doi: 10.1161/01.STR.0000120312.26163.EC. [DOI] [PubMed] [Google Scholar]
- 26.Wong EC. Vessel-encoded arterial spin-labeling using pseudocontinuous tagging. Magn Reson Med. 2007 Dec;58(6):1086–91. doi: 10.1002/mrm.21293. [DOI] [PubMed] [Google Scholar]
- 27.Lanzman RS, Kropil P, Schmitt P, et al. Nonenhanced ECG-gated time-resolved 4D steady-state free precession (SSFP) MR angiography (MRA) of cerebral arteries: comparison at 1.5T and 3T. European journal of radiology. 2012 Apr;81(4):e531–5. doi: 10.1016/j.ejrad.2011.06.044. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


